-
PDF
- Split View
-
Views
-
Cite
Cite
Megan S Barker, Reena T Gottesman, Masood Manoochehri, Silvia Chapman, Brian S Appleby, Danielle Brushaber, Katrina L Devick, Bradford C Dickerson, Kimiko Domoto-Reilly, Julie A Fields, Leah K Forsberg, Douglas R Galasko, Nupur Ghoshal, Jill Goldman, Neill R Graff-Radford, Murray Grossman, Hilary W Heuer, Ging-Yuek Hsiung, David S Knopman, John Kornak, Irene Litvan, Ian R Mackenzie, Joseph C Masdeu, Mario F Mendez, Belen Pascual, Adam M Staffaroni, Maria Carmela Tartaglia, Bradley F Boeve, Adam L Boxer, Howard J Rosen, Katherine P Rankin, Stephanie Cosentino, Katya Rascovsky, Edward D Huey, ALLFTD Consortium , Proposed research criteria for prodromal behavioural variant frontotemporal dementia, Brain, Volume 145, Issue 3, March 2022, Pages 1079–1097, https://doi.org/10.1093/brain/awab365
Close - Share Icon Share
Abstract
At present, no research criteria exist for the diagnosis of prodromal behavioural variant frontotemporal dementia (bvFTD), though early detection is of high research importance. Thus, we sought to develop and validate a proposed set of research criteria for prodromal bvFTD, termed ‘mild behavioural and/or cognitive impairment in bvFTD’ (MBCI-FTD).
Participants included 72 participants deemed to have prodromal bvFTD; this comprised 55 carriers of a pathogenic mutation known to cause frontotemporal lobar degeneration, and 17 individuals with autopsy-confirmed frontotemporal lobar degeneration. All had mild behavioural and/or cognitive changes, as judged by an evaluating clinician.
Based on extensive clinical workup, the prodromal bvFTD group was divided into a Development Group (n = 22) and a Validation Group (n = 50). The Development Group was selected to be the subset of the prodromal bvFTD group for whom we had the strongest longitudinal evidence of conversion to bvFTD, and was used to develop the MBCI-FTD criteria. The Validation Group was the remainder of the prodromal bvFTD group and was used as a separate sample on which to validate the criteria. Familial non-carriers were included as healthy controls (n = 165). The frequencies of behavioural and neuropsychiatric features, neuropsychological deficits, and social cognitive dysfunction in the prodromal bvFTD Development Group and healthy controls were assessed.
Based on sensitivity and specificity analyses, seven core features were identified: apathy without moderate-severe dysphoria, behavioural disinhibition, irritability/agitation, reduced empathy/sympathy, repetitive behaviours (simple and/or complex), joviality/gregariousness, and appetite changes/hyperorality. Supportive features include a neuropsychological profile of impaired executive function or naming with intact orientation and visuospatial skills, reduced insight for cognitive or behavioural changes, and poor social cognition. Three core features or two core features plus one supportive feature are required for the diagnosis of possible MBCI-FTD; probable MBCI-FTD requires imaging or biomarker evidence, or a pathogenic genetic mutation.
The proposed MBCI-FTD criteria correctly classified 95% of the prodromal bvFTD Development Group, and 74% of the prodromal bvFTD Validation Group, with a false positive rate of <10% in healthy controls. Finally, the MBCI-FTD criteria were tested on a cohort of individuals with prodromal Alzheimer’s disease, and the false positive rate of diagnosis was 11–16%. Future research will need to refine the sensitivity and specificity of these criteria, and incorporate emerging biomarker evidence.
Introduction
Detecting the earliest clinical features of neurodegenerative diseases is important for appropriate clinical trial enrolment and optimal patient care. Clinical prodromes of dementia due to Alzheimer’s disease and dementia with Lewy bodies have been previously defined, with published diagnostic criteria for mild cognitive impairment (MCI) due to Alzheimer’s disease,1 and prodromal dementia with Lewy bodies.2 To date, no formal criteria have been generated for prodromal behavioural variant frontotemporal dementia (bvFTD).
Frontotemporal dementia (FTD) is the fourth most common dementia, and disproportionately affects younger individuals. The median onset is during the sixth decade,3 though onset may be as early as the third decade.4 Between 15–30% of all FTD cases follow an autosomal dominant pattern of inheritance. The majority of genetic FTD is caused by mutations in the microtubule-associated protein tau (MAPT) or progranulin (GRN) genes, or a hexanucleotide repeat expansion in chromosome 9 open reading frame 72 (C9orf72), though a number of other disease-causing mutations have been decribed.5,MAPT, GRN and C9orf72 mutations are highly penetrant.6 Carriers of autosomal dominant pathogenic mutations represent a valuable cohort for characterizing the disease prodrome because disease onset is, to some degree, predictable, allowing the earliest stages of disease to be tracked.7,8 Furthermore, neuropathology in genetic mutation carriers can be predicted. Sporadic cases of bvFTD tend to come to the attention of specialists after the prodromal phase, and require autopsy data (neuropathological evaluation) to confirm disease pathology.
Early symptoms of bvFTD
The behavioural variant is the most frequent clinical phenotype of sporadic and genetic FTD, and is characterized by changes in behaviour, social conduct, and personality.9,10 In the most recent bvFTD diagnostic criteria, Rascovsky et al.10 specify that the earliest symptoms (presenting within the first 3 years of the illness) include apathy, behavioural disinhibition, loss of sympathy or empathy, and perseverative behaviours. Likewise, in genetic mutation carriers destined to develop FTD, apathy and disinhibition are reported to be among the first behavioural symptoms.11,12 Psychotic symptoms, such as hallucinations and delusional beliefs, have also been reported in the years prior to diagnosis, particularly in C9orf72 expansion carriers.13-15
The neuropsychological profile of bvFTD tends to be dysexecutive (i.e. deficits in higher-order cognitive skills such as reasoning, planning, abstraction, word generation), with relative preservation of episodic memory and visuospatial functions.10,16 However, early changes have been documented in the domains of language, attention, memory, and social cognition, as well as executive function.7,8,11,17–19 Informant- and patient-reported memory complaints are also common in preclinical bvFTD.11,20 Cognitive changes may precede behavioural symptoms in a subset of patients who go on to develop bvFTD.19
The current study
To date, the earliest clinical features of bvFTD have been described in small cohorts, but no diagnostic criteria exist. Early detection will likely optimize treatment efficacy, and is important for early counselling and guidance, as well as the implementation of management strategies. Parallel lines of research have described behavioural symptoms (‘mild behavioural impairment’) in prodromal neurodegenerative diseases, not specifically FTD, and a checklist is available21 that operationalizes the most recent mild behavioural impairment criteria.22 However, these criteria were not developed with bvFTD as the focus.23 Thus, the aim of the current study was to develop research diagnostic criteria for prodromal bvFTD, in a cohort of early symptomatic (‘prodromal’) individuals carrying a MAPT, GRN, or C9orf72 pathogenic mutation that progressed to overt bvFTD, as compared to healthy control subjects, defined here as non-mutation carrier family members. We then aimed to validate the criteria on a separate group, which included pathogenic mutation carriers and/or individuals with pathology-confirmed frontotemporal lobar degeneration (FTLD) on autopsy, all of whom had received a clinical diagnosis indicative of a behavioural phenotype (bvFTD or ‘mild behavioural impairment’). Finally, we tested the criteria in a group of individuals with pathology-confirmed Alzheimer’s disease, who were seen during their prodromal disease stage, to establish the specificity of the criteria to FTD. In these criteria, we have opted to use the term ‘mild behavioural and/or cognitive impairment in bvFTD’ (MBCI-FTD), to acknowledge that both behavioural/neuropsychiatric symptoms and cognitive impairments might be present during the disease prodrome.
Materials and methods
Study data
We used data from the Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL; U54 NS092089) and Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS; U01 AG045390) North American consortia [now the ARTFL LEFFTDS Longitudinal Frontotemporal Lobar Degeneration consortium (ALLFTD; U19 AG063911)] (January 2020 data freeze), the National Alzheimer’s Coordinating Center (NACC; U24 AG072122), and the Examination of the Earliest Symptoms and Biomarkers of FTLD in MAPT Carriers (ESFTLD; R01 NS076837) study based at Columbia University. Participants in the ARTFL/LEFFTDS studies are evaluated yearly at one of 18 sites across North America, with a standardized clinical evaluation including a neurological exam, neuropsychological testing, informant interviews, blood draw, brain MRI scan, and optional lumbar puncture. Full study procedures, including genotyping of FTLD-associated genes, have been published elsewhere.24–32 Similarly, the Alzheimer’s Disease Research Centers (ADRCs) that contribute data to NACC evaluate participants longitudinally and collect clinical, neuropsychological, and diagnostic data using the Uniform Data Set (UDS). A subset of participants receive a neuropathological evaluation at autopsy. Full descriptions of NACC, UDS, and neuropathological evaluation are available elsewhere.33–36 The current study used data from 31 ADRCs, collected between 2005 and 2018. The ESFTLD study is a longitudinal study of families with known pathogenic MAPT mutations, with similar assessments to ARTFL/LEFFTDS; study procedures are described elsewhere.17,37,38 The ARTFL/LEFFTDS/ALLFTD study, NACC participating ADRCs, and ESFTLD study received ethical approval, and all participants or their surrogates provided informed written consent.
Participant selection
Prodromal bvFTD group
To define the prodromal features of bvFTD, we selected participants from the ARTFL/LEFFTDS, ESFTLD, and NACC datasets, with either (i) a confirmed pathogenic FTLD-associated genetic mutation as a proxy for FTLD neuropathology; or (ii) pathologically-confirmed FTLD on autopsy. All participants included in this group were assigned a behavioural clinical phenotype (bvFTD or ‘mild behavioural impairment’) at some point during their participation in the study; thus, those with pure or predominant primary progressive aphasia, amyotrophic lateral sclerosis, or parkinsonian syndromes were excluded. Participants were defined as ‘prodromal’ based on the clinician-assigned Clinical Dementia Rating (CDR®) Dementia Staging Instrument plus NACC FTLD Behaviour and Language Domains (CDR®+NACC FTLD) global score. This score is calculated after rating impairment (0 = none, 0.5 = questionable/very mild, 1 = mild, 2 = moderate, 3 = severe) across eight domains: Memory, Orientation, Judgement and Problem Solving, Community Affairs, Home and Hobbies, Personal Care,39 Behaviour, and Language.40 Domain ratings are combined using an algorithm to calculate the CDR®+NACC FTLD global score (0–3),28 which is sensitive to changes in early FTLD.27,40 The first visit at which a CDR®+NACC FTLD global score of 0.5 was assigned, indicating very mild features consistent with ‘questionable’ or prodromal dementia, was considered the ‘prodromal visit’. At this evaluation, none of the prodromal bvFTD group met Rascovsky et al.10 criteria for bvFTD based on clinician-recorded symptoms; this was to ensure that all participants could be validly deemed ‘prodromal’.
From the ARTFL/LEFFTDS dataset, n = 38 prodromal pathogenic mutation carriers were included. In addition, three MAPT mutation carriers from the Columbia University ESFTLD study and 14 genetic mutation carriers from NACC were included, rendering a final prodromal genetic mutation carrier sample of n = 55 (MAPT = 20, GRN = 7, C9orf72 = 17, C9orf72+GRN = 1, unspecified mutation = 10). From NACC, 17 participants with pathology confirmed FTLD, who had ultimately received a clinical diagnosis of bvFTD and who were seen by a clinician during their prodromal disease stage, were also identified for inclusion. These participants were presumed to be sporadic, as no known genetic mutation was recorded. In total, the prodromal bvFTD sample was n = 72 (see Supplementary Fig. 1 for full details). Staff evaluating the participants were blinded to genetic status whenever possible. This was not always possible if the participant was referred from a clinical setting in which they had been evaluated by a member of the research team, or if the research participant self-disclosed. In these cases, effort was taken to preserve the blinding of other staff members.
Healthy control group
Healthy control participants were defined as familial non-carriers (participants with a known FTLD-associated genetic mutation in the family but who were not carriers themselves). Healthy controls were drawn from the ARTFL/LEFFTDS dataset (n = 165), because this study had the most comprehensive assessments available. Healthy controls were older than 30 years, to match the prodromal bvFTD group in age (Supplementary Fig. 1).
Alzheimer’s Test Group
A cohort of n = 301 individuals with pathology-confirmed Alzheimer’s disease were drawn from the NACC dataset. All participants were evaluated by a clinician during their prodromal disease phase, at which point they received a global CDR® of 0.5 and a diagnosis of MCI due to presumed Alzheimer’s disease (47% single-domain amnestic MCI, 47% multiple-domain amnestic MCI, 6% non-amnestic MCI). On autopsy, all individuals in this group had intermediate or high Alzheimer disease neuropathological changes based on NIA-AA criteria,41,42 and 38% had evidence of Lewy body pathology [5% brainstem-predominant, 22% limbic (transitional) or amygdala predominant, 11% neocortical (diffuse)].
Measures
Behavioural and neuropsychiatric features
Participants underwent detailed clinical evaluation, and additional measures were obtained via interview with informants. Assessments included the NACC UDS and FTLD module.43 All clinicians, study personnel, patients, and informants were blind to study aims at the time of the evaluations.
Standard clinician forms
The clinician forms included the following features to be evaluated: apathy/withdrawal/inertia, depressed mood, psychosis (visual/auditory hallucinations, abnormal/false/delusional beliefs), behavioural disinhibition, irritability, agitation, personality change, REM sleep behaviour disorder, anxiety, hyperorality, loss of sympathy or empathy, and ritualistic or compulsive behaviours. All features were marked as present or absent by the clinician at the evaluation.
Behavioural/neuropsychiatric questionnaires
The Neuropsychiatric Inventory Questionnaire (NPI-Q)44 was completed with the informant and includes the following items: apathy/indifference, depression/dysphoria, delusions, hallucinations, disinhibition, irritability/lability, agitation/aggression, anxiety, night time behaviours, elation/euphoria, motor disturbance, and appetite/eating changes. All features were marked as present (mild, moderate or severe) or absent. The 15-item version of the Geriatric Depression Scale (GDS),45 a measure of depressive symptoms, especially dysphoria, was administered to participants. The Interpersonal Reactivity Index46 (Empathic Concern and Perspective Taking subscales) was included as a measure of informant-reported empathy.
Clinical notes
A goal of the study was to capture features that are beyond the scope of the current bvFTD diagnostic criteria. In the ARTFL/LEFFTDS dataset, the richest source of this information was free text clinical notes [e.g. Clinical Global Impressions of Change form (CGI-C)]. The CGI-C is a structured interview such that the patient and the informant answer the same questions regarding behavioural and cognitive changes.47 Clinical notes were available for 26 participants from the prodromal bvFTD group. Each study site that contributed participants to the prodromal bvFTD group provided at least one CGI-C clinical note. Based on the most frequently endorsed features in these notes, as well as the bvFTD literature, the following features were extracted: apathy, disinhibition, irritability, loss of empathy/sympathy, repetitive behaviours [simple (e.g. pacing) and complex (e.g. rituals)], hyperorality, depression, sleep problems, joviality/gregariousness, delusions, hallucinations, emotional blunting, and reduced insight. Features were considered ‘present’ if they were reported by the informant, subject, or clinician. Reduced insight was marked as ‘present’ if the informant reported behavioural or cognitive problems that were not endorsed by the patient. Retrospective coding of the free text clinical notes was completed by two independent raters (M.S.B and R.T.G), with an interrater reliability of 0.89; disagreements were adjudicated by a third rater (M.M.). Raters were not blind to study objectives, but were instructed to be comprehensive in identifying features noted in the free text, with little a priori guidance as to the specific features themselves. The goal was to identify features that may be missed on the standardized clinician and informant forms, which only capture a relatively narrow range of symptoms.
Neuropsychological assessment
All participants completed a standardized neuropsychological assessment (NACC UDS v. 1.1, 1.2, 2.0 or 3.0 batteries).33–35,43 The Craft Story or Logical Memory immediate and delayed recall scores measured verbal episodic memory. The Benson Complex Figure copy and delayed recall assessed visuospatial skills and non-verbal episodic memory, respectively. The Montreal Cognitive Assessment (MoCA)48 cube drawing or Mini-Mental State Examination (MMSE) pentagon drawing tests49 assessed visuospatial skills when the Benson Figure copy was not available. The Multilingual Naming Test (MINT) or Boston Naming Test (BNT, 30-item odd) measured visual confrontation naming. The Digit or Number Span Forward and Backward tests gauged auditory attention and working memory, respectively. Verbal initiation and generation were measured with verbal fluency tasks, with both category (animals) and letter (F, L) cues. Trail Making A measured psychomotor speed, and Trail Making B assessed set-shifting, a facet of executive function. The MoCA or MMSE provided a brief global assessment of cognition.
Social cognition assessment
Questionnaires from the NACC FTLD module assessing social cognition were administered to all ARTFL/LEFFTDS and Columbia ESFTLD study participants. The Social Norms Questionnaire (SNQ), which gauges awareness of social expectations,50 was completed by the participant. Two types of errors can be made on the SNQ: breaking a social norm (‘break score’, e.g. saying it is okay to eat pasta with your fingers) and over-adhering to social norms (‘over-adhere score’, e.g. saying it is not okay to eat ribs with your fingers). The SNQ is based on mainstream North American culture. The Revised Self-Monitoring Scale (RSMS), a questionnaire that measures socioemotional sensitivity,51 was completed by the informant.
Procedure
We aimed to first develop a set of criteria, and then validate and test the proposed criteria. Thus, the study was broadly separated into a development phase and a validation and testing phase. Subsets of the prodromal bvFTD and healthy control groups were included in both the development and validation phases, while the Alzheimer’s cohort was used only in the testing phase.
Prodromal bvFTD Development Group and Validation Group
In order to develop and validate the MBCI-FTD criteria, we divided the prodromal bvFTD cohort (n = 72) into a Development Group (n = 22) and a Validation Group (n = 50) (Fig. 1). The purpose of creating these groups was to define a cohort to use to develop the criteria (Development Group) in which we had the highest confidence that the features observed and reported were part of a bvFTD prodrome, and a separate more heterogeneous cohort on which to validate the criteria (Validation Group). All participants included in the Development Group showed clear longitudinal disease progression towards bvFTD (clinical details are provided in Supplementary Table 1). In brief, the Development Group included participants with all of the following: (i) at least one follow-up visit subsequent to their prodromal visit; (ii) evidence of disease progression following their prodromal visit, as measured by an increase in the CDR®+NACC FTLD Sum of Boxes score; (iii) an increase over time in behavioural symptoms consistent with bvFTD; and (iv) a minimum of three Rascovsky et al.10 BvFTD diagnostic criteria features present at their most recent study visit, two of which had to be predominant features of the clinical presentation. Participants from NACC were not included in the Development Group as the available measures were more limited (see Supplementary Table 2 for the specific assessment measures available in each study, and for the n’s drawn from each study). The final Development Group included n = 22 prodromal bvFTD participants from the ARTFL/LEFFTDS and Columbia ESFTLD studies (MAPT = 12, GRN = 2, C9orf72 = 7, C9orf72+GRN = 1; Fig. 1).
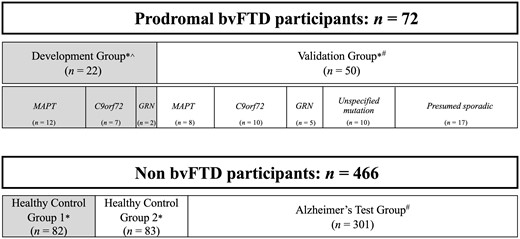
Schematic depicting data used in the development and validation/testing phases of the study. Grey shaded boxes represent the participants included in the development phase, unshaded boxes represent the participants included in the validation and testing phase. The prodromal bvFTD participants were assigned to the Development Group based on longitudinal evidence of disease progression and strong evidence of phenoconversion to bvFTD (see ‘Materials and methods’ section). Healthy controls were randomly assigned to Healthy Control Group 1 and Healthy Control Group 2. *ARTFL/LEFFTDS study data. ^Columbia ESFTLD study data. #NACC data. The prodromal bvFTD Development Group included one additional participant not depicted in the subgroups of the figure, who carries both a GRN mutation and a C9orf72 repeat expansion.
The Validation Group included: (i) the remainder of the prodromal bvFTD group from ARTFL/LEFFTDS who (a) only had a single study visit; or (b) had multiple study visits, but the evaluation at follow-up visits did not meet behavioural criteria for the Development Group (i.e. clinician indicated a bvFTD phenotype but explicit documentation of the Rascovsky et al.10 diagnostic criteria was not available); or (ii) participants drawn from the NACC dataset. The inclusion of the Validation Group as an independent (i.e. no overlapping subjects) validation sample was based on the assumption that the vast majority would go on to meet full Rascovsky et al.10 diagnostic criteria for bvFTD at some point during their disease course, but with less certainty than the Development Group. The final Validation Group included n = 50 prodromal bvFTD participants from ARTFL/LEFFTDS and NACC (MAPT = 8, GRN = 5, C9orf72 = 10, unspecified mutation = 10, presumed sporadic = 17; Fig. 1).
Development phase
Half of the healthy control group was randomly selected as a comparison for the prodromal bvFTD Development Group, to be used in developing the criteria (Healthy Control Group 1; n = 82) (Fig. 1).
From the clinician forms, informant forms, and clinical notes, a list of potential behavioural or neuropsychiatric features was created: apathy/withdrawal/indifference, disinhibition, irritability/lability, agitation, reduced empathy or sympathy, depression, anxiety, psychosis (delusions or hallucinations), repetitive behaviours, sleep disturbances, elation/euphoria, appetite changes/hyperorality, joviality/gregariousness, reduced insight, and emotional blunting. All features were either ‘present’ or ‘absent’ per clinician or informant rating. In the case of the Interpersonal Reactivity Index, reduced empathy was considered ‘present’ if the participant was rated ≥ 1.5 standard deviations (SD) below age- and sex-specific means (see Supplementary Table 3 for cut-off scores). In constructing this list, the following a priori decisions were made: (i) apathy was only considered present if there was no evidence of moderate-severe dysphoria as reported on the GDS (>6/15), to allow the MBCI-FTD criteria to distinguish between major depressive disorder and bvFTD; (ii) informant-reported irritability was included in analyses if it reached a moderate level, to maximize specificity against neurologically healthy controls in whom mild irritability is common (this decision was supported post hoc by the clinical notes in which examples of moderate-severe irritability and labile mood were frequent); and (iii) personality change was excluded from current analyses as its definition was determined to be too broad to be useful in the context of criteria (decided by clinical consensus).
Impairments on neuropsychological tests were coded as ‘present’ or ‘absent’ based on non-linear age-, sex- and education-adjusted z-scores,26 and impairment was defined as z ≤ −1.5. With regard to the social cognition assessment, the questionnaires are designed to have substantial variability in performance below the normal range; thus, Youden’s J index was calculated to identify optimal cut-off scores that would discriminate between the prodromal bvFTD and control groups. Impairment was defined as performance below the Youden cut-off (Supplementary Fig. 2).
Validation and testing phase
Once a set of criteria had been developed, we aimed to establish their utility, by testing whether they were able to correctly classify a separate cohort of prodromal bvFTD participants (Validation Group), and healthy controls (Healthy Control Group 2; n = 83), as well as a cohort of individuals with prodromal Alzheimer’s disease (Alzheimer’s Test Group; n = 301). The Validation Group of prodromal bvFTD participants allowed us to determine whether the criteria were generalizable to a more heterogenous prodromal bvFTD cohort, including presumably sporadic cases. Testing the criteria in the healthy controls and prodromal Alzheimer’s disease allowed us to determine whether the criteria would correctly classify non-FTD individuals.
Statistical analyses
All statistical analyses were performed using JASP version 0.11.1.0 or custom scripts in Python version 3.6. Analyses conducted to establish the criteria used data from the prodromal bvFTD Development Group and Healthy Control Group 1 only. Analyses conducted to validate and test the criteria used data from the prodromal bvFTD Validation Group, Healthy Control Group 2, and the Alzheimer’s Test Group only.
To assess whether the prodromal bvFTD Development Group was demographically different from Healthy Control Group 1 (used in the development phase of the criteria) and from the prodromal bvFTD Validation Group, we conducted two-tailed independent groups t-tests to compare age, education, and disease severity (as measured by the CDR®+NACC FTLD Sum of Boxes, where applicable), and two-proportions z-tests to compare sex and handedness (α = 0.05).
All candidate features, including behavioural/neuropsychiatric features, neuropsychological impairments, and social cognition impairments, were dichotomous (present versus absent). We calculated sensitivity and specificity for each feature using 2 × 2 matrices. Sensitivity is the proportion of the prodromal bvFTD group who had the feature present [sensitivity = true positives / (true positives + false negatives)]. Specificity is the proportion of healthy controls who did not display the feature [specificity = true negatives / (true negatives + false positives)]. Confidence intervals (95% CI) are reported.
Finally, sensitivity and specificity were calculated for the criteria as a whole, first on the Development Group and Healthy Control Group 1, and then on the Validation Group, Healthy Control Group 2, and the Alzheimer’s Test Group.
Data availability
ARTFL/LEFFTDS data are available upon request from the ALLFTD Executive Committee at https://www.allftd.org/. Columbia ESFTLD study data are available upon request from the corresponding author (E.D.H). NACC data are available upon request at https://naccdata.org/. Sensitive genetic information prevents public archiving of the ARTFL/LEFFTDS and ESFTLD datasets.
Results
Demographic information
All prodromal bvFTD participants and healthy controls were 31–80 years old; group means spanned 49–56 years (Table 1). The Alzheimer’s Test Group was older on average (mean = 80.5 years), as would be expected. All groups were highly educated on average (15–16 years of education). Over 97% of prodromal bvFTD participants and healthy control subjects, and 92% of the Alzheimer’s Test Group, self-identified as white/Caucasian.
Demographic and clinical characteristics of the prodromal bvFTD group (including Development and Validation Groups), healthy controls (Healthy Control Groups 1 and 2), and the Alzheimer’s Test Group
| . | Total prodromal bvFTD group (n = 72) . | Development Group (n = 22) . | Validation Group (n = 50) . | Healthy Control Group 1 (n = 82) . | Healthy Control Group 2 (n = 83) . | Alzheimer’s Test Group (n = 301) . |
|---|---|---|---|---|---|---|
| Genetic status, n (%) | N/A | N/A | N/A | |||
| MAPT | 20 (28%) | 12 (55%) | 8 (16%) | |||
| GRN | 7 (10%) | 2 (9%) | 5 (10%) | |||
| C9orf72 | 17 (24%) | 7 (32%) | 10 (20%) | |||
| C9orf72+GRN | 1 (1%) | 1 (5%) | 0 (0%) | |||
| Unspecified mutation | 10 (14%) | 0 (0%) | 10 (20%) | |||
| Presumed sporadic | 17 (24%) | 0 (0%) | 17 (34%) | |||
| Age | ||||||
| Mean (SD) | 56.53 (11.31) | 56.18 (11.17) | 56.68 (11.48) | 49.41 (12.09) | 51.59 (11.08) | 80.49 (9.87) |
| Range | 32–80 | 36–80 | 32–76 | 31–80 | 32–76 | 35–100 |
| Education | ||||||
| Mean (SD) | 15.11 (2.30) | 15.23 (2.41) | 15.06 (2.27) | 15.07 (2.55) | 16.02 (2.40) | 15.94 (3.06)a |
| Range | 12–21 | 12–20 | 12–21 | 11–20 | 12–20 | 2–25 |
| Sex (M:F) | 44:28 | 10:12 | 34:16 | 30:52 | 38:45 | 152:149 |
| Handedness (R:L:A) | 62:9:1 | 20:2:0 | 42:7:1 | 69:9:3b | 71:8:4 | 268:24:8b |
| CDR®+NACC FTLD Sum of Boxes | ||||||
| Mean (SD) | 2.00 (0.94) | 1.89 (0.87) | 2.05 (0.98) | 0.16 (0.52) | 0.07 (0.37) | 1.43 (0.93)c |
| Range | 0.5–3.5 | 0.5–3.5 | 0.5–3.5 | 0.0–3.0 | 0.0–3.0 | 0.5–4.5c |
| . | Total prodromal bvFTD group (n = 72) . | Development Group (n = 22) . | Validation Group (n = 50) . | Healthy Control Group 1 (n = 82) . | Healthy Control Group 2 (n = 83) . | Alzheimer’s Test Group (n = 301) . |
|---|---|---|---|---|---|---|
| Genetic status, n (%) | N/A | N/A | N/A | |||
| MAPT | 20 (28%) | 12 (55%) | 8 (16%) | |||
| GRN | 7 (10%) | 2 (9%) | 5 (10%) | |||
| C9orf72 | 17 (24%) | 7 (32%) | 10 (20%) | |||
| C9orf72+GRN | 1 (1%) | 1 (5%) | 0 (0%) | |||
| Unspecified mutation | 10 (14%) | 0 (0%) | 10 (20%) | |||
| Presumed sporadic | 17 (24%) | 0 (0%) | 17 (34%) | |||
| Age | ||||||
| Mean (SD) | 56.53 (11.31) | 56.18 (11.17) | 56.68 (11.48) | 49.41 (12.09) | 51.59 (11.08) | 80.49 (9.87) |
| Range | 32–80 | 36–80 | 32–76 | 31–80 | 32–76 | 35–100 |
| Education | ||||||
| Mean (SD) | 15.11 (2.30) | 15.23 (2.41) | 15.06 (2.27) | 15.07 (2.55) | 16.02 (2.40) | 15.94 (3.06)a |
| Range | 12–21 | 12–20 | 12–21 | 11–20 | 12–20 | 2–25 |
| Sex (M:F) | 44:28 | 10:12 | 34:16 | 30:52 | 38:45 | 152:149 |
| Handedness (R:L:A) | 62:9:1 | 20:2:0 | 42:7:1 | 69:9:3b | 71:8:4 | 268:24:8b |
| CDR®+NACC FTLD Sum of Boxes | ||||||
| Mean (SD) | 2.00 (0.94) | 1.89 (0.87) | 2.05 (0.98) | 0.16 (0.52) | 0.07 (0.37) | 1.43 (0.93)c |
| Range | 0.5–3.5 | 0.5–3.5 | 0.5–3.5 | 0.0–3.0 | 0.0–3.0 | 0.5–4.5c |
Age and education are reported in years. Genetic status refers to FTLD-associated genetic mutations. CDR®+NACC FTLD Sum of Boxes score calculated by summing the six CDR® domain rating scores, plus two supplemental domains of behaviour and language. M = male; F = female, self-reported. R = right-handed; L = left-handed; A = ambidextrous.
aEducation values missing for three participants in the Alzheimer’s Test Group.
bTraditional CDR® sum of boxes score, calculated by summing only the six CDR® domain rating scores, appropriate for Alzheimer’s disease.
cHandedness was unknown for one healthy control and one participant in the Alzheimer’s Test Group.
Demographic and clinical characteristics of the prodromal bvFTD group (including Development and Validation Groups), healthy controls (Healthy Control Groups 1 and 2), and the Alzheimer’s Test Group
| . | Total prodromal bvFTD group (n = 72) . | Development Group (n = 22) . | Validation Group (n = 50) . | Healthy Control Group 1 (n = 82) . | Healthy Control Group 2 (n = 83) . | Alzheimer’s Test Group (n = 301) . |
|---|---|---|---|---|---|---|
| Genetic status, n (%) | N/A | N/A | N/A | |||
| MAPT | 20 (28%) | 12 (55%) | 8 (16%) | |||
| GRN | 7 (10%) | 2 (9%) | 5 (10%) | |||
| C9orf72 | 17 (24%) | 7 (32%) | 10 (20%) | |||
| C9orf72+GRN | 1 (1%) | 1 (5%) | 0 (0%) | |||
| Unspecified mutation | 10 (14%) | 0 (0%) | 10 (20%) | |||
| Presumed sporadic | 17 (24%) | 0 (0%) | 17 (34%) | |||
| Age | ||||||
| Mean (SD) | 56.53 (11.31) | 56.18 (11.17) | 56.68 (11.48) | 49.41 (12.09) | 51.59 (11.08) | 80.49 (9.87) |
| Range | 32–80 | 36–80 | 32–76 | 31–80 | 32–76 | 35–100 |
| Education | ||||||
| Mean (SD) | 15.11 (2.30) | 15.23 (2.41) | 15.06 (2.27) | 15.07 (2.55) | 16.02 (2.40) | 15.94 (3.06)a |
| Range | 12–21 | 12–20 | 12–21 | 11–20 | 12–20 | 2–25 |
| Sex (M:F) | 44:28 | 10:12 | 34:16 | 30:52 | 38:45 | 152:149 |
| Handedness (R:L:A) | 62:9:1 | 20:2:0 | 42:7:1 | 69:9:3b | 71:8:4 | 268:24:8b |
| CDR®+NACC FTLD Sum of Boxes | ||||||
| Mean (SD) | 2.00 (0.94) | 1.89 (0.87) | 2.05 (0.98) | 0.16 (0.52) | 0.07 (0.37) | 1.43 (0.93)c |
| Range | 0.5–3.5 | 0.5–3.5 | 0.5–3.5 | 0.0–3.0 | 0.0–3.0 | 0.5–4.5c |
| . | Total prodromal bvFTD group (n = 72) . | Development Group (n = 22) . | Validation Group (n = 50) . | Healthy Control Group 1 (n = 82) . | Healthy Control Group 2 (n = 83) . | Alzheimer’s Test Group (n = 301) . |
|---|---|---|---|---|---|---|
| Genetic status, n (%) | N/A | N/A | N/A | |||
| MAPT | 20 (28%) | 12 (55%) | 8 (16%) | |||
| GRN | 7 (10%) | 2 (9%) | 5 (10%) | |||
| C9orf72 | 17 (24%) | 7 (32%) | 10 (20%) | |||
| C9orf72+GRN | 1 (1%) | 1 (5%) | 0 (0%) | |||
| Unspecified mutation | 10 (14%) | 0 (0%) | 10 (20%) | |||
| Presumed sporadic | 17 (24%) | 0 (0%) | 17 (34%) | |||
| Age | ||||||
| Mean (SD) | 56.53 (11.31) | 56.18 (11.17) | 56.68 (11.48) | 49.41 (12.09) | 51.59 (11.08) | 80.49 (9.87) |
| Range | 32–80 | 36–80 | 32–76 | 31–80 | 32–76 | 35–100 |
| Education | ||||||
| Mean (SD) | 15.11 (2.30) | 15.23 (2.41) | 15.06 (2.27) | 15.07 (2.55) | 16.02 (2.40) | 15.94 (3.06)a |
| Range | 12–21 | 12–20 | 12–21 | 11–20 | 12–20 | 2–25 |
| Sex (M:F) | 44:28 | 10:12 | 34:16 | 30:52 | 38:45 | 152:149 |
| Handedness (R:L:A) | 62:9:1 | 20:2:0 | 42:7:1 | 69:9:3b | 71:8:4 | 268:24:8b |
| CDR®+NACC FTLD Sum of Boxes | ||||||
| Mean (SD) | 2.00 (0.94) | 1.89 (0.87) | 2.05 (0.98) | 0.16 (0.52) | 0.07 (0.37) | 1.43 (0.93)c |
| Range | 0.5–3.5 | 0.5–3.5 | 0.5–3.5 | 0.0–3.0 | 0.0–3.0 | 0.5–4.5c |
Age and education are reported in years. Genetic status refers to FTLD-associated genetic mutations. CDR®+NACC FTLD Sum of Boxes score calculated by summing the six CDR® domain rating scores, plus two supplemental domains of behaviour and language. M = male; F = female, self-reported. R = right-handed; L = left-handed; A = ambidextrous.
aEducation values missing for three participants in the Alzheimer’s Test Group.
bTraditional CDR® sum of boxes score, calculated by summing only the six CDR® domain rating scores, appropriate for Alzheimer’s disease.
cHandedness was unknown for one healthy control and one participant in the Alzheimer’s Test Group.
Development versus Validation Group
Statistical comparisons were conducted to assess demographic differences between the prodromal bvFTD Development and Validation Groups (Table 2). The groups appeared similarly distributed with respect to age, education, and disease severity (per CDR®+NACC FTLD Sum of Boxes). The balance of males to females appeared consistent across groups (0.45 versus 0.68), as did handedness (0.91 versus 0.85). There was a statistically significant higher proportion of MAPT mutation carriers in the Development Group than the Validation Group (0.55 versus 0.16); this is addressed in the ‘Validation and testing phase’ results section.
Statistical comparisons of demographic and clinical characteristics in the prodromal bvFTD Development and Validation Groups, and Healthy Control Group 1
| . | Mean difference . | 95% CI Lower, upper . | P-value . |
|---|---|---|---|
| Development Group versus Validation Group | |||
| Age | −0.50 | −6.31, 5.31 | 0.865 |
| Education | 0.17 | −1.01, 1.35 | 0.778 |
| Sex | −0.23 | −0.50, 0.05 | 0.122 |
| Handedness | 0.07 | −0.12, 0.26 | 0.681 |
| CDR®+NACC FTLD Sum of Boxes | −0.16 | −0.65, 0.32 | 0.501 |
| Proportion of MAPT mutation carriers | 0.28 | 0.12, 0.65 | 0.002 |
| Development Group versus Healthy Control Group 1 | |||
| Age | 6.77 | 1.10, 12.44 | 0.020 |
| Education | 0.15 | −1.05, 1.34 | 0.800 |
| Sex | 0.09 | −0.17, 0.35 | 0.608 |
| Handedness | 0.07 | −0.10, 0.24 | 0.645 |
| . | Mean difference . | 95% CI Lower, upper . | P-value . |
|---|---|---|---|
| Development Group versus Validation Group | |||
| Age | −0.50 | −6.31, 5.31 | 0.865 |
| Education | 0.17 | −1.01, 1.35 | 0.778 |
| Sex | −0.23 | −0.50, 0.05 | 0.122 |
| Handedness | 0.07 | −0.12, 0.26 | 0.681 |
| CDR®+NACC FTLD Sum of Boxes | −0.16 | −0.65, 0.32 | 0.501 |
| Proportion of MAPT mutation carriers | 0.28 | 0.12, 0.65 | 0.002 |
| Development Group versus Healthy Control Group 1 | |||
| Age | 6.77 | 1.10, 12.44 | 0.020 |
| Education | 0.15 | −1.05, 1.34 | 0.800 |
| Sex | 0.09 | −0.17, 0.35 | 0.608 |
| Handedness | 0.07 | −0.10, 0.24 | 0.645 |
Italic text denotes statistically significantly difference between groups at α = 0.05. Development Group comprised n = 22 prodromal bvFTD participants, Validation Group comprised n = 50 prodromal bvFTD participants, Healthy Control Group 1 comprised n = 82 familial non-carrier controls.
Statistical comparisons of demographic and clinical characteristics in the prodromal bvFTD Development and Validation Groups, and Healthy Control Group 1
| . | Mean difference . | 95% CI Lower, upper . | P-value . |
|---|---|---|---|
| Development Group versus Validation Group | |||
| Age | −0.50 | −6.31, 5.31 | 0.865 |
| Education | 0.17 | −1.01, 1.35 | 0.778 |
| Sex | −0.23 | −0.50, 0.05 | 0.122 |
| Handedness | 0.07 | −0.12, 0.26 | 0.681 |
| CDR®+NACC FTLD Sum of Boxes | −0.16 | −0.65, 0.32 | 0.501 |
| Proportion of MAPT mutation carriers | 0.28 | 0.12, 0.65 | 0.002 |
| Development Group versus Healthy Control Group 1 | |||
| Age | 6.77 | 1.10, 12.44 | 0.020 |
| Education | 0.15 | −1.05, 1.34 | 0.800 |
| Sex | 0.09 | −0.17, 0.35 | 0.608 |
| Handedness | 0.07 | −0.10, 0.24 | 0.645 |
| . | Mean difference . | 95% CI Lower, upper . | P-value . |
|---|---|---|---|
| Development Group versus Validation Group | |||
| Age | −0.50 | −6.31, 5.31 | 0.865 |
| Education | 0.17 | −1.01, 1.35 | 0.778 |
| Sex | −0.23 | −0.50, 0.05 | 0.122 |
| Handedness | 0.07 | −0.12, 0.26 | 0.681 |
| CDR®+NACC FTLD Sum of Boxes | −0.16 | −0.65, 0.32 | 0.501 |
| Proportion of MAPT mutation carriers | 0.28 | 0.12, 0.65 | 0.002 |
| Development Group versus Healthy Control Group 1 | |||
| Age | 6.77 | 1.10, 12.44 | 0.020 |
| Education | 0.15 | −1.05, 1.34 | 0.800 |
| Sex | 0.09 | −0.17, 0.35 | 0.608 |
| Handedness | 0.07 | −0.10, 0.24 | 0.645 |
Italic text denotes statistically significantly difference between groups at α = 0.05. Development Group comprised n = 22 prodromal bvFTD participants, Validation Group comprised n = 50 prodromal bvFTD participants, Healthy Control Group 1 comprised n = 82 familial non-carrier controls.
Development Group versus Healthy Control Group 1
As the two groups involved in the development of the criteria, statistical comparisons were conducted to test for demographic differences between the Development Group and Healthy Control Group 1 (Table 2). The groups appeared similarly distributed in education. The Development Group was estimated to be slightly older, P = 0.020, but this difference of ∼7 years (56 versus 49 years) was not considered clinically meaningful. The sex balance was reasonably consistent across groups (0.45 versus 0.36), as was handedness (0.91 versus 0.84).
Development phase
Behavioural and neuropsychiatric features
Table 3 presents the frequencies, as well as sensitivity and specificity, of the behavioural and neuropsychiatric features in the prodromal bvFTD Development Group, reported on the clinician and informant forms (n = 22), and frequencies of the features extracted from the clinical notes [available for 68% (n = 15) of the Development Group]. The most frequent clinician-rated features were apathy (59%), disinhibition (55%), irritability (41%), and reduced empathy/sympathy (50%). Likewise, apathy (64%), disinhibition (55%), irritability (64%), and reduced empathy/sympathy (50%), were the most frequent informant-endorsed features. Agitation was endorsed similarly highly by informants (55%), but in only 5% of cases by clinicians. This may be due to differences in how the question is worded; the clinician form defines agitation as ‘trouble sitting still’ and/or ‘shouting/kicking/hitting’, while the informant form asks about being ‘hard to handle’ or ‘resistive to help’. In addition, agitation might present differently in home versus clinical settings, clinicians and caregivers may differently classify a given behaviour (e.g. if a patient becomes agitated if stopped from performing a repetitive behaviour), or clinicians may have a higher threshold of what constitutes agitation. In the clinical notes, apathy and disinhibition were endorsed in two-thirds of the Development Group, and irritability in one-third. Overall, when combining clinician and informant reports, apathy, disinhibition, irritability, and reduced empathy/sympathy had good sensitivity and specificity, with each reported in ≥70% of the prodromal bvFTD Development Group and ≤13% of healthy control subjects (Table 3).
Behavioural and neuropsychiatric features of the prodromal bvFTD Development Group and Healthy Control Group 1
| . | Clinician indicated n = 22 . | Informant report n = 22 . | Clinical notes n = 15 . | Total number in Development Group with feature n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . |
|---|---|---|---|---|---|---|
| Apathy/withdrawal/indifference without dysphoria | 13 (59%) | 14 (64%) | 10 (67%) | 18 (82%) | 0.82 [0.60, 0.95] | 0.94 [0.86, 0.98] |
| Disinhibition | 12 (55%) | 12 (55%) | 10 (67%) | 18 (82%) | 0.82 [0.60, 0.95] | 0.91 [0.83, 0.96] |
| Irritability/lability | 9 (41%) | 14 (64%) | 5 (33%) | 16 (73%) | 0.73 [0.50, 0.89] | 0.90 [0.82, 0.96] |
| Agitation | 1 (5%) | 12 (55%) | N/A | 13 (59%) | 0.59 [0.36, 0.79] | 0.93 [0.85, 0.97] |
| Reduced empathy or sympathy | 11 (50%) | 11 (50%) | 7 (47%) | 16 (73%) | 0.73 [0.50, 0.89] | 0.88 [0.79, 0.94] |
| Depression | 5 (23%) | 9 (41%) | 0 (0%) | 10 (45%) | 0.45 [0.24, 0.68] | 0.69 [0.58, 0.79] |
| Anxiety | 2 (9%) | 10 (45%) | 1 (7%) | 10 (45%) | 0.45 [0.24, 0.68] | 0.74 [0.64, 0.83] |
| Psychosis (delusions + hallucinations) | 3 (14%) | 2 (9%) | 3 (20%) | 3 (14%) | 0.14 [0.03, 0.35] | 0.99 [0.93, 1.00] |
| Repetitive behaviours (simple + complex) | 4 (18%) | 4 (18%) | 3 (20%) | 8 (36%) | 0.36 [0.17, 0.59] | 0.95 [0.88. 0.99] |
| REM sleep disorder/night time behaviours | 0 (0%) | 8 (36%) | 1 (6%) | 8 (36%) | 0.36 [0.17, 0.59] | 0.72 [0.61, 0.81] |
| Elation/euphoria | N/A | 5 (23%) | N/A | 5 (23%) | 0.23 [0.08, 0.45] | 0.98 [0.91, 1.00] |
| Appetite changes/hyperorality | 1 (5%) | 8 (36%) | 2 (13%) | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] |
| Joviality/gregariousness | N/A | N/A | 7 (47%) | 7/15a (47%) | 0.47 [0.21, 0.73] | N/A |
| Reduced insight | N/A | N/A | 9 (60%) | 9/15a (60%) | 0.60 [0.32, 0.84] | N/A |
| Emotional blunting | N/A | N/A | 1 (7%) | 1/15a (7%) | 0.07 [0.00, 0.32] | N/A |
| . | Clinician indicated n = 22 . | Informant report n = 22 . | Clinical notes n = 15 . | Total number in Development Group with feature n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . |
|---|---|---|---|---|---|---|
| Apathy/withdrawal/indifference without dysphoria | 13 (59%) | 14 (64%) | 10 (67%) | 18 (82%) | 0.82 [0.60, 0.95] | 0.94 [0.86, 0.98] |
| Disinhibition | 12 (55%) | 12 (55%) | 10 (67%) | 18 (82%) | 0.82 [0.60, 0.95] | 0.91 [0.83, 0.96] |
| Irritability/lability | 9 (41%) | 14 (64%) | 5 (33%) | 16 (73%) | 0.73 [0.50, 0.89] | 0.90 [0.82, 0.96] |
| Agitation | 1 (5%) | 12 (55%) | N/A | 13 (59%) | 0.59 [0.36, 0.79] | 0.93 [0.85, 0.97] |
| Reduced empathy or sympathy | 11 (50%) | 11 (50%) | 7 (47%) | 16 (73%) | 0.73 [0.50, 0.89] | 0.88 [0.79, 0.94] |
| Depression | 5 (23%) | 9 (41%) | 0 (0%) | 10 (45%) | 0.45 [0.24, 0.68] | 0.69 [0.58, 0.79] |
| Anxiety | 2 (9%) | 10 (45%) | 1 (7%) | 10 (45%) | 0.45 [0.24, 0.68] | 0.74 [0.64, 0.83] |
| Psychosis (delusions + hallucinations) | 3 (14%) | 2 (9%) | 3 (20%) | 3 (14%) | 0.14 [0.03, 0.35] | 0.99 [0.93, 1.00] |
| Repetitive behaviours (simple + complex) | 4 (18%) | 4 (18%) | 3 (20%) | 8 (36%) | 0.36 [0.17, 0.59] | 0.95 [0.88. 0.99] |
| REM sleep disorder/night time behaviours | 0 (0%) | 8 (36%) | 1 (6%) | 8 (36%) | 0.36 [0.17, 0.59] | 0.72 [0.61, 0.81] |
| Elation/euphoria | N/A | 5 (23%) | N/A | 5 (23%) | 0.23 [0.08, 0.45] | 0.98 [0.91, 1.00] |
| Appetite changes/hyperorality | 1 (5%) | 8 (36%) | 2 (13%) | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] |
| Joviality/gregariousness | N/A | N/A | 7 (47%) | 7/15a (47%) | 0.47 [0.21, 0.73] | N/A |
| Reduced insight | N/A | N/A | 9 (60%) | 9/15a (60%) | 0.60 [0.32, 0.84] | N/A |
| Emotional blunting | N/A | N/A | 1 (7%) | 1/15a (7%) | 0.07 [0.00, 0.32] | N/A |
Feature may be endorsed by clinician, informant or patient to be included in the ‘Total’ column. ‘Without dysphoria’ refers to a lack of moderate-to-severe dysphoria per self-report on the GDS. CI = confidence interval, binomial calculation, lower and upper bounds shown. Prodromal bvFTD Development Group comprised n = 22 with clinician and informant report data (MAPT = 12, GRN = 2, C9orf72 = 7, C9orf72+GRN = 1) and n = 15 with clinical notes (MAPT = 10, GRN = 1, C9orf72 = 3, C9orf72+GRN = 1). Informant report was based on the Neuropsychiatric Inventory Questionnaire (NPI-Q) except in the case of empathy/sympathy, which was based on the Interpersonal Reactivity Index (IRI) total score; sensitivity and specificity for the IRI subscale scores are: Empathic Concern = 0.27, 0.89; Perspective Taking = 0.45, 0.93. Clinical notes were based on both patient and informant report, as well as clinical impression. Specificity calculated in Healthy Control Group 1, n = 82. N/A = not available.
aClinical notes are the only source of information for this feature, n = 15.
Behavioural and neuropsychiatric features of the prodromal bvFTD Development Group and Healthy Control Group 1
| . | Clinician indicated n = 22 . | Informant report n = 22 . | Clinical notes n = 15 . | Total number in Development Group with feature n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . |
|---|---|---|---|---|---|---|
| Apathy/withdrawal/indifference without dysphoria | 13 (59%) | 14 (64%) | 10 (67%) | 18 (82%) | 0.82 [0.60, 0.95] | 0.94 [0.86, 0.98] |
| Disinhibition | 12 (55%) | 12 (55%) | 10 (67%) | 18 (82%) | 0.82 [0.60, 0.95] | 0.91 [0.83, 0.96] |
| Irritability/lability | 9 (41%) | 14 (64%) | 5 (33%) | 16 (73%) | 0.73 [0.50, 0.89] | 0.90 [0.82, 0.96] |
| Agitation | 1 (5%) | 12 (55%) | N/A | 13 (59%) | 0.59 [0.36, 0.79] | 0.93 [0.85, 0.97] |
| Reduced empathy or sympathy | 11 (50%) | 11 (50%) | 7 (47%) | 16 (73%) | 0.73 [0.50, 0.89] | 0.88 [0.79, 0.94] |
| Depression | 5 (23%) | 9 (41%) | 0 (0%) | 10 (45%) | 0.45 [0.24, 0.68] | 0.69 [0.58, 0.79] |
| Anxiety | 2 (9%) | 10 (45%) | 1 (7%) | 10 (45%) | 0.45 [0.24, 0.68] | 0.74 [0.64, 0.83] |
| Psychosis (delusions + hallucinations) | 3 (14%) | 2 (9%) | 3 (20%) | 3 (14%) | 0.14 [0.03, 0.35] | 0.99 [0.93, 1.00] |
| Repetitive behaviours (simple + complex) | 4 (18%) | 4 (18%) | 3 (20%) | 8 (36%) | 0.36 [0.17, 0.59] | 0.95 [0.88. 0.99] |
| REM sleep disorder/night time behaviours | 0 (0%) | 8 (36%) | 1 (6%) | 8 (36%) | 0.36 [0.17, 0.59] | 0.72 [0.61, 0.81] |
| Elation/euphoria | N/A | 5 (23%) | N/A | 5 (23%) | 0.23 [0.08, 0.45] | 0.98 [0.91, 1.00] |
| Appetite changes/hyperorality | 1 (5%) | 8 (36%) | 2 (13%) | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] |
| Joviality/gregariousness | N/A | N/A | 7 (47%) | 7/15a (47%) | 0.47 [0.21, 0.73] | N/A |
| Reduced insight | N/A | N/A | 9 (60%) | 9/15a (60%) | 0.60 [0.32, 0.84] | N/A |
| Emotional blunting | N/A | N/A | 1 (7%) | 1/15a (7%) | 0.07 [0.00, 0.32] | N/A |
| . | Clinician indicated n = 22 . | Informant report n = 22 . | Clinical notes n = 15 . | Total number in Development Group with feature n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . |
|---|---|---|---|---|---|---|
| Apathy/withdrawal/indifference without dysphoria | 13 (59%) | 14 (64%) | 10 (67%) | 18 (82%) | 0.82 [0.60, 0.95] | 0.94 [0.86, 0.98] |
| Disinhibition | 12 (55%) | 12 (55%) | 10 (67%) | 18 (82%) | 0.82 [0.60, 0.95] | 0.91 [0.83, 0.96] |
| Irritability/lability | 9 (41%) | 14 (64%) | 5 (33%) | 16 (73%) | 0.73 [0.50, 0.89] | 0.90 [0.82, 0.96] |
| Agitation | 1 (5%) | 12 (55%) | N/A | 13 (59%) | 0.59 [0.36, 0.79] | 0.93 [0.85, 0.97] |
| Reduced empathy or sympathy | 11 (50%) | 11 (50%) | 7 (47%) | 16 (73%) | 0.73 [0.50, 0.89] | 0.88 [0.79, 0.94] |
| Depression | 5 (23%) | 9 (41%) | 0 (0%) | 10 (45%) | 0.45 [0.24, 0.68] | 0.69 [0.58, 0.79] |
| Anxiety | 2 (9%) | 10 (45%) | 1 (7%) | 10 (45%) | 0.45 [0.24, 0.68] | 0.74 [0.64, 0.83] |
| Psychosis (delusions + hallucinations) | 3 (14%) | 2 (9%) | 3 (20%) | 3 (14%) | 0.14 [0.03, 0.35] | 0.99 [0.93, 1.00] |
| Repetitive behaviours (simple + complex) | 4 (18%) | 4 (18%) | 3 (20%) | 8 (36%) | 0.36 [0.17, 0.59] | 0.95 [0.88. 0.99] |
| REM sleep disorder/night time behaviours | 0 (0%) | 8 (36%) | 1 (6%) | 8 (36%) | 0.36 [0.17, 0.59] | 0.72 [0.61, 0.81] |
| Elation/euphoria | N/A | 5 (23%) | N/A | 5 (23%) | 0.23 [0.08, 0.45] | 0.98 [0.91, 1.00] |
| Appetite changes/hyperorality | 1 (5%) | 8 (36%) | 2 (13%) | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] |
| Joviality/gregariousness | N/A | N/A | 7 (47%) | 7/15a (47%) | 0.47 [0.21, 0.73] | N/A |
| Reduced insight | N/A | N/A | 9 (60%) | 9/15a (60%) | 0.60 [0.32, 0.84] | N/A |
| Emotional blunting | N/A | N/A | 1 (7%) | 1/15a (7%) | 0.07 [0.00, 0.32] | N/A |
Feature may be endorsed by clinician, informant or patient to be included in the ‘Total’ column. ‘Without dysphoria’ refers to a lack of moderate-to-severe dysphoria per self-report on the GDS. CI = confidence interval, binomial calculation, lower and upper bounds shown. Prodromal bvFTD Development Group comprised n = 22 with clinician and informant report data (MAPT = 12, GRN = 2, C9orf72 = 7, C9orf72+GRN = 1) and n = 15 with clinical notes (MAPT = 10, GRN = 1, C9orf72 = 3, C9orf72+GRN = 1). Informant report was based on the Neuropsychiatric Inventory Questionnaire (NPI-Q) except in the case of empathy/sympathy, which was based on the Interpersonal Reactivity Index (IRI) total score; sensitivity and specificity for the IRI subscale scores are: Empathic Concern = 0.27, 0.89; Perspective Taking = 0.45, 0.93. Clinical notes were based on both patient and informant report, as well as clinical impression. Specificity calculated in Healthy Control Group 1, n = 82. N/A = not available.
aClinical notes are the only source of information for this feature, n = 15.
Results were less clear for mood symptoms. Depression was moderately endorsed by clinicians (23%) and informants (41%), but self-reported depressed mood/dysphoria was minimal (GDS). In fact, the GDS mean was 2.8/15 (SD = 2.95), with only one individual from the prodromal bvFTD Development Group scoring >6, and two scoring 6, commensurate with mild depression.45 Notably, reports of depressed mood were absent from all free text clinical notes. Anxiety was moderately commonly endorsed by informants (45%), but clinician endorsement was only 9%, and was only mentioned in one clinical note. These discrepancies highlight the difficulty in parsing out neuropsychiatric features; for example, a patient who is pacing might be seen as anxious by family members despite denying feelings of anxiety, or an apathetic patient who watches television all day may be described as depressed by family members but is not experiencing dysphoria. Since anxiety and depression had low specificity, with symptoms reported in 25–30% of healthy controls, we decided that these features did not adequately discriminate between the groups.
Other features present in the Development Group included appetite changes or hyperorality (41%), elation/euphoria (23%), and repetitive behaviours (simple, e.g. pacing; or complex, e.g. ritualistic behaviours) (36%). Although sensitivity was lower than other features, specificity was excellent, with each feature present in <10% of healthy controls. Night time behaviours (e.g. awakening during night) were noted relatively frequently by informants and in the clinical notes (36%); REM sleep behaviour disorder was not noted in any of the participants. However, night time behaviours had poor specificity, occurring in 28% of control subjects. Contrary to expectations,52 psychosis was rare in our sample. Hallucinations were reported in only two participants in the prodromal bvFTD Development Group (9%), and those same two participants plus one other reported delusions (14%) (n = 2 MAPT, n = 1 C9orf72).
Several additional features were extracted from the clinical notes: reduced insight, joviality/gregariousness, and emotional blunting. Interestingly, reduced insight was present in 60% of the group for whom we had clinical notes, which was almost as frequent as reports of apathy and disinhibition in the clinical notes (Table 3). Joviality/gregariousness was endorsed in 47%. Emotional blunting was only noted in one individual. Specificity analyses could not be conducted on these data as clinical notes were not available for healthy controls. However, due to the value and richness of this information, we opted to include these data to ensure we were not missing any features that would optimize the sensitivity of the criteria.
Neuropsychological assessment
Table 4 presents the neuropsychological deficits in the prodromal bvFTD Development Group. Executive dysfunction was defined as clinical impairment (z ≤ −1.5) on either Trails B time or Letter fluency, or ≥2 errors on Trails B.53 This was the most common domain of impairment, occurring in 50% of the Development Group. The second most frequent impairment was in naming, which was present in 45%. Semantic generation impairments (animal fluency) were present in 41% of the Development Group. Episodic memory impairments (delayed free recall) were present more frequently in the verbal domain (36%) than the non-verbal domain (18%). Psychomotor speed was clinically slowed in 36% of the Development Group. Simple auditory attention was intact in the majority (impairments in 9%), while working memory impairments were present in 23%. Interestingly, 23% of the prodromal bvFTD Development Group scored below the clinical z-score cut-off in the visuospatial domain, but inspection of the raw scores revealed that no one scored less than 13/17, indicating that visuospatial skills were largely preserved. Orientation remained intact (≥5/6) in everyone in the prodromal bvFTD Development Group.
Neuropsychological characteristics of the prodromal bvFTD Development Group and Healthy Control Group 1
| Cognitive domain . | Neuropsychological tests used . | Number in Development Group with impairment n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . |
|---|---|---|---|---|
| Executive function | Trails B time, Trails B errors, letter fluency (F, L) | 11 (50%) | 0.50 [0.28, 0.72] | 0.74 [0.64, 0.83] |
| Naming | MINT or BNT | 10 (45%) | 0.45 [0.24, 0.68] | 0.79 [0.69, 0.87] |
| Semantic generation | Category fluency (animals) | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] |
| Attention | Number span forward | 2 (9%) | 0.09 [0.01, 0.29] | 0.95 [0.88, 0.99] |
| Working memory | Number span backward | 5 (23%) | 0.23 [0.08, 0.45] | 0.88 [0.79, 0.94] |
| Visuospatial skills | Benson Figure copy | 5 (23%) | 0.23 [0.08, 0.45] | 0.93 [0.85, 0.97] |
| Verbal episodic memory | Craft Story or Logical Memory delayed recall | 8 (36%) | 0.36 [0.17, 0.59] | 0.86 [0.77, 0.93] |
| Nonverbal episodic memory | Benson Figure delayed recall | 4 (18%) | 0.18 [0.05, 0.40] | 0.88 [0.79, 0.94] |
| Psychomotor speed | Trails A | 8 (36%) | 0.36 [0.17, 0.59] | 0.86 [0.77, 0.93] |
| Orientation | MoCA orientation | 0 (0%) | 0.0 [0.0, 0.15]a | 1.0 [0.96, 1.0]a |
| Cognitive domain . | Neuropsychological tests used . | Number in Development Group with impairment n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . |
|---|---|---|---|---|
| Executive function | Trails B time, Trails B errors, letter fluency (F, L) | 11 (50%) | 0.50 [0.28, 0.72] | 0.74 [0.64, 0.83] |
| Naming | MINT or BNT | 10 (45%) | 0.45 [0.24, 0.68] | 0.79 [0.69, 0.87] |
| Semantic generation | Category fluency (animals) | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] |
| Attention | Number span forward | 2 (9%) | 0.09 [0.01, 0.29] | 0.95 [0.88, 0.99] |
| Working memory | Number span backward | 5 (23%) | 0.23 [0.08, 0.45] | 0.88 [0.79, 0.94] |
| Visuospatial skills | Benson Figure copy | 5 (23%) | 0.23 [0.08, 0.45] | 0.93 [0.85, 0.97] |
| Verbal episodic memory | Craft Story or Logical Memory delayed recall | 8 (36%) | 0.36 [0.17, 0.59] | 0.86 [0.77, 0.93] |
| Nonverbal episodic memory | Benson Figure delayed recall | 4 (18%) | 0.18 [0.05, 0.40] | 0.88 [0.79, 0.94] |
| Psychomotor speed | Trails A | 8 (36%) | 0.36 [0.17, 0.59] | 0.86 [0.77, 0.93] |
| Orientation | MoCA orientation | 0 (0%) | 0.0 [0.0, 0.15]a | 1.0 [0.96, 1.0]a |
Impairment defined as at least 1.5 SD below mean (z ≤ −1.5), based on age-, sex- and education adjusted norms.26 Exceptions include: Trails B errors where impairment was defined as ≥2 errors; orientation where impairment was defined as <5/6 on MoCA orientation questions. CI = confidence interval, binomial calculation, lower and upper bounds shown. Development Group comprised n = 22 prodromal bvFTD participants (MAPT = 12, GRN = 2, C9orf72 = 7, C9orf72+GRN = 1). Specificity calculated in Healthy Control Group 1, n = 82. BNT = Boston Naming Test; MINT = Multilingual Naming Test.
One-sided 97.5% CI.
Neuropsychological characteristics of the prodromal bvFTD Development Group and Healthy Control Group 1
| Cognitive domain . | Neuropsychological tests used . | Number in Development Group with impairment n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . |
|---|---|---|---|---|
| Executive function | Trails B time, Trails B errors, letter fluency (F, L) | 11 (50%) | 0.50 [0.28, 0.72] | 0.74 [0.64, 0.83] |
| Naming | MINT or BNT | 10 (45%) | 0.45 [0.24, 0.68] | 0.79 [0.69, 0.87] |
| Semantic generation | Category fluency (animals) | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] |
| Attention | Number span forward | 2 (9%) | 0.09 [0.01, 0.29] | 0.95 [0.88, 0.99] |
| Working memory | Number span backward | 5 (23%) | 0.23 [0.08, 0.45] | 0.88 [0.79, 0.94] |
| Visuospatial skills | Benson Figure copy | 5 (23%) | 0.23 [0.08, 0.45] | 0.93 [0.85, 0.97] |
| Verbal episodic memory | Craft Story or Logical Memory delayed recall | 8 (36%) | 0.36 [0.17, 0.59] | 0.86 [0.77, 0.93] |
| Nonverbal episodic memory | Benson Figure delayed recall | 4 (18%) | 0.18 [0.05, 0.40] | 0.88 [0.79, 0.94] |
| Psychomotor speed | Trails A | 8 (36%) | 0.36 [0.17, 0.59] | 0.86 [0.77, 0.93] |
| Orientation | MoCA orientation | 0 (0%) | 0.0 [0.0, 0.15]a | 1.0 [0.96, 1.0]a |
| Cognitive domain . | Neuropsychological tests used . | Number in Development Group with impairment n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . |
|---|---|---|---|---|
| Executive function | Trails B time, Trails B errors, letter fluency (F, L) | 11 (50%) | 0.50 [0.28, 0.72] | 0.74 [0.64, 0.83] |
| Naming | MINT or BNT | 10 (45%) | 0.45 [0.24, 0.68] | 0.79 [0.69, 0.87] |
| Semantic generation | Category fluency (animals) | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] |
| Attention | Number span forward | 2 (9%) | 0.09 [0.01, 0.29] | 0.95 [0.88, 0.99] |
| Working memory | Number span backward | 5 (23%) | 0.23 [0.08, 0.45] | 0.88 [0.79, 0.94] |
| Visuospatial skills | Benson Figure copy | 5 (23%) | 0.23 [0.08, 0.45] | 0.93 [0.85, 0.97] |
| Verbal episodic memory | Craft Story or Logical Memory delayed recall | 8 (36%) | 0.36 [0.17, 0.59] | 0.86 [0.77, 0.93] |
| Nonverbal episodic memory | Benson Figure delayed recall | 4 (18%) | 0.18 [0.05, 0.40] | 0.88 [0.79, 0.94] |
| Psychomotor speed | Trails A | 8 (36%) | 0.36 [0.17, 0.59] | 0.86 [0.77, 0.93] |
| Orientation | MoCA orientation | 0 (0%) | 0.0 [0.0, 0.15]a | 1.0 [0.96, 1.0]a |
Impairment defined as at least 1.5 SD below mean (z ≤ −1.5), based on age-, sex- and education adjusted norms.26 Exceptions include: Trails B errors where impairment was defined as ≥2 errors; orientation where impairment was defined as <5/6 on MoCA orientation questions. CI = confidence interval, binomial calculation, lower and upper bounds shown. Development Group comprised n = 22 prodromal bvFTD participants (MAPT = 12, GRN = 2, C9orf72 = 7, C9orf72+GRN = 1). Specificity calculated in Healthy Control Group 1, n = 82. BNT = Boston Naming Test; MINT = Multilingual Naming Test.
One-sided 97.5% CI.
Social cognition assessment
For the SNQ, a Break score of ≥2 and an over-adhere score of ≥3 optimally discriminated between the groups. An optimal cut-off of ≤36 was identified for the RSMS, which is largely consistent with scores obtained in known bvFTD cohorts.54
SNQ over-adhere errors were more frequent than break errors in the Development Group (54% and 27%, respectively). However, break errors had much higher specificity (0.96) than over-adhere errors (0.76) (Table 5). The RSMS was frequently below the Youden cut-off in the prodromal bvFTD Development Group (60%), and specificity was reasonable (0.86).
Social cognition questionnaire results in the prodromal bvFTD Development Group and Healthy Control Group 1
| . | Number in Development Group with impairment n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . | Youden’s J . |
|---|---|---|---|---|
| SNQ | ||||
| Break score (≥2) | 6 (27%) | 0.27 [0.11, 0.50] | 0.96 [0.90, 0.99] | 0.235 |
| Over-adhere score (≥3) | 12 (54%) | 0.54 [0.32, 0.76] | 0.76 [0.65, 0.84] | 0.292 |
| RSMS Total (≤36) | 12/20a (60%) | 0.60 [0.36, 0.81] | 0.86 [0.76, 0.93] | 0.495 |
| . | Number in Development Group with impairment n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . | Youden’s J . |
|---|---|---|---|---|
| SNQ | ||||
| Break score (≥2) | 6 (27%) | 0.27 [0.11, 0.50] | 0.96 [0.90, 0.99] | 0.235 |
| Over-adhere score (≥3) | 12 (54%) | 0.54 [0.32, 0.76] | 0.76 [0.65, 0.84] | 0.292 |
| RSMS Total (≤36) | 12/20a (60%) | 0.60 [0.36, 0.81] | 0.86 [0.76, 0.93] | 0.495 |
Impairment defined based on Youden cut-off. CI = confidence interval, binomial calculation, lower and upper bounds shown. Development Group comprised n = 22 prodromal bvFTD participants (MAPT = 12, GRN = 2, C9orf72 = 7, C9orf72+GRN = 1). Specificity calculated in Healthy Control Group 1, n = 82.
an = 20 from the Development Group had RSMS data (MAPT = 12, GRN = 2, C9orf72 = 5, C9orf72+GRN = 1).
Social cognition questionnaire results in the prodromal bvFTD Development Group and Healthy Control Group 1
| . | Number in Development Group with impairment n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . | Youden’s J . |
|---|---|---|---|---|
| SNQ | ||||
| Break score (≥2) | 6 (27%) | 0.27 [0.11, 0.50] | 0.96 [0.90, 0.99] | 0.235 |
| Over-adhere score (≥3) | 12 (54%) | 0.54 [0.32, 0.76] | 0.76 [0.65, 0.84] | 0.292 |
| RSMS Total (≤36) | 12/20a (60%) | 0.60 [0.36, 0.81] | 0.86 [0.76, 0.93] | 0.495 |
| . | Number in Development Group with impairment n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . | Youden’s J . |
|---|---|---|---|---|
| SNQ | ||||
| Break score (≥2) | 6 (27%) | 0.27 [0.11, 0.50] | 0.96 [0.90, 0.99] | 0.235 |
| Over-adhere score (≥3) | 12 (54%) | 0.54 [0.32, 0.76] | 0.76 [0.65, 0.84] | 0.292 |
| RSMS Total (≤36) | 12/20a (60%) | 0.60 [0.36, 0.81] | 0.86 [0.76, 0.93] | 0.495 |
Impairment defined based on Youden cut-off. CI = confidence interval, binomial calculation, lower and upper bounds shown. Development Group comprised n = 22 prodromal bvFTD participants (MAPT = 12, GRN = 2, C9orf72 = 7, C9orf72+GRN = 1). Specificity calculated in Healthy Control Group 1, n = 82.
an = 20 from the Development Group had RSMS data (MAPT = 12, GRN = 2, C9orf72 = 5, C9orf72+GRN = 1).
Creating the MBCI-FTD criteria from the development phase
In creating the MBCI-FTD criteria, we prioritized specificity over sensitivity, because we aimed to develop a diagnostic tool rather than a screening test. Thus, for inclusion as a core feature of the criteria, specificity was required to be >0.85. We also required the feature to be present in at least 30% (sensitivity ≥0.3); any feature less frequent was considered to potentially lack clinical utility. Several behavioural/neuropsychiatric features met these requirements and were included as core features: apathy without dysphoria, disinhibition, irritability/lability, loss of empathy/sympathy, repetitive behaviours, joviality/gregariousness, and appetite changes. Agitation was combined with irritability for parsimony. Similarly, informant-reported elation/euphoria was combined with joviality/gregariousness, because the questionnaire asks about acting excessively happy, similar to the clinical reports of joviality. Table 6 provides final sensitivity and specificity values for each feature.
Evaluation of the MBCI-FTD features in the prodromal bvFTD Development and Validation Groups and healthy controls
| . | Criteria development . | Criteria validation . | ||||
|---|---|---|---|---|---|---|
| Number in Development Group with feature n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . | Number in Validation Group with feature n = 50 . | Sensitivity [95% CI] . | Specificity [95% CI] . | |
| Core Features | ||||||
| Apathy without dysphoria | 18 (82%) | 0.82 [0.60, 0.95] | 0.94 [0.86, 0.98] | 27 (54%) | 0.54 [0.39, 0.68] | 0.97 [0.91, 0.99] |
| Disinhibition | 18 (82%) | 0.82 [0.60, 0.95] | 0.91 [0.83, 0.96] | 26 (52%) | 0.52 [0.37, 0.66] | 0.95 [0.88, 0.99] |
| Irritability/lability/agitation | 17 (77%) | 0.77 [0.55, 0.92] | 0.87 [0.77, 0.93] | 26 (52%) | 0.52 [0.37, 0.66] | 0.91 [0.83, 0.96] |
| Reduced sympathy or empathy | 16 (73%) | 0.73 [0.50, 0.89] | 0.88 [0.79, 0.94] | 17 (34%) | 0.34 [0.21, 0.49] | 0.84 [0.75, 0.91] |
| Repetitive behaviours (simple + complex) | 8 (36%) | 0.36 [0.17, 0.59] | 0.95 [0.88. 0.99] | 16 (32%) | 0.32 [0.20, 0.47] | 0.94 [0.86, 0.98] |
| Joviality/gregariousness | 10 (45%) | 0.45 [0.24, 0.68] | 0.98 [0.91, 1.00] | 11 (22%) | 0.22 [0.12, 0.36] | 1.00 [0.96, 1.0]a |
| Appetite changes/hyperorality | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] | 20 (40%) | 0.40 [0.26, 0.55] | 0.94 [0.86, 0.98] |
| Supportive Features | ||||||
| Neuropsychological profile | 16 (73%) | 0.73 [0.50, 0.89] | 0.60 [0.48, 0.70] | 20 (40%) | 0.40 [0.26, 0.55] | 0.75 [0.64, 0.84] |
| Reduced insight | 9/15a (60%) | 0.60 [0.32, 0.84] | N/A | 8/11b (73%) | 0.73 [0.39, 0.94] | N/A |
| Poor social cognition | 13 (59%) | 0.59 [0.36, 0.79] | 0.87 [0.77, 0.93] | 9/23c (39%) | 0.39 [0.20, 0.61] | 0.81 [0.70, 0.88] |
| Overall: Meet MBCI-FTD criteria | 21 (95%) | 0.95 [0.77, 1.00] | 0.90 [0.82, 0.96] | 37 (74%) | 0.74 [0.60, 0.85] | 0.93 [0.85, 0.97] |
| . | Criteria development . | Criteria validation . | ||||
|---|---|---|---|---|---|---|
| Number in Development Group with feature n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . | Number in Validation Group with feature n = 50 . | Sensitivity [95% CI] . | Specificity [95% CI] . | |
| Core Features | ||||||
| Apathy without dysphoria | 18 (82%) | 0.82 [0.60, 0.95] | 0.94 [0.86, 0.98] | 27 (54%) | 0.54 [0.39, 0.68] | 0.97 [0.91, 0.99] |
| Disinhibition | 18 (82%) | 0.82 [0.60, 0.95] | 0.91 [0.83, 0.96] | 26 (52%) | 0.52 [0.37, 0.66] | 0.95 [0.88, 0.99] |
| Irritability/lability/agitation | 17 (77%) | 0.77 [0.55, 0.92] | 0.87 [0.77, 0.93] | 26 (52%) | 0.52 [0.37, 0.66] | 0.91 [0.83, 0.96] |
| Reduced sympathy or empathy | 16 (73%) | 0.73 [0.50, 0.89] | 0.88 [0.79, 0.94] | 17 (34%) | 0.34 [0.21, 0.49] | 0.84 [0.75, 0.91] |
| Repetitive behaviours (simple + complex) | 8 (36%) | 0.36 [0.17, 0.59] | 0.95 [0.88. 0.99] | 16 (32%) | 0.32 [0.20, 0.47] | 0.94 [0.86, 0.98] |
| Joviality/gregariousness | 10 (45%) | 0.45 [0.24, 0.68] | 0.98 [0.91, 1.00] | 11 (22%) | 0.22 [0.12, 0.36] | 1.00 [0.96, 1.0]a |
| Appetite changes/hyperorality | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] | 20 (40%) | 0.40 [0.26, 0.55] | 0.94 [0.86, 0.98] |
| Supportive Features | ||||||
| Neuropsychological profile | 16 (73%) | 0.73 [0.50, 0.89] | 0.60 [0.48, 0.70] | 20 (40%) | 0.40 [0.26, 0.55] | 0.75 [0.64, 0.84] |
| Reduced insight | 9/15a (60%) | 0.60 [0.32, 0.84] | N/A | 8/11b (73%) | 0.73 [0.39, 0.94] | N/A |
| Poor social cognition | 13 (59%) | 0.59 [0.36, 0.79] | 0.87 [0.77, 0.93] | 9/23c (39%) | 0.39 [0.20, 0.61] | 0.81 [0.70, 0.88] |
| Overall: Meet MBCI-FTD criteria | 21 (95%) | 0.95 [0.77, 1.00] | 0.90 [0.82, 0.96] | 37 (74%) | 0.74 [0.60, 0.85] | 0.93 [0.85, 0.97] |
Features defined as per development phase. Development Group comprised n = 22 prodromal bvFTD participants (MAPT = 12, GRN = 2, C9orf72 = 7, C9orf72+GRN = 1), Validation Group comprised n = 50 prodromal bvFTD participants (MAPT = 8, GRN = 5, C9orf72 = 10, unspecified mutation = 10, presumed sporadic = 17). Specificity calculated in Healthy Control Group 1 (n = 82) for criteria development, and Healthy Control Group 2 for criteria validation (n = 83). CI = confidence interval, binomial calculation, upper and lower bounds shown. n = 2 participants in the Development Group were aged 70+ years at the time of their prodromal visit; with these participants excluded, the overall results did not change [sensitivity = 0.95 (19/20)]. n = 10 participants in the Validation Group were aged 70+ years at the time of their prodromal visit; with these participants excluded, the overall results did not meaningfully change [sensitivity = 0.75 (30/40)]. N/A = not available.
aOne-sided 97.5% CI.
bInsight measure available for n = 15 participants in the Development Group (MAPT = 10, GRN = 1, C9orf72 = 3, C9orf72+GRN = 1) and n = 11 participants in the Validation Group (MAPT = 4, GRN = 3, C9orf72 = 4).
cSocial cognition assessed in n = 23 participants in the Validation Group (MAPT = 8, GRN = 5, C9orf72 = 10).
Evaluation of the MBCI-FTD features in the prodromal bvFTD Development and Validation Groups and healthy controls
| . | Criteria development . | Criteria validation . | ||||
|---|---|---|---|---|---|---|
| Number in Development Group with feature n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . | Number in Validation Group with feature n = 50 . | Sensitivity [95% CI] . | Specificity [95% CI] . | |
| Core Features | ||||||
| Apathy without dysphoria | 18 (82%) | 0.82 [0.60, 0.95] | 0.94 [0.86, 0.98] | 27 (54%) | 0.54 [0.39, 0.68] | 0.97 [0.91, 0.99] |
| Disinhibition | 18 (82%) | 0.82 [0.60, 0.95] | 0.91 [0.83, 0.96] | 26 (52%) | 0.52 [0.37, 0.66] | 0.95 [0.88, 0.99] |
| Irritability/lability/agitation | 17 (77%) | 0.77 [0.55, 0.92] | 0.87 [0.77, 0.93] | 26 (52%) | 0.52 [0.37, 0.66] | 0.91 [0.83, 0.96] |
| Reduced sympathy or empathy | 16 (73%) | 0.73 [0.50, 0.89] | 0.88 [0.79, 0.94] | 17 (34%) | 0.34 [0.21, 0.49] | 0.84 [0.75, 0.91] |
| Repetitive behaviours (simple + complex) | 8 (36%) | 0.36 [0.17, 0.59] | 0.95 [0.88. 0.99] | 16 (32%) | 0.32 [0.20, 0.47] | 0.94 [0.86, 0.98] |
| Joviality/gregariousness | 10 (45%) | 0.45 [0.24, 0.68] | 0.98 [0.91, 1.00] | 11 (22%) | 0.22 [0.12, 0.36] | 1.00 [0.96, 1.0]a |
| Appetite changes/hyperorality | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] | 20 (40%) | 0.40 [0.26, 0.55] | 0.94 [0.86, 0.98] |
| Supportive Features | ||||||
| Neuropsychological profile | 16 (73%) | 0.73 [0.50, 0.89] | 0.60 [0.48, 0.70] | 20 (40%) | 0.40 [0.26, 0.55] | 0.75 [0.64, 0.84] |
| Reduced insight | 9/15a (60%) | 0.60 [0.32, 0.84] | N/A | 8/11b (73%) | 0.73 [0.39, 0.94] | N/A |
| Poor social cognition | 13 (59%) | 0.59 [0.36, 0.79] | 0.87 [0.77, 0.93] | 9/23c (39%) | 0.39 [0.20, 0.61] | 0.81 [0.70, 0.88] |
| Overall: Meet MBCI-FTD criteria | 21 (95%) | 0.95 [0.77, 1.00] | 0.90 [0.82, 0.96] | 37 (74%) | 0.74 [0.60, 0.85] | 0.93 [0.85, 0.97] |
| . | Criteria development . | Criteria validation . | ||||
|---|---|---|---|---|---|---|
| Number in Development Group with feature n = 22 . | Sensitivity [95% CI] . | Specificity [95% CI] . | Number in Validation Group with feature n = 50 . | Sensitivity [95% CI] . | Specificity [95% CI] . | |
| Core Features | ||||||
| Apathy without dysphoria | 18 (82%) | 0.82 [0.60, 0.95] | 0.94 [0.86, 0.98] | 27 (54%) | 0.54 [0.39, 0.68] | 0.97 [0.91, 0.99] |
| Disinhibition | 18 (82%) | 0.82 [0.60, 0.95] | 0.91 [0.83, 0.96] | 26 (52%) | 0.52 [0.37, 0.66] | 0.95 [0.88, 0.99] |
| Irritability/lability/agitation | 17 (77%) | 0.77 [0.55, 0.92] | 0.87 [0.77, 0.93] | 26 (52%) | 0.52 [0.37, 0.66] | 0.91 [0.83, 0.96] |
| Reduced sympathy or empathy | 16 (73%) | 0.73 [0.50, 0.89] | 0.88 [0.79, 0.94] | 17 (34%) | 0.34 [0.21, 0.49] | 0.84 [0.75, 0.91] |
| Repetitive behaviours (simple + complex) | 8 (36%) | 0.36 [0.17, 0.59] | 0.95 [0.88. 0.99] | 16 (32%) | 0.32 [0.20, 0.47] | 0.94 [0.86, 0.98] |
| Joviality/gregariousness | 10 (45%) | 0.45 [0.24, 0.68] | 0.98 [0.91, 1.00] | 11 (22%) | 0.22 [0.12, 0.36] | 1.00 [0.96, 1.0]a |
| Appetite changes/hyperorality | 9 (41%) | 0.41 [0.21, 0.64] | 0.91 [0.83, 0.96] | 20 (40%) | 0.40 [0.26, 0.55] | 0.94 [0.86, 0.98] |
| Supportive Features | ||||||
| Neuropsychological profile | 16 (73%) | 0.73 [0.50, 0.89] | 0.60 [0.48, 0.70] | 20 (40%) | 0.40 [0.26, 0.55] | 0.75 [0.64, 0.84] |
| Reduced insight | 9/15a (60%) | 0.60 [0.32, 0.84] | N/A | 8/11b (73%) | 0.73 [0.39, 0.94] | N/A |
| Poor social cognition | 13 (59%) | 0.59 [0.36, 0.79] | 0.87 [0.77, 0.93] | 9/23c (39%) | 0.39 [0.20, 0.61] | 0.81 [0.70, 0.88] |
| Overall: Meet MBCI-FTD criteria | 21 (95%) | 0.95 [0.77, 1.00] | 0.90 [0.82, 0.96] | 37 (74%) | 0.74 [0.60, 0.85] | 0.93 [0.85, 0.97] |
Features defined as per development phase. Development Group comprised n = 22 prodromal bvFTD participants (MAPT = 12, GRN = 2, C9orf72 = 7, C9orf72+GRN = 1), Validation Group comprised n = 50 prodromal bvFTD participants (MAPT = 8, GRN = 5, C9orf72 = 10, unspecified mutation = 10, presumed sporadic = 17). Specificity calculated in Healthy Control Group 1 (n = 82) for criteria development, and Healthy Control Group 2 for criteria validation (n = 83). CI = confidence interval, binomial calculation, upper and lower bounds shown. n = 2 participants in the Development Group were aged 70+ years at the time of their prodromal visit; with these participants excluded, the overall results did not change [sensitivity = 0.95 (19/20)]. n = 10 participants in the Validation Group were aged 70+ years at the time of their prodromal visit; with these participants excluded, the overall results did not meaningfully change [sensitivity = 0.75 (30/40)]. N/A = not available.
aOne-sided 97.5% CI.
bInsight measure available for n = 15 participants in the Development Group (MAPT = 10, GRN = 1, C9orf72 = 3, C9orf72+GRN = 1) and n = 11 participants in the Validation Group (MAPT = 4, GRN = 3, C9orf72 = 4).
cSocial cognition assessed in n = 23 participants in the Validation Group (MAPT = 8, GRN = 5, C9orf72 = 10).
With regard to the neuropsychological profile, from a clinical utility perspective, we were interested in finding the profile that was characteristic of the Development Group. The most frequent impairments were in executive function and naming (∼50%). This is consistent with existing literature, including sporadic cases.10,19 However, executive function and naming deficits were also present in 21–26% of healthy controls, indicating low specificity. Therefore, this neuropsychological profile was included as a supportive feature rather than a core feature. In line with bvFTD diagnostic criteria,10 visuospatial skills appeared intact in the Development Group. Everyone in the prodromal bvFTD Development Group also had intact time/place orientation. Thus, the deficit in executive function and/or naming needs to be in the context of relatively preserved orientation and visuospatial skills to fit the MBCI-FTD neuropsychological profile criterion (sensitivity = 0.73, specificity = 0.60; Table 6).
Despite reasonable sensitivity and specificity, impairments on animal fluency, verbal episodic memory, and psychomotor speed were not included in the MBCI-FTD criteria, due to poor specificity against other neurodegenerative diseases. Category fluency and episodic memory are compromised in early Alzheimer’s disease dementia,55–57 while psychomotor speed problems are characteristic of dementia with Lewy bodies. We also note that the semantic and verbal episodic memory weaknesses present in the prodromal bvFTD Development Group may be inflated due to an overrepresentation of MAPT mutation carriers in this group.18,58,59
In terms of social cognition, both the SNQ break score (≥2) and RSMS total score (≤36) had reasonably high specificity (>0.85). These were combined into an ‘impaired social cognition’ criterion, defined as impaired appreciation of social expectations or reduced socio-emotional sensitivity upon assessment (sensitivity = 0.59, specificity = 0.87; Table 6). Though these social cognition questionnaires show reasonable diagnostic potential, we decided that since they are not widely used in clinical settings, this criterion should be included as a supportive feature. Further, we have opted to use the term ‘social cognition’ to define this criterion, but acknowledge that this is a broad umbrella term covering a complex, multi-dimensional domain. In the current study our measurement tools allowed us to examine the ability to understand social norm violations (SNQ) and the ability to detect and respond to people’s subtle social cues in real life situations (RSMS), but we did not examine other facets of social cognition such as emotion processing or theory of mind. Thus, for consistency with the literature we use the term ‘social cognition’, but note that in the MBCI-FTD criteria this is specifically operationalized per Appendix I.
Finally, reduced insight was included due to the frequency with which it was reported in the clinical notes, approaching that of apathy and disinhibition. However, reduced insight was included as a supportive feature rather than a core feature, since we could not calculate specificity.
Depression, anxiety, and sleep problems were endorsed in 25–30% of healthy control subjects, so were not considered specific enough for inclusion in the MBCI-FTD criteria. Hallucinations and delusions were present in too few individuals in the Development Group to be considered a feature, although we note that psychosis may be present in a subset. Similarly, emotional blunting was only explicitly reported in one individual and was therefore not included; it may be that it is a difficult characteristic to capture with existing measures.
MBCI-FTD criteria
We propose a set of criteria for MBCI-FTD (Table 7, features defined in Appendix I). The core features include: apathy without moderate-severe dysphoria, behavioural disinhibition, irritability/agitation, reduced empathy/sympathy, repetitive behaviours (simple and/or complex), joviality/gregariousness, and appetite changes/hyperorality. Supportive features include a neuropsychological profile of executive dysfunction or impaired naming in the context of preserved visuospatial skills and orientation, reduced insight for cognitive or behavioural changes, and impaired social cognition. Importantly, any MBCI-FTD supportive feature has to be paired with the presence of at least two core features. Multiple supportive features cannot replace core features for diagnosis. Thus, three core features or two core features plus one supportive feature are required for a diagnosis of MBCI-FTD (Table 7). Overall, the proposed MBCI-FTD criteria correctly classify 95% (21/22) of the Development Group, with a false positive rate in Healthy Control Group 1 of 10% (8/82) (sensitivity = 0.96, specificity = 0.90). The decision to require three features for diagnosis was based on a sensitivity-specificity trade-off: decreasing the number of features required to two resulted in decreased specificity (i.e. higher false positive rate in healthy controls, >20%).
| Definition of MBCI-FTD . |
|---|
| A clinical syndrome defined by the presence of persistent and progressive decline in behaviour and/or cognition for more than six months based on observation or history provided by knowledgeable informant. |
| 1. Must be present to diagnose MBCI-FTD |
| A.Concern regarding behavioural and/or cognitive change from previous functioning, per informant, clinician, or patient |
| B.Preserved instrumental activities of daily living (unless due to physical impairment, e.g. motor neuron disease or parkinsonism) |
| C.>18 years old |
| 2. Possible MBCI-FTD |
| At least three of the following core features (A–G) are sufficient, and must represent a change from previous behaviour, to diagnose possible MBCI-FTD |
| A. Apathy without moderate-severe dysphoria |
| B. Behavioural disinhibition |
| C. Irritability or agitation |
| D. Loss of empathy or sympathy |
| E. Repetitive behaviours (either E1 or E2) |
| E1. Simple: Aberrant motor behaviour, or restlessness (e.g. pacing, fidgeting, tapping) |
| E2. Complex: Perseverative, compulsive or ritualistic behaviour (e.g. rigidity, rituals, hoarding) |
| F. Joviality or gregariousness |
| G. Appetite changes/hyperorality |
| If only two of the above core features (A–G) are present, then at least one of the following (H or I or J) must also be present to diagnose Possible MBCI-FTD: |
| H. Neuropsychological deficits in context of intact or relatively preserved time/place orientation and visuospatial skills (one of H1–H2 must be present) |
| H1. Clinical impairment or clinically significant decline on executive tasks (e.g. verbal generation, set-shifting, etc.) |
| H2. Clinical impairment or clinically significant decline on naming tests |
| I. Reduced insight for at least one aspect of behavioural or cognitive change |
| J. Impairments on standardized measures of social cognition (one of J1-J2 must be present) |
| J1. Reduced understanding or awareness of social expectations |
| J2. Low socioemotional sensitivity |
| 3. Probable MBCI-FTD |
| Both of the following (A–B) must be present to diagnose Probable MBCI-FTD: |
| A. Meets criteria for Possible MBCI-FTD |
| B. Genetic or biomarker evidence of FTLD (at least one of B1–B3 must be present) |
| B1. Presence of a known pathogenic mutation |
| B2. Imaging evidence of FTD |
| B2.1 Frontal and/or anterior temporal atrophy on MRI or CT |
| B2.2 Frontal and/or anterior temporal hypoperfusion or hypometabolism on PET or SPECT |
| B3. Other plasma/CSF biomarkers indicative of FTLD pathology |
| 4. Exclusionary criteria for MBCI-FTD |
| A. History of sudden onset or other medical conditions severe enough to account for symptoms (e.g. cerebrovascular, infectious, toxic, inflammatory, or metabolic disorders, traumatic brain injury or brain tumour) |
| B. Plasma or CSF or molecular imaging biomarkers more consistent with Alzheimer’s disease than FTLD |
| C. Meets diagnostic criteria for Probable bvFTD |
| Definition of MBCI-FTD . |
|---|
| A clinical syndrome defined by the presence of persistent and progressive decline in behaviour and/or cognition for more than six months based on observation or history provided by knowledgeable informant. |
| 1. Must be present to diagnose MBCI-FTD |
| A.Concern regarding behavioural and/or cognitive change from previous functioning, per informant, clinician, or patient |
| B.Preserved instrumental activities of daily living (unless due to physical impairment, e.g. motor neuron disease or parkinsonism) |
| C.>18 years old |
| 2. Possible MBCI-FTD |
| At least three of the following core features (A–G) are sufficient, and must represent a change from previous behaviour, to diagnose possible MBCI-FTD |
| A. Apathy without moderate-severe dysphoria |
| B. Behavioural disinhibition |
| C. Irritability or agitation |
| D. Loss of empathy or sympathy |
| E. Repetitive behaviours (either E1 or E2) |
| E1. Simple: Aberrant motor behaviour, or restlessness (e.g. pacing, fidgeting, tapping) |
| E2. Complex: Perseverative, compulsive or ritualistic behaviour (e.g. rigidity, rituals, hoarding) |
| F. Joviality or gregariousness |
| G. Appetite changes/hyperorality |
| If only two of the above core features (A–G) are present, then at least one of the following (H or I or J) must also be present to diagnose Possible MBCI-FTD: |
| H. Neuropsychological deficits in context of intact or relatively preserved time/place orientation and visuospatial skills (one of H1–H2 must be present) |
| H1. Clinical impairment or clinically significant decline on executive tasks (e.g. verbal generation, set-shifting, etc.) |
| H2. Clinical impairment or clinically significant decline on naming tests |
| I. Reduced insight for at least one aspect of behavioural or cognitive change |
| J. Impairments on standardized measures of social cognition (one of J1-J2 must be present) |
| J1. Reduced understanding or awareness of social expectations |
| J2. Low socioemotional sensitivity |
| 3. Probable MBCI-FTD |
| Both of the following (A–B) must be present to diagnose Probable MBCI-FTD: |
| A. Meets criteria for Possible MBCI-FTD |
| B. Genetic or biomarker evidence of FTLD (at least one of B1–B3 must be present) |
| B1. Presence of a known pathogenic mutation |
| B2. Imaging evidence of FTD |
| B2.1 Frontal and/or anterior temporal atrophy on MRI or CT |
| B2.2 Frontal and/or anterior temporal hypoperfusion or hypometabolism on PET or SPECT |
| B3. Other plasma/CSF biomarkers indicative of FTLD pathology |
| 4. Exclusionary criteria for MBCI-FTD |
| A. History of sudden onset or other medical conditions severe enough to account for symptoms (e.g. cerebrovascular, infectious, toxic, inflammatory, or metabolic disorders, traumatic brain injury or brain tumour) |
| B. Plasma or CSF or molecular imaging biomarkers more consistent with Alzheimer’s disease than FTLD |
| C. Meets diagnostic criteria for Probable bvFTD |
| Definition of MBCI-FTD . |
|---|
| A clinical syndrome defined by the presence of persistent and progressive decline in behaviour and/or cognition for more than six months based on observation or history provided by knowledgeable informant. |
| 1. Must be present to diagnose MBCI-FTD |
| A.Concern regarding behavioural and/or cognitive change from previous functioning, per informant, clinician, or patient |
| B.Preserved instrumental activities of daily living (unless due to physical impairment, e.g. motor neuron disease or parkinsonism) |
| C.>18 years old |
| 2. Possible MBCI-FTD |
| At least three of the following core features (A–G) are sufficient, and must represent a change from previous behaviour, to diagnose possible MBCI-FTD |
| A. Apathy without moderate-severe dysphoria |
| B. Behavioural disinhibition |
| C. Irritability or agitation |
| D. Loss of empathy or sympathy |
| E. Repetitive behaviours (either E1 or E2) |
| E1. Simple: Aberrant motor behaviour, or restlessness (e.g. pacing, fidgeting, tapping) |
| E2. Complex: Perseverative, compulsive or ritualistic behaviour (e.g. rigidity, rituals, hoarding) |
| F. Joviality or gregariousness |
| G. Appetite changes/hyperorality |
| If only two of the above core features (A–G) are present, then at least one of the following (H or I or J) must also be present to diagnose Possible MBCI-FTD: |
| H. Neuropsychological deficits in context of intact or relatively preserved time/place orientation and visuospatial skills (one of H1–H2 must be present) |
| H1. Clinical impairment or clinically significant decline on executive tasks (e.g. verbal generation, set-shifting, etc.) |
| H2. Clinical impairment or clinically significant decline on naming tests |
| I. Reduced insight for at least one aspect of behavioural or cognitive change |
| J. Impairments on standardized measures of social cognition (one of J1-J2 must be present) |
| J1. Reduced understanding or awareness of social expectations |
| J2. Low socioemotional sensitivity |
| 3. Probable MBCI-FTD |
| Both of the following (A–B) must be present to diagnose Probable MBCI-FTD: |
| A. Meets criteria for Possible MBCI-FTD |
| B. Genetic or biomarker evidence of FTLD (at least one of B1–B3 must be present) |
| B1. Presence of a known pathogenic mutation |
| B2. Imaging evidence of FTD |
| B2.1 Frontal and/or anterior temporal atrophy on MRI or CT |
| B2.2 Frontal and/or anterior temporal hypoperfusion or hypometabolism on PET or SPECT |
| B3. Other plasma/CSF biomarkers indicative of FTLD pathology |
| 4. Exclusionary criteria for MBCI-FTD |
| A. History of sudden onset or other medical conditions severe enough to account for symptoms (e.g. cerebrovascular, infectious, toxic, inflammatory, or metabolic disorders, traumatic brain injury or brain tumour) |
| B. Plasma or CSF or molecular imaging biomarkers more consistent with Alzheimer’s disease than FTLD |
| C. Meets diagnostic criteria for Probable bvFTD |
| Definition of MBCI-FTD . |
|---|
| A clinical syndrome defined by the presence of persistent and progressive decline in behaviour and/or cognition for more than six months based on observation or history provided by knowledgeable informant. |
| 1. Must be present to diagnose MBCI-FTD |
| A.Concern regarding behavioural and/or cognitive change from previous functioning, per informant, clinician, or patient |
| B.Preserved instrumental activities of daily living (unless due to physical impairment, e.g. motor neuron disease or parkinsonism) |
| C.>18 years old |
| 2. Possible MBCI-FTD |
| At least three of the following core features (A–G) are sufficient, and must represent a change from previous behaviour, to diagnose possible MBCI-FTD |
| A. Apathy without moderate-severe dysphoria |
| B. Behavioural disinhibition |
| C. Irritability or agitation |
| D. Loss of empathy or sympathy |
| E. Repetitive behaviours (either E1 or E2) |
| E1. Simple: Aberrant motor behaviour, or restlessness (e.g. pacing, fidgeting, tapping) |
| E2. Complex: Perseverative, compulsive or ritualistic behaviour (e.g. rigidity, rituals, hoarding) |
| F. Joviality or gregariousness |
| G. Appetite changes/hyperorality |
| If only two of the above core features (A–G) are present, then at least one of the following (H or I or J) must also be present to diagnose Possible MBCI-FTD: |
| H. Neuropsychological deficits in context of intact or relatively preserved time/place orientation and visuospatial skills (one of H1–H2 must be present) |
| H1. Clinical impairment or clinically significant decline on executive tasks (e.g. verbal generation, set-shifting, etc.) |
| H2. Clinical impairment or clinically significant decline on naming tests |
| I. Reduced insight for at least one aspect of behavioural or cognitive change |
| J. Impairments on standardized measures of social cognition (one of J1-J2 must be present) |
| J1. Reduced understanding or awareness of social expectations |
| J2. Low socioemotional sensitivity |
| 3. Probable MBCI-FTD |
| Both of the following (A–B) must be present to diagnose Probable MBCI-FTD: |
| A. Meets criteria for Possible MBCI-FTD |
| B. Genetic or biomarker evidence of FTLD (at least one of B1–B3 must be present) |
| B1. Presence of a known pathogenic mutation |
| B2. Imaging evidence of FTD |
| B2.1 Frontal and/or anterior temporal atrophy on MRI or CT |
| B2.2 Frontal and/or anterior temporal hypoperfusion or hypometabolism on PET or SPECT |
| B3. Other plasma/CSF biomarkers indicative of FTLD pathology |
| 4. Exclusionary criteria for MBCI-FTD |
| A. History of sudden onset or other medical conditions severe enough to account for symptoms (e.g. cerebrovascular, infectious, toxic, inflammatory, or metabolic disorders, traumatic brain injury or brain tumour) |
| B. Plasma or CSF or molecular imaging biomarkers more consistent with Alzheimer’s disease than FTLD |
| C. Meets diagnostic criteria for Probable bvFTD |
We opted to include two levels of certainty in the MBCI-FTD diagnosis framework: ‘possible’ and ‘probable’. Possible MBCI-FTD may be diagnosed based on behavioural and cognitive features alone, while a pathogenic mutation or biomarker is required for a diagnosis of probable MBCI-FTD. Possible MBCI-FTD is intended to capture cases in which biomarkers or genetic testing may be unavailable, and we felt it was appropriate to acknowledge this lower level of certainty in diagnosis. This framework is largely in line with the Rascovsky et al.10 bvFTD diagnostic criteria, although there is no ‘definite’ certainty level for MBCI-FTD, because, even if a genetic mutation is present, the nature of this prodromal phase is that it is somewhat ‘questionable’.
Validation and testing phase
When evaluated on the prodromal bvFTD Validation Group and Healthy Control Group 2, the criteria correctly classified 74% (37/50) of the Validation Group as MBCI-FTD, with a false positive rate of 7% (6/83) (sensitivity = 0.74, specificity = 0.93) in healthy controls. Evaluating the specific MBCI-FTD features on the Validation Group and Healthy Control Group 2 yielded the results in Table 6, and the results broken down by mutation status and type are available in Supplementary Table 4. In brief, the criteria classified 63% (5/8) of MAPT mutation carriers, 100% (5/5) of GRN mutation carriers, 70% (7/10) of C9orf72 expansion carriers, 8/10 (80%) of mutation carriers with an unspecified mutation, and 12/17 (71%) of presumed sporadic cases as MBCI-FTD, indicating that they are not strongly biased in favour of one genetic mutation, and are applicable to presumably sporadic cases. We highlight that we were not able to assess social cognition or insight in the NACC participants (Supplementary Table 2), so the sensitivity results in the Validation Group are likely artificially lowered, as 31/50 participants were from NACC. We also conducted an informal post hoc review of the available data for all prodromal bvFTD Validation Group participants, to assess whether any clinical features were ‘missed’ in the relatively smaller Development Group sample. Upon this review, no clinical features were candidates for post hoc inclusion in the criteria, as none were present in >20% of the Validation Group.
When tested in the Alzheimer’s Test Group (n = 301), the MBCI-FTD criteria correctly classified 84–89% of participants (false positive rate of 11–16%; specificity = 0.84–0.89). Ranges are provided as the Alzheimer’s group data were from NACC, and social cognition and insight were not assessed in this group. The upper limit of the false positive rate is a conservative estimate, as it assumes both lack of insight and impaired social cognition would be present in all participants. Finally, we divided the Alzheimer’s Test Group into those with and without Lewy body pathology. In the Alzheimer’s disease without Lewy body group (n = 178) the criteria correctly classified 84–89% (false positive rate of 9–13%; specificity = 0.87–0.91), and in the Alzheimer’s disease with Lewy body group (n = 114) the criteria correctly classified 80–86% (false positive rate of 14–20%; specificity = 0.80–0.86). Finally, it is possible that Lewy body pathology would have only contributed to the clinical presentation in participants with neocortical (diffuse) Lewy bodies, as all participants had Braak stage ≥3.60 In this subset (n = 32), 78–87% were correctly classified by the MBCI-FTD criteria (false positive rate of 13–22%; specificity = 0.78–0.87).
Discussion
In this study, we developed and tested a proposed set of diagnostic criteria for MBCI-FTD, representing the clinical prodrome of bvFTD. We emphasize that all features must represent a change from previous functioning, per informant report or multiple evaluations.
Unsurprisingly, the criteria bear strong similarity to the Rascovsky et al.10 bvFTD diagnostic criteria. However, we encourage clinicians to impose a somewhat lower threshold for accepting a feature as ‘present’ for MBCI-FTD diagnosis. Rascovsky et al.10 specified that symptoms should be ‘persistent’ or ‘recurrent’, but with patients in the very earliest symptomatic stages it is difficult to have confidence that a feature has been ‘persistent’, or existed over a period of time. Nevertheless, features should be represented by more than single or rare events, so repeated behaviours, even if mild or questionable, should be included (except in the case of irritability/agitation, which should be significant or moderate, per Appendix I).
There are several other key differences between the Rascovsky et al.10 criteria and the MBCI-FTD criteria worth highlighting. First, the apathy criterion in MBCI-FTD was only considered ‘present’ when it occurred in the absence of moderate-severe dysphoria. This was an a priori decision, as early bvFTD is often misdiagnosed as depression.61 A handful of participants in the prodromal bvFTD Validation Group did display moderate-severe dysphoria, so this should not be an exclusion criterion, but we hope that requiring the presence of apathy to be qualified by a lack of dysphoria will aid in differentiating early bvFTD from depression. Further, recent evidence indicates that apathy and anhedonia are correlated but separable symptoms in FTD.62 In the current study we did not measure anhedonia, but future research should assess the related symptoms of dysphoria, apathy, and anhedonia in bvFTD, and how dissociable they are during the prodromal phase. It may be that anhedonia is a key early feature, perhaps even more relevant than apathy.
Another feature not typically associated with early bvFTD is irritability/agitation, though this was one of the most frequently reported features in our prodromal bvFTD group. Indeed, reviewing the clinical notes revealed multiple descriptions of patients being ‘quick to anger’ and ‘flying off the handle’. This is an interesting finding that should be investigated; it may be that this feature peaks during the prodrome, before a more blunted emotional presentation predominates.
Reduced insight into behavioural or cognitive changes was present in both previous iterations of the FTD criteria (Lund/Manchester; Neary et al.9), but was removed from the Rascovsky et al.10 criteria. We included it as a supportive feature for two main reasons. First, although we could only obtain this information from the clinical notes, it was almost as common as apathy and disinhibition, indicating that it is an important feature. Second, insight or concern may be a key feature in discriminating bvFTD from psychiatric disorders, such that insight is generally reduced in the former and preserved in the latter.63 Features such as a lack of insight or lack of concern, for which we have some evidence of clinical utility and specificity, should be prospectively objectively measured in FTD cohorts. If a reliable informant is not available, the clinician should exercise caution in making inferences about insight. We also note that although insight is intact in some patients, those with poor insight might be unreliable in their reports of neuropsychiatric symptoms. Self-report, affected by insight, is the gold standard method of assessment for several important neuropsychiatric symptoms, including mood (e.g. dysphoria), anxiety, and obsessions. The interaction between insight and self-reported neuropsychiatric symptoms in bvFTD is an important direction for future prospective research.
In line with Rascovsky et al.10 we found a dysexecutive neuropsychological profile to be most characteristic of the prodromal bvFTD Development Group. Naming impairments were also frequently observed, while time/place orientation and visuospatial skills remained intact. If multiple assessments are available, a clinically significant decline in executive functioning or naming may be judged by a clinical neuropsychologist as sufficient for the patient to meet this criterion. Unlike the Rascovsky et al.10 and Neary et al.9 criteria, the relative preservation of episodic memory is not an MBCI-FTD requirement. Inspection of the neuropsychological testing revealed amnestic impairments in a subset of participants, and there are many reports of memory problems in early bvFTD.7,17,29 Memory complaints are common in early bvFTD,11 but may not necessarily be reflective of true amnesia (e.g. word finding difficulties are often reported as memory problems, even if they reflect language dysfunction). We included the neuropsychological profile as a supportive feature, given its relatively low specificity. Additional neuropsychological tests of executive function and language (e.g. Hayling Test,64 spontaneous speech), or clinical use of more sensitive cognitive tests (e.g. NIH-EXAMINER),65 would perhaps allow for a better defined neuropsychological profile more specific to bvFTD.
With regard to social cognition: quantifying social cognitive deficits in bvFTD remains a flourishing research endeavour. It is challenging because there is wide variability in the general population, leading to difficulty establishing normative cut-offs; recent studies suggest that longitudinal changes on tests such as the RSMS may be more informative than a score at a single point in time.54 In addition, many tests are highly specific to certain cultures, rendering poor generalizability. In the current study, we only had access to a limited set of questionnaires, and therefore, in the MBCI-FTD criteria, we suggest that reduced social cognition can be at least partially captured by tests gauging understanding of social expectation violations or sensitivity to socio-emotional cues. However, we did not have any measures gauging other aspects of social cognition such as emotion processing, or indeed any non-questionnaire tests of social cognition, many of which are useful in quantifying social cognitive deficits in bvFTD. Examples include the Social Cognition and Emotional Assessment (SEA or mini-SEA),66,67 The Awareness of Social Inference Test,68–70 or Theory of Mind tests (e.g. Frith-Happé animations71,72). We leave open the possibility that the social cognition criterion (Criterion 2: J) may in the future include objective impairments in other facets of social cognition, or on other measures capturing the aspects of social cognition examined in the current study.
Unexpectedly, hallucinations or delusions did not appear to be a feature of prodromal bvFTD in our cohort. Psychotic features are relatively common in FTD patients with C9orf72 or GRN mutations,14,52,73–79 and psychosis may precede other FTD symptoms by years.76,80,81 However, in the Development Group only three participants reported delusions and/or hallucinations. Even in the Validation Group, which was more heterogeneous than the Development Group, none of the participants experienced hallucinations, and only two (4%) had delusional thoughts. This should be explored further, and regional differences should be taken into account (e.g. UK versus USA). The presence of adult-onset psychotic features should not necessarily guide clinicians away from an FTD workup.
We emphasize that the ideal definitions of the neuropsychiatric/behavioural features remain unknown, and different instruments define features differently. Broad features may end up as a ‘catch all’ for many behavioural changes: for example, disinhibition may include gambling, reckless driving, approaching strangers, offensive joking, wearing malodorous clothing, and stealing. In some cases, focal features may be included under the umbrella of the broader feature (e.g. excessive joviality classified as disinhibition). We separated out features where we could, as a movement away from broad definitions and towards more specific, and potentially more clinically useful, definitions of pathological behaviour. Refining definitions will aid in distinguishing FTD from psychiatric disorders (e.g. disinhibition in bipolar disorder is different from disinhibition in FTD),82 as well as other neurodegenerative disorders, as neuropsychiatric features are common across neurodegenerative diseases.83,84 Similarly, more refined operational definitions (e.g. apathy versus anhedonia62) may aid the development of precise measures of these features, and indeed more accurate clinicopathological correlations.
These preliminary criteria come with caveats regarding their sensitivity and specificity. First, the relatively small Development Group sample size, and the limited availability of the clinical notes, opens the possibility that some features were ‘missed’. We attempted to mitigate this by conducting full reviews of all available data in the whole prodromal bvFTD group, but the possibility remains. The sample size also contributed to uncertainty in the estimated sensitivity values (i.e. wide confidence intervals). Thus, it will be of high importance to establish the sensitivity of these criteria in larger cohorts of prodromal bvFTD, such as the GENFI study, and particularly in sporadic disease. Our sample of presumed sporadic bvFTD cases was small because we required pathological confirmation of FTLD and a clinical evaluation during the disease prodrome, and such cases are rare. Nonetheless, the results were promising as the criteria performed well in classifying this sample (71%). Furthermore, although we established the specificity of the criteria against prodromal Alzheimer’s disease, a longer-term goal should be to establish their utility in differentiating between prodromal bvFTD and other rarer neurodegenerative diseases, as well as primary psychiatric disorders. Positive and negative predictive value of these criteria will need to be prospectively determined in various clinical settings. We were bound by the available data in this study, and that led to a dichotomous present versus absent scoring system; it is possible that an ‘uncertain’ rating might have improved the sensitivity of the criteria.
It is also worth highlighting that there is significant overlap in clinical features between FTLD phenotypes, and that the boundaries between diagnostic categories are often blurred; for example, disinhibition and social cognitive dysfunction are commonly present in primary progressive aphasia and progressive supranuclear palsy, and motor dysfunction (e.g. parkinsonism) may develop in bvFTD.85 The MBCI-FTD criteria were developed using patients who went on to be diagnosed with bvFTD per the Rascovsky et al.10 criteria, but there is a high chance that the MBCI-FTD criteria will diagnose individuals who will ultimately also end up with other FTLD disorders. Thus, although we use the term ‘bvFTD’ in discussing this prodromal state, as this is the currently accepted terminology in the field, we expect the criteria to be applicable to individuals exhibiting the behavioural syndrome associated with FTD, and not necessarily limited to the subset who will receive a ‘pure’, categorical diagnosis of bvFTD.
Finally, we were not able to evaluate the role of biomarkers in this study, and the biomarker criterion (Criterion 3: B3) is intended to capture a wide range of biomarkers that may become available in the future. As biomarker research continues to rapidly advance, these criteria can undergo revision, and clinical feature requirements may be relaxed; for example, two clinical features may be sufficient for an MBCI-FTD diagnosis in the context of strong biomarker evidence. This is a proposition especially worth considering for clinical trial enrolment when a genetic mutation is present (see Supplementary Table 5 for sensitivity and specificity analyses of the criteria when two features are required).
We have proposed the first preliminary diagnostic criteria for prodromal bvFTD, or MBCI-FTD, leveraging data from one of the largest prodromal bvFTD cohorts reported to date. The criteria correctly classified 95% of the prodromal bvFTD group on which they were developed, and 74% of a separate, more heterogeneous cohort (Validation Group). False positive rates were low, in both healthy controls (7–10%) and individuals with prodromal Alzheimer’s disease (11–16%). These criteria represent a step towards defining a clinical prodrome of bvFTD, and will be valuable for clinical trial enrolment, and for early diagnosis and counselling. Future research should prioritize validation in other, larger cohorts.
Acknowledgements
The authors acknowledge the invaluable contributions of the study participants and families as well as the assistance of the support staffs at each of the participating sites. The manuscript has been reviewed by the ALLFTD Executive Committee for scientific content.
Funding
Data collection and dissemination of the data presented in this manuscript were supported by the ALLFTD Consortium (U19 AG063911, PIs B.F.B., H.J.R., A.L.B.) funded by the National Institute on Aging and the National Institute of Neurological Disorders and Stroke, and the former ARTFL & LEFFTDS Consortia (ARTFL: U54 NS092089, PI A.L.B., funded by the National Institute of Neurological Disorders and Stroke and National Center for Advancing Translational Sciences; LEFFTDS: U01 AG045390, PIs B.F.B., H.J.R., funded by the National Institute on Aging and the National Institute of Neurological Disorders and Stroke), as well as the ESFTLD study (R01 NS076837, PIs S.C. and E.D.H., funded by the National Institute of Neurological Disorders and Stroke). The NACC database is funded by National Institute on Aging/National Institutes of Health Grant U24 AG072122. NACC data are contributed by the National Institute on Aging funded ADRCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Competing interests
B.S.A. has received research funding from CDC, National Institutes of Health, Ionis, & Alector. Consulting for Acadia and Ionis. Royalties from Wolters Kluwer. B.C.D.: Research support from the National Institutes of Health, Alzheimer’s Drug Discovery Foundation, consulting for Acadia, Arkuda, Axovant, Lilly, Biogen, Merck, Novartis, Wave LifeSciences. Editorial duties with payment for Elsevier (Neuroimage: Clinical and Cortex). Royalties from Oxford University Press and Cambridge University Press. K.D-R. receives research support from the National Institutes of Health and Lawson Health Research Institute. Receives speaker fees from MedBridge. J.A.F. receives research funding from the National Institutes of Health. D.R.G. is a paid consultant for Biogen, vTv Pharmaceuticals, Cognition Theraptutics, Fujirebio and Amprion and received payment as a journal Editor from Springer. N.G. has participated or is currently participating in clinical trials of anti-dementia drugs sponsored by the following companies: Bristol Myers Squibb, Lilly/Avid Radiopharmaceuticals, Janssen, Novartis, Pfizer, Wyeth, SNIFF (The Study of Nasal Insulin to Fight Forgetfulness) study, and A4 (The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease) trial. She receives research support from Tau Consortium and Association for Frontotemporal Dementia and is funded by the National Institutes of Health. J.G. receives research support from the National Institutes of Health, Huntington’s Disease Society of America, New York State Department of Health (RFA #1510130358). N.R.G-R. takes part in multicenter studies funded by Biogen, AbbVie, and Lilly. G-Y.H. has received research support as a clinical trials site investigator from Anavax, Biogen, Eli Lilly and Roche; and has received research funding from the CIHR, Alzheimer Society of Canada, and the National Institutes of Health. D.S.K. has served on a Data Safety Monitoring Board for the DIAN study. He serves on a Data Safety monitoring Board for a tau therapeutic for Biogen, but receives no personal compensation. He is a site investigator in the Biogen aducanumab trials. He is an investigator in a clinical trials sponsored by Lilly Pharmaceuticals and the University of Southern California. He serves as a consultant for Samus Therapeutics, Third Rock, Roche and Alzeca Biosciences but receives no personal compensation. He receives research support from the National Institutes of Health. J.K. has provided expert witness testimony for 1) Teva Pharmaceuticals in Forest Laboratories Inc. et al. v. Teva Pharmaceuticals USA, Inc., Case Nos. 1:14-cv-00121 and 1:14-cv-00686 (D. Del. filed 31 Jan. 2014 and 30 May 2014) regarding the drug Memantine; 2) for Apotex/HEC/Ezra in Novartis AG et al. v. Apotex Inc., No. 1:15-cv-975 (D. Del. filed Oct. 26, 2015, regarding the drug Fingolimod; 3) on behalf of Puma Biotechnology in Hsingching Hsu et al, versus Puma Biotechnology, INC., et al. 2018 regarding the drug Neratinib and 4) on behalf of Hikma Pharmaceuticals in Amarin Pharma, Inc versus Hikma Pharmaceuticals in 2019. He receives research support from the NIH. I.L.: Research is supported by the National Institutes of Health; Parkinson Study Group, Michael J Fox Foundation, Parkinson Foundation, Lewy Body Association, Parkinson Foundation, Roche, Abbvie, Biogen, EIP-Pharma and Biohaven Pharmaceuticals. She was member of a Lundbeck Advisory Board and Corticobasal Degeneration Solutions. She receives her salary from the University of California San Diego and as Chief Editor of Frontiers in Neurology. I.R.M.: Scientific advisory board member for Prevail Therapeutics. J.C.M. receives personal fees from GE Healthcare, grants and personal fees from Eli Lilly, grants from Acadia, Avanir, Biogen, Eisai, Janssen, NIH, Novartis, with no relation to the submitted work. A.M.S. receives research funding from the NIA-NIH, the Bluefield Project to Cure Frontotemporal Dementia, and the Larry L. Hillblom Foundation. B.F.B. has served as an investigator for clinical trials sponsored by GE Healthcare and Axovant. He receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from the National Institutes of Health, the Mayo Clinic Dorothy and Harry T. Mangurian Jr Lewy Body Dementia Program and the Little Family Foundation. A.L.B. receives research support from the National Institutes of Health, the Tau Research Consortium, the Association for Frontotemporal Degeneration and the Bluefield Project to Cure Frontotemporal Dementia. He has served as a consultant for AGTC, Alector, Arkuda, Arvinas, Bioage, Ionis, Lundbeck, Passage BIO, Samumed, Ono, Sangamo, Stealth, Transposon, UCBand Wave, and received research support from Avid, Eisai, Biogen and Roche. H.R. has received research support from Biogen Pharmaceuticals, has consulting agreements with Wave Neuroscience and Ionis Pharmaceuticals, and receives research support from the National Institutes of Health. K.P.R. reports funding from the National Institutes of Health, Quest Diagnostics, the Rainwater Charitable Foundation, and the Marcus Foundation. The other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Appendix I
Definition and operationalization of each feature included in the proposed MBCI-FTD criteria.
| Feature . | MBCI-FTD criterion definition . |
|---|---|
| . | Important that each of these features represents a change from previous functioning . |
| Apathy without moderate-severe dysphoria | Apathy is defined as a lack of interest in or indifference towards usual or previously rewarding activities (e.g. the patient may no longer be interested in hobbies), reduced interest in the activities of others, a loss of motivation, a lack of spontaneity, decreased initiation of activities or social interactions (e.g. the patient may require prompting to finish a task, does not begin or sustain conversations with family or friends), social withdrawal, a loss of drive. This criterion should not be considered present if the patient reports moderate-severe dysphoria (per self-report or self-completed questionnaire, such as the Geriatric Depression Scale or the Beck Depression Inventory). We strongly caution against using caregiver or informant reports of depression here, because a lack of motivation may be interpreted by family members as depressed mood. |
| Behavioural disinhibition | Behavioural disinhibition may occur in or out of the social context, and may manifest as: impulsive, rash or careless actions (e.g. extreme spending, gambling, reckless driving, stealing, sharing confidential information such as a credit card number, talking to strangers as though they are friends, touching strangers), socially inappropriate behaviour (e.g. using inappropriate coarse or rude language, inappropriate laughing, offensive jokes, sexually-explicit or hurtful comments), a loss of manners or decorum (e.g. cutting in line, belching, picking teeth), or a disregard for personal hygiene (e.g. wearing stained clothing). Behavioural disinhibition applies even if one understands and regrets an action. Note that if the patient is displaying excessive joviality, this should not be counted under disinhibition but rather as its own feature. |
| Irritability/agitation | Irritable or agitated patients tend to be overreactive, labile, impatient, or ‘cranky’. They may be resistant to help and hard to handle at times, and may shout at family members or others, or even hit or kick. Patients experiencing irritability or agitation may have difficulty coping with delays or waiting for planned activities. Caregivers might describe labile patients as being ‘quick to anger’ or ‘flying off the handle’. Mild irritability that does not represent a significant decline or change in behaviour should not be included here. This is distinct from pseudobulbar affect, which is not part of this criterion. |
| Repetitive behaviours (simple and complex) | Repetitive behaviours may be simple or complex in nature. Simple perseverative behaviours might include: tapping, pacing, fidgeting, wrapping string, handling buttons or other small objects, rubbing, clapping, humming, rocking, lip pursing, lip smacking, picking or scratching, throat clearing. Complex perseverative behaviours include compulsive and/or ritualistic behaviours. Examples include: collecting objects, hoarding, counting, cleaning rituals, walking fixed routes, lining up objects in a particular order, checking. |
| Joviality/gregariousness | Patients who display joviality or gregariousness may be described as being more jocular, outgoing, friendly, or jolly than usual. The patient may act excessively happy or be overly sociable, and may appear to ‘feel too good’. |
| Appetite changes/hyperorality | In the MBCI-FTD criteria, appetite changes may be present in either direction [hyperphagia (overeating), or hypophagia (undereating)], as per the Neuropsychiatric Inventory. However, based on clinical experience, we highlight that hyperphagia is the more common appetite change in prodromal bvFTD, and it is rare for a prodromal bvFTD patient to be hypophagic, unless there is concomitant amyotrophic lateral sclerosis (ALS). Appetite changes may manifest as an increased preference for certain types of food, particularly sweet foods or carbohydrates, or may display rigid food preferences. Patients may engage in binge eating, and in some cases gain significant amounts of weight. (Note, however, that in cases of ALS weight loss may be observed.). Patients may increase their consumption of alcohol or cigarettes. Hyperorality, or the tendency to want to put objects in the mouth, may also be observed. |
| Reduced empathy or sympathy | Reduced empathy or sympathy is defined as a reduced ability to read others’ emotional cues or understand another’s point of view. It may manifest as a diminished responsiveness to others’ feelings or needs, or a lack or personal warmth. Patients may appear indifferent to the feelings of others, or display a lack of regard for others’ distress (affective empathy). The ability to take the perspective of others is an important aspect of empathy (cognitive empathy), and therefore patients who have difficulty ‘seeing things from someone else’s point of view’ are considered to have poor empathy. Reduced social engagement is also a common presentation of reduced empathy, though care should be taken to ensure that this is not simply due to apathy (a lack of motivation to engage). Caregivers may report that the patient who lacks empathy is ‘emotionally distant’. In terms of gathering this information in a clinical context, we strongly suggest that reduced empathy or sympathy is ascertained from clinical interview with a caregiver or informant. Questionnaires (such as the Interpersonal Reactivity Index Empathic Concern or Perspective Taking subscale) may be used for informant report, but we highlight that scores should be interpreted against appropriate normative data. |
| Reduced insight | Reduced insight can be ascertained by a discrepancy between the reports of caregivers or informants and patients themselves. The patient may exhibit poor insight for cognitive changes, behavioural changes, or both. Patients with motor symptoms (e.g. from ALS) may deny or minimize these symptoms. A lack of insight into any behavioural or cognitive change is enough for this feature to be present. Importantly, the clinician should make a judgement on the reliability of the informant; if the informant has very little contact with the patient, or if it appears they may be overestimating symptoms because of their own mental state (e.g. high stress or anxiety over diagnosis), their report may be given less weight. If no informant is available, clinicians should be careful in marking this criterion as present. |
| Neuropsychological profile | The neuropsychological profile of MBCI-FTD is defined as a clinical impairment on executive function tests (e.g. set-shifting, letter fluency, cognitive inhibition, abstract reasoning, planning, etc.) or naming tests, in the context of intact or relatively preserved time/place orientation and visuospatial skills. If there is an impairment or relative weakness in orientation or visuospatial functioning, this criterion is not met. We acknowledge that although in the MBCI-FTD criteria a clinical impairment on at least one test is required (demographically-adjusted z-score ≤ −1.5), clinically significant relative impairments, especially if observed across multiple tests within the same domain, should not be discounted. Likewise, change from previous cognitive functioning or from estimated prior functioning should be considered. However, this judgment should only be made by trained clinical neuropsychologists. We also caution against using screening tests, such as the Mini-Mental State Examination, as the sole tool to determine the neuropsychological profile of the patient. Finally, given the pervasiveness of executive dysfunction, and its tendency to affect performance in other cognitive domains (e.g. complex figure drawing, memory testing), we strongly advise that this criterion be applied based on the judgment of a clinical neuropsychologist, and not subject or informant complaints. |
| Poor social cognition | The ‘poor social cognition’ criterion should only be applied if there is meaningfully reduced performance on a validated measure of social cognition. In developing the MBCI-FTD criteria, we examined only two aspects of social cognition: understanding of social expectations and socioemotional sensitivity. Reduced understanding of social expectations refers to a lack of ‘social semantic knowledge’, or a lack of knowledge of the contexts in which certain behaviours are appropriate, and specifically refers to a tendency to break social rules. For example, indicating that it is acceptable to laugh when someone else trips and falls. The endorsement of breaking multiple social norms is particularly specific to FTD. In the current study we have used the Social Norms Questionnaire, but note that this instrument is highly specific to North American culture and is not necessarily suitable for use outside North America unless it is adapted. Poor socioemotional sensitivity refers to a sensitivity and responsiveness to subtle emotional expressions during face-to-face interactions, for example having the ability to control how one comes across to others depending on the impression they want to give. Socioemotional sensitivity can be measured with the Revised Self-Monitoring Scale. We highlight that it is likely that there are other aspects of social cognition (e.g. theory of mind) that will prove to be useful for this criterion, and we strongly recommend that future studies consider using other social cognition tools in the context of the MBCI-FTD criteria. This criterion will benefit from future refinement, and we intend for this criterion to encompass impairments on social cognition tasks beyond the two tests we had access to in the current study Caution is recommended when assessing social cognition, as this ability varies widely in the general population; therefore, special care must be taken to ensure that this represents a change from previous functioning. |
| Feature . | MBCI-FTD criterion definition . |
|---|---|
| . | Important that each of these features represents a change from previous functioning . |
| Apathy without moderate-severe dysphoria | Apathy is defined as a lack of interest in or indifference towards usual or previously rewarding activities (e.g. the patient may no longer be interested in hobbies), reduced interest in the activities of others, a loss of motivation, a lack of spontaneity, decreased initiation of activities or social interactions (e.g. the patient may require prompting to finish a task, does not begin or sustain conversations with family or friends), social withdrawal, a loss of drive. This criterion should not be considered present if the patient reports moderate-severe dysphoria (per self-report or self-completed questionnaire, such as the Geriatric Depression Scale or the Beck Depression Inventory). We strongly caution against using caregiver or informant reports of depression here, because a lack of motivation may be interpreted by family members as depressed mood. |
| Behavioural disinhibition | Behavioural disinhibition may occur in or out of the social context, and may manifest as: impulsive, rash or careless actions (e.g. extreme spending, gambling, reckless driving, stealing, sharing confidential information such as a credit card number, talking to strangers as though they are friends, touching strangers), socially inappropriate behaviour (e.g. using inappropriate coarse or rude language, inappropriate laughing, offensive jokes, sexually-explicit or hurtful comments), a loss of manners or decorum (e.g. cutting in line, belching, picking teeth), or a disregard for personal hygiene (e.g. wearing stained clothing). Behavioural disinhibition applies even if one understands and regrets an action. Note that if the patient is displaying excessive joviality, this should not be counted under disinhibition but rather as its own feature. |
| Irritability/agitation | Irritable or agitated patients tend to be overreactive, labile, impatient, or ‘cranky’. They may be resistant to help and hard to handle at times, and may shout at family members or others, or even hit or kick. Patients experiencing irritability or agitation may have difficulty coping with delays or waiting for planned activities. Caregivers might describe labile patients as being ‘quick to anger’ or ‘flying off the handle’. Mild irritability that does not represent a significant decline or change in behaviour should not be included here. This is distinct from pseudobulbar affect, which is not part of this criterion. |
| Repetitive behaviours (simple and complex) | Repetitive behaviours may be simple or complex in nature. Simple perseverative behaviours might include: tapping, pacing, fidgeting, wrapping string, handling buttons or other small objects, rubbing, clapping, humming, rocking, lip pursing, lip smacking, picking or scratching, throat clearing. Complex perseverative behaviours include compulsive and/or ritualistic behaviours. Examples include: collecting objects, hoarding, counting, cleaning rituals, walking fixed routes, lining up objects in a particular order, checking. |
| Joviality/gregariousness | Patients who display joviality or gregariousness may be described as being more jocular, outgoing, friendly, or jolly than usual. The patient may act excessively happy or be overly sociable, and may appear to ‘feel too good’. |
| Appetite changes/hyperorality | In the MBCI-FTD criteria, appetite changes may be present in either direction [hyperphagia (overeating), or hypophagia (undereating)], as per the Neuropsychiatric Inventory. However, based on clinical experience, we highlight that hyperphagia is the more common appetite change in prodromal bvFTD, and it is rare for a prodromal bvFTD patient to be hypophagic, unless there is concomitant amyotrophic lateral sclerosis (ALS). Appetite changes may manifest as an increased preference for certain types of food, particularly sweet foods or carbohydrates, or may display rigid food preferences. Patients may engage in binge eating, and in some cases gain significant amounts of weight. (Note, however, that in cases of ALS weight loss may be observed.). Patients may increase their consumption of alcohol or cigarettes. Hyperorality, or the tendency to want to put objects in the mouth, may also be observed. |
| Reduced empathy or sympathy | Reduced empathy or sympathy is defined as a reduced ability to read others’ emotional cues or understand another’s point of view. It may manifest as a diminished responsiveness to others’ feelings or needs, or a lack or personal warmth. Patients may appear indifferent to the feelings of others, or display a lack of regard for others’ distress (affective empathy). The ability to take the perspective of others is an important aspect of empathy (cognitive empathy), and therefore patients who have difficulty ‘seeing things from someone else’s point of view’ are considered to have poor empathy. Reduced social engagement is also a common presentation of reduced empathy, though care should be taken to ensure that this is not simply due to apathy (a lack of motivation to engage). Caregivers may report that the patient who lacks empathy is ‘emotionally distant’. In terms of gathering this information in a clinical context, we strongly suggest that reduced empathy or sympathy is ascertained from clinical interview with a caregiver or informant. Questionnaires (such as the Interpersonal Reactivity Index Empathic Concern or Perspective Taking subscale) may be used for informant report, but we highlight that scores should be interpreted against appropriate normative data. |
| Reduced insight | Reduced insight can be ascertained by a discrepancy between the reports of caregivers or informants and patients themselves. The patient may exhibit poor insight for cognitive changes, behavioural changes, or both. Patients with motor symptoms (e.g. from ALS) may deny or minimize these symptoms. A lack of insight into any behavioural or cognitive change is enough for this feature to be present. Importantly, the clinician should make a judgement on the reliability of the informant; if the informant has very little contact with the patient, or if it appears they may be overestimating symptoms because of their own mental state (e.g. high stress or anxiety over diagnosis), their report may be given less weight. If no informant is available, clinicians should be careful in marking this criterion as present. |
| Neuropsychological profile | The neuropsychological profile of MBCI-FTD is defined as a clinical impairment on executive function tests (e.g. set-shifting, letter fluency, cognitive inhibition, abstract reasoning, planning, etc.) or naming tests, in the context of intact or relatively preserved time/place orientation and visuospatial skills. If there is an impairment or relative weakness in orientation or visuospatial functioning, this criterion is not met. We acknowledge that although in the MBCI-FTD criteria a clinical impairment on at least one test is required (demographically-adjusted z-score ≤ −1.5), clinically significant relative impairments, especially if observed across multiple tests within the same domain, should not be discounted. Likewise, change from previous cognitive functioning or from estimated prior functioning should be considered. However, this judgment should only be made by trained clinical neuropsychologists. We also caution against using screening tests, such as the Mini-Mental State Examination, as the sole tool to determine the neuropsychological profile of the patient. Finally, given the pervasiveness of executive dysfunction, and its tendency to affect performance in other cognitive domains (e.g. complex figure drawing, memory testing), we strongly advise that this criterion be applied based on the judgment of a clinical neuropsychologist, and not subject or informant complaints. |
| Poor social cognition | The ‘poor social cognition’ criterion should only be applied if there is meaningfully reduced performance on a validated measure of social cognition. In developing the MBCI-FTD criteria, we examined only two aspects of social cognition: understanding of social expectations and socioemotional sensitivity. Reduced understanding of social expectations refers to a lack of ‘social semantic knowledge’, or a lack of knowledge of the contexts in which certain behaviours are appropriate, and specifically refers to a tendency to break social rules. For example, indicating that it is acceptable to laugh when someone else trips and falls. The endorsement of breaking multiple social norms is particularly specific to FTD. In the current study we have used the Social Norms Questionnaire, but note that this instrument is highly specific to North American culture and is not necessarily suitable for use outside North America unless it is adapted. Poor socioemotional sensitivity refers to a sensitivity and responsiveness to subtle emotional expressions during face-to-face interactions, for example having the ability to control how one comes across to others depending on the impression they want to give. Socioemotional sensitivity can be measured with the Revised Self-Monitoring Scale. We highlight that it is likely that there are other aspects of social cognition (e.g. theory of mind) that will prove to be useful for this criterion, and we strongly recommend that future studies consider using other social cognition tools in the context of the MBCI-FTD criteria. This criterion will benefit from future refinement, and we intend for this criterion to encompass impairments on social cognition tasks beyond the two tests we had access to in the current study Caution is recommended when assessing social cognition, as this ability varies widely in the general population; therefore, special care must be taken to ensure that this represents a change from previous functioning. |
| Feature . | MBCI-FTD criterion definition . |
|---|---|
| . | Important that each of these features represents a change from previous functioning . |
| Apathy without moderate-severe dysphoria | Apathy is defined as a lack of interest in or indifference towards usual or previously rewarding activities (e.g. the patient may no longer be interested in hobbies), reduced interest in the activities of others, a loss of motivation, a lack of spontaneity, decreased initiation of activities or social interactions (e.g. the patient may require prompting to finish a task, does not begin or sustain conversations with family or friends), social withdrawal, a loss of drive. This criterion should not be considered present if the patient reports moderate-severe dysphoria (per self-report or self-completed questionnaire, such as the Geriatric Depression Scale or the Beck Depression Inventory). We strongly caution against using caregiver or informant reports of depression here, because a lack of motivation may be interpreted by family members as depressed mood. |
| Behavioural disinhibition | Behavioural disinhibition may occur in or out of the social context, and may manifest as: impulsive, rash or careless actions (e.g. extreme spending, gambling, reckless driving, stealing, sharing confidential information such as a credit card number, talking to strangers as though they are friends, touching strangers), socially inappropriate behaviour (e.g. using inappropriate coarse or rude language, inappropriate laughing, offensive jokes, sexually-explicit or hurtful comments), a loss of manners or decorum (e.g. cutting in line, belching, picking teeth), or a disregard for personal hygiene (e.g. wearing stained clothing). Behavioural disinhibition applies even if one understands and regrets an action. Note that if the patient is displaying excessive joviality, this should not be counted under disinhibition but rather as its own feature. |
| Irritability/agitation | Irritable or agitated patients tend to be overreactive, labile, impatient, or ‘cranky’. They may be resistant to help and hard to handle at times, and may shout at family members or others, or even hit or kick. Patients experiencing irritability or agitation may have difficulty coping with delays or waiting for planned activities. Caregivers might describe labile patients as being ‘quick to anger’ or ‘flying off the handle’. Mild irritability that does not represent a significant decline or change in behaviour should not be included here. This is distinct from pseudobulbar affect, which is not part of this criterion. |
| Repetitive behaviours (simple and complex) | Repetitive behaviours may be simple or complex in nature. Simple perseverative behaviours might include: tapping, pacing, fidgeting, wrapping string, handling buttons or other small objects, rubbing, clapping, humming, rocking, lip pursing, lip smacking, picking or scratching, throat clearing. Complex perseverative behaviours include compulsive and/or ritualistic behaviours. Examples include: collecting objects, hoarding, counting, cleaning rituals, walking fixed routes, lining up objects in a particular order, checking. |
| Joviality/gregariousness | Patients who display joviality or gregariousness may be described as being more jocular, outgoing, friendly, or jolly than usual. The patient may act excessively happy or be overly sociable, and may appear to ‘feel too good’. |
| Appetite changes/hyperorality | In the MBCI-FTD criteria, appetite changes may be present in either direction [hyperphagia (overeating), or hypophagia (undereating)], as per the Neuropsychiatric Inventory. However, based on clinical experience, we highlight that hyperphagia is the more common appetite change in prodromal bvFTD, and it is rare for a prodromal bvFTD patient to be hypophagic, unless there is concomitant amyotrophic lateral sclerosis (ALS). Appetite changes may manifest as an increased preference for certain types of food, particularly sweet foods or carbohydrates, or may display rigid food preferences. Patients may engage in binge eating, and in some cases gain significant amounts of weight. (Note, however, that in cases of ALS weight loss may be observed.). Patients may increase their consumption of alcohol or cigarettes. Hyperorality, or the tendency to want to put objects in the mouth, may also be observed. |
| Reduced empathy or sympathy | Reduced empathy or sympathy is defined as a reduced ability to read others’ emotional cues or understand another’s point of view. It may manifest as a diminished responsiveness to others’ feelings or needs, or a lack or personal warmth. Patients may appear indifferent to the feelings of others, or display a lack of regard for others’ distress (affective empathy). The ability to take the perspective of others is an important aspect of empathy (cognitive empathy), and therefore patients who have difficulty ‘seeing things from someone else’s point of view’ are considered to have poor empathy. Reduced social engagement is also a common presentation of reduced empathy, though care should be taken to ensure that this is not simply due to apathy (a lack of motivation to engage). Caregivers may report that the patient who lacks empathy is ‘emotionally distant’. In terms of gathering this information in a clinical context, we strongly suggest that reduced empathy or sympathy is ascertained from clinical interview with a caregiver or informant. Questionnaires (such as the Interpersonal Reactivity Index Empathic Concern or Perspective Taking subscale) may be used for informant report, but we highlight that scores should be interpreted against appropriate normative data. |
| Reduced insight | Reduced insight can be ascertained by a discrepancy between the reports of caregivers or informants and patients themselves. The patient may exhibit poor insight for cognitive changes, behavioural changes, or both. Patients with motor symptoms (e.g. from ALS) may deny or minimize these symptoms. A lack of insight into any behavioural or cognitive change is enough for this feature to be present. Importantly, the clinician should make a judgement on the reliability of the informant; if the informant has very little contact with the patient, or if it appears they may be overestimating symptoms because of their own mental state (e.g. high stress or anxiety over diagnosis), their report may be given less weight. If no informant is available, clinicians should be careful in marking this criterion as present. |
| Neuropsychological profile | The neuropsychological profile of MBCI-FTD is defined as a clinical impairment on executive function tests (e.g. set-shifting, letter fluency, cognitive inhibition, abstract reasoning, planning, etc.) or naming tests, in the context of intact or relatively preserved time/place orientation and visuospatial skills. If there is an impairment or relative weakness in orientation or visuospatial functioning, this criterion is not met. We acknowledge that although in the MBCI-FTD criteria a clinical impairment on at least one test is required (demographically-adjusted z-score ≤ −1.5), clinically significant relative impairments, especially if observed across multiple tests within the same domain, should not be discounted. Likewise, change from previous cognitive functioning or from estimated prior functioning should be considered. However, this judgment should only be made by trained clinical neuropsychologists. We also caution against using screening tests, such as the Mini-Mental State Examination, as the sole tool to determine the neuropsychological profile of the patient. Finally, given the pervasiveness of executive dysfunction, and its tendency to affect performance in other cognitive domains (e.g. complex figure drawing, memory testing), we strongly advise that this criterion be applied based on the judgment of a clinical neuropsychologist, and not subject or informant complaints. |
| Poor social cognition | The ‘poor social cognition’ criterion should only be applied if there is meaningfully reduced performance on a validated measure of social cognition. In developing the MBCI-FTD criteria, we examined only two aspects of social cognition: understanding of social expectations and socioemotional sensitivity. Reduced understanding of social expectations refers to a lack of ‘social semantic knowledge’, or a lack of knowledge of the contexts in which certain behaviours are appropriate, and specifically refers to a tendency to break social rules. For example, indicating that it is acceptable to laugh when someone else trips and falls. The endorsement of breaking multiple social norms is particularly specific to FTD. In the current study we have used the Social Norms Questionnaire, but note that this instrument is highly specific to North American culture and is not necessarily suitable for use outside North America unless it is adapted. Poor socioemotional sensitivity refers to a sensitivity and responsiveness to subtle emotional expressions during face-to-face interactions, for example having the ability to control how one comes across to others depending on the impression they want to give. Socioemotional sensitivity can be measured with the Revised Self-Monitoring Scale. We highlight that it is likely that there are other aspects of social cognition (e.g. theory of mind) that will prove to be useful for this criterion, and we strongly recommend that future studies consider using other social cognition tools in the context of the MBCI-FTD criteria. This criterion will benefit from future refinement, and we intend for this criterion to encompass impairments on social cognition tasks beyond the two tests we had access to in the current study Caution is recommended when assessing social cognition, as this ability varies widely in the general population; therefore, special care must be taken to ensure that this represents a change from previous functioning. |
| Feature . | MBCI-FTD criterion definition . |
|---|---|
| . | Important that each of these features represents a change from previous functioning . |
| Apathy without moderate-severe dysphoria | Apathy is defined as a lack of interest in or indifference towards usual or previously rewarding activities (e.g. the patient may no longer be interested in hobbies), reduced interest in the activities of others, a loss of motivation, a lack of spontaneity, decreased initiation of activities or social interactions (e.g. the patient may require prompting to finish a task, does not begin or sustain conversations with family or friends), social withdrawal, a loss of drive. This criterion should not be considered present if the patient reports moderate-severe dysphoria (per self-report or self-completed questionnaire, such as the Geriatric Depression Scale or the Beck Depression Inventory). We strongly caution against using caregiver or informant reports of depression here, because a lack of motivation may be interpreted by family members as depressed mood. |
| Behavioural disinhibition | Behavioural disinhibition may occur in or out of the social context, and may manifest as: impulsive, rash or careless actions (e.g. extreme spending, gambling, reckless driving, stealing, sharing confidential information such as a credit card number, talking to strangers as though they are friends, touching strangers), socially inappropriate behaviour (e.g. using inappropriate coarse or rude language, inappropriate laughing, offensive jokes, sexually-explicit or hurtful comments), a loss of manners or decorum (e.g. cutting in line, belching, picking teeth), or a disregard for personal hygiene (e.g. wearing stained clothing). Behavioural disinhibition applies even if one understands and regrets an action. Note that if the patient is displaying excessive joviality, this should not be counted under disinhibition but rather as its own feature. |
| Irritability/agitation | Irritable or agitated patients tend to be overreactive, labile, impatient, or ‘cranky’. They may be resistant to help and hard to handle at times, and may shout at family members or others, or even hit or kick. Patients experiencing irritability or agitation may have difficulty coping with delays or waiting for planned activities. Caregivers might describe labile patients as being ‘quick to anger’ or ‘flying off the handle’. Mild irritability that does not represent a significant decline or change in behaviour should not be included here. This is distinct from pseudobulbar affect, which is not part of this criterion. |
| Repetitive behaviours (simple and complex) | Repetitive behaviours may be simple or complex in nature. Simple perseverative behaviours might include: tapping, pacing, fidgeting, wrapping string, handling buttons or other small objects, rubbing, clapping, humming, rocking, lip pursing, lip smacking, picking or scratching, throat clearing. Complex perseverative behaviours include compulsive and/or ritualistic behaviours. Examples include: collecting objects, hoarding, counting, cleaning rituals, walking fixed routes, lining up objects in a particular order, checking. |
| Joviality/gregariousness | Patients who display joviality or gregariousness may be described as being more jocular, outgoing, friendly, or jolly than usual. The patient may act excessively happy or be overly sociable, and may appear to ‘feel too good’. |
| Appetite changes/hyperorality | In the MBCI-FTD criteria, appetite changes may be present in either direction [hyperphagia (overeating), or hypophagia (undereating)], as per the Neuropsychiatric Inventory. However, based on clinical experience, we highlight that hyperphagia is the more common appetite change in prodromal bvFTD, and it is rare for a prodromal bvFTD patient to be hypophagic, unless there is concomitant amyotrophic lateral sclerosis (ALS). Appetite changes may manifest as an increased preference for certain types of food, particularly sweet foods or carbohydrates, or may display rigid food preferences. Patients may engage in binge eating, and in some cases gain significant amounts of weight. (Note, however, that in cases of ALS weight loss may be observed.). Patients may increase their consumption of alcohol or cigarettes. Hyperorality, or the tendency to want to put objects in the mouth, may also be observed. |
| Reduced empathy or sympathy | Reduced empathy or sympathy is defined as a reduced ability to read others’ emotional cues or understand another’s point of view. It may manifest as a diminished responsiveness to others’ feelings or needs, or a lack or personal warmth. Patients may appear indifferent to the feelings of others, or display a lack of regard for others’ distress (affective empathy). The ability to take the perspective of others is an important aspect of empathy (cognitive empathy), and therefore patients who have difficulty ‘seeing things from someone else’s point of view’ are considered to have poor empathy. Reduced social engagement is also a common presentation of reduced empathy, though care should be taken to ensure that this is not simply due to apathy (a lack of motivation to engage). Caregivers may report that the patient who lacks empathy is ‘emotionally distant’. In terms of gathering this information in a clinical context, we strongly suggest that reduced empathy or sympathy is ascertained from clinical interview with a caregiver or informant. Questionnaires (such as the Interpersonal Reactivity Index Empathic Concern or Perspective Taking subscale) may be used for informant report, but we highlight that scores should be interpreted against appropriate normative data. |
| Reduced insight | Reduced insight can be ascertained by a discrepancy between the reports of caregivers or informants and patients themselves. The patient may exhibit poor insight for cognitive changes, behavioural changes, or both. Patients with motor symptoms (e.g. from ALS) may deny or minimize these symptoms. A lack of insight into any behavioural or cognitive change is enough for this feature to be present. Importantly, the clinician should make a judgement on the reliability of the informant; if the informant has very little contact with the patient, or if it appears they may be overestimating symptoms because of their own mental state (e.g. high stress or anxiety over diagnosis), their report may be given less weight. If no informant is available, clinicians should be careful in marking this criterion as present. |
| Neuropsychological profile | The neuropsychological profile of MBCI-FTD is defined as a clinical impairment on executive function tests (e.g. set-shifting, letter fluency, cognitive inhibition, abstract reasoning, planning, etc.) or naming tests, in the context of intact or relatively preserved time/place orientation and visuospatial skills. If there is an impairment or relative weakness in orientation or visuospatial functioning, this criterion is not met. We acknowledge that although in the MBCI-FTD criteria a clinical impairment on at least one test is required (demographically-adjusted z-score ≤ −1.5), clinically significant relative impairments, especially if observed across multiple tests within the same domain, should not be discounted. Likewise, change from previous cognitive functioning or from estimated prior functioning should be considered. However, this judgment should only be made by trained clinical neuropsychologists. We also caution against using screening tests, such as the Mini-Mental State Examination, as the sole tool to determine the neuropsychological profile of the patient. Finally, given the pervasiveness of executive dysfunction, and its tendency to affect performance in other cognitive domains (e.g. complex figure drawing, memory testing), we strongly advise that this criterion be applied based on the judgment of a clinical neuropsychologist, and not subject or informant complaints. |
| Poor social cognition | The ‘poor social cognition’ criterion should only be applied if there is meaningfully reduced performance on a validated measure of social cognition. In developing the MBCI-FTD criteria, we examined only two aspects of social cognition: understanding of social expectations and socioemotional sensitivity. Reduced understanding of social expectations refers to a lack of ‘social semantic knowledge’, or a lack of knowledge of the contexts in which certain behaviours are appropriate, and specifically refers to a tendency to break social rules. For example, indicating that it is acceptable to laugh when someone else trips and falls. The endorsement of breaking multiple social norms is particularly specific to FTD. In the current study we have used the Social Norms Questionnaire, but note that this instrument is highly specific to North American culture and is not necessarily suitable for use outside North America unless it is adapted. Poor socioemotional sensitivity refers to a sensitivity and responsiveness to subtle emotional expressions during face-to-face interactions, for example having the ability to control how one comes across to others depending on the impression they want to give. Socioemotional sensitivity can be measured with the Revised Self-Monitoring Scale. We highlight that it is likely that there are other aspects of social cognition (e.g. theory of mind) that will prove to be useful for this criterion, and we strongly recommend that future studies consider using other social cognition tools in the context of the MBCI-FTD criteria. This criterion will benefit from future refinement, and we intend for this criterion to encompass impairments on social cognition tasks beyond the two tests we had access to in the current study Caution is recommended when assessing social cognition, as this ability varies widely in the general population; therefore, special care must be taken to ensure that this represents a change from previous functioning. |
Appendix II
ALLFTD Consortium members
ALLFTD consortium members who are not named authors are listed below. Full details are provided in the Supplementary material.
Tatiana Foroud, Daniel Kaufer, Walter Kremers, Gabriel Leger, Chiadi Onyike, Aaron Ritter, Erik D. Roberson, Sandra Weintraub.
References
Abbreviations
- ARTFL
Advancing Research and Treatment for Frontotemporal Lobar Degeneration
- (bv)FTD
(behavioural variant) frontotemporal dementia
- CDR
Clinical Dementia Rating;
- ESFTLD
Examination of the Earliest Symptoms and Biomarkers of Frontotemporal Lobar Degeneration in MAPT Carriers
- FTLD
frontotemporal lobar degeneration
- GDS
Geriatric Depression Scale
- LEFFTDS
Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects
- MBCI-FTD
mild behavioural and/or cognitive impairment in behavioural variant frontotemporal dementia
- MoCA
Montreal Cognitive Assessment
- NACC
National Alzheimer’s Coordinating Center
- RSMS
Revised Self-Monitoring Scale
- SNQ
Social Norms Questionnaire


