-
PDF
- Split View
-
Views
-
Cite
Cite
Kishore R Kumar, Andrea Cortese, Susan E Tomlinson, Stephanie Efthymiou, Melina Ellis, Danqing Zhu, Marion Stoll, Natalia Dominik, Stephen Tisch, Michel Tchan, Kathy H C Wu, Sophie Devery, Penelope J Spring, Simon Hawke, Phillip Cremer, Karl Ng, Mary M Reilly, Garth A Nicholson, Henry Houlden, Marina Kennerson, RFC1 expansions can mimic hereditary sensory neuropathy with cough and Sjögren syndrome, Brain, Volume 143, Issue 10, October 2020, Page e82, https://doi.org/10.1093/brain/awaa244
Close - Share Icon Share
We read with great interest the article by Cortese and colleagues (2020) describing 100 carriers of the RFC1 expansion. This study explores the phenotypic spectrum of RFC1 expansions, identified as a cause of cerebellar ataxia, neuropathy, and vestibular areflexia syndrome (CANVAS) and late onset ataxia (Cortese et al., 2019; Rafehi et al., 2019). They concluded that RFC1 should be considered in all cases of sensory ataxic neuropathy, especially if there are manifestations of cerebellar impairment, vestibular dysfunction, and cough.
In collaboration with this group, we assessed for RFC1 expansions in a cohort from Sydney, Australia, to further explore the phenotypic spectrum. We recruited probands without a genetic diagnosis and with a phenotype of hereditary cerebellar ataxia (HCA), hereditary peripheral neuropathy, or CANVAS, including archival cases. Participants were recruited through the Molecular Medicine Laboratory, Concord Repatriation General Hospital. Subjects’ consent was obtained according to the Declaration of Helsinki. The study was approved by the Sydney Local Health District Human Ethics Committee (HREC/17/CRGH/8).
DNA was extracted using standard techniques and forwarded for RFC1 analysis to UCL Queen Square Institute of Neurology and The National Hospital for Neurology, London, UK. Individuals had repeat primed PCR to detect the pathogenic (AAGGG) and non-pathogenic (AAAGG or AAAAG) repeat expansions, as described (Cortese et al., 2019). Confirmation of the repeat expansion and determination of the size was performed using Southern blot analysis.
We investigated 17 probands with a variety of initial diagnoses including HCA without sensory neuropathy (n = 5), HCA with sensory neuropathy (n = 2), hereditary sensory neuropathy (HSN, n = 4), CANVAS (n = 2), Sjögren’s ataxic sensory neuropathy (n = 1), paraneoplastic neuropathy (n = 1), inherited spastic ataxia with neuropathy (n = 1), and sensory ataxic neuropathy (n = 1). The age at onset was 56.5 ± 11.6 years, age at examination was 66.1 ± 10.3 years (mean ± standard deviation). The sample included eight males and nine females. There was a positive family history in 10/17, sensory neuronopathy on clinical examination in 11/17, sensory neuronopathy on nerve conduction studies in 11/17 (11/14 tested), documented abolished vestibuloocular reflex on head impulse test (HIT) in 7/17, documented bilateral vestibular areflexia on vestibular testing in 4/17, cerebellar dysfunction on clinical examination in 13/17, cerebellar atrophy on MRI in 6/17, cough documented in 8/17 and sural biopsies performed in 2/17. An affected relative was available for study in one subject only (proband Patient R190103).
We identified a biallelic pathogenic AAGGG expansion in 5 of 17 individuals using repeat primed PCR, confirmed on Southern blot analysis (Fig. 1).
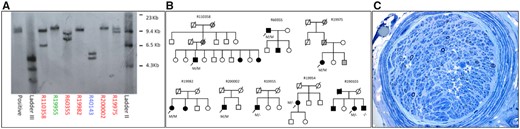
Southern blot results, pedigrees and sural nerve biopsy findings. (A) Southern blot results confirming RCF1 biallelic AAGGG expansions. The number of repeats for each allele are shown in parentheses. Patients R19975 (821, 1015), R19982 (1070, 1070), R110358 (292, 1192), R60355 (526, 821), and R200002 (1128, 1128). Patient R19955 was heterozygous for the AAGGG expansion (1325). Patient R40143 was positive for the repeat primed (AAAAG) PCR, which are non-pathogenic expansions. We considered pathogenic repeat expansions >250. (B) Pedigrees of families with biallelic pathogenic RCF1 expansions. Black filled symbols indicate affected individuals. Grey filled symbols indicate family members whose affected status is uncertain. Arrow indicates proband. Diagonal line indicates deceased individuals. M = allele with pathogenic RFC1 expansion; a dash indicates an allele without pathogenic RFC1 expansion. (C) Right sural nerve biopsy (Toluidine blue) from Patient R19977 showing a severe reduction in myelinated fibre density with only a few large myelinated fibres remaining in most fascicles. There were no inflammatory cell infiltrates. On the teased fibre preparation most fibres were of small diameter and thinly remyelinated. Ten per cent of fibres show evidence of previous demyelination/remyelination. Immunofluorescence stains for immunoglobulin, complement, fibrinogen and the Congo red stain for amyloid were negative. The findings are consistent with severe axonal loss consistent with a neuropathy or ganglionopathy.
The five individuals with biallelic expansions had several diagnoses at presentation, highlighting that RFC1 expansions can mimic a variety of disorders (Table 1 and Fig. 1). Despite different diagnoses at presentation, four of five patients had their diagnosis revised to CANVAS and fulfilled proposed criteria (Szmulewicz et al., 2016) for clinically possible, probable or definite CANVAS on retrospective review. Notably, all probands with biallelic RFC1 expansions had evidence of sensory neuropathy, as well as a positive HIT and cough when tested (four of five cases). In comparison, those probands without biallelic RFC1 expansions frequently did not have a HIT performed (8/12) or it was negative (1/12) and were rarely questioned on cough (9/12), reflecting limitations in data collection. Biallelic RFC1 expansions were not found in HCA although the majority (5/7) did not have sensory neuropathy.
| ID . | Gender . | Age at onset . | Age at examination . | Sensory neuronopathy (clinical) . | Sensory neuronopathy (NCS) . | Bilateral vestibular areflexia (clinical - abolished VOR at HIT) . | Bilateral vestibular areflexia (vestibular testing) . | Cerebellar dysfunction (clinical) . | Cerebellar atrophy on MRI . | Cough . | Initial diagnosis considered . | Revised diagnosis . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects with biallelic pathogenic expansions | ||||||||||||
| R110358 | M | 50 | 60 | Yes | Yes | Yes | NA | Yes | NA | Yes | HSN with cough | CANVAS |
| R60355 | M | 41 | 62 | Yes | Yes | NA | NA | No | NA | Yes | HSN with cough and gastro-oesophageal reflux | |
| R19975 | F | 60 | 63 | Yes | Yes | Yes | Yes, cVEMP not obtainable, ocular VEMPs not obtainable on the right side | Yes | No | NA | Sjögren’s ganglionopathy | CANVAS |
| R19982 | F | 54 | 67 | Yes | Yes | Yes | NA | Yes | Yes | Yes | Inherited spastic ataxia with neuropathy | CANVAS |
| R200002 | M | 49 | 55 | Yes | Yes | Yes | VEMPs absent | Yes | NA | Yes | CANVAS | |
| Subjects with a single heterozygous pathogenic expansion | ||||||||||||
| R19955 | M | 58 | 73 | Yes | Yes | Yes | VEMPs, caloric testing responses absent bilaterally | Yes | Yes | Yes | Neuropathy related to insulin resistance, paraneoplastic neuropathy | CANVAS |
| R19954 | F | 63 | 79 | No | No | NA | VEMPs normal | Yes | No | NA | Hereditary cerebellar ataxia | |
| R190103 | F | 41 | 60 | No | NA | NA | NA | Yes | Yes | NA | Hereditary cerebellar ataxia | |
| ID . | Gender . | Age at onset . | Age at examination . | Sensory neuronopathy (clinical) . | Sensory neuronopathy (NCS) . | Bilateral vestibular areflexia (clinical - abolished VOR at HIT) . | Bilateral vestibular areflexia (vestibular testing) . | Cerebellar dysfunction (clinical) . | Cerebellar atrophy on MRI . | Cough . | Initial diagnosis considered . | Revised diagnosis . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects with biallelic pathogenic expansions | ||||||||||||
| R110358 | M | 50 | 60 | Yes | Yes | Yes | NA | Yes | NA | Yes | HSN with cough | CANVAS |
| R60355 | M | 41 | 62 | Yes | Yes | NA | NA | No | NA | Yes | HSN with cough and gastro-oesophageal reflux | |
| R19975 | F | 60 | 63 | Yes | Yes | Yes | Yes, cVEMP not obtainable, ocular VEMPs not obtainable on the right side | Yes | No | NA | Sjögren’s ganglionopathy | CANVAS |
| R19982 | F | 54 | 67 | Yes | Yes | Yes | NA | Yes | Yes | Yes | Inherited spastic ataxia with neuropathy | CANVAS |
| R200002 | M | 49 | 55 | Yes | Yes | Yes | VEMPs absent | Yes | NA | Yes | CANVAS | |
| Subjects with a single heterozygous pathogenic expansion | ||||||||||||
| R19955 | M | 58 | 73 | Yes | Yes | Yes | VEMPs, caloric testing responses absent bilaterally | Yes | Yes | Yes | Neuropathy related to insulin resistance, paraneoplastic neuropathy | CANVAS |
| R19954 | F | 63 | 79 | No | No | NA | VEMPs normal | Yes | No | NA | Hereditary cerebellar ataxia | |
| R190103 | F | 41 | 60 | No | NA | NA | NA | Yes | Yes | NA | Hereditary cerebellar ataxia | |
CANVAS = cerebellar ataxia, neuropathy, and vestibular areflexia syndrome; cVEMP = vestibular evoked myogenic potentials elicited from the sternocleidomastoid muscle (cervical); F = female; HIT = head impulse test; HSN = hereditary sensory neuropathy; M = male; NA = not available; NCS = nerve conduction study; VEMP = vestibular evoked myogenic potential; VOR = vestibulo-ocular reflex.
| ID . | Gender . | Age at onset . | Age at examination . | Sensory neuronopathy (clinical) . | Sensory neuronopathy (NCS) . | Bilateral vestibular areflexia (clinical - abolished VOR at HIT) . | Bilateral vestibular areflexia (vestibular testing) . | Cerebellar dysfunction (clinical) . | Cerebellar atrophy on MRI . | Cough . | Initial diagnosis considered . | Revised diagnosis . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects with biallelic pathogenic expansions | ||||||||||||
| R110358 | M | 50 | 60 | Yes | Yes | Yes | NA | Yes | NA | Yes | HSN with cough | CANVAS |
| R60355 | M | 41 | 62 | Yes | Yes | NA | NA | No | NA | Yes | HSN with cough and gastro-oesophageal reflux | |
| R19975 | F | 60 | 63 | Yes | Yes | Yes | Yes, cVEMP not obtainable, ocular VEMPs not obtainable on the right side | Yes | No | NA | Sjögren’s ganglionopathy | CANVAS |
| R19982 | F | 54 | 67 | Yes | Yes | Yes | NA | Yes | Yes | Yes | Inherited spastic ataxia with neuropathy | CANVAS |
| R200002 | M | 49 | 55 | Yes | Yes | Yes | VEMPs absent | Yes | NA | Yes | CANVAS | |
| Subjects with a single heterozygous pathogenic expansion | ||||||||||||
| R19955 | M | 58 | 73 | Yes | Yes | Yes | VEMPs, caloric testing responses absent bilaterally | Yes | Yes | Yes | Neuropathy related to insulin resistance, paraneoplastic neuropathy | CANVAS |
| R19954 | F | 63 | 79 | No | No | NA | VEMPs normal | Yes | No | NA | Hereditary cerebellar ataxia | |
| R190103 | F | 41 | 60 | No | NA | NA | NA | Yes | Yes | NA | Hereditary cerebellar ataxia | |
| ID . | Gender . | Age at onset . | Age at examination . | Sensory neuronopathy (clinical) . | Sensory neuronopathy (NCS) . | Bilateral vestibular areflexia (clinical - abolished VOR at HIT) . | Bilateral vestibular areflexia (vestibular testing) . | Cerebellar dysfunction (clinical) . | Cerebellar atrophy on MRI . | Cough . | Initial diagnosis considered . | Revised diagnosis . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects with biallelic pathogenic expansions | ||||||||||||
| R110358 | M | 50 | 60 | Yes | Yes | Yes | NA | Yes | NA | Yes | HSN with cough | CANVAS |
| R60355 | M | 41 | 62 | Yes | Yes | NA | NA | No | NA | Yes | HSN with cough and gastro-oesophageal reflux | |
| R19975 | F | 60 | 63 | Yes | Yes | Yes | Yes, cVEMP not obtainable, ocular VEMPs not obtainable on the right side | Yes | No | NA | Sjögren’s ganglionopathy | CANVAS |
| R19982 | F | 54 | 67 | Yes | Yes | Yes | NA | Yes | Yes | Yes | Inherited spastic ataxia with neuropathy | CANVAS |
| R200002 | M | 49 | 55 | Yes | Yes | Yes | VEMPs absent | Yes | NA | Yes | CANVAS | |
| Subjects with a single heterozygous pathogenic expansion | ||||||||||||
| R19955 | M | 58 | 73 | Yes | Yes | Yes | VEMPs, caloric testing responses absent bilaterally | Yes | Yes | Yes | Neuropathy related to insulin resistance, paraneoplastic neuropathy | CANVAS |
| R19954 | F | 63 | 79 | No | No | NA | VEMPs normal | Yes | No | NA | Hereditary cerebellar ataxia | |
| R190103 | F | 41 | 60 | No | NA | NA | NA | Yes | Yes | NA | Hereditary cerebellar ataxia | |
CANVAS = cerebellar ataxia, neuropathy, and vestibular areflexia syndrome; cVEMP = vestibular evoked myogenic potentials elicited from the sternocleidomastoid muscle (cervical); F = female; HIT = head impulse test; HSN = hereditary sensory neuropathy; M = male; NA = not available; NCS = nerve conduction study; VEMP = vestibular evoked myogenic potential; VOR = vestibulo-ocular reflex.
Highlighting the diversity of presentations of RFC1, we identified biallelic expansions in two of four families who were initially diagnosed with HSN (although not in a family with HSN with cough linked to chromosome 3p22-p24) (Kok et al., 2003; Spring et al., 2005).
This included a large Lebanese family in which the proband (Patient R110358) presented at the age of 60 years with a 10-year history of recurrent cough, preceding a 5-year history of burning feet. He had severe coughing fits, in which his face would go red, as well as a positive HIT. He had two affected sisters; he also had a possibly affected mother and maternal grandmother with a cough, but these family members were deceased and therefore unavailable for further assessment.
A 62-year-old male diagnosed with HSN with cough and gastro-oesophageal reflux was also found to have biallelic RFC1 expansions (Patient R60355). He had a cough that began 20 years prior to assessment and his sensory symptoms began 4–5 years prior; the cough was attributed to reflux and was treated with a Nissen fundoplication.
We further describe biallelic RFC1 expansions in a female (Patient R19977) initially diagnosed as Sjögren syndrome. She presented at 60 years of age with a 6-month history of gait imbalance with recurrent falls and paraesthesias in the feet and a tendency to inadvertently burn her hands, and a cough attributed to gastro-oesophageal reflux treated with a Nissen fundoplication. Examination revealed evidence of cerebellar signs, distal sensory neuropathy, ataxic gait, and positive Romberg’s sign. Nerve conduction studies showed absent sensory responses, and a sural biopsy showed severe axonal loss (Fig. 1). She was diagnosed with Sjögren’s sensory ataxic neuropathy, although there was uncertainty given the absence of sicca features and a negative extractable nuclear antigen. She was treated with azathioprine, mycophenolate, and intravenous immunoglobulin with no improvement. Further assessment 3 years after presentation revealed a bilaterally abnormal HIT consistent with CANVAS. Of potential interest, she was diagnosed with sensorineural hearing loss (Table 1).
Additionally, we identified three individuals who carried a single pathogenic RFC1 expansion (Patients R19955, R19954 and R190103). They included a 73-year-old male (Patient R19955) with peripheral neuropathy and a persistent cough for over 6 years with an unremarkable CT chest, in whom the diagnosis of paraneoplastic neuropathy was considered but with negative antineuronal antibodies. He was later found to have bilateral vestibular dysfunction consistent with CANVAS (Table 1 and Supplementary Video 1). This individual was heterozygous for both the pathogenic AAGGG and non-pathogenic AAAGG expansion. For another proband, Patient R190103, a heterozygous pathogenic expanded allele did not segregate in her affected brother (Patient R190104). Proband Patient R19954 had a heterozygous pathogenic expansion and had sporadic cerebellar ataxia without sensory neuropathy.
Our study highlights that RFC1 expansions can present with a variety of disorders, such as HSN with cough and Sjörgen’s ataxic neuropathy, consistent with the findings of Cortese and colleagues (2020).
We note that in many instances, the cough may precede the observation of peripheral neuropathy by many years, in keeping with findings of Cortese and colleagues. Individuals with RFC1 expansions may have severe, paroxysmal bouts of coughing (proband Patient R110358), and in other cases may describe a dry, tickly cough occurring at night and disturbing sleep (Patient R60355). The cough may be brought to the attention of a range of medical specialties who may not immediately draw the association with peripheral neuropathy, leading to investigation for primary lung pathology or attribution to gastro-oesphageal reflux pathology (Patients R60355 and R19977).
Furthermore, although vestibular dysfunction was universally present in biallelic RFC1 expansion carriers in this cohort, there was often a delay before a HIT and/or vestibular testing were performed and vestibular impairment was detected, highlighted by the finding that the majority of probands (52.9%, 9/17) did not have a HIT recorded.
We note that in at least one proband, an early diagnosis of CANVAS may have prevented treatments with potentially serious adverse effects, such as immunotherapy for Sjögren syndrome. Furthermore, this individual had a sural nerve biopsy, a test that may have serious complications, and which may be avoided in the future with greater awareness of this condition.
Additionally, three families with RFC1 expansions had a multigenerational family history suggestive of autosomal dominant inheritance (Patients R110358, R60355 and R19975). In these cases, the children, parents or grandparents of the proband had some features suggestive of CANVAS (e.g. cough or gait abnormalities) but were not available for assessment. We suspect that in some instances RFC1 may show a pseudodominant pattern of inheritance given the relatively high population frequency of the pathogenic expanded allele (Cortese et al., 2019) and this may complicate interpretation of the mode of inheritance.
Furthermore, one individual (Patient R19955) had a definite diagnosis of CANVAS and was heterozygous for both the AAGGG pathogenic expansion as well as the non-pathogenic AAAGG expansion. A possible explanation is that there may be another mutation type on the other allele (e.g. a sequencing variant), but we acknowledge the difficulty of definitive confirmation of RFC1 expansion testing which requires sequencing of the repeats, as highlighted by a recent study (Akcimen et al., 2019).
In summary, clinicians should be aware that RFC1 expansions may masquerade as a variety of different disorders, and that early detection of vestibular impairment and genetic testing may be critical to the diagnosis and have management implications. Biallelic pathogenic RFC1 expansions were identified for a relatively high percentage of cases (29.4%, 5/17), and we agree that RFC1-related disorders are frequently under-recognized or misdiagnosed. Cortese and colleagues highlight the importance of a thorough clinical examination; consistent with this we recommend routinely performing a HIT and assessing for cough in individuals with neuropathy in order to look for clues to the presence of an RFC1 expansion-related disorder.
Data availability
Data are available from the authors upon reasonable request.
Acknowledgements
K.R.K. is supported by a philanthropic grant from the Paul Ainsworth Family Foundation and receives an award from the Aligning Science Across Parkinson’s disease initiative through the Michael J. Fox Foundation. H.H. thanks the MRC, Wellcome Trust, MSA Trust and NIHR UCLH BRC, Andrea Cortese thanks Medical Research Council, (MR/T001712/1) and Fondazione CARIPLO (2019-1836) for grant support.
Funding
No funding was received towards this work.
Competing interests
The authors report no competing interests.
References
Author notes
Kishore R. Kumar, Andrea Cortese, Henry Houlden, and Marina Kennerson contributed equally to this work.


