-
PDF
- Split View
-
Views
-
Cite
Cite
Radha Desai, Ann E. Frazier, Romina Durigon, Harshil Patel, Aleck W. Jones, Ilaria Dalla Rosa, Nicole J. Lake, Alison G. Compton, Hayley S. Mountford, Elena J. Tucker, Alice L. R. Mitchell, Deborah Jackson, Abdul Sesay, Miriam Di Re, Lambert P. van den Heuvel, Derek Burke, David Francis, Sebastian Lunke, George McGillivray, Simone Mandelstam, Fanny Mochel, Boris Keren, Claude Jardel, Anne M. Turner, P. Ian Andrews, Jan Smeitink, Johannes N. Spelbrink, Simon J. Heales, Masakazu Kohda, Akira Ohtake, Kei Murayama, Yasushi Okazaki, Anne Lombès, Ian J. Holt, David R. Thorburn, Antonella Spinazzola, ATAD3 gene cluster deletions cause cerebellar dysfunction associated with altered mitochondrial DNA and cholesterol metabolism, Brain, Volume 140, Issue 6, June 2017, Pages 1595–1610, https://doi.org/10.1093/brain/awx094
Close - Share Icon Share
Abstract
Although mitochondrial disorders are clinically heterogeneous, they frequently involve the central nervous system and are among the most common neurogenetic disorders. Identifying the causal genes has benefited enormously from advances in high-throughput sequencing technologies; however, once the defect is known, researchers face the challenge of deciphering the underlying disease mechanism. Here we characterize large biallelic deletions in the region encoding the ATAD3C, ATAD3B and ATAD3A genes. Although high homology complicates genomic analysis of the ATAD3 defects, they can be identified by targeted analysis of standard single nucleotide polymorphism array and whole exome sequencing data. We report deletions that generate chimeric ATAD3B/ATAD3A fusion genes in individuals from four unrelated families with fatal congenital pontocerebellar hypoplasia, whereas a case with genomic rearrangements affecting the ATAD3C/ATAD3B genes on one allele and ATAD3B/ATAD3A genes on the other displays later-onset encephalopathy with cerebellar atrophy, ataxia and dystonia. Fibroblasts from affected individuals display mitochondrial DNA abnormalities, associated with multiple indicators of altered cholesterol metabolism. Moreover, drug-induced perturbations of cholesterol homeostasis cause mitochondrial DNA disorganization in control cells, while mitochondrial DNA aggregation in the genetic cholesterol trafficking disorder Niemann-Pick type C disease further corroborates the interdependence of mitochondrial DNA organization and cholesterol. These data demonstrate the integration of mitochondria in cellular cholesterol homeostasis, in which ATAD3 plays a critical role. The dual problem of perturbed cholesterol metabolism and mitochondrial dysfunction could be widespread in neurological and neurodegenerative diseases.
Introduction
Mutations in mtDNA and in the nuclear-encoded factors required for mtDNA maintenance and expression result in a broad range of human diseases, most of which affect the CNS (Area-Gomez and Schon, 2014). Originally believed to float free in the mitochondrial matrix unfettered by packaging proteins, it is now established that mtDNA is organized in nucleoprotein complexes, called nucleoids, which are associated with the inner mitochondrial membrane (Spelbrink, 2010). The apparatus mediating the interaction with the inner mitochondrial membrane is predicted to have a role in mtDNA organization and distribution, and its dysfunction might result in alteration of nucleoid structure and composition, which in turn could adversely affect mtDNA segregation and membrane architecture, leading to disease. Cholesterol co-sediments with the mtDNA (Gerhold et al., 2015) and if the association is physiologically meaningful, perturbed cholesterol homeostasis should result in mtDNA abnormalities, as should defects in factors effecting cholesterol–mtDNA interactions. A candidate to participate in such interactions is ATAD3, ATPase family, AAA+ domain containing 3, which was assigned as a detergent-resistant component of mitochondrial nucleoids with potential roles in mtDNA organization and segregation (He et al., 2007) and enhancing hormonal-induced steroidogenesis (Issop et al., 2015). Moreover, ATAD3 co-purifies with SPTLC1 and SPTLC2 (serine palmitoyltransferase, long chain base subunits 1 and 2) (He et al., 2012) that synthesize sphingolipids, which form membrane micro-domains with cholesterol, and a small fraction of ATAD3 forms an 800 kDa complex that co-migrates with a labelled cholesterol probe (Rone et al., 2012). Hence, ATAD3 may have overlapping roles in cholesterol distribution in mitochondria and mtDNA organization. ATAD3 has also been linked to adipogenesis and lipid metabolism (Hoffmann et al., 2009), mitochondrial translation (He et al., 2012) and iron and heme homeostasis (van den Ecker et al., 2015).
Most species have a single ATAD3 gene but hominids have a cluster of three genes arranged in tandem close to the telomere of chromosome 1p: ATAD3C, ATAD3B and ATAD3A. A recurrent de novo dominant missense mutation in ATAD3A was recently shown to cause a phenotype comprising global developmental delay, hypotonia, optic atrophy, axonal neuropathy, and hypertrophic cardiomyopathy in five unrelated subjects (Harel et al., 2016). That study also identified a homozygous ATAD3A missense mutation in siblings with congenital cataract, ataxia and seizures plus biallelic deletions of ATAD3A and adjacent ATAD3 genes in one subject with severe cerebellar hypoplasia and neonatal death. A dominant mutation in ATAD3A was later described to cause hereditary spastic paraplegia and axonal neuropathy (Cooper et al., 2017). Here, we report six subjects with cerebellar pathology, all associated with biallelic genomic rearrangements affecting the ATAD3 gene cluster. In four of five families, the affected individuals had fatal congenital pontocerebellar hypoplasia with a simplified gyral pattern, and the single adult case had cerebellar atrophy with dystonia and ataxia. At the cellular level, we demonstrated that ATAD3 deficiency causes aberrant mtDNA organization and is associated with elevated free cholesterol and increased expression of genes involved in cholesterol metabolism. We also show that genetic or pharmacological perturbations of cellular cholesterol homeostasis perturb mtDNA organization. The consequences of the ATAD3 deletions for mtDNA organization and cholesterol metabolism offer a pathogenetic explanation for the disorder.
Materials and methods
Determination of deletion and breakpoints
Molecular karyotyping of DNA was performed with the Illumina HumanCytoSNP-12 (version 2.1) or Infinium CoreExome-24 arrays, as previously described (Bruno et al., 2011). Automated detection of long contiguous segments of homozygosity was performed with the CNVPartition v3.1.6 algorithm in KaryoStudio software. SNP genotypes were generated in GenomeStudio software (Illumina) with data from a set of 102 intra-run samples.
A custom comparative genomic hybridization (CGH) NimbleGen 12 × 135 K array (Roche Diagnostics) was designed to densely tile 1034 MitoExome genes encoding known mitochondrial proteins (Calvo et al., 2012). Targeted exonic regions were tiled to an average probe spacing of 50 bp, intronic regions to an average of 900 bp, and regions directly upstream and downstream of targeted genes were covered forming a low-resolution backbone tiled at 3600 bp. CGH arrays were performed, scanned and analysed in accordance with manufacturer’s recommendations using gender matched, pooled DNA from seven unaffected individuals as a control.
Intragenic deletions were further investigated by long-range PCR amplification and sequencing of the junction region (BigDye® v3.1 terminators; Applied Biosystems) to better define the breakpoints. The primer sequences used in this study and their binding sites within the ATAD3 region are shown in Supplementary Fig. 1.
Gene-specific RNA studies
RNA was extracted from cultured fibroblasts using the Illustra RNAspin Mini Kit (GE healthcare) and cDNA was generated using the SuperScript® III First strand synthesis system (Invitrogen) as per manufacturers’ protocols. For analysis of nonsense-mediated decay and mRNA splicing, fibroblasts were cultured in medium with and without 100 ng/μl cycloheximide for 24 h before RNA preparation (Lamande et al., 1998). Quantitative reverse transcription (qRT)-PCR analysis of fibroblast ATAD3A and ATAD3B expression was performed as previously described for other genes (Tucker et al., 2013), with the following modifications; each 20 μl PCR reaction contained 2.5 μl of cDNA synthesized from 800 ng of mRNA, 10 μl of SensiFAST™ SYBR® Green (Bioline) and 0.5 μM each of forward and reverse primers, and was measured in triplicate on each plate. Nucleotide variation between ATAD3A and ATAD3B transcripts and primers used are highlighted in Supplementary Fig. 1B. Results were normalized to HPRT expression (primers 5’-CCTGGCGTCGTGATTAGTGA and 5’-CGAGCAAGACGTTCAGTCCT) and Sanger sequencing of amplicons confirmed specificity.
RNA-Seq analysis
For each sample, total RNA was extracted from ∼8 × 106 fibroblasts using TRIzol® reagent (Sigma), quantified by NanoDrop and quality checked using the Agilent Bioanalyzer. Samples that showed minimal degradation as measured by RNA integrity number (RIN) > 8.0 were further processed for Illumina sequencing library preparation with the TruSeq Stranded mRNA HT Sample Prep Kit (Illumina, Part# RS-122-2103). Libraries were generated from 1 μg of total RNA and sequenced on the Illumina HiSeq 4000, using the paired-end 101 bp dual indexing protocol. FastQ files were generated using CASAVA BCL to FastQ (version 2.16). Sequencing yield was typically ∼70 million strand-specific paired-end reads. The RSEM package (version 1.2.29) (Li and Dewey, 2011) in conjunction with the STAR alignment algorithm (version 2.5.1b) (Dobin et al., 2013) was used for the mapping and subsequent gene-level counting of the sequenced reads with respect to hg19 Ensembl genes downloaded from the UCSC Table Browser (Karolchik et al., 2004) on 14 April 2016. The ‘–forward-prob’ parameter was set to ‘0’ and all other parameters were kept as default. Differential expression analysis was performed with the DESeq2 package (version 1.10.1) (Love et al., 2014) within the R programming environment (version 3.2.3) (R Core Team, 2015). An adjusted P-value of ≤ 0.05 was used as the significance threshold for the identification of differentially expressed genes. All raw RNA-Seq sequence data and per sample transcript per million counts generated by RSEM can be accessed via GEO (GEO ID GSE86550).
Pathway analysis
Gene set enrichment analysis for differentially expressed genes was performed by Gene Ontology Pathway and Biological processes using GeneGo MetaCore (https://portal.genego.com/).
Gene set enrichment analysis
Genes from each given pairwise comparison were ranked using the Wald statistic. Gene set enrichment analysis (GSEA; Subramanian et al., 2005) pre-ranked analysis was performed with respect to MSigDB (version 5.1) C2 canonical pathways and C5 GO biological process. All parameters were kept as default except for enrichment statistic (classic), min size (5) and max size (50 000). Gene signatures with a false discovery rate (FDR) q-value of ≤ 0.05 were considered to be significant.
Cell culture, DNA and enzyme analysis
Primary fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies) supplemented with 10% foetal bovine serum (Hyclone), 1% penicillin and streptomycin (PS, Life Technologies) at 37°C in a 5% CO2 atmosphere. All cells were negative for mycoplasma based on regular screening using LookOut® Mycoplasma PCR Detection Kit (Sigma). Total DNA was isolated from cultured human fibroblasts using DNeasy® Blood and Tissue Kit (QIAGEN), or from fibroblasts or blood using a NucleoBond® CB20 DNA Extraction kit (Scientifix), according to the manufacturer’s protocol. Estimation of mtDNA copy number was performed by quantitative PCR, as described previously for tissue biopsies (Pagnamenta et al., 2006) and cultured fibroblasts (Dalla Rosa et al., 2016).
Spectrophotometric enzyme assays assessing mitochondrial OXPHOS enzyme activities were performed in cultured fibroblast mitochondria and skeletal muscle or liver biopsy post-nuclear supernatants from Subjects S1a, S1b and S3 as described previously (Frazier and Thorburn, 2012). OXPHOS enzymes in skeletal muscle post-nuclear supernatants from Subject S5 were assayed as described elsewhere (Medja et al., 2009).
Immunoblotting
Protein fractionation, transfer and immuno-detection were performed as described (Dalla Rosa et al., 2014), with some modifications. Muscle and liver samples were prepared as previously described (Cooper et al., 2003). Cells were lysed on ice in phosphate-buffered saline (PBS), 0.1% n-dodecyl β-d-maltoside (DDM), 1% SDS, 1 × protease inhibitor cocktail (Roche), 50 U Benzonase® and phosphatase inhibitor complexes (Cell signalling), or in RIPA buffer containing 1× protease inhibitor cocktail (Roche). Protein concentration was measured by Lowry assay (DC™ Reagent, Bio-Rad) or BCA assay, and 10 μg of lysate analysed per lane. Primary antibodies were: mouse anti-GAPDH (1:20 000, Abcam), mouse anti-NDUFB8 (1:1000, Abcam), mouse anti-COX II (1:2000, Abcam), mouse anti-VDAC1 (Porin) (1:10000, Merck), rabbit anti-ATAD3 (1:60 000, gift from John Walker), rabbit anti-SREBF2 (1:1000, Abcam), rabbit anti-CES-1 (1:2000, Proteintech).
Immunocytochemistry and cell imaging
Fibroblast cultures were incubated with 20 μM BrdU for 8 h and fixed with 2% paraformaldehyde for 15 min at room temperature, then treated with PBS containing 0.2% Triton™ X-100. After a 5 min PBS wash, cells were incubated for 90 min at 40°C in 2N HCl. Cells were blocked with 5% goat serum in PBS for 1 h, then incubated with primary antibody in PBS at 4°C overnight and subsequently with secondary antibodies for 2 h at ambient temperature. A 90 min incubation at room temperature with Alexa Fluor® 488 conjugated streptavidin (Invitrogen) was followed with a final set of PBS washes. The coverslips were mounted on glass slides using Progold with DAPI. For staining that did not include BrdU the HCl antigen retrieval step was omitted. Primary antibodies: mouse anti-DNA Progen (1:200, AC-30-10); rat anti-BrdU Bio-Rad (1:200, MCA2060); rabbit anti-Tom20 (1:400 Santa Cruz). Secondary antibodies: Alexa Fluor® 488 goat anti-mouse (1:500); Alexa Fluor® 488 goat anti-rat (1:500); Alexa Fluor® 568 goat anti-rabbit (1:1000). Unesterified cholesterol in fibroblasts was stained with filipin, using a cholesterol assay kit (Abcam), detected by wide-field fluorescence microscopy and quantified using ImageJ.
Mitochondrial DNA foci sizing and counting
Nucleoid size was analysed using 3D confocal images of human fibroblast cells stained with anti-DNA antibody. Images were acquired using parameters set for a control field of cells at the beginning of each microscopy session. Individual files were put through particle analysis using Fiji (ImageJ), with a fluorescence intensity threshold set manually, such that only signal above background was considered. This threshold was maintained for all images. Then the Fiji plugin, 3D particle count, was used to count the number of particles across the stack of optical sections. The plug-in counts each particle and defines its size, generating datasets based on ∼1500–5000 particles. The particle information was transferred to a spreadsheet and arranged in ascending order by size. The total number of particles were then binned into 20 size categories (bins) and presented as a histogram. No attempt was made to create parameter settings that would produce an accurate estimate of the total number of mtDNA foci (nucleoid number) in a cell.
Results
Clinical and biochemical features associated with deletions in the ATAD3 gene cluster
Clinical and laboratory findings in individuals with deletions in the ATAD3 gene cluster
| Subject ID . | Sex Ethnicity Consanguinity . | ATAD3 deletion . | Age at onset . | Age at death . | Symptoms . | Brain imaging . | Mitochondrial investigations . |
|---|---|---|---|---|---|---|---|
| S1a |
|
| 38 weeks gestation | Day 5 | Polyhydramnios, reduced foetal and no postnatal spontaneous movements, required ventilation, dysmorphic features, severe encephalopathy | Cerebellar and brainstem hypoplasia with simplified anterior gyral pattern anteriorly, diffuse white matter signal abnormality, marked ex vacuo ventricular dilatation consistent with global atrophy | Plasma lactate elevated up to 6.2 mM, borderline low CI, CIII, CIV in skeletal muscle but normal in skin fibroblasts. Decreased steady state levels of a critical CI and CIV subunit in fibroblasts and RNASeq results indicative of decreased expression of a broad range of OXPHOS factors. |
| S1b |
|
| 33 weeks gestation | Day 1 | Reduced foetal movements, foetus died intrapartum during a lengthy labour | Not performed | Borderline-low CI, CIII, CIV in skeletal muscle and borderline- low CI, CII, CIII in liver. |
| S2 |
|
| 33 weeks gestation | Day 5 | Polyhydramnios and lack of foetal movements. Caesarean section at 33 weeks due to foetal distress. No spontaneous movements, required ventilation, dysmorphic features, severe encephalopathy | Cerebellar hypoplasia, abnormalities of cortical gyration and periventricular white matter abnormalities | Plasma lactate elevated (2.7 to 4.4 mM), elevated urine 3-methylglutaconate and 3-methylglutatarate. Decreased CI, CIII, CIV in skin fibroblasts but normal in skeletal muscle and liver. |
| S3 |
|
| 34 weeks gestation | Day 2 | Polyhydramnios. Caesarean section at 33 weeks due to foetal distress. Required ventilation, dysmorphic features, severe encephalopathy | Pontocerebellar hypoplasia, supratentorial white matter and cortical abnormalities, ventricular dilatation, simplified gyral pattern | Plasma lactate levels slightly elevated at 2.6 mM. |
| S4 |
|
| 37 weeks gestation | 7 months and 10 days | Reduced foetal movements, spontaneous delivery, cyanosis, no spontaneous respiration with Apgar score of 6/6, required ventilation throughout his life. Progressive cardiac hypertrophy | Cerebral, cerebellar and brain stem atrophy, simplified gyral pattern in the frontal lobes, haemorrhage from bilateral lateral ventricles | CSF lactate elevated (3.4 mM). Low oxygen consumption rate in skin fibroblasts but activities of CI, CII, CII+III, CIII and CIV were normal. |
| S5 |
|
| Childhood | Alive at 30 years | Moderate mental retardation since childhood, dystonia, psychiatric problems, cerebellar ataxia since about 25 years | Cerebellar atrophy | Normal metabolic investigations. Normal histological analysis of muscle biopsy. In fibroblasts, decreased steady state levels of a critical CI and CIV subunit, and RNASeq results indicative of decreased expression of a broad range of OXPHOS factors (albeit less pronounced than for Subject S1a). |
| Subject ID . | Sex Ethnicity Consanguinity . | ATAD3 deletion . | Age at onset . | Age at death . | Symptoms . | Brain imaging . | Mitochondrial investigations . |
|---|---|---|---|---|---|---|---|
| S1a |
|
| 38 weeks gestation | Day 5 | Polyhydramnios, reduced foetal and no postnatal spontaneous movements, required ventilation, dysmorphic features, severe encephalopathy | Cerebellar and brainstem hypoplasia with simplified anterior gyral pattern anteriorly, diffuse white matter signal abnormality, marked ex vacuo ventricular dilatation consistent with global atrophy | Plasma lactate elevated up to 6.2 mM, borderline low CI, CIII, CIV in skeletal muscle but normal in skin fibroblasts. Decreased steady state levels of a critical CI and CIV subunit in fibroblasts and RNASeq results indicative of decreased expression of a broad range of OXPHOS factors. |
| S1b |
|
| 33 weeks gestation | Day 1 | Reduced foetal movements, foetus died intrapartum during a lengthy labour | Not performed | Borderline-low CI, CIII, CIV in skeletal muscle and borderline- low CI, CII, CIII in liver. |
| S2 |
|
| 33 weeks gestation | Day 5 | Polyhydramnios and lack of foetal movements. Caesarean section at 33 weeks due to foetal distress. No spontaneous movements, required ventilation, dysmorphic features, severe encephalopathy | Cerebellar hypoplasia, abnormalities of cortical gyration and periventricular white matter abnormalities | Plasma lactate elevated (2.7 to 4.4 mM), elevated urine 3-methylglutaconate and 3-methylglutatarate. Decreased CI, CIII, CIV in skin fibroblasts but normal in skeletal muscle and liver. |
| S3 |
|
| 34 weeks gestation | Day 2 | Polyhydramnios. Caesarean section at 33 weeks due to foetal distress. Required ventilation, dysmorphic features, severe encephalopathy | Pontocerebellar hypoplasia, supratentorial white matter and cortical abnormalities, ventricular dilatation, simplified gyral pattern | Plasma lactate levels slightly elevated at 2.6 mM. |
| S4 |
|
| 37 weeks gestation | 7 months and 10 days | Reduced foetal movements, spontaneous delivery, cyanosis, no spontaneous respiration with Apgar score of 6/6, required ventilation throughout his life. Progressive cardiac hypertrophy | Cerebral, cerebellar and brain stem atrophy, simplified gyral pattern in the frontal lobes, haemorrhage from bilateral lateral ventricles | CSF lactate elevated (3.4 mM). Low oxygen consumption rate in skin fibroblasts but activities of CI, CII, CII+III, CIII and CIV were normal. |
| S5 |
|
| Childhood | Alive at 30 years | Moderate mental retardation since childhood, dystonia, psychiatric problems, cerebellar ataxia since about 25 years | Cerebellar atrophy | Normal metabolic investigations. Normal histological analysis of muscle biopsy. In fibroblasts, decreased steady state levels of a critical CI and CIV subunit, and RNASeq results indicative of decreased expression of a broad range of OXPHOS factors (albeit less pronounced than for Subject S1a). |
Clinical and laboratory findings in individuals with deletions in the ATAD3 gene cluster
| Subject ID . | Sex Ethnicity Consanguinity . | ATAD3 deletion . | Age at onset . | Age at death . | Symptoms . | Brain imaging . | Mitochondrial investigations . |
|---|---|---|---|---|---|---|---|
| S1a |
|
| 38 weeks gestation | Day 5 | Polyhydramnios, reduced foetal and no postnatal spontaneous movements, required ventilation, dysmorphic features, severe encephalopathy | Cerebellar and brainstem hypoplasia with simplified anterior gyral pattern anteriorly, diffuse white matter signal abnormality, marked ex vacuo ventricular dilatation consistent with global atrophy | Plasma lactate elevated up to 6.2 mM, borderline low CI, CIII, CIV in skeletal muscle but normal in skin fibroblasts. Decreased steady state levels of a critical CI and CIV subunit in fibroblasts and RNASeq results indicative of decreased expression of a broad range of OXPHOS factors. |
| S1b |
|
| 33 weeks gestation | Day 1 | Reduced foetal movements, foetus died intrapartum during a lengthy labour | Not performed | Borderline-low CI, CIII, CIV in skeletal muscle and borderline- low CI, CII, CIII in liver. |
| S2 |
|
| 33 weeks gestation | Day 5 | Polyhydramnios and lack of foetal movements. Caesarean section at 33 weeks due to foetal distress. No spontaneous movements, required ventilation, dysmorphic features, severe encephalopathy | Cerebellar hypoplasia, abnormalities of cortical gyration and periventricular white matter abnormalities | Plasma lactate elevated (2.7 to 4.4 mM), elevated urine 3-methylglutaconate and 3-methylglutatarate. Decreased CI, CIII, CIV in skin fibroblasts but normal in skeletal muscle and liver. |
| S3 |
|
| 34 weeks gestation | Day 2 | Polyhydramnios. Caesarean section at 33 weeks due to foetal distress. Required ventilation, dysmorphic features, severe encephalopathy | Pontocerebellar hypoplasia, supratentorial white matter and cortical abnormalities, ventricular dilatation, simplified gyral pattern | Plasma lactate levels slightly elevated at 2.6 mM. |
| S4 |
|
| 37 weeks gestation | 7 months and 10 days | Reduced foetal movements, spontaneous delivery, cyanosis, no spontaneous respiration with Apgar score of 6/6, required ventilation throughout his life. Progressive cardiac hypertrophy | Cerebral, cerebellar and brain stem atrophy, simplified gyral pattern in the frontal lobes, haemorrhage from bilateral lateral ventricles | CSF lactate elevated (3.4 mM). Low oxygen consumption rate in skin fibroblasts but activities of CI, CII, CII+III, CIII and CIV were normal. |
| S5 |
|
| Childhood | Alive at 30 years | Moderate mental retardation since childhood, dystonia, psychiatric problems, cerebellar ataxia since about 25 years | Cerebellar atrophy | Normal metabolic investigations. Normal histological analysis of muscle biopsy. In fibroblasts, decreased steady state levels of a critical CI and CIV subunit, and RNASeq results indicative of decreased expression of a broad range of OXPHOS factors (albeit less pronounced than for Subject S1a). |
| Subject ID . | Sex Ethnicity Consanguinity . | ATAD3 deletion . | Age at onset . | Age at death . | Symptoms . | Brain imaging . | Mitochondrial investigations . |
|---|---|---|---|---|---|---|---|
| S1a |
|
| 38 weeks gestation | Day 5 | Polyhydramnios, reduced foetal and no postnatal spontaneous movements, required ventilation, dysmorphic features, severe encephalopathy | Cerebellar and brainstem hypoplasia with simplified anterior gyral pattern anteriorly, diffuse white matter signal abnormality, marked ex vacuo ventricular dilatation consistent with global atrophy | Plasma lactate elevated up to 6.2 mM, borderline low CI, CIII, CIV in skeletal muscle but normal in skin fibroblasts. Decreased steady state levels of a critical CI and CIV subunit in fibroblasts and RNASeq results indicative of decreased expression of a broad range of OXPHOS factors. |
| S1b |
|
| 33 weeks gestation | Day 1 | Reduced foetal movements, foetus died intrapartum during a lengthy labour | Not performed | Borderline-low CI, CIII, CIV in skeletal muscle and borderline- low CI, CII, CIII in liver. |
| S2 |
|
| 33 weeks gestation | Day 5 | Polyhydramnios and lack of foetal movements. Caesarean section at 33 weeks due to foetal distress. No spontaneous movements, required ventilation, dysmorphic features, severe encephalopathy | Cerebellar hypoplasia, abnormalities of cortical gyration and periventricular white matter abnormalities | Plasma lactate elevated (2.7 to 4.4 mM), elevated urine 3-methylglutaconate and 3-methylglutatarate. Decreased CI, CIII, CIV in skin fibroblasts but normal in skeletal muscle and liver. |
| S3 |
|
| 34 weeks gestation | Day 2 | Polyhydramnios. Caesarean section at 33 weeks due to foetal distress. Required ventilation, dysmorphic features, severe encephalopathy | Pontocerebellar hypoplasia, supratentorial white matter and cortical abnormalities, ventricular dilatation, simplified gyral pattern | Plasma lactate levels slightly elevated at 2.6 mM. |
| S4 |
|
| 37 weeks gestation | 7 months and 10 days | Reduced foetal movements, spontaneous delivery, cyanosis, no spontaneous respiration with Apgar score of 6/6, required ventilation throughout his life. Progressive cardiac hypertrophy | Cerebral, cerebellar and brain stem atrophy, simplified gyral pattern in the frontal lobes, haemorrhage from bilateral lateral ventricles | CSF lactate elevated (3.4 mM). Low oxygen consumption rate in skin fibroblasts but activities of CI, CII, CII+III, CIII and CIV were normal. |
| S5 |
|
| Childhood | Alive at 30 years | Moderate mental retardation since childhood, dystonia, psychiatric problems, cerebellar ataxia since about 25 years | Cerebellar atrophy | Normal metabolic investigations. Normal histological analysis of muscle biopsy. In fibroblasts, decreased steady state levels of a critical CI and CIV subunit, and RNASeq results indicative of decreased expression of a broad range of OXPHOS factors (albeit less pronounced than for Subject S1a). |
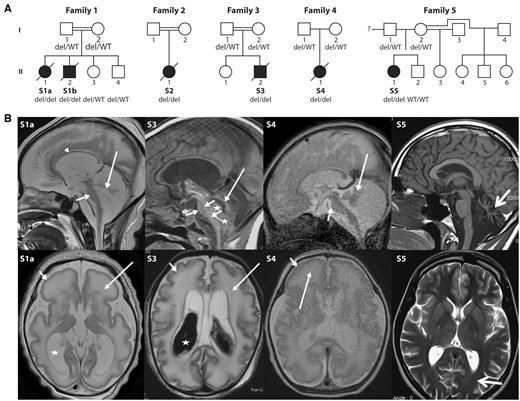
Pedigrees and brain MRI from five unrelated families with cerebellar disorders. (A) Pedigrees and ATAD3 genotypes for available members of Families 1–5. (B) Brain MRI of Subjects S1a, S3, S4 and S5. Top row: Sagittal images of Subjects S1a, S3 and S4 in the neonatal period and Subject S5 at 22 years of age. The neonates have severe brainstem and cerebellar hypoplasia with flat pons (short arrows) and tiny cerebellar vermis (long arrows). There is increase of the tegmento-vermian angle and ex vacuo enlargement of the posterior fossa CSF spaces. Arrowheads indicate the thin corpus callosum. Stars in Subject S3 show isointense blood products within and below the fourth ventricle. Subject S5 presents with severe hypoplasia/atrophy of the cerebellar vermis (thick arrow) with ex vacuo enlargement of the fourth ventricle; brainstem and normal corpus callosum are normal. Bottom row: Axial T2-weighted images show simplified sulcation and gyration more marked frontally (short arrow) and diffuse white matter T2 signal abnormality (long arrow) in Subjects S1a and S4. Similar but less severe changes are seen in Subject S3 with shallow simplified sulcation. Both subjects had a thin cortical ribbon, decreased white matter volumes with marked T2 hyperintensity, ex vacuo ventriculomegaly (stars) and prominence of the extra-axial CSF spaces in keeping with brain atrophy. Hypointense material within the lateral ventricles of Subject S3 is haemorrhage. Subject S5 has normal ventricles and subtle ‘frosted glass’ aspect of the posterior periventricular white matter (thick arrow). del = ATAD3 deletion; WT = wild-type.
ATAD3 gene cluster deletions
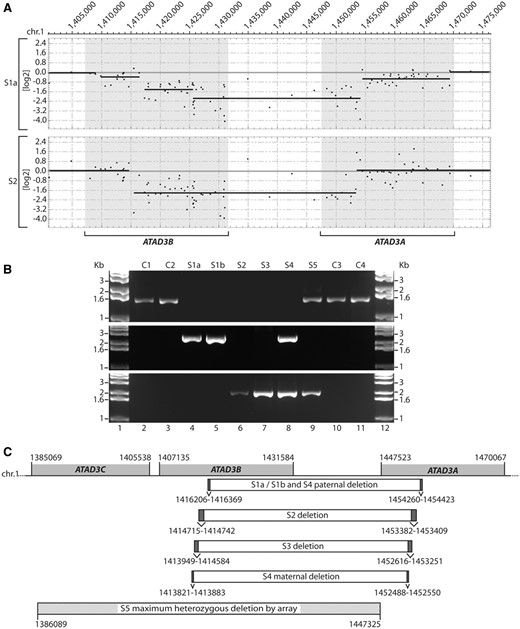
Identification of genomic ATAD3 deletions. (A) A custom CGH array was used to delineate homozygous deletions detected on chromosome 1 p in DNA from Subjects S1a and S2. Shaded boxes indicate the location of the ATAD3C, ATAD3B and ATAD3A genes. Details of deleted regions predicted by SNP and CGH arrays are summarized in Supplementary Table 2. (B) Long-range PCRs were performed on genomic DNA from subjects and controls. Primers OT472 and OT473 (middle panel) flank the ATAD3 deletion breakpoints predicted in Subject S1a by array CGH. Primers OT572 and OT575 (bottom panel) flank the ATAD3 deletion breakpoints predicted in S2 by array CGH. As a control, primers OT570 and OT575 (top panel) were used since primer OT570 is located within the predicted deleted ATAD3B/ATAD3A region. (C) Genomic DNA sequencing of the breakpoint-spanning PCR products determined the ATAD3B/ATAD3A deletion boundaries in each subject, with chromosome 1 coordinates indicated (hg19). Ambiguous regions flanking the deletion boundaries that have identical sequence in ATAD3B and ATAD3A are identified by dark grey boxes. The deletion predicted by high-density SNP array for Subject S5 is indicated, with maximum and minimum deletion boundaries labelled by hatched boxes.
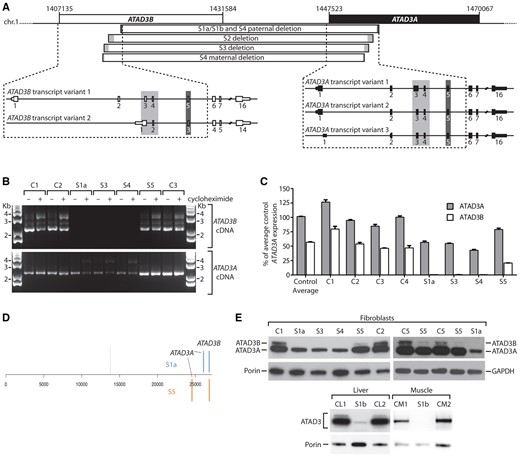
ATAD3 gene cluster deletions result in decreased expression of ATAD3A, and the loss of ATAD3B at the mRNA and protein level. (A) ATAD3 deletions in relation to exon structure of full length ATAD3B and ATAD3A isoforms for Subjects S1a, S1b, S2 and S3. (B) Full length ATAD3A and ATAD3B isoforms were amplified from control and subject cDNA prepared from fibroblasts grown ± cycloheximide. Primers OT441 and OT443 were used to amplify ATAD3A, while primers OT441 and OT445 were used to amplify ATAD3B. The ATAD3A product amplified in S1a, S3 and S4 represents the fusion cDNA due to cross-hybridization of primer OT441. (C) The relative expression levels of ATAD3A versus ATAD3B were determined by qRT-PCR from four controls and Subjects S1a, S3, S4 and S5. Results were normalized to HPRT expression and presented as percent of average control ATAD3A expression; n = 3, error = SEM. Two-way ANOVA comparing all controls and subjects showed ATAD3B expression was significantly reduced in all subjects compared to individual controls (P < 0.0001), as was ATAD3A in S1a, S3 and S4 (P < 0.0001). For Subject S5, the reduction in ATAD3A was not significant in comparison to all controls. (D) Ranked gene expression list showing the positions of ATAD3A and ATAD3B in Subjects S1a and S5 versus control. The dotted line represents the position of genes that are expressed at the same level in subject and control samples; to the left, genes expressed more highly in the subjects; to the right, genes expressed less than the control. Total number of genes 26 689. S5 fibroblasts manifested reduced expression of ATAD3B, with log2 fold change (FC) = −1.31; and to a lesser extent the intact ATAD3A log2FC = −0.73. The transcripts of the ATAD3B/ATAD3A chimera of Subject S1a registered as decreased expression of both original genes; ATAD3B, log2FC−3.0, ATAD3A, log2FC = −1.50. (E) ATAD3 proteins detected by immunoblotting using a pan-specific antibody in tissues and fibroblasts from subjects, relative to controls. Porin or GAPDH was used as a loading control.
High density SNP array analysis of Subject S5 indicated that one allele carried an ATAD3C/ATAD3B deletion of 43 to 61 kbp in size, while long-range PCR suggested the second allele had a similar ATAD3B/ATAD3A deletion to Subjects S2 and S3 (Fig. 2B and C). However, RNA analyses showed that the second allele is more complicated than a simple fusion gene. Unlike in Subjects S2 and S3, full length ATAD3B cDNA could be amplified from S5 fibroblasts but there was no cDNA sequence corresponding to the ATAD3B/ATAD3A fusion transcripts (Fig. 3B and Supplementary Fig. 4D). Sequencing of ATAD3A cDNA suggested one allele had at least one exon (exon 8) replaced by ATAD3B sequence, resulting in a minimum of two missense variants in the ATAD3A isoform 2 sequence (p.L269A and p.A271T, Supplementary Fig. 5C and E). Transcriptome analysis of Subjects S1a, S5 and control fibroblasts suggested the ATAD3C gene is not expressed in fibroblasts and indicated that ATAD3A and ATAD3B were among the most altered of the full set of expressed genes in both Subjects S1a and S5 (Fig. 3D); qRT-PCR confirmed that ATAD3B, and to a lesser extent ATAD3A, expression were decreased in Subject S5 (Fig. 3C). The precise genomic rearrangement on the second ATAD3 allele has not been defined but the simplest explanation that reconciles the DNA and RNA results is that both the ATAD3A and ATAD3B genes on the second allele have some sequence replaced by elements of the corresponding gene via interlocus gene conversion; as documented for other disease mutations, particularly between pairs of genes with high homology (Casola et al., 2012; Dumont, 2015).
Immunoblotting of ATAD3 detected two species in control samples, as previously described (He et al., 2007), the lower one being compatible with the 66 kDa ATAD3A isoform 2. Only one species of ∼65 kDa was detected in Subjects’ S1a, S3 and S4 samples, at much lower abundance than control cell lines. A more marked loss of the lower band was seen in liver and muscle of Subject S1b compared with fibroblasts (Fig. 3E). Thus, the protein data are fully concordant with the genomic mapping that predicted an ATAD3A/ATAD3B fusion protein indistinguishable in size from ATAD3A. Moreover, because ATAD3A is invariably more highly expressed than ATAD3B [see for example, He et al. (2007) and Fig. 3D], the decrease in expression can be attributed largely to the fusion gene being under the control of the ATAD3B promoter. In Subject S5, the band of the size expected for ATAD3B (72.6 kDa) was barely detectable (Fig. 3E), the residual signal can be explained by low expression of ATAD3B, or expression of the 71.4 kDa ATAD3A isoform 1. ATAD3A isoform 2 was less abundant than in controls, but greatly exceeded that of the fatal neonatal subjects (Fig. 3E), providing a ready explanation for the milder disease phenotype of Subject S5.
Mitochondrial DNA abnormalities associated with ATAD3 deletions
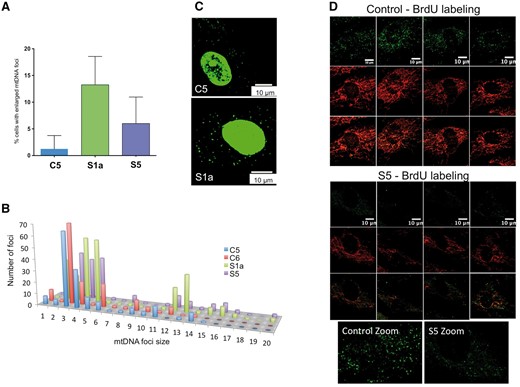
ATAD3 deficiency is associated with mtDNA abnormalities. The DNA of human fibroblasts was stained with anti-DNA antibody and the number of cells with enlarged mtDNAs was scored for cell lines C1, S1a and S5. (A) A minimum of 150 cells was counted for each cell line (n = 4 independent experiments, error bars are 1 standard deviation from the mean). See Supplementary Fig. 6 for representative images for Subject S5. (B) The signals of foci in the cytoplasm (mtDNA) plotted as frequency distribution after particle point analysis of a minimum of 20 cells. To smooth the data, signals were sorted into 20 ‘bins’ based on intensity. Foci falling in bins 1–6 all correspond to one mtDNA molecule based on detailed study of the images, including ones subjected to deconvolution analysis (Akman et al., 2016), and the fact that most mtDNAs are organized as single copies (Kukat et al., 2011), with the variation across the range expected to be the result of differences in condensation (packing) and efficiency of antibody access and coating. Many of the foci of bins 7–9 could be resolved to two or three separate or overlapping smaller dots indicating they contained two or three mtDNA molecules, whereas larger foci in bins 10 and above contained more than three copies of mtDNA. Very large mtDNA foci (bins > 11) were significantly more numerous in Subjects S1a (P < 0.0001) and S5 (P < 0.0001) fibroblasts than controls. (C) PicoGreen® staining of mtDNA reveals larger foci in cells of S1a than those of controls. Representative images showing the PicoGreen® stained structures in the cytoplasm (mtDNA). Nuclear DNA staining with PicoGreen® is highly variable (often it is undetectable) (He et al., 2007) and so cannot serve as a reference. Microscope settings and image capture parameters were identical. PicoGreen® staining was as previously described (Ashley et al., 2005). (D) BrdU incorporation into mtDNA detected by immunofluorescence. Single confocal optical sections of C1 and S5 fibroblasts treated with 20 μM BrdU for 8 h and with anti-BrdU antibody (green) and anti-ATAD3 antibody (red).
Pharmacological perturbation of cholesterol metabolism induces mtDNA clustering
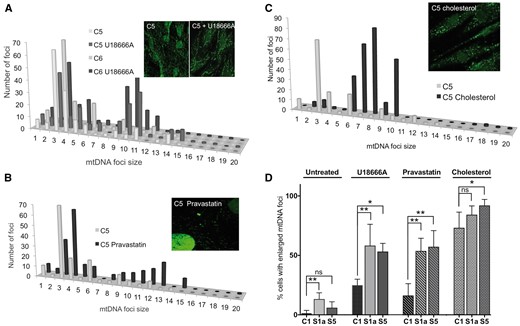
U186666A, pravastatin and cholesterol increase the size of mitochondrial nucleoids in human fibroblasts. The DNA of control human fibroblasts was stained with anti-DNA antibody after treating cells for 7 days without or with 5 μM U18666A (A), or pravastatin (B); or for 5 days with 5 mM cholesterol (C). P-values for the difference between large mtDNA foci (bins > 8) of treated versus untreated cells were P = 0.0116 (U18666A); P = 0.0011 (pravastatin); P = 0.0002 (cholesterol). The data are plotted as frequency distributions after particle point analysis. Insets are representative images. (D) The proportion of fibroblasts with enlarged mtDNA foci, after treatment with U18666A, pravastatin, or cholesterol, compared to untreated cells, based on a minimum count of 50 cells from each of three independent experiments, error bars are 1 standard deviation from the mean. Fibroblasts of Subjects S1a and S5 carry deletions in the ATAD3 gene cluster. Data for the ‘untreated’ cells, derived from experiments carried out in parallel, are reproduced from Fig. 4A. Probability was determined using a one-way ANOVA test (uncorrected Fisher’s LSD). *P < 0.05; **P < 0.01, ***P < 0.001.
U18666A or pravastatin treatment of ATAD3-deficient fibroblasts increased the number of cells with enlarged mtDNA foci, maintaining or amplifying the distinction from control cells (Fig. 5D). There were also marked effects on the size of individual foci, particularly in Subject S1a (Supplementary Fig. 7A and B). In contrast to these drugs, cholesterol supplementation reduced the mtDNA differences between ATAD3-deficient and control fibroblasts (Fig. 5D and Supplementary Fig. 7C).
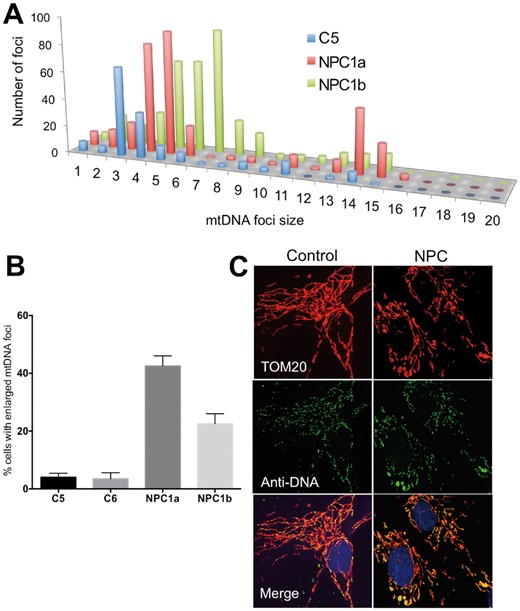
The cholesterol trafficking disorder, Niemann-Pick disease type C, is associated with mtDNA aggregation
Niemann-Pick type C disease is associated with mtDNA disorganization. (A) Frequency distribution of mtDNA foci size in fibroblasts from a control and two individuals with NPC1 defects. (B) Proportion of cells with mtDNA clusters, error bars are 1 standard deviation from the mean. (C) Representative images. Anti-DNA (green) and anti-TOM20 labelling of fibroblasts. Yellow foci, and green foci bounded by red, are mtDNAs.
ATAD3-deficient cells display perturbed cholesterol and lipid metabolism
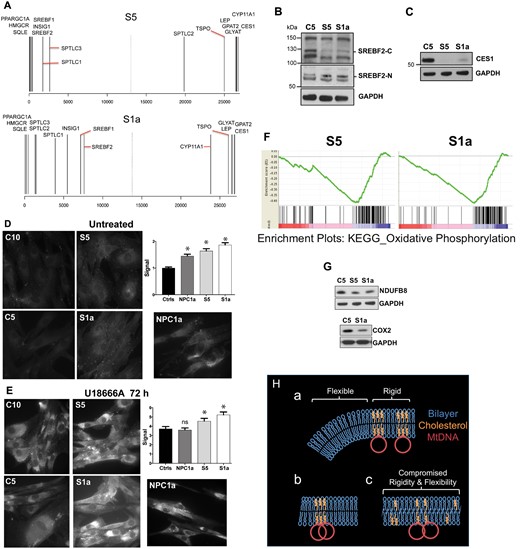
Abnormal cholesterol homeostasis and reduced expression of OXPHOS factors associated with ATAD3 gene cluster deletions. (A) Rank gene expression list showing the position of selected factors for Subjects S1a and S5 versus control (see also Supplementary Figs 8–10 and Supplementary material). (B and C) Proteins from fibroblasts of controls and S1a and S5 were fractionated by SDS-PAGE and probed with the indicated antibodies after blot transfer. There was no increase in the steady-state level of the larger isoforms of SREBF2 (located in the cytoplasm, SREBF2-C) among the subject samples but a SCAP processed SREBF2 isoform, which translocates to the nucleus (SREBF2-N) and resolves at ∼55 kDa, was increased in S1a and S5 samples (this particular species may be a phosphorylated form of SREBF2 (Krycer et al., 2012); CES1 was greatly diminished. (D and E) Free cholesterol detected by filipin labelling of fibroblasts exposed to (D) no treatment, or (E) 5 μM U18666A for 72 h. *P < 0.05, ns = not significant. (F) Gene set enrichment plots for OXPHOS in Subjects S1a and S5. Each vertical black line represents an mRNA, with the most positively correlated to the left (in the red zone). Clustering at the negatively correlated end of the spectrum (blue zone) indicates the pathway is repressed compared to the reference. Consistent changes across a gene set give sharp curves (green lines) and S1a gave the sharper curve of the two. Moreover, for S1a OXPHOS was the third most negatively differentially expressed pathway or process, whereas it was 17th for S5. These observations are consistent with greater OXPHOS impairment in S1a than S5. (G) Immunoblots of respiratory chain components of complex IV (COX2) and complex I (NDUFB8). (H) Proposed arrangement of cholesterol in the mitochondrial inner membrane, the mtDNA in particular is not to scale. (a) In normal conditions localized high concentrations of cholesterol impart rigidity to the membrane for optimal organization and segregation of the mtDNA, whereas other regions require greater flexibility to form the highly invaginated membranes characteristic of the inner mitochondrial membrane. (b) If cholesterol is scarce there is insufficient sterol to permit mtDNA segregation. (c) Alternatively, if cholesterol is present in normal amounts but dispersed, both rigidity and flexibility are suboptimal; hence, a key role of ATAD3 may be to concentrate cholesterol where the mtDNA is located. Such membrane abnormalities (b or c) could cause the increase in mitochondrial turnover (mitophagy) reported for other ATAD3 mutants (Harel et al., 2016).
GSEA analysis (Subramanian et al., 2005) corroborated the Meta-Core results, highlighting lipid metabolism and cholesterol and sterol biosynthesis as altered in ATAD3-deficient cells, especially those of Subject S5 (Supplementary Fig. 10). In addition, GSEA revealed that highly significant decreases in the OXPHOS system in the ATAD3 deletion cells (FDR q-value < 1 × 10−16 for Subjects S1a and S5) correlated with disease severity (Fig. 7F). Individual OXPHOS components tested were slightly decreased at the protein level (Fig. 7G), and thus were concordant with the GSEA.
Discussion
Defects in mitochondrial function have been linked to pontocerebellar hypoplasia previously, particularly mutations in the RARS2 tRNA synthetase gene (Edvardson et al., 2007) and occasional cases with a mtDNA deletion (Biancheri et al., 2011) or OXPHOS enzyme defect (as reported for Subject S2; de Koning et al., 1999). We describe four unrelated families in whom chimeric ATAD3B/ATAD3A gene fusions result in congenital pontocerebellar hypoplasia. A family with a similar deletion and clinical presentation was also recently reported (Harel et al., 2016). In a cohort of 169 cases of pontocerebellar hypoplasia, 106 were linked to mutations in four genes (TSEN54, TSEN2, TSEN34 and RARS2) (Namavar et al., 2011). The identification of five pontocerebellar hypoplasia families with different ethnicities and ATAD3B/ATAD3A gene fusions suggests ATAD3 defects could underlie a significant portion of the remaining cases lacking a molecular diagnosis, especially those with associated cortical abnormalities.
The ATAD3 gene cluster is likely to have been generated via consecutive gene duplication events and the extensive regions of direct repeats predispose to non-allelic homologous recombination and an increased frequency of genomic re-arrangements such as deletions and interlocus gene conversion. The highly repetitive sequences can result in erroneous calling of copy number in SNP arrays due to a paucity of unique SNP markers and cross hybridization of these few markers. Similar issues complicate mapping and detection of ATAD3 single nucleotide variants and copy number changes by whole exome sequencing, where standard pipelines do not routinely detect most copy number variants. Indeed, SNP array and whole exome sequencing of Subject S3 DNA in a diagnostic laboratory were initially reported as normal. Following diagnosis of Subject S1a, SNP array data for the 1p36.33 region from over 50 000 individuals were reanalysed, flagging the likely homozygous deletion in Subject S3.
Hence, the detection of pathogenic mutations in the ATAD3 genes is compromised and re-interrogation of previous SNP or exome data for this region is warranted for unsolved cases with relevant phenotypes. Homozygous deletions in the region can be identified from exome sequencing by visual inspection of the aligned read data through absence of coverage (Supplementary Fig. 11), indicating that more specialized calling algorithms should be able to recognize the abnormality in the future. Base level resolution of the breakpoints will, however, in most cases require long read sequencing technology to overcome homology and mapping issues.
Aberrant mtDNA organization and cholesterol homeostasis in CNS defects associated with ATAD3 deficiency
Neurological dysfunction and developmental impairment are features of many mitochondrial disorders, but the pathogenic mechanisms are poorly understood. The current findings suggest that cholesterol could be a key factor in the pathology linked to ATAD3 defects, a view supported by the cerebellar dysfunction present in cholesterol-related genetic diseases. Hypoplasia of the cerebellum is classically associated with Smith-Lemli-Opitz syndrome (MIM 270400), a recessive disorder of cholesterol synthesis, and cerebellar dysfunction is a feature of Niemann-Pick disease Type C. On the other hand, the mtDNA abnormalities evident in Niemann-Pick disease Type C (Fig. 6) and ATAD3 deletion disorders (Fig. 4) suggest these could be the primary driver of the cerebellar pathologies.
Mitochondria also play an important role in steroidogenesis. Purkinje cells are the main sites of neurosteroid production in the brain (Tsutsui and Yamazaki, 1995; Usui et al., 1995) and progesterone and its metabolite, allopregnanolone, are high in neonatal life, when cerebellar circuits form in mammals to promote dendritic growth, spine formation and maintenance of the Purkinje cells (Sakamoto et al., 2002). Hence, a shortage of the products of cholesterol provides a plausible alternative explanation for the cerebellar pathology accompanying the ATAD3 deletions.
In contrast to the fatal infantile ATAD3B/ATAD3A gene fusion defects, recessive and de novo dominant ATAD3A missense mutations are associated with milder forms of cerebellar dysfunction and a broader range of neurological and multisystem disorders (Harel et al., 2016; Cooper et al., 2017). This suggests that ATAD3A missense mutations result in loss of a subset of ATAD3’s functions, or cause it to malfunction, rather than the severe protein deficiency caused by biallelic deletions in the ATAD3A locus. Subject S5 differs from other reported individuals with ATAD3 defects in having biallelic genomic rearrangements of the ATAD3 locus but with a phenotype more similar to that caused by ATAD3A missense mutations. This presumably results from the complicated rearrangement in one allele allowing expression of one wild-type ATAD3A allele but affecting expression of both ATAD3B alleles.
Mitochondria, ATAD3 and cholesterol homeostasis
The perturbations of cholesterol homeostasis associated with ATAD3 deficiency suggest mitochondria are a key organelle affecting cholesterol metabolism and that ATAD3 regulates the supply of cholesterol to the mitochondria. That the mitochondrial signal for cholesterol biosynthesis precipitated by ATAD3 deficiency overrides those of the remainder of the cell, where there is no apparent shortage of cholesterol, speaks to the importance of maintaining mitochondrial cholesterol levels within strict limits. The impact on the mtDNA of perturbed cholesterol metabolism (Figs 4–6) suggests insertion of the sterol in the inner mitochondrial membrane is important for mtDNA replication and segregation, which occur at mitochondrial–endoplasmic reticulum junctions (Murley et al., 2013; Lewis et al., 2016). Given the central role of the endoplasmic reticulum in cholesterol metabolism (Iaea and Maxfield, 2015), such contact sites are the obvious route for cholesterol channelling to mitochondria, and the ATAD3 associated with the mtDNA and the inner mitochondrial membrane (He et al., 2007) is ideally placed to ensure cholesterol is concentrated here. Low or dispersed cholesterol in the inner mitochondrial membrane would be expected to alter its rigidity and thereby impede mtDNA segregation (Fig. 7H).
Cholesterol and mitochondrial integrity have both been linked to neurodegeneration (Karasinska and Hayden, 2011). Hitherto these were seen as distinct ideas, which can now be accommodated in a single hypothesis, where defects in either cholesterol metabolism or mitochondrial dysfunction will adversely impact the other. It will therefore be of considerable interest to learn how mtDNA organization and maintenance operate in a broad range of neurodegenerative disorders.
Acknowledgements
We acknowledge Prof Frank Baas and Prof Richard Leventer for clinical review of the MRI images of Subject S3, Drs Michelle Farrar and Chee Hiew for assistance with MRI images from Subject S1a, Dr Kazuyuki Ito for assistance with clinical review of Subject S4 and Yoshihito Kishita for technical assistance.
Funding
In Australia this work was supported by grants from the Australian National Health and Medical Research Council (NHMRC) (1068409 and 1107094 to D.R.T.), the Australian Mitochondrial Disease Foundation, an NHMRC Principal Research Fellowship (1102896 to D.R.T.), an NHMRC Early Career Fellowship (1054432 to E.J.T.), an NHMRC scholarship (H.S.M.), an Australian Postgraduate Award (N.J.L.) and the Victorian Government’s Operational Infrastructure Support Program. In the UK, the study was funded by the Medical Research Council [intramural award to I.J.H. (to April 2016), and a senior non-clinical fellowship to A.S., MC_PC_13029). In France, the work was supported by grants from the FRM (Fondation pour la Recherche Médicale) grant DPM20121125550 to A.L.; from the Association Française contre les Myopathies (AFM) to A.L. and Association contre les Maladies Mitochondriales (AMMi) to A.L.. In the Netherlands, the work was supported by a CSBB grant (853.00.130) from NWO-ALW to J.S.. In Japan, the work was supported by a Strategic Research Center in Private Universities grant from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and by the Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development (AMED).
Supplementary material
Supplementary material is available at Brain online.
References
Author notes
*,#These authors contributed equally to this work.


