-
PDF
- Split View
-
Views
-
Cite
Cite
Wolf-Julian Neumann, Andrea A. Kühn, Reply: Role of cortico-pallidal connectivity in the pathophysiology of dystonia, Brain, Volume 139, Issue 9, September 2016, Page e49, https://doi.org/10.1093/brain/aww105
Close - Share Icon Share
Sir,
We appreciate the opportunity to comment on the relevant Letter to the Editor by Cacciola and colleagues (2016). A great effort has been made throughout recent decades to define the various anatomical structures that are implicated in the pathophysiology of dystonia (Quartarone and Hallett, 2013). Dystonia is now considered to be a network disorder defining basal ganglia, cerebral cortex, cerebellum, thalamus and brainstem as major nodes (Lehericy et al., 2013). The characterization of abnormal neuronal activation patterns in dystonia at different levels of the nervous system including the loss of inhibition, enhanced plasticity and abnormal sensorimotor integration, have shaped our view on the pathophysiology of dystonia. However, the functional roles of the different brain areas and their interaction within the sensorimotor network on the expression of variable dystonic symptoms remain to be elucidated (Lehericy et al., 2013). Thus, we believe that the research focus should now shift from characterizing the involved structural components of the dystonia network to investigating network interactions and their role for dystonic symptoms and therapeutic effects. Argyelan and colleagues (2009) implicated a loss of structural connectivity with the cerebellum through analysis of fibre integrity in the pathophysiology of dystonia and could show a negative correlation between structural cerebellar connectivity and motor cortex activation. Recently, a direct connection between the cortex and the pallidum has been proposed by Milardi et al. (2015). Following this, the letter from Cacciola et al. (2016) has inspired us to reanalyse our data in this regard. We would like to take advantage of the opportunity to demonstrate the resulting correlation analysis in this letter. We showed previously that pallido-cerebellar alpha band coupling is negatively correlated with dystonic symptom severity, but no correlation was found for the motor cortex that is connected to the internal globus pallidus (GPi) through beta band oscillations (Neumann et al., 2015). However, in supplementary analysis of the previously published local field potential-magnetoencephalography data we looked at correlations of spectral power from the extracted sources (for methods see Neumann et al., 2015) that represent nodes in the network and have found a negative correlation between cerebellar alpha band power (7–13 Hz) and motor cortical beta band power (13–30 Hz; Spearman’s Rho = −0.74; P = 0.023; Fig. 1A).
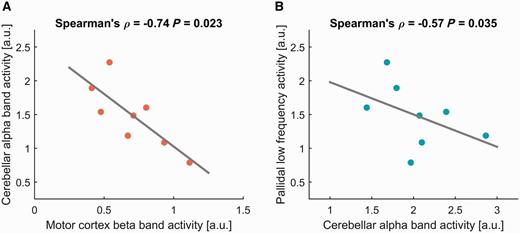
Averaged cerebellar alpha oscillatory activity (7–13 Hz) from source-extracted data correlate negatively with motor cortex beta band activity (A; 13–30 Hz) and pallidal low frequency activity (B; 4–12 Hz) across eight patients with dystonia.
Thus, a reduction of cerebellar alpha band activity, which may be caused by a loss of structural fibre integrity, could lead to increased motor cortical beta oscillatory activity in patients with dystonia. Furthermore, pallidal low frequency activity has been consistently shown in local field potential recordings from patients with dystonia (Silberstein et al., 2003; Chen et al., 2006a, b; Liu et al., 2006, 2008; Chen and Brown, 2007; Sharott et al., 2008; Barow et al., 2014) that can be suppressed by high frequency stimulation (Barow et al., 2014). In the present correlation analysis, we also found a negative correlation between pallidal low frequency activity and cerebellar alpha band power in the cohort (Spearman’s Rho = −0.57; P = 0.035; Fig. 1B). Hence, enhanced pallidal low frequency activity could be an adaptive phenomenon that is caused by the loss of alpha band power in the cerebellum. Therefore, we propose the hypothesis that cerebellar alpha band activity and coupling could be a crucial influence on normal basal ganglia-cortex function. Loss of functional connectivity here could lead to dysfunctional interactions in the cortex-basal ganglia loop and could eventually be related to a modulation of motor cortical synaptic plasticity (Quartarone et al., 2003). The newly described super direct pathway (Milardi et al., 2015) is a tempting candidate through which these interactions could be mediated, but other indirect pathways are also possible. The aim to bring these different pieces of the puzzle together now calls for multimodal experimental approaches. Cortical plasticity has been proposed to be enhanced in dystonia patients (Quartarone et al., 2003; Edwards et al., 2006) and variable effects of pallidal deep brain stimulation on synaptic plasticity using paired associative stimulation have been described (Tisch et al., 2007; Ruge et al., 2011a, b) but the relation between abnormal plasticity and pallidal oscillatory activity remains unclear. Combined transcranial magnetic stimulation and local field potential-magnetoencephalography studies could shed light on these interactions. Furthermore, we should aim to investigate the functional role of the different oscillatory networks and their structural representation through a combination of electrophysiological and neuroimaging approaches. Finally, deep brain stimulation offers a powerful tool to manipulate these networks and pathophysiological phenomena and we should aim to use this tool to investigate not only its therapeutic mechanism, but also to paint a global picture of the pathophysiology of dystonia.
Funding
This work has been funded by the German Federal Ministry of Education and Research (BMBF) grant ‘Dystract 01GM1514D’ and the German research foundation (DFG) grant ‘KFO247’.
References


