-
PDF
- Split View
-
Views
-
Cite
Cite
Alastair Compston, Acute poliomyelitis. By F. E. Batten MD Cantab FRCP Lond. The Lumleian Lectures for 1916 delivered before the Royal College of Physicians. Brain 1916; 39: 115–211 , Brain, Volume 139, Issue 5, May 2016, Pages 1615–1620, https://doi.org/10.1093/brain/aww073
Close - Share Icon Share

The early twentieth century saw worldwide epidemics of poliomyelitis. In 1916, in an article in Brain , Frederick Batten told the story of the disease and anticipated much that happened later. Alastair Compston revisits Batten’s paper and highlights the emerging understanding that would lead ultimately to immunological therapies and vaccines.
‘I was driving home after watching the hospital rugger-team play when my attack of poliomyelitis began’. A few days later, Dr Ronald Henson (1915–94: deputy editor of Brain , 1975–82) develops headache and vomiting, and finds that he cannot lift his arm. Soon after he has difficulty with speech and swallowing and the weakness spreads so that, for a while, he is left with bilateral involvement of the arms and shoulder girdles, bulbar palsy, and weakness of the intercostal and trunk muscles. His mental state is altered with confusion and flight of ideas. He feels as though he is sitting in his own medulla oblongata watching the fallout of cells from the nucleus ambiguus. His perception of extra-personal space is distorted. Sleep is disturbed and he craves, but is denied, adequate sedation. His muscles fasciculate and he develops myoclonus in the weak arms. Slow spontaneous improvement begins after 10 days and continues for the next 6 months. Generalized fatigue and inability to sustain exercise last much longer. Physiotherapy and active exercises seem to help. The nursing is excellent: he concludes that ‘it is difficult to imagine a more terrifying predicament than that of the patient with bulbar poliomyelitis who lies in the care of the ignorant’. And as any physician who has been unwell recognizes, ‘my illness gave … an opportunity to show the way in which the sick should be treated … doctoring will never be quite the same again [Anon (R. Henson) Poliomyelitis, Lancet 1953; i: 1196–7].
Adolph Kussmaul (1822–1902) first used the term ‘poliomyelitis anterior acuta’ to refer to an illness that had laboured under a variety of names since the 18th century. No less tortuous was the naming of the causative agent, identified as viral by Karl Landsteiner (1868–1943) in 1908, and attributed to three strains of ‘poliovirus’, now known to be one of the enteroviruses, 40 years later. The virologist John (Rodman) Paul (1893–1971), involved from the 1930s in clinical epidemiology and the development of treatments for the disease, has written A history of poliomyelitis (1971). Cases are probably included in accounts of illness from ancient times but the first definite description starts with Michael Underwood (1737–1820) in the second edition of A treatise on the diseases of children etc. (1789). Further sporadic descriptions were made during the 19th century, including the work of Jacob Heine (1800–79) after whom the disease was, for a while, named in association with (Karl Oskar) Medin (1847–1927). Particularly brilliant was the suggestion by James Abercrombie [(1781–1844): Pathological and practical researches on diseases of the brain and spinal cord , 1828] that—with resolution of the spat between Charles Bell (1744–1842) and François Magendie (1783–1855) on the anterior versus posterior nature of the ‘way in’ and the ‘way out’ of the spinal cord—a disease causing paralysis with sensation intact must selectively affect the anterior horn cells or nerve roots. (Jean-Martin) Charcot (1825–93) added to this pathological insight, recognition that the anterolateral columns are also preferentially involved. Dr Paul traces emerging ideas on poliomyelitis as an epidemic infectious disease, mainly of children, across the first decades of the 20th century and the contribution made by applying knowledge from the emerging discipline of immunology. By 1916, the use of intrathecal injection of convalescent serum was being subjected to clinical trials but these were poorly designed and gave ambiguous results other than offering the truism that early treatment is preferable to dealing with advanced cases. The use of human and horse immune serum continued until the mid-1930s when it became clear that addition of convalescent serum added nothing to the concentration of antibody produced early in the illness by the patient’s own immune system. Only after World War II, during which poliomyelitis was intermittently epidemic and of much military significance in the Middle East, did this approach change with the use of concentrated immune globulin. By then, supportive treatment with assisted ventilation in an ‘iron lung’, developed by Dr Philip Drinker (1894–1972) in Boston, had saved lives; and various orthopaedic techniques were used to immobilize the paralysed limbs until this practice was discredited by a nursing sister, Elizabeth Kenny (1890–1952). By 1935, ideas on vaccination were developing and these gained momentum with the systematic evaluation of the levels of immunity required to protect from infection; the introduction of methods for virus inactivation; and successful isolation of the poliovirus in tissue culture in the laboratory of John (Franklin) Enders (1897–1985). Eventually, the frequency of poliomyelitis was reduced through the work of Hilary Koprowski (1916–2013); Jonas Salk (1914–95) who perfected the safe and effective use of inactivated virus for mass immunization (Salk et al. Journal of the American Medical Association 1953; 151: 1081–98) despite the 1955 Cutter episode in which live virus was inadvertently administered to some children; and Albert Sabin (1906–93) who introduced oral attenuated live virus vaccine, also briefly blighted in 1962 by the development of paralytic poliomyelitis caused by type 3 virus in a few adults (Sabin et al. Annals of the New York Academy of Sciences 1955; 61: 924–38). As a result, from 1955 to 1965, the annual frequency of cases in the USA reduced by >99%.
One hundred years ago, in his Lumleian lectures delivered before the Royal College of Physicians and published in the issue of Brain for June 1916, Frederick Batten (1865–1918) told the story of poliomyelitis and anticipated much that happened later. Batten qualified in medicine from Cambridge (1887) and St Bartholomew’s Hospital (1891): he was appointed to the consultant staff of the National Hospital for the Paralysed and Epileptic (pathologist, 1899; physician, 1900–1918; dean, 1908–1918) and he served as the first paediatric neurologist at the Hospital for Sick Children, Great Ormond Street, London (c1900–1918): Sir Gordon Holmes (1876–1965) recalls that:
With others Batten described subacute combined degeneration of the spinal cord in 1900; ‘familial or juvenile amaurotic idiocy’ [cerebromacular degeneration or Batten’s disease, in which he may have been preceded in 1897 by his brother, Rayner (1835–1909)] in 1903; and a form of congenital myopathy (designated Batten-Turner disease) also in 1903. For many years, the ward that cared for cases of poliomyelitis and other acute neurological illnesses at Queen Square was named after him.‘Batten’s approach was scientific … interpret[ing] symptoms as disturbances of function and determin[ing] the changes in structure … to which they were due. His honesty, simplicity and directness impressed all who came in contact with him’.
Batten opens his lecture by pointing out that, in the decade since Farquhar Buzzard (1871–1945) touched upon poliomyelitis is his Goulstonian Lecture for 1907, the disease has become epidemic across the globe. Although the British Isles have been relatively spared, poliomyelitis became notifiable following an outbreak in Cumberland during 1910 and with compulsory notification imposed by the Local Government Board on the recommendation of the Royal College of Physicians from September 1912. Bearing some similarities to rabies but spreading like meningococcal meningitis, and most accurately described as ‘meningo-myelo-encephalitis-disseminata’, poliomyelitis is an acute specific fever affecting any part of the CNS for which:
‘No effectual remedy, other than prevention, will be found for a disease which is so fulminating in attack and so destructive in its effects’.
In a previous paper in Brain (1911; 34: 45–69), Batten rehearsed details of 61 worldwide epidemics starting in Sweden in 1881, spreading to Italy in 1883, and reappearing intermittently in northern Europe a few years later with particularly widespread, large and persistent outbreaks in Norway and Sweden from 1899, notably that described by (Ivar) Wickman (1872–1914) in 1905. Epidemic poliomyelitis occurred in North America from 1894, the outbreak in Vermont reported by Dr Charles Caverly (1856–1918)] affecting 132 cases. The illness has become more prevalent over time in the USA: 5093 cases were recognized during 1910 alone carrying a 14% mortality.
Dr Paul tells us that later, during the severe epidemic of 1916 also in the north-eastern part of the United States, isolation and quarantine and a ban on travel were first instituted to good effect. Five years later Franklin Delano Roosevelt (1882–1945) developed poliomyelitis leaving him with paralysed legs. This did not prevent Roosevelt from serving four terms as President of the United States; in the late 1920s, ‘FDR’ founded a treatment centre for people with poliomyelitis in Warm Springs, Georgia which he often visited himself for hydrotherapy in the 89 °F (32 °C) natural waters.
Batten writes that epidemics were observed in England from 1897; and with apparent increasing frequency and irregular geographic distribution in each year from 1912—Lancashire and the Midlands taking an especially heavy toll in 1912, and Gloucestershire in 1915. Over this period, up to 823 cases occurred annually with a 13% mortality and 50% permanently disabled. Unusually, the 1913 epidemic in Barrow-in-Furness affected very young children perhaps because the more susceptible had already been affected in 1910 leaving ‘thirty-seven cripples’; and with the rest of the population:
‘Naturally immune or [with] acquired immunity … exhausting the susceptible material of the population’.
However, the ‘spot-map’ of cases overlapped in 1910 and 1913 suggesting that environmental factors were similar and implicating the presence of a ‘virus carrier’. Detailed scrutiny, after the epidemics had resolved, failed to identify a common environmental factor responsible for the disease. Dr Batten pays close attention to cases in London—the boroughs of Stepney, Islington, Hackney (and Wandsworth) being particularly vulnerable so that the London Hospital has accumulated unusual experience of managing the disease. The epidemiological pattern of poliomyelitis shows seasonality peaking in August and September or equivalent months in the southern hemisphere where ethnic susceptibility and resistance were apparent in the 1909 outbreak affecting the south Pacific island of Nauru. Age is important with children proving especially vulnerable. Infectivity is high, the illness showing a short incubation period of 1–4 days with spread by contact at school or through lines of communication and transport.
Farquhar Buzzard had concluded that poliomyelitis:
‘Is an acute specific fever occurring sporadically and epidemically; its essential lesion is an inflammation of the interstitial tissue of the central nervous system, due to the presence of micro-organisms, or their toxin, probably in the blood, but possibly in the lymph, circulating within that system’.
In his historical survey, Batten starts with the 1860 account of Heine, updating his earlier book Observations on paralytic conditions of the lower extremities and their treatment (1840). Heine attached no particular significance to atrophy of the anterior horn cells; but this feature was emphasized by (Edmé Félix Alfred) Vulpian (1826–87) and (Jacob Augustus) Lockhart Clarke (1817–80). Gradually there emerged a concept that poliomyelitis is due to inflammatory injury of the grey matter of the spinal cord with secondary effects on the interstitial tissue:
‘The inflammation travels along the perivascular lymphatics; the perineural lymphatics probably carry the infection from the site of inoculation to the spinal cord’.
But this analysis is not universally accepted and the alternative view that poliomyelitis is a diffuse disorder of interstitial tissue continues to be debated.
Batten’s experience is that children dying within a few days of onset show some enlargement of lymph tissue. The CSF is usually normal but may show a raised protein and modest mononuclear cell reaction. An increase in polymorphonuclear cells is sometimes seen in peripheral blood. The absence of lymphocytosis is ‘because these [cells] are constantly being withdrawn to meet the invasion of the virus at its various points of attack’. Macroscopically, the brain feels normal but the cord may be swollen and the grey matter softened or gelatinous in appearance. In longer standing cases, the cord is shrunken and the grey matter visibly atrophied ( Fig. 1 ). Other parts of the CNS show congestion or areas of softening. Batten has studied the microscopic changes and supports the view that, in the acute stage, there is perivascular lymphocytic infiltration centred on the anterior region of the cord with interstitial glial changes and evidence for phagocytosis of anterior horn cells by neuroglia ( Fig. 2 ). Batten’s impression that the posterior root ganglion cells are also affected makes for difficulty in distinguishing poliomyelitis from herpes zoster infection; and the confusion is not helped by the fact that herpes zoster shows a similar seasonal and geographical distribution to poliomyelitis and may cause localized paralysis in addition to the sensory changes. There is microscopic involvement of the medulla and mid-brain even in cases of poliomyelitis without relevant clinical symptoms or signs during life. With more prolonged survival, Batten has observed demyelination and degeneration of fibres in the efferent portion of the anterior root and of the antero-lateral tracts in the spinal cord. Later still, the anterior horns and antero-lateral tracts appear atrophic.
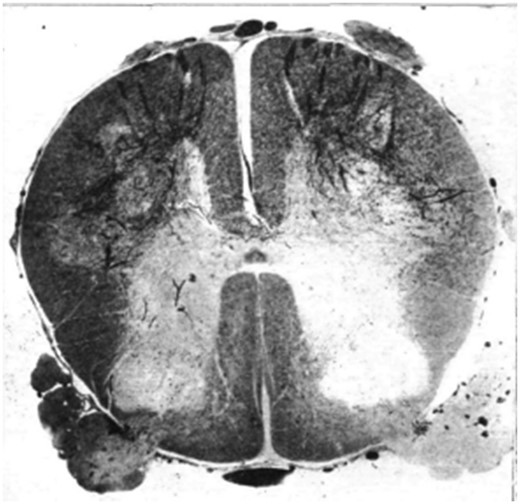
Section of spinal cord of a child who died six weeks after the onset of the disease, stained by Marchi method, showing degeneration of the efferent fibres of the anterior roots.
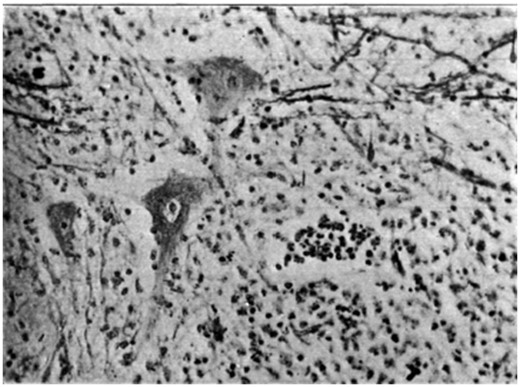
Photograph of the ganglion cells of the anterior horn showing varying degree of ‘neuronophagia’.
The evidence for a poliomyelitis-like illness occurring spontaneously in animals is anecdotal. Experimental work has shown that the disease can be transmitted to monkeys, and occasionally rabbits but no other animals, most predictably by intracerebral inoculation of emulsified spinal cord although the intraperitoneal route may also be successful. The incubation period is 8 to 14 days. Injection into a peripheral nerve leads to paralysis of that limb but the further spread is prevented if the nerve is immediately divided proximal to the site of injection. Intravenous injection does not work and the organism seems unable to enter the brain and spinal cord from this route. The agent passes through a porcelain filter and is not destroyed by glycerine or prolonged drying but it is susceptible to 0.2% potassium permanganate or 6% peroxide, and temperatures above 55°. In these respects, the ‘virus is distinct from rabies’.
Although John Paul attaches less significance to the work in his history of poliomyelitis, writing in 1916 Batten is impressed by the demonstration of (Simon) Flexner (1863–1946) and (Hideyo) Noguchi (1876–1928) who cultured a microorganism appearing as globoid bodies 0.15 to 0.3 μm in diameter arranged in pairs, chains or masses, and transmitted the disease with this agent. To the modern reader, Batten is loose in his microbial terminology referring variously to this organism—which fulfils the conditions required for the establishment of a causal relationship to disease—as bacterium, protozoan, parasite or virus. Whatever its nature, the microorganism responsible for transmission ‘bears an [a]etiological relationship to epidemic poliomyelitis in the human subject’. Significantly, it has been shown that the ‘virus’ passes through the gastro-intestinal mucosa and the nasal mucous membranes; and, in the Swedish epidemic of 1911, nasal and intestinal washings from deceased and living patients (not all affected indicating that there are carriers of the microorganism) were used successfully to transmit the disease to monkeys by intraperitoneal injection. It follows that ‘the virus is always present in the…nose, mouth…and intestine of persons affected by poliomyelitis during the acute stage of the disease’. As for the vector responsible for transmission, Batten considers it unlikely that poliomyelitis can be transmitted ‘by the means of fleas, lice, bugs and flies’. Later, it became clear that poliomyelitis is indeed transmitted between humans by nasal or gastrointestinal mucosal fluids. The suggestion that intra-uterine infection can occur is hard to separate from the frequent examples of birth trauma and perinatal cerebral haemorrhage. Virulence varies between different epidemics and changes over time. These features account, perhaps, for the onset, spread and fading of epidemics better than the moderating effect of a second, entirely hypothetical, subsidiary microorganism that co-operates and allows the causative poliovirus to ‘ripen’ and fade.
Batten considers that a patient who survives is immune and does not develop a second attack of poliomyelitis. However, he acknowledges the occurrence of progressive muscular atrophy long after recovery from poliomyelitis. This concept in clinical neurology, aligned with Gowers’ abiotropy, has never been fully resolved. Batten is impressed that monkeys are protected from intracerebral inoculation by incubation of spinal cord homogenate with convalescent serum from typical paralytic cases but not unaffected contacts. Anticipating the work of Landsteiner and research on poliomyelitis carried out over the next three decades, Batten assumes that this is explained by the presence of antibodies although:
‘The use of an extract of organs containing the virus as antigen does not permit the discovery of antibodies in the serum or cerebrospinal fluid’.
This absence of ‘amboceptor’ is similar to that observed in rabies—the diagnosis of poliomyelitis then being made by association with weakness in the limbs or circumstantial evidence for a recent outbreak of the disease.
Chapter VI of Batten’s monograph deals at length with the clinical features of poliomyelitis. Typically there is flaccid paralysis of one or more limbs. Sometimes this ascends progressively from the legs, and at an irregular pace, eventually affecting all muscles below the neck causing death from respiratory failure. These are the ‘Landry’ or jump cases, in some of whom temporary improvement seems to occur before the illness again progresses. Less common is a descending form of poliomyelitis. Children with isolated trunk weakness show marked scoliosis; and, in other cases, the differential weakness leads to abnormal posture and fixed deformities of the trunk or limbs ( Fig. 3 ). Batten attributes ophthalmoplegia and blindness to poliomyelitis is some cases. Examples of transverse poliomyelitis, as described by Batten, with flaccid paralysis, loss of sphincter control and a sensory level developing brisk reflexes and clonus now seem inappropriately classified. And, unlike Dr Batten, we would doubt the diagnosis of poliomyelitis in a young patient with acute bilateral optic neuritis and a spinal cord lesion. In fact, one senses that he is happy to diagnose poliomyelitis in any acute neurological disease affecting children including hemiplegia with convulsions or tremor, athetosis, chorea, ataxia and other movement disorders for which no other cause is forthcoming. To us the purely meningitic form seems indistinguishable from cases of viral meningitis with neck stiffness, Kernig’s sign and active CSF in which no organism is detected. Batten’s justification for including these cases is that, in rare instances where the children do not survive, histological changes consistent with poliomyelitis are present at autopsy. Although describing mental defect as a possible manifestation of the disease, even Batten is cautious about attributing this to poliomyelitis. And he is even more sceptical about the neuritic form recognizing that this is unlike poliomyelitis; depends on spurious features such as occurrence in the month of August; and, lacking pathological confirmation, cannot readily be distinguished from other forms of toxic neuritis.
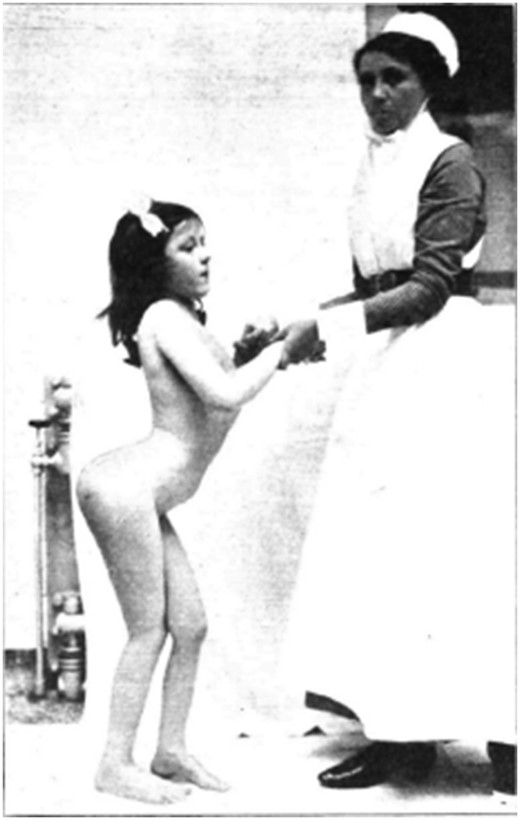
Girl with extensive poliomyelitis and bilateral contraction of the flexors of the hip joints. In the attempt to assume the erect position a lordosis of the spine is produced.
The best that can be offered for treatment is to isolate the patient although spread throughout the hospital ward by personal contact is improbable. The nose and mouth are cleaned with potassium permanganate or chlorine water, and pain relief given as necessary. Despite the practical difficulties and uncertain results, Batten does advocate use in the preparalytic phase of immune serum, obtained from 30 cc peripheral blood in a patient who has recovered from poliomyelitis and given as 10 cc by daily intrathecal injection for 3–4 days. But the mainstay of treatment is rest in bed for at least 3 weeks with the legs or arms immobilized using detachable plaster of Paris splints, or a walking machine in a supine or erect position but not sitting ( Fig. 4 ). This prevents excessive muscle tone in agonists and antagonists and makes for advantages with subsequent re-education of the paralysed muscles ( Fig. 5 ). Management is helped by hydrotherapy, massage and Galvanic but not Faradic electricity. These measures are all designed to prevent the subsequent need for tendon lengthening, transplantation, fixation, nerve-grafting and arthrodeses.
![Photo[graph] of child with flaccid paralysis of both legs in walking machine. The early stage of the re-education of the child in walking.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/139/5/10.1093_brain_aww073/2/m_aww073f4p.jpeg?Expires=1748125821&Signature=Pl97awISUPyJNRo8bB4udNJbZTskAznCi9MMKcGiLl2JgG3rEeXFw5U6E8Qer3n7l~OV1qiqSs02DgHdd7BRG3EgEdwvwDydW~em4uD4Q02PfSb0-vbLMQipAGct3Tmo7FNI~iyk~W-gzMLIH8MCq7KED-~gzyOvFDJUGuoiqWTYt6UAFwPPv0cudEVYo4Eadb09G-Dy02eJkPlrQKrR2Tw29M54LqczJ-9SZygj-Oey1OkQZw7sS409zD07owuN-HSAEMqAu45-AxOi0Scmmco9bSqWZsu4oKBsQzx4sVurffr8iEPaNWExTLw4SOxAiFozZAmpo9UVK593x7cLOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Photo[graph] of child with flaccid paralysis of both legs in walking machine. The early stage of the re-education of the child in walking.
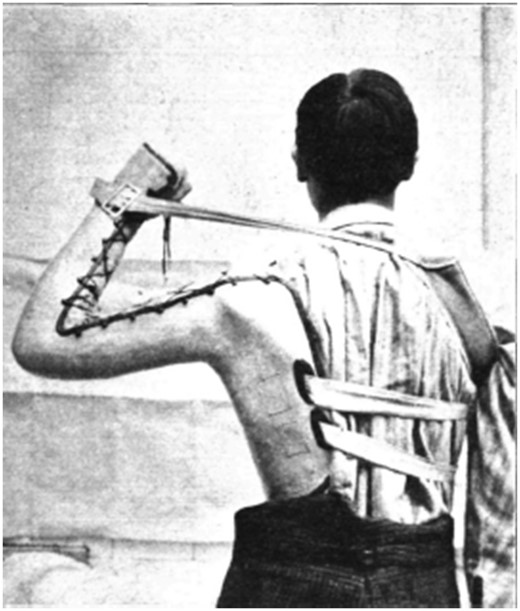
Man with poliomyelitis of the shoulder muscles of the left arm. The splint has an additional support from a strap and band around the right shoulder.
Frederick Batten’s Lumleian lectures provide an exhaustive review of the literature on poliomyelitis, meticulously researched and sourced. There may not be much that is original or previously unpublished by others but he has observed, and thought about, a large number of cases. To the modern reader, his boundaries for the clinical spectrum of neurological disease caused by poliomyelitis are wide. Even if he confuses the many different species of microorganism, it is remarkable how prescient were his ideas on the role of immunity in the pathogenesis and management of the disease. Batten anticipates developments that eventually led to a full understanding of the pathogenesis and the introduction of novel immunological and vaccine therapies. But even now there are loose ends. Fifty-two years after Ronald Henson wrote about his personal experience of poliomyelitis in the Lancet , that journal continues to report original work aimed at achieving even better immunological response to immunization with bivalent type 1 and type 3 and inactivated poliovirus vaccine (Sutter et al. Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label randomised controlled trial, Lancet 2015: 386; 2413–21).


