-
PDF
- Split View
-
Views
-
Cite
Cite
James L. Stone, James T. Goodrich, The craniopagus malformation: classification and implications for surgical separation, Brain, Volume 129, Issue 5, May 2006, Pages 1084–1095, https://doi.org/10.1093/brain/awl065
Close - Share Icon Share
Abstract
Craniopagus twins (CPT) are an uncommon, highly fascinating accident of nature. The clinical pathology of this complex entity is reviewed and placed in perspective. A logical classification aids understanding of the anomaly, and is essential to gauge outcome from separation attempts. ‘Partial forms’ lack significant shared dural venous sinuses (SDVS) and ‘Total forms’ with SDVS also exhibit more severe compressional brain distortion. Our classification consists of Partial Angular (PA), Partial Vertical (PV), Total Angular (TA) and Total Vertical (TV, formerly O'Connell Types I–III). Total vertical has a continuous cranium, and inter-twin axial facial rotation <40° (Type I), 140–180° (Type II) or intermediate (Type III). The term ‘Angular’ denotes an inter-twin longitudinal angle below 140°, regardless of axial rotation. Our review categorized 64 well-delineated CPT, including 41 operative separation attempts in small children since initial success in 1952. Just over one-half were TV, almost one-third TA, and partial forms accounted for the remaining one-sixth. About 30% of CPT had shared or fused brain tissue, and a similar percentage of TA twins shared a posterior fossa. Partial forms had significantly higher birth weights, were separated at an earlier age (6 versus 11 months) and had lower mortality and better outcome compared with Total forms. A multi-staged surgical separation for Total CPT had a significantly better mortality than single-staged separation. Discussion emphasizes embryological, anatomical and clinical aspects of the malformation, with emphasis upon obstacles to a successful outcome.
Introduction
Craniopagus twins (CPT) joined at the head are an uncommon malformation found once in ∼2.5 million live births and represent only 2–6% of conjoined twins (Edmonds and Layde, 1982; Baldwin, 1998; Spencer, 2003; Spitz and Kiely, 2003). Approximately 40% of conjoined twins are stillborn and an additional one-third die within 24 h, usually from congenital organ anomalies, leaving perhaps 25% to be considered for surgical separation (Baldwin, 1998; Spitz and Kiely, 2003; Kaufman, 2004). Nevertheless, a handful of CPT separation attempts occur yearly worldwide. In the last half-century, with the many advances in medicine including brain imaging, neuroanaesthesia and neurosurgical techniques, a successful outcome is possible following separation of total CPT.
In CPT, the face and foramen magnum are not primarily involved, the skulls are usually joined in roughly homologous regions but asymmetries are common, and both vertical and non-vertical or angular forms are found (Potter, 1952; Guttmacher and Nichols, 1967; Winston, 1987; Spencer, 2003). A number of reviews have detailed the complex anatomical and surgical problems in separating total forms of CPT—notably shared dural venous sinuses (SDVS) and the subsequent negative implication towards survival and quality of life (Grossman et al., 1953; Todorov, 1974; O'Connell, 1976; Roberts, 1985; Bucholz et al., 1987; Gaist et al., 1987; Winston, 1987; Jansen et al., 1998; Spencer, 2003; Goh, 2004; van Ouwererk et al., 2004; Walker and Browd, 2004). Partial forms of CPT also exist and can generally be separated with much less risk (O'Connell, 1976; Winston, 1987). At present no existing CPT classification system is available to adequately categorize the entity, evaluate the risk of separation and subsequently allow a realistic analysis of outcome.
Our review suggests a practical four category system that breaks down the CPT anomaly on the basis of a vertical or angular configuration, and the presence or absence of significant SDVS. This simple scheme was retrospectively applied to 64 adequately described sets of CPT twins reported over the last 86 years. Our survey rationalizes the discussion of CPT by yielding a relative frequency of the categories, emphasizes the most challenging total vertical (TV) and total angular (TA) forms and allows us to present an outcome analysis from surgical separation in 41 operated twins from 1952 to date.
The spectrum of craniopagus
Confusion surrounds the severity of the CPT anomaly especially in relation to the difficulty of separation and subsequent outcome. Published cases of highly successful separation have included those with apparently more localized or somewhat extensive cranial conjoining, but analysis of these cases revealed a lack of significant SDVS (Baldwin and Dekaban, 1958; Wolfowitz et al., 1968; Pertuiset, 1975; Konovalov and Vaichis, 1991; Sathekge et al., 1998; Di Rocco et al., 2004). This contrasted sharply with the more common CPT separation attempts in the presence of SDVS, frequently accompanied by high morbidity and mortality. Outcome in cases with SDVS was often related to venous blood loss, loss of venous outflow, cerebral swelling, haemorrhage or ischaemia and the possibility of intraoperative venous sinus air embolism (Leiter, 1932; Grossman et al., 1953; O'Connell, 1962, 1964, 1968; Kohama et al., 1972; Cywes et al., 1982; Roberts, 1984, 1985, 1986; Bucholz et al., 1987; Gaist et al., 1987; Winston et al., 1987; Khan et al., 1999; Frazee et al., 2004; Walker and Browd, 2004). In addition, many CPT are premature with systemic cardiac or urological anomalies, prone to aspiration pneumonia, generally fragile and may tolerate both anaesthesia and blood loss poorly (Leiter, 1932; Barbosa, 1949; Robertson, 1953; Boin, 1964; Marcinski et al., 1978; Wong et al., 1980, 2003; Georges et al., 1987; Winston, 1987; Hoffman, 1997; Campbell et al., 2002, 2004; Yang and Xu, 2002; Huang et al., 2004).
Partial forms of CPT
O'Connell, a British neurosurgeon who had particular interest in the venous sinuses and experience with CPT in the 1960s, clearly differentiated the less common partial or localized types from the more common total types (O'Connell, 1934, 1976). In Partial CPT, the unions are usually frontal (Baldwin and Dekaban, 1958, 1965; Klar, 1963; Wolfowitz et al., 1968; Lansdell, 1999; Di Rocco et al., 2004), and less commonly occipital (Stanley et al., 1983; Sathekge et al., 1998; Campbell et al., 2002; Campbell, 2004) or vertical biparietal (Voris et al., 1957; Voris, 1963; Pertuiset, 1975, 1976; Marcinski et al., 1978; Pertuiset et al., 1989; Konovalov and Vaichis, 1991) (Fig. 1). The junctional diameter is often smaller in the partial forms and occasionally an incomplete layer of bone may be present between the twins (Voris et al., 1957; Stanley et al., 1983; Di Rocco et al., 2004). In Partial CPT, each child largely maintains independent calvarial convexities except at the common area of skull junction, and cerebral deformities and compaction tend to be local and mild. The dura of both children may be intact or deficient and the cortical gyri may interdigitate, but leptomeninges are usually separable. However, conjoined or shared brain tissue with associated leptomeningeal vessels has been reported (in 3 of 11 cases), and subsequent division may lead to disability (Baldwin and Dekaban, 1958; Klar, 1963; Konovalov and Vaichis, 1991). Intracranial dural venous cross-circulation (SDVS) is absent or negligible on preoperative studies and at surgery. In addition, Partial CPT variety children tend to be hardier; they usually undergo successful separation at an earlier age than Total CPT, and the separation more often results in survival of both children who may lead normal lives. Nevertheless, technical difficulties or anomalies of other organ systems may jeopardize outcome or life (Voris et al., 1957; Klar, 1963; Marcinski et al., 1978; Stanley et al., 1983; Campbell et al., 2002; Campbell, 2004). Voris of Chicago in 1955 was the first to separate a set of CPT twins (Partial) with long-term survival of both—one being normal, the other severely impaired (Voris et al., 1957; Voris, 1963; Spencer, 2003).
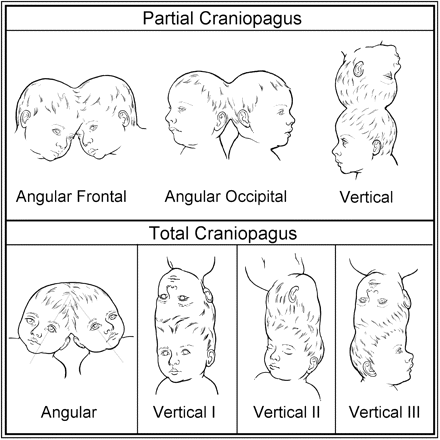
Classification of craniopagus. ‘Partial’ and ‘Total’ forms of the craniopagus malformation.
Total vertical CPT
The TV or O'Connell types consist of a longitudinal (stove-pipe) arrangement with the general appearance of one common continuous cranium housing four cerebral hemispheres (O'Connell, 1976; Roberts, 1984, 1985, 1986; Walker and Browd, 2004) (Fig. 1). Minor longitudinal tilting between the twins is common with a longitudinal inter-twin axis or angle of ∼140–180° (O'Connell, 1976). An incomplete or complete, single-layered transverse dural septum typically separates the flattened cerebral hemispheres, and the falx cerebri in both twins is usually absent or anomalous deep to the conjoined region (Robertson, 1953; O'Connell, 1976). In TV CPT, the major cerebral arterial supply is usually confined to each respective twin (O'Connell, 1976; Winston, 1987). On occasion, in addition to small interconnecting leptomeningeal vessels, conjoined brain tissue may contain a larger artery requiring division to effect separation (O'Connell, 1964, 1976; Roberts, 1986; Hoffman, 1997; Walker and Browd, 2004).
The anatomy of the peripheral dural shelf at the conjoined cerebral hemispheric zone and the enclosed circumferential venous sinus (CVS) have been well described (Sonnenburg, 1919; Grossman and Sugar, 1953; O'Connell, 1976; Roberts, 1984) (Fig. 2). The CVS had been noted in a post-mortem cerebral angiogram on a set of TV CPT (Sonnenburg, 1919) and was first angiographically visualized in vivo on similar twins by Sugar and Grossman in 1952 (Grossman et al., 1953; Sugar et al., 1953). In TV CPT, the CVS generally traverses at least the hemi-circumference of the conjoined region and replaces the absent superior saggital sinus (O'Connell, 1976; Roberts, 1984; Winston, 1987; Walker and Browd, 2004). The CVS usually drains the homolateral superior cerebral hemispheres of each twin, may be associated with venous ‘lakes’ and empties into a common or asymmetrically shared posterior confluence of sinuses. In TV CPT, with increasing inter-axial rotation (see below), the CVS assumes an oblique configuration conforming to the lateral hemispheric cleavage plane, drains the ipsilateral superior hemispheres of both twins and, although highly variable, may connect the lateral sinus of one twin to the lateral sinus of the other twin (O'Connell, 1976; Roberts, 1986; Walker and Browd, 2004). Often, one twin is anatomically predisposed to keep the CVS with its dural shelf and the transverse dural septum (Fig. 2). In this situation, multiple, staged surgical procedures gradually divide venous contributions from the other twin to minimize blood loss over stages and induce development of collateral basal venous drainage in the child deprived of superior venous drainage (Sugar et al., 1953; Goodrich and Staffenberg, 2004; Walker and Browd, 2004; Staffenberg and Goodrich, 2005). Both the CVS and SDVS are always present in TV CPT forms, making successful surgical separation particularly hazardous and perhaps impossible (O'Connell, 1976; Walker and Browd, 2004).
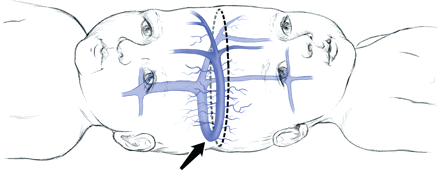
The CVS (arrow) is typically contained within the peripheral dural shelf of one child, but drains the homolateral hemisphere of each child. The dural shelf is usually in continuity with an incomplete or complete layer of dura separating the twins' hemispheres. Surgical separation strategy may entail sequential division of venous sinus branches from the right twin (dashed line), allowing the anatomically predisposed left twin to keep the major CVS and associated dura. In the right twin, subsequent reversal of venous drainage to collateral basal channels may be induced.
As initially described by O'Connell (O'Connell, 1976) (Fig. 1), in TV CPT Type I, both children face in the same general directional axis, with an inter-twin axial rotation <40° (Sonnenburg, 1919; Cameron, 1928; Blumensaat, 1932; Leiter, 1932; Grossman et al., 1953; Robertson, 1953; Todorov, 1974; Wong et al., 1980; Roberts, 1984, 1985; Schindler and Hajek, 1988; Aquino et al., 1997; Hoffman, 1997; Piza-Katzer, 2002; Goodrich and Staffenberg, 2004; Walker and Browd, 2004). The Type II TV deformity shows 140–180° of axial rotation with the children's faces on the opposite side of the conjoined skull (Kafka, 1920; O'Connell, 1962, 1968; Tan et al., 1971; Roberts, 1986; Sakala, 1986; Georges et al., 1987; Wong et al., 2003; Frazee, 2004; Goh, 2004). In the Type III variety, axial rotation (40 to <140) degrees is intermediate between that in the other types (Trenkner, 1938; Barbosa, 1949; Dill, 1953; O'Connell, 1964; Kohama et al., 1972; Abad, 1974; Jain, 1979; Bonderson and Allen, 1989; Loverro et al., 1991; Khan et al., 1999; Swift et al., 2004).
The cerebral hemispheres in TV Type I CPT show relatively symmetric superior biparietal or vertex compressional flattening, and the posterior fossa tends to be small (Fig. 3). The anterior and middle fossae are spacious (Robertson, 1953; O'Connell, 1976), slight tilting or inter-twin axial rotation leads to some facial asymmetry and later positional plagiocephaly is common (Staffenberg and Goodrich, 2005). Progressive axial rotation between the twins during development in TV Types III and II produces a progressively marked obliquity of the conjoined junction and extremely severe anteroposterior cerebral hemispheric compressional distortion (O'Connell, 1964, 1968, 1976) (Fig. 3). This includes gross craniofacial, middle and posterior fossa deformities. The marked bone, dural and cerebral asymmetries in TV Types III and II CPT would be expected to compound the difficulties of intraoperative localization and surgical separation (O'Connell, 1964, 1968, 1976; Tan et al., 1971; Kohama et al., 1972; Roberts, 1986; Georges et al., 1987; Khan et al., 1999; Wong et al., 2003; Frazee et al., 2004; Goh, 2004; Swift et al., 2004).
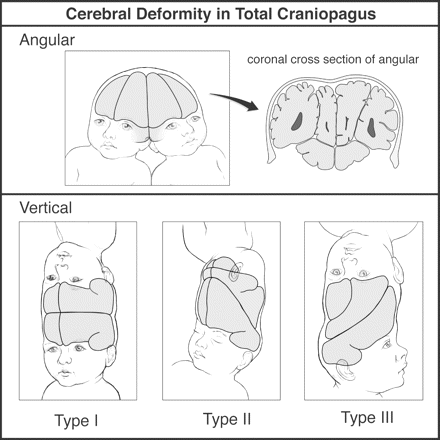
Cerebral deformity in total craniopagus. Angular and vertical Types I, II and III.
In addition to compressed cerebral tissue in TV CPT, conjoined (inseparable, fused) cerebral cortex and underlying white mater was found at surgery in six sets of TV twins (O'Connell, 1964, 1968; Wong et al., 1980; Hoffman, 1997; Walker and Browd, 2004; Staffenberg and Goodrich, 2005). If conjoined brain tissue is known to be present, it may be wise to divide it as early as possible to maximize cerebral plasticity (Bucholz et al., 1987; Walker and Browd, 2004). It has been suggested that surgical separation of TV CPT should ideally be within 9–12 months of age to allow optimal psychomotor development (Franklin, 1964; O'Connell, 1968, 1976).
Total angular CPT
We found that a group of CPT twins existed with both more acute inter-twin longitudinal angulation and SDVS accompanied by complex interconnecting venous channels (or CVS), and markedly distorted cerebral hemispheres (Wilson and Storer, 1957; Boin, 1964; Sapinski and Pawlicki, 1966; Lenard and Schulte, 1972; Duhamel, 1975; Villarejo et al., 1981; Cywes et al., 1982; Hughes and Fino, 1984; Schultz et al., 1986; Bucholz et al., 1987; Gaist et al., 1987; Winston et al., 1987, Lahmeyer, 1988; O'Neill et al., 1988; Cameron et al., 1989; Drummond et al., 1991; Jansen et al., 1998; Yang and Xu, 2002; Campbell, 2004; Campbell et al., 2004; Huang et al., 2004; van Ouwerkerk et al., 2004) (Figs 1 and 3). Comparable with TV CPT, cerebral compaction, distortion and displacement may result in secondary skull base deformity. Most of these complex, or, as we prefer to term them, TA, forms of CPT are joined asymmetrically, and inter-twin axial rotation may be present as well, but some are roughly symmetric (Boin, 1964; Duhamel, 1975; Cywes et al., 1982; Cameron et al., 1989; Drummond et al., 1991; Jansen et al., 1998; Huang et al., 2004). As noted in other forms of CPT, conjoined brain tissue was reported to be present at surgery in three sets of TA twins (Duhamel, 1975; Bucholz et al., 1987; Winston et al., 1987), and cerebral arterial cross-filling in five sets of TA twins (Lenard and Schulte, 1972; Bucholz et al., 1987; Winston et al., 1987; O'Neill et al., 1988; Jansen et al., 1998).
In the absence of the typical TV or longitudinal type of configuration, these TA twins could not be well evaluated as to inherent risk for separation, and were at least as challenging to separate as TV CPT (Wilson and Storer, 1957; Boin, 1964; Duhamel, 1975; Villarejo et al., 1981; Cywes et al., 1982; Hughes and Fino, 1984; Bucholz et al., 1987; Gaist et al., 1987; Winston et al., 1987; Cameron et al., 1989; Drummond et al., 1991; Campbell, 2004; Campbell et al., 2004). It was suggested that non-linear angular forms of CPT embryologically began as TV parietal types, and physical forces resulted in secondary acute angulation (O'Connell, 1976; Gaist et al., 1987). In our opinion, although nearly all reported TA twins had involvement of the parietal area in one or both children, the majority were sufficiently asymmetric or lateral/posterior in their union to argue against an initial vertical biparietal orientation (see below).
Cranially conjoined twins have traditionally been classified according to the area of junction, but additional modifiers are clearly necessary to define severity (Winston, 1987; Spencer, 2003). Recognizing limitations, Winston proposed a partial to complete A–D grading system, which emphasized the deepest shared structures depicted by progressive dural and arachnoidal loss with inseparable cerebral tissue (Winston, 1987). The presence and importance of SDVS was discussed and depicted in Types C and D, and although it was noted that axial rotation and angulation were important (Todorov et al., 1974), these were not included in that system. Unfortunately, inconsistency is present in the literature regarding the reported grades, and successfully separated Partial cases without SDVS but other challenging problems such as difficult cerebral adhesions or fused convolutions have been classified Type D, the most serious form of CPT (Konovalov and Vaichis, 1991). Several authors related outcome after surgical separation to the degree of preoperative venous sharing, and found improved outcome utilizing multiple stages (Bucholz et al., 1987; Walker and Browd, 2004).
Some lessons of CPT surgical separation
From the 1920s until 1950, several sets of CPT underwent unsuccessful exploratory separation attempts in an urgent fashion fearing the death of one twin (Cameron, 1928; Leiter, 1932; Barbosa, 1949; Robertson, 1953). The modern era for CPT surgical separation began in 1952 when Oscar Sugar and associates working in Chicago were the first to be successful in that one child survived (Grossman et al., 1953; Sugar et al., 1953). Specifics of the neurosurgical management and long-term outcome are now described below (Fig. 4A and B).
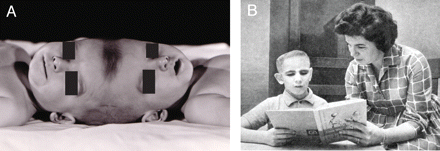
(A) The Brodie twins separated in 1952. (B) Rodney Brodie at age 10 being home schooled. (Photographs provided by Dr Herbert J. Grossman, with permission).
The Brodie male TV Type I CPT were born full term on September 16, 1951 by uncomplicated delivery with a combined weight of 5335 g. Air studies and cerebral angiography suggested separate brains, and the venogram disclosed a CVS on one side. Between ages 9 and 15 months, a five-staged neurosurgical approach sought to delineate whether a dural septum existed between the brains and whether it could be split, and the existing pattern of venous drainage. Two procedures included both circumferential craniectomy and attempted venous sinography, and three intradural stages employed section of separating dural membranes and veins, and placement of polyethylene sheets to maintain planes and minimize cerebral adhesions. A shared unilateral CVS and two prominent parasagittal veins in the transverse dural septum communicated anteriorly and drained into a common torcula posteriorly. These dural sinuses and the transverse (separating) dural septum stayed with the ultimate survivor (Rodney). The initially intact transverse dural septum was incised over Rodney's right parietal region during the first intradural exploration. The final separation (December 17, 1952) took over 10 h, and required 6000 cc of whole blood transfusion, equivalent to about three complete exchanges in each child—then the longest operation ever performed in a child and the most blood replaced. The twin without superior veins quickly developed cerebral swelling, never awoke and died in ∼1 month. Polymyxin B combated local and systemic pseudomonas infection in Rodney, and multi-stage scalp rotation flaps and dural grafting eventually provided watertight dural closure. By 1 year after surgery, Rodney was ambulatory; postoperative left haemiparesis and astereognosis (probably related to the right parietal surgical defect in the transverse dural septum) had completely resolved, but he exhibited mild to moderate psychomotor retardation. At age 3 years, a spontaneous right parietal intracerebral haemorrhage required evacuation. The haematoma cavity communicated with the lateral ventricle, which showed areas of haemosiderosis in the walls. Coagulation of the choroid plexus bilaterally failed to completely control hydrocephalus, but intermittent subgaleal taps were beneficial over a period of years. Seizures were easily controlled with phenobarbital. Despite moderate mental retardation, this very verbal and cheerful boy reached the third grade equivalent by age 8 or 9 years. Progressive ambulatory problems followed by gradual demise without subgaleal fluid build-up led to death in 1963 at age 11 ½ years. Autopsy was not performed.
Through the late 1970s, multi-staged surgical separation attempts for TV CPT were largely patterned after Sugar's technique of gradual venous separation and the use of polyethylene sheets to maintain cleavage planes between stages. The result was survival of the child who received the CVS and bulk of the superior dural venous sinuses, usually with significant disability, or the death of both children (Wilson and Storer, 1957; O'Connell, 1962, 1964, 1968; Boin, 1964; Kohama et al., 1972; Abad, 1974; Todorov et al., 1974; Duhamel, 1975; Winston et al., 1987). Further success was achieved by Roberts and Walker of Salt Lake City, Utah, in 1979 with the multi-staged separation of TV Type I CPT accompanied by survival of both children with some disability (Wong et al., 1980; Roberts, 1984, 1986), and in 1984 they used similar techniques to separate TV Type II twins, with survival of both children (Roberts, 1986; Georges et al., 1987; Walker and Browd, 2004).
In 1980, an unusual case of CPT was referred to Sugar in Chicago, who was assisted by one of the present authors (J.L.S.) (Fig. 5). These twins were remarkably asymmetric and angular in their attachment, and both died from venous sinus blood loss during the first stage of neurosurgical separation. A plastic surgery report was published regarding the novel use of models (Schultz et al., 1986), as well as electroencephalalographical studies (Hughes and Fino, 1984; Lahmeyer, 1988). The neurosurgical and anatomical findings are now reported as pivotal to our understanding of the spectrum of CPT and the evolution of our classification scheme.
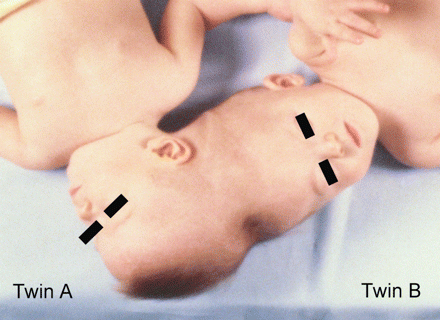
Total angular asymmetric twins treated at the University of Illinois in Chicago in 1981 (Photograph from the author's collection, J.L. Stone).
Female CPT twins were born on January 3, 1980 by C-section for breech presentation at 28 weeks gestation and found to have extensive occipitoparietal (Twin A) to vertex (Twin B) fusion (Fig. 5). Combined birth weight was 2043 g, and mild hyaline membrane disease resulted in episodes of intubation and re-intubation as birth weight doubled at 3 months. Enhanced CT scan at age 5 months showed multiple dural septae between the brains and a large midline arachnoid cyst in B. Cerebral angiography at age 8 months showed arterial filling confined to each twin, but venous drainage posteriorly disclosed multiple peripheral CVSs that shared height-dependent internal connections of the lateral and confluence of sinuses. In the non-conjoined midline anterior vertex regions, superior sagittal sinuses were seen. Localized pneumonia and atelectasis persisted in A, the smaller twin, and never completely resolved. Plastic surgery mobilized myocutaneous flaps at 13 months and bronchoscopy improved the respiratory condition of A, who had developed an enlarged but normal functioning heart. The girls had progressed well, smiled, recognized relatives and spoke monosyllables. At age 18 months (June 10, 1981), craniotomy was undertaken as a first stage of separation, but the twins died from dural venous sinus haemorrhage during exploration. Autopsy found that they shared multiple common intracranial venous channels, dural partitioning between the brains was nearly complete and they shared no common neural structures. Physical contiguity was present in that A's cerebellar hemispheres were housed in an incomplete posterior fossa within B's cranium. Separated by arachnoid, A's cerebellar hemispheres deformed and displaced B's medial parieto-occipital lobes. Twin A had absence of the cerebellar vermis, absence of the splenium and thinning of the posterior corpus callosum, shortened olfactory tracts, polymicrogyria and a larger right cerebral hemisphere. Twin B had a large arachnoid cyst above the corpus callosum near the midline, absence of the right sylvian fissure and many areas of polymicrogyria, especially the left superior and middle temporal gyri. The heart size of A was twice the normal size, and bronchiolar changes were present in its lungs.
As noted earlier, we became aware of asymmetric or symmetric TA CPT cases with SDVS and CVS or similar interconnecting veins. As in the above case, the posterior fossa is occasionally shared in TA CPT (Lenard and Schulte, 1972; Bucholz et al., 1987; Jansen et al., 1998; Yang and Xu, 2002; van Ouwerkerk et al., 2004), and in one instance conjoined abnormal cerebellar tissue required division (Bucholz et al., 1987). It was our impression that TA CPT were at least as challenging to separate as TV CPT and perhaps more so (see Table 2).
Conjoined cerebral tissue has been found in all forms of CPT. This nervous tissue can be associated with absence of an arachnoid and pial cleavage plane between the gyri, and shared gyral vasculature, and constitute overtly conjoined cerebral cortex and white matter (Fig. 6). It is uncertain if this represents a primary developmental phenomena, or if fusion is secondary to cerebral opposition, pressure or adhesive forces. The extent of conjoined brain is usually limited relative to the total size of the junction, and perhaps cannot be regarded as part of the congenital defect itself. Section of this tissue may contribute to deficits (O'Connell, 1964, 1968; Wong et al., 1980).
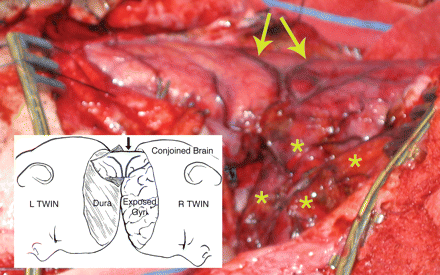
Total vertical Type I twins, prone position, near conclusion of the final surgical separation stage. As in Fig. 2, the left twin was predisposed to retain the major superior venous drainage (CVS) including the incomplete transverse dural septum between the hemispheres. A retractor spreads the cleavage plane between hemispheres, and the circular defect in the dural septum was incised to further inspect the 3 cm diameter segment of conjoined parietal cortex with enclosed white matter before division. Arrows point to homologous diverging veins at the site of cortical fusion, and asterisks indicate exposed flattened superior gyri of the right twin (Photograph from the author's collection, J.L. Stone and J.T. Goodrich).
In any case of CPT, semi-urgent surgical separation is occasionally necessitated by persistent aspiration or airway problems (Khan et al., 1999), threatened blindness (Di Rocco et al., 2004) or the impending death of one twin (Cameron, 1928; Robertson, 1953; Duhamel, 1975; Marcinski et al., 1978; Jain, 1979; Villarejo et al., 1981; Hoffman, 1997; Campbell et al., 2004). Any non-elective surgical separation procedure in conjoined twins is well known to have increased morbidity (O'Neill et al., 1988; Spencer, 2003; Spitz and Kiely, 2003). Other potentially life-threatening aspects of CPT separation include adequate dural closure with autologous, cadaveric or synthetic dural substitutes, and scalp coverage by delayed pedicle flaps or the use of subgaleal tissue expansion (Bucholz, et al., 1987; Winston et al., 1987; Piza-Katzer, 2002; Staffenberg and Goodrich, 2004; Swift et al., 2004). Any method has the potential for cerebrospinal fluid (CSF) leakage and meningitis (Georges et al., 1987; Winston et al., 1987; Cameron et al., 1989; Frazee et al., 2004). CSF shunting for hydrocephalus or to promote wound healing may be necessary, and focal or generalized seizures, although likely under-reported, may follow separation (O'Connell, 1964; Roberts, 1986; Goh, 2004; Walker and Browd, 2004). The operative microscope was first used in CPT separation in 1974 (Pertuiset, 1975).
In recent years, MRI including arterial and venous phases, frameless stereotactic localization and functional MRI have been utilized in CPT (Schindler and Hajek, 1988; Jansen et al., 1998; Campbell et al., 2002; Yang and Xu, 2002; Di Rocco et al., 2004; Frazee et al., 2004; Goh, 2004; van Ouwerkerk et al., 2004; Walker and Browd, 2004; Ho et al., 2005). To better depict surgical anatomy, neuroradiological imaging has also produced life-sized three-dimensional transparent plastic models and holograms depicting the complex intracranial vascular anatomy in relation to the skull and brain (Goh, 2004; Goodrich and Staffenberg, 2004; Swift et al., 2004; Staffenberg and Goodrich, 2005).
From the late 1980s through 2004, we saw the advent of single-stage surgical separations for Total CPT lasting at times 22–100 h (Cameron et al., 1989; Sathekge et al., 1998; Wong et al., 2003; Campbell et al., 2004; Frazee et al., 2004; Goh, 2004; Huang et al., 2004; Swift et al., 2004). Although surgical results for Total CPT were gradually improving over the decades, our impression was that single-stage results may be no better than the earlier developed multi-staged separation procedures (Sugar et al., 1953; O'Connell, 1962, 1964, 1968; Roberts, 1984; Bucholz et al., 1987; Drummond et al., 1991; Walker and Browd, 2004; Staffenberg and Goodrich, 2005). However, no adequate classification system of CPT existed to comparatively evaluate outcomes among different types and also capture the challenging TA form.
Proposed classification of CPT
All types of the CPT anomaly may have severe life-threatening congenital anomalies outside of the central nervous system as well as brain distortions, dural defects between the twins and adherent brain tissue. Developmental cerebral and cortical dysplasias, aberrant cerebrospinal fluid-filled cystic structures and cerebral arterial interconnections may be present as well. Dural leaf anomalies are frequent, as are craniofacial asymmetries. However, it is clear that critical inter-twin dural venous sinus sharing (SDVS, CVS) and gross brain compressional anomalies are generally more severe in the ‘Total’ forms compared with the ‘Partial’ forms of craniopagus.
We propose the word ‘Total’ in our CPT classification to designate the presence of significant SDVS invariably accompanied by a CVS or similar anastomosing venous sinuses. The four categories are Partial Angular (PA), Partial Vertical (PV), Total Vertical (TV) and Total Angular (TA) (Fig. 1). The TV category is further broken down into Types I, II and III (Fig. 1).
Case Reports
The determination of the presence of SDVS (Total CPT) was made from radiological, surgical or autopsy findings, or a clear illustration demonstrating a common conjoined total vertical cranium. As outlined above, the common cranium TV forms are always associated with SDVS, and this observation conformed to our survey. O'Connell considered the inter-twin longitudinal angle in TV CPT to be ∼140–180°, so we elected to consider an angle < 140° to qualify for TA (O'Connell, 1976). In our survey, we noted only one case resembling TV, but with an inter-twin longitudinal angulation in the range of 120–130°, and classified it as TA, as SDVS was documented. In all non-vertical, angular forms of CPT, we used radiological, surgical or autopsy findings of SDVS to differentiate TA from PA. The relatively uncommon PV forms lack significant SDVS and present a vertex or semi-vertical biparietal ‘stacked appearance’—each child with well-maintained skull convexities beyond the conjoined regions (Fig. 1).
Following an extensive literature review, we retrospectively applied our classification to 64 adequately characterized sets of CPT twins reported in the medical literature since 1919. Several instructive examples of aborted (Loverro et al., 1991; Aquino et al., 1997) and parasitic (Bonderson and Allen, 1989) CPT were included.
From within this survey, we also analysed 41 published operative separation attempts in infants or small children (up to age 3 years) since success in 1952. This included 30 sets of Total (TV+TA) CPT and all 11 reported Partial (PA+PV) CPT, one of whom underwent a two-staged surgical separation (Baldwin and Dekaban, 1958).
Surgery for Total CPT was single staged in 19 twins (Wilson and Storer, 1957; Kohama et al., 1972; Abad et al., 1974; Duhamel, 1975; Villarejo et al., 1981; Cywes et al., 1982; Schultz et al., 1986; Gaist et al., 1987; Cameron et al., 1989; Hoffman, 1997; Khan, 1999; Piza-Katzer, 2002; Wong et al., 2003; Campbell et al., 2004; Frazee et al., 2004; Goh, 2004; Huang et al., 2004; Swift et al., 2004) and multi-staged in 11 (Grossman et al., 1953; Sugar et al., 1953; O'Connell, 1962, 1964, 1968; Wong et al., 1980; Roberts, 1984, 1985, 1986; Bucholz et al., 1987; Georges et al., 1987; Winston et al., 1987; Drummond et al., 1991; Goodrich and Staffenberg, 2004; Walker and Browd, 2004; Staffenberg and Goodrich, 2005).
Outcome, as determined from published reports, only included long-term follow-up in a minority of cases (Voris, 1963; Baldwin and Dekaban, 1965; Pertuiset et al., 1989; Lansdell, 1999; Spencer, 2003) and often lacked in detail. Outcome categories were normal, mild to moderate and severe disability.
A female predominance was noted in 69% of CPT, in line with a reported 70% frequency of females in large series of conjoined twins (Baldwin, 1998; Spitz and Kiely, 2003). Fifty-two per cent of CPT were TV, 31% TA (Total forms of CPT accounted for 83%), 11% PA and 6% PV (Partial forms of CPT accounted for 17%). Total vertical was Type I in 42%, Type III 33% and Type II in 24%. In 86% per cent of all CPT, the parietal area of both or one twin was involved (TV, PV and 90% of TA). Total angular CPT were asymmetric in 65%, roughly symmetric in 35% and shared a posterior fossa in 30%. Birth weights were higher for Partial forms (PA + PV) compared with Total forms (TA + TV) of CPT (P < 0.01), and also higher for TV compared with TA CPT (Table 1). Angiographically present cerebral arterial cross-circulation between twins was present in two PA, four TV and five TA sets of twins.
| Craniopagus twins (CPT) . | No. of twins (%) . | Sex . | . | . | Gestation weeks . | . | Birth weight (grams) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Female . | Male . | ? . | No. of twins . | Average . | No. of twins . | Average . | ||||
| Partial angular (PA) | 7 (11) | 6 | 1 | 6 | 36.5 | 5 | 4483 | |||||
| Partial verticle (PV) | 4 (6) | 3 | 1 | 0 | 1 | 5902 | ||||||
| All ‘partials’ (PA + PV) | 11 (17) | 9 (82% female) | 2 | 6 | 36.5 | 6 | 4719* | |||||
| Total angular (TA) | 20 (31) | 15 | 4 | 1 | 8 | 37.1 | 10 | 4001 | ||||
| Total vertical (TV) | ||||||||||||
| I | 14 (22) | 8 | 5 | 1 | 3 | 38 | 3 | 4919 | ||||
| II | 8 (13) | 7 | 1 | 5 | 36.6 | 2 | 4625 | |||||
| III | 11 (17) | 4 | 7 | 3 | 37.3 | 3 | 3717 | |||||
| All TV | 33 (52) | 19 (59% female) | 13 | 1 | 11 | 37.2 | 8 | 4395† | ||||
| All ‘totals’ (TA + TV) | 53 (83) | 34 (67% female) | 17 | 2 | 19 | 37.2 | 18 | 4176*,† | ||||
| All CPT | 64 (100) | 43 (69% female) | 19 | 2 | 25 | 37 | 24 | 4312 | ||||
| Craniopagus twins (CPT) . | No. of twins (%) . | Sex . | . | . | Gestation weeks . | . | Birth weight (grams) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Female . | Male . | ? . | No. of twins . | Average . | No. of twins . | Average . | ||||
| Partial angular (PA) | 7 (11) | 6 | 1 | 6 | 36.5 | 5 | 4483 | |||||
| Partial verticle (PV) | 4 (6) | 3 | 1 | 0 | 1 | 5902 | ||||||
| All ‘partials’ (PA + PV) | 11 (17) | 9 (82% female) | 2 | 6 | 36.5 | 6 | 4719* | |||||
| Total angular (TA) | 20 (31) | 15 | 4 | 1 | 8 | 37.1 | 10 | 4001 | ||||
| Total vertical (TV) | ||||||||||||
| I | 14 (22) | 8 | 5 | 1 | 3 | 38 | 3 | 4919 | ||||
| II | 8 (13) | 7 | 1 | 5 | 36.6 | 2 | 4625 | |||||
| III | 11 (17) | 4 | 7 | 3 | 37.3 | 3 | 3717 | |||||
| All TV | 33 (52) | 19 (59% female) | 13 | 1 | 11 | 37.2 | 8 | 4395† | ||||
| All ‘totals’ (TA + TV) | 53 (83) | 34 (67% female) | 17 | 2 | 19 | 37.2 | 18 | 4176*,† | ||||
| All CPT | 64 (100) | 43 (69% female) | 19 | 2 | 25 | 37 | 24 | 4312 | ||||
Birth weights—All ‘Partials’ versus All ‘Totals’, x2 = 7.56, 1 df, P < 0.01;
Birth weight—TV versus TA–NS.
| Craniopagus twins (CPT) . | No. of twins (%) . | Sex . | . | . | Gestation weeks . | . | Birth weight (grams) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Female . | Male . | ? . | No. of twins . | Average . | No. of twins . | Average . | ||||
| Partial angular (PA) | 7 (11) | 6 | 1 | 6 | 36.5 | 5 | 4483 | |||||
| Partial verticle (PV) | 4 (6) | 3 | 1 | 0 | 1 | 5902 | ||||||
| All ‘partials’ (PA + PV) | 11 (17) | 9 (82% female) | 2 | 6 | 36.5 | 6 | 4719* | |||||
| Total angular (TA) | 20 (31) | 15 | 4 | 1 | 8 | 37.1 | 10 | 4001 | ||||
| Total vertical (TV) | ||||||||||||
| I | 14 (22) | 8 | 5 | 1 | 3 | 38 | 3 | 4919 | ||||
| II | 8 (13) | 7 | 1 | 5 | 36.6 | 2 | 4625 | |||||
| III | 11 (17) | 4 | 7 | 3 | 37.3 | 3 | 3717 | |||||
| All TV | 33 (52) | 19 (59% female) | 13 | 1 | 11 | 37.2 | 8 | 4395† | ||||
| All ‘totals’ (TA + TV) | 53 (83) | 34 (67% female) | 17 | 2 | 19 | 37.2 | 18 | 4176*,† | ||||
| All CPT | 64 (100) | 43 (69% female) | 19 | 2 | 25 | 37 | 24 | 4312 | ||||
| Craniopagus twins (CPT) . | No. of twins (%) . | Sex . | . | . | Gestation weeks . | . | Birth weight (grams) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Female . | Male . | ? . | No. of twins . | Average . | No. of twins . | Average . | ||||
| Partial angular (PA) | 7 (11) | 6 | 1 | 6 | 36.5 | 5 | 4483 | |||||
| Partial verticle (PV) | 4 (6) | 3 | 1 | 0 | 1 | 5902 | ||||||
| All ‘partials’ (PA + PV) | 11 (17) | 9 (82% female) | 2 | 6 | 36.5 | 6 | 4719* | |||||
| Total angular (TA) | 20 (31) | 15 | 4 | 1 | 8 | 37.1 | 10 | 4001 | ||||
| Total vertical (TV) | ||||||||||||
| I | 14 (22) | 8 | 5 | 1 | 3 | 38 | 3 | 4919 | ||||
| II | 8 (13) | 7 | 1 | 5 | 36.6 | 2 | 4625 | |||||
| III | 11 (17) | 4 | 7 | 3 | 37.3 | 3 | 3717 | |||||
| All TV | 33 (52) | 19 (59% female) | 13 | 1 | 11 | 37.2 | 8 | 4395† | ||||
| All ‘totals’ (TA + TV) | 53 (83) | 34 (67% female) | 17 | 2 | 19 | 37.2 | 18 | 4176*,† | ||||
| All CPT | 64 (100) | 43 (69% female) | 19 | 2 | 25 | 37 | 24 | 4312 | ||||
Birth weights—All ‘Partials’ versus All ‘Totals’, x2 = 7.56, 1 df, P < 0.01;
Birth weight—TV versus TA–NS.
Adequate information was available in 41 sets of twin children who underwent surgical separation from 1952 to date (Table 2). The age at surgical separation ranged from 5 days (Marcinski et al., 1978) to 36 months (Hoffman, 1997; Piza-Katzer, 2002), but approximated 6 months of age for Partial and 11 months for Total CPT (P value < 0.05). Total CPT had increased operative mortality (48% died) and fewer normal or mild to moderate recoveries (25%), compared with Partial CPT (14% died, 73% normal or mild to moderate outcome) (P values < 0.01 and 0.001). In two TA and one TV survivor, the outcome was not adequately given in the reports. Although long-term published outcome reports were infrequent, a normal outcome after separation was deemed present in 12 Partial CPT children and only 1 Total CPT child (Bucholz et al., 1987). Total angular had increased mortality (57 versus 41%) and fewer mild to moderate recoveries compared with TV, but this as well as mortality for asymmetry versus symmetry of TA was not statistically significant. Surgical separation for Total CPT children carried a mortality of 23% for multiple staged and 63% for single-staged separation (P < 0.01), wide disparity apparent in both TA and TV groups. Although multiple stages are not usually required for Partial CPT separation, the pooled CPT results would support such a plan. Conjoined (inseparable, fused) brain was found at surgery in six sets of TV, three sets of TA and three sets of Partial CPT for a total incidence of 29%. Following surgical separation, ventriculoperitoneal or lumboperitoneal shunting for hydrocephalus or CSF wound leakage was necessary in seven TV and six TA survivors (42% of TV + TA). Also, focal or generalized seizures were noted in four TV and two TA survivors (19% of TV + TA), and meningitis in four TV and one TA survivor (16% of TV + TA). Shunting, seizures and meningitis were not reported after Partial CPT separation.
| Craniopagus twins (CPT) . | Totals . | . | Age at final separation (months) . | Mortality . | Mild-moderate or normal outcome . | Severe disability . | Single stage . | . | Multiple stage . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | No. of Twins (%) . | No. of Children . | . | No. died (%) . | No. of children (%) . | No. of children (%) . | No. of children (%) . | No. died . | No. of children (%) . | No. died . | |||
| Partial angular (PA) | 7 (17) | 14 | 5.2 | 2 (14) | 10 (71) | 2 (14) | 12 (17) | 2 | 2 | 0 | |||
| Partial verticle (PV) | 4 (10) | 8 | 7.8 | 1 (12.5) | 6 (75) | 1 (12.5) | 8 (12.5) | 1 | 0 | 0 | |||
| All ‘partials’ (PA + PV) | 11 (27) | 22 | 6.1a | 3 (14c) | 16 (73e) | 3 (14) | 20 (15) | 3 | 2 (0) | 0 | |||
| Total angular (TA) | 14 (34) | 28 | 6.2b | 16 (57d) | 6 (21f) | 4 (14) | 22 (68) | 15 | 6 (17) | 1 | |||
| Total Vertical (TV) | |||||||||||||
| I | 6 (15) | 12 | 26.2 | 4 (33) | 5 (42) | 2 (17) | 4 | 3 | 8 | 1 | |||
| II | 5 (12) | 10 | 10.2 | 2 (20) | 4 (40) | 4 (40) | 4 | 0 | 6 | 2 | |||
| III | 5 (12) | 10 | 9.1 | 7 (70) | 0 (0) | 3 (30) | 8 | 6 | 2 | 1 | |||
| All TV | 16 (39) | 32 | 15.8b | 13 (41d) | 9 (28f) | 9 (28) | 16 (56) | 9 | 16 (25) | 4 | |||
| All ‘Totals’ (TA + TV) | 30 (73) | 60 | 11.4a | 29 (48c) | 15 (25e) | 13 (22) | 38 (63) | 24g | 22 (23) | 5g | |||
| All surgical CPT | 41 (100) | 82 | 10.0 | 32 (39) | 31 (38) | 16 (20) | 58 (47) | 27h | 24 (21) | 5h | |||
| Craniopagus twins (CPT) . | Totals . | . | Age at final separation (months) . | Mortality . | Mild-moderate or normal outcome . | Severe disability . | Single stage . | . | Multiple stage . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | No. of Twins (%) . | No. of Children . | . | No. died (%) . | No. of children (%) . | No. of children (%) . | No. of children (%) . | No. died . | No. of children (%) . | No. died . | |||
| Partial angular (PA) | 7 (17) | 14 | 5.2 | 2 (14) | 10 (71) | 2 (14) | 12 (17) | 2 | 2 | 0 | |||
| Partial verticle (PV) | 4 (10) | 8 | 7.8 | 1 (12.5) | 6 (75) | 1 (12.5) | 8 (12.5) | 1 | 0 | 0 | |||
| All ‘partials’ (PA + PV) | 11 (27) | 22 | 6.1a | 3 (14c) | 16 (73e) | 3 (14) | 20 (15) | 3 | 2 (0) | 0 | |||
| Total angular (TA) | 14 (34) | 28 | 6.2b | 16 (57d) | 6 (21f) | 4 (14) | 22 (68) | 15 | 6 (17) | 1 | |||
| Total Vertical (TV) | |||||||||||||
| I | 6 (15) | 12 | 26.2 | 4 (33) | 5 (42) | 2 (17) | 4 | 3 | 8 | 1 | |||
| II | 5 (12) | 10 | 10.2 | 2 (20) | 4 (40) | 4 (40) | 4 | 0 | 6 | 2 | |||
| III | 5 (12) | 10 | 9.1 | 7 (70) | 0 (0) | 3 (30) | 8 | 6 | 2 | 1 | |||
| All TV | 16 (39) | 32 | 15.8b | 13 (41d) | 9 (28f) | 9 (28) | 16 (56) | 9 | 16 (25) | 4 | |||
| All ‘Totals’ (TA + TV) | 30 (73) | 60 | 11.4a | 29 (48c) | 15 (25e) | 13 (22) | 38 (63) | 24g | 22 (23) | 5g | |||
| All surgical CPT | 41 (100) | 82 | 10.0 | 32 (39) | 31 (38) | 16 (20) | 58 (47) | 27h | 24 (21) | 5h | |||
Age at final separation, All ‘Partials’ versus All ‘Totals’, x2 = 4.01, 1 df, P < 0.05; bAge at final separation, TA versus TV–NS; cMortality All Partials' versus all Totals', x2 = 8.14, 1 df, P < 0.01; dMortality TA versus TV–NS; eFunctional Outcome—All ‘Partials' versus All ‘Totals' x2 = 15.56, 1 df, P < 0.001;fFunctional Outcome—TA versus TV–NS;gMortality—All Total (TA +TV) CPT, single stage versus multiple stage separation x2 = 9.121, 1 df, P < 0.01;hMortality—All CPT, single stage versus multiple stage separation x2 = 4.72, 1 df, P < 0.05.
| Craniopagus twins (CPT) . | Totals . | . | Age at final separation (months) . | Mortality . | Mild-moderate or normal outcome . | Severe disability . | Single stage . | . | Multiple stage . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | No. of Twins (%) . | No. of Children . | . | No. died (%) . | No. of children (%) . | No. of children (%) . | No. of children (%) . | No. died . | No. of children (%) . | No. died . | |||
| Partial angular (PA) | 7 (17) | 14 | 5.2 | 2 (14) | 10 (71) | 2 (14) | 12 (17) | 2 | 2 | 0 | |||
| Partial verticle (PV) | 4 (10) | 8 | 7.8 | 1 (12.5) | 6 (75) | 1 (12.5) | 8 (12.5) | 1 | 0 | 0 | |||
| All ‘partials’ (PA + PV) | 11 (27) | 22 | 6.1a | 3 (14c) | 16 (73e) | 3 (14) | 20 (15) | 3 | 2 (0) | 0 | |||
| Total angular (TA) | 14 (34) | 28 | 6.2b | 16 (57d) | 6 (21f) | 4 (14) | 22 (68) | 15 | 6 (17) | 1 | |||
| Total Vertical (TV) | |||||||||||||
| I | 6 (15) | 12 | 26.2 | 4 (33) | 5 (42) | 2 (17) | 4 | 3 | 8 | 1 | |||
| II | 5 (12) | 10 | 10.2 | 2 (20) | 4 (40) | 4 (40) | 4 | 0 | 6 | 2 | |||
| III | 5 (12) | 10 | 9.1 | 7 (70) | 0 (0) | 3 (30) | 8 | 6 | 2 | 1 | |||
| All TV | 16 (39) | 32 | 15.8b | 13 (41d) | 9 (28f) | 9 (28) | 16 (56) | 9 | 16 (25) | 4 | |||
| All ‘Totals’ (TA + TV) | 30 (73) | 60 | 11.4a | 29 (48c) | 15 (25e) | 13 (22) | 38 (63) | 24g | 22 (23) | 5g | |||
| All surgical CPT | 41 (100) | 82 | 10.0 | 32 (39) | 31 (38) | 16 (20) | 58 (47) | 27h | 24 (21) | 5h | |||
| Craniopagus twins (CPT) . | Totals . | . | Age at final separation (months) . | Mortality . | Mild-moderate or normal outcome . | Severe disability . | Single stage . | . | Multiple stage . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | No. of Twins (%) . | No. of Children . | . | No. died (%) . | No. of children (%) . | No. of children (%) . | No. of children (%) . | No. died . | No. of children (%) . | No. died . | |||
| Partial angular (PA) | 7 (17) | 14 | 5.2 | 2 (14) | 10 (71) | 2 (14) | 12 (17) | 2 | 2 | 0 | |||
| Partial verticle (PV) | 4 (10) | 8 | 7.8 | 1 (12.5) | 6 (75) | 1 (12.5) | 8 (12.5) | 1 | 0 | 0 | |||
| All ‘partials’ (PA + PV) | 11 (27) | 22 | 6.1a | 3 (14c) | 16 (73e) | 3 (14) | 20 (15) | 3 | 2 (0) | 0 | |||
| Total angular (TA) | 14 (34) | 28 | 6.2b | 16 (57d) | 6 (21f) | 4 (14) | 22 (68) | 15 | 6 (17) | 1 | |||
| Total Vertical (TV) | |||||||||||||
| I | 6 (15) | 12 | 26.2 | 4 (33) | 5 (42) | 2 (17) | 4 | 3 | 8 | 1 | |||
| II | 5 (12) | 10 | 10.2 | 2 (20) | 4 (40) | 4 (40) | 4 | 0 | 6 | 2 | |||
| III | 5 (12) | 10 | 9.1 | 7 (70) | 0 (0) | 3 (30) | 8 | 6 | 2 | 1 | |||
| All TV | 16 (39) | 32 | 15.8b | 13 (41d) | 9 (28f) | 9 (28) | 16 (56) | 9 | 16 (25) | 4 | |||
| All ‘Totals’ (TA + TV) | 30 (73) | 60 | 11.4a | 29 (48c) | 15 (25e) | 13 (22) | 38 (63) | 24g | 22 (23) | 5g | |||
| All surgical CPT | 41 (100) | 82 | 10.0 | 32 (39) | 31 (38) | 16 (20) | 58 (47) | 27h | 24 (21) | 5h | |||
Age at final separation, All ‘Partials’ versus All ‘Totals’, x2 = 4.01, 1 df, P < 0.05; bAge at final separation, TA versus TV–NS; cMortality All Partials' versus all Totals', x2 = 8.14, 1 df, P < 0.01; dMortality TA versus TV–NS; eFunctional Outcome—All ‘Partials' versus All ‘Totals' x2 = 15.56, 1 df, P < 0.001;fFunctional Outcome—TA versus TV–NS;gMortality—All Total (TA +TV) CPT, single stage versus multiple stage separation x2 = 9.121, 1 df, P < 0.01;hMortality—All CPT, single stage versus multiple stage separation x2 = 4.72, 1 df, P < 0.05.
Overall, we found this classification scheme unambiguous, easy to apply and practical. Our experience was that an adequate illustration, and the occasional use of a protractor to estimate the longitudinal inter-twin angle, regardless of the axial inter-twin angle, clearly differentiated angular from vertical forms.
A modern perspective
Conjoined twins or ‘double monsters’ are a most intriguing and provocative anomaly that has fascinated mankind over the millennia. Aristotle is said to have remarked ‘most of them are due to the embryos growing together’ (Guttmacher and Nichols, 1967). Embryologists have traditionally attributed identical or monovular twinning to ‘splitting or fission’ of either the inner cell mass of pleuripotential cells, or the early embryonic disc at 13–14 days of gestation, just before or upon appearance of the primitive streak (Potter, 1952; Langman, 1969; Baldwin, 1998; Kaufman, 2004). By correlation, incomplete fission is theorized to result in conjoined twins (Kaufman, 2004). Bolstering fission at a slightly later primitive streak stage is evidence that conjoined twins are in the minority of monovular twins having monochorionic, monoamniotic placentation and resultant higher foetal mortality (Sakala et al., 1986; Aquino et al., 1997; Baldwin, 1998; Kaufman, 2004). Circulatory impairment secondary to umbilical cord intertwining probably contributes to lowered birth weight, high foetal mortality and parasitic forms of conjoined twins (Potter, 1952; Aquino et al., 1997; Baldwin, 1998). Almost one-half of the more common conjoined twins joined at the trunk or abdomen (but not CPT) have situs inversus or mirror-image laterality organ defects (Levin et al., 1996; Kaufman, 2004). Laterality defective conjoined twins have recently been experimentally produced in initially single vertebrate embryos by molecular genetic interference with body-plan/lateral induction organizational centres adjacent to the primitive streak (Levin et al., 1996).
Alternatively, the controversial ‘fusion or collision’ theory has again been proposed to account for some conjoined twins, especially those asymmetrically oriented, or attached in areas of later exposed ectoderm such as the future cerebral hemispheres (Spencer, 2003). Conjoined twins have also been produced in lower animals by attachment of previously separate twin embryos (Willis, 1962; Guttmacher and Nichols, 1967; Patten, 1968). However, rather than ‘fission’ or ‘fusion’, the defect leading to conjoined twins may well be a coalescence by overlapping of closely contiguous twin embryonic axis formative fields within a single embryonic disc (Potter, 1952; Willis, 1962; Beckwith, 2003). It is likely that future understanding of embryonic induction and organizational centres may radically change how we envision the initial development of this complex anomaly.
The orderly process of brain development begins on the dorsal aspect of the embryo under the inductive influence of the notochord and chordal mesoderm forming the neural plate at about the 18th day of gestation (Langman, 1970; Lemire et al., 1975). This influence then forms the neural folds and tube with closure of the anterior neuropore at ∼24 days. Disturbance of either programmed development or inductive events during this early period results in craniorachischisis or anencephaly (Harding and Copp, 2002). Interaction of the neural tube with the surrounding mesoderm and notochord gives rise at 32 days to the meninx primitiva, a mesoderm derivative that forms the meninges and surrounds the neural tube by 36 days gestation (Lemire et al., 1975). External to this, and during the same time period, parachordal mesenchyme forms the cranial roof responsible for membranous calvarial skull ossification in the following months (Lemire et al., 1975). In CPT, interference by cerebral opposition of the other twin probably leads to subsequent failure of dural tension bands at 10–16 weeks of gestation required for the formation of a normal falx, sagittal venous sinuses, superior dura and skull (Robertson, 1953; Smith, 1978).
On the ventral aspect of the embryo around 4–5 weeks of gestation, the inductive influence of the notochord and prechordal mesoderm results in formation of the face and forebrain or prosencephalon (Lemire et al., 1975; Volpe, 2001; Harding and Copp, 2002). Further ventral induction in the following weeks results in the development of the telencephalic cerebral vesicles and early corpus callosum. By the end of 10 weeks, the basic neopallial shape and lobes have been determined and they greatly enlarge in the following months. Occurring simultaneously is periventricular neuronal and glial proliferation (3–4 months), and cortical migration with gyral formation (3–5 months) (Lemire et al., 1975). Polymicrogyria, hypoplasia of the corpus callosum and cerebellar vermian aplasia—as noted in our 1981 TA twins—reflect either a primary developmental or a secondary encephaloclastic process from various local and non-specific insults frequented by mental retardation and seizures (Friede, 1975; Volpe, 2001; Harding and Copp, 2002). The second trimester stages of brain development build on the integrity of earlier stages and continue for years after birth. These include final migration, alignment and layering of neurons, establishment of circuitry and myelinaton (Lemire et al., 1975; Harding and Copp, 2002). In CPT, and especially the total forms with significant brain compression, the cerebral vesicle or telencephalon of one twin opposed to that of the other probably represents an obstacle to normal hemisphere structural development, organization, growth and function.
Over the last half-century, CPT twins have challenged the resolve and capability of neurological surgeons riding a wave of technological improvements in neurovascular and brain imaging, localization and surgical techniques. In addition to the absence of significant sharing of venous outflow, arguments for a less complex developmental anomaly in Partial (PA + PV) forms of CPT are the significantly higher birth weights, younger age at separation, less complicated separation and better outcome compared with Total (TV + TA) CPT. Similarly, the TA twins with lower birth weights, less predictable cerebral distortion and posterior fossa involvement in 30% may prove even more challenging than TV, as more cases are accumulated and classified. Nevertheless, an optimistic note is that statistical significance supports a multiple staged rather than single-staged surgical separation for Total forms of CPT children. Conjoined brain tissue was present in almost 30% of all operated twins and, if identified, and separation believed possible, this tissue should be divided at the earliest possible stage. However, some children, especially those with Total CPT, may suffer developmental delay and psychomotor retardation from cerebral hemispheric structural and migrational anomalies, compressional growth forces and social-interactive isolation before surgical separation is feasible to undertake. A realistic goal in that case would be separation without a new superimposed neurological deficit—an outcome recently achieved in TV Type I CPT by one of the authors (J.T.G.) (Goodrich and Staffenberg, 2004; Staffenberg and Goodrich, 2005). In some cases, successful separation may not be possible, as risks outweigh the benefits of conjoined life and such decisions ultimately rest with parents after information provided by knowledgeable physicians.
In this review we have elaborated on embryological, anatomical, neurological and surgical aspects of the CPT anomaly. We suggest a relatively simple four-category classification scheme based upon the presence or absence of significant shared venous sinuses and a vertical or angular configuration of the twins. Application of this scheme to 64 reported CPT cases in the medical literature allowed us to provide an approximate frequency of the forms of CPT and capture a ‘Total Angular’ form that accounts for almost one-third of all CPT. Just over one-half of CPT are of the ‘Total Vertical’ form with variable degrees of rotation between twins, and the remaining one-sixth ‘Partial CPT’ with the absence of shared venous sinuses. The outcome in 41 published and adequately described surgical separation attempts since initial success in 1952 was also analysed according to our scheme. We believe this classification provides for a more rational dialogue of the CPT entity, and allows analysis of comparative risks and outcome following surgical separation.
We dedicate this paper to Dr Oscar Sugar, the surgical pioneer of craniopagus separation, and the inspiration behind this effort. His thoughtful approach and warm human qualities remain an example to those fortunate to know him. We also thank Drs Herbert J. Grossman, Theodore S. Roberts, Marion Walker, Willy Lopez, David Staffenberg and Carol Macmillan for helpful insights. Ms Marjorie Brodie was most generous with her time and energy, as were John Fino and Peg Kilmartin. Ms Christa Wellman provided the illustrations, and Ms Tobi McCauley and Ernestine Daniels assisted with preparation of the manuscript. We also appreciate the assistance of Mr Douglas Bicknese of Special Collections at the Library of the Health Sciences, University of Illinois at Chicago. Funding to pay the Open Access publication charges for this article was provided by the Ralph and Marian Falk Foundation.
References
Abad JM, Mejia A, Peguero G, Navarro C, Lafuente J, Gracia AL. Observacion de una pareja de gemelos craneopagos parietals.
Aquino DB, Timmons C, Burns D, Lowichik A. Craniopagus parasiticus: a case illustrating its relationship to craniopagus conjoined twinning.
Baldwin M, Dekaban A. The surgical separation of Siamese twins conjoined by the head followed by normal development.
Baldwin M, Dekaban A. Cephalopagus twins seven years after separation. Follow-up of a case.
Baldwin VJ. Pathology of multiple pregnancy. In: Wigglesworth JS and Singer DB, editors. Textbook of fetal and perinatal pathology, 2nd edn. Oxford, England: Blackwell Science;
Beckwith JB. Conjoined twins: developmental malformations and clinical implications.
Blumensaat C. Kraniopagus parieto-frontalis bilateralis mit liquorkommunikation.
Boin S. A propos de quatre cas d'anesthesie-reanimation chez des jumeaux conjoints.
Bonderson J, Allen E. Craniopagus parasiticus. Everard Home's two-headed boy of Bengal and some other cases.
Bucholz RD, Yoon KW, Shively RE. Temporoparietal craniopagus: case report and review of the literature.
Cameron DE, Reitz BA, Carson BS, Long DM, Dufresne CR, Vander Kolk CA, et al. Separation of craniopagus Siamese twins using cardiopulmonary bypass and hypothermic circulatory arrest.
Campbell S. Separation of craniopagus twins: the Brisbane experience.
Campbell S, Theile R, Stuart G, Cheng E, Sinnott S, Pritchard G, et al. Separation of craniopagus joined at the occiput.
Campbell S, Theile R, Stuart G, McDonald M, Sinnott S, Frawley K, et al. Craniopagus: second Brisbane case.
Cywes S, Davies MRQ, Rode H. Conjoined twins—the Red Cross War Memorial Children's Hospital experience.
Di Rocco C, Caldarelli M, Tamburrini G, Koutzoglou M, Massimi L, DiRocco F, et al. Craniopagus: the Thessaloniki-Rome experience.
Drummond G, Scott P, Mackay D, Lipschitz R. Separation of the Baragwanath craniopagus twins.
Frazee J, Fried I, Kawamoto H, Lazareff J, Nieto J, Patel S, et al. The separation of Guatemalan craniopagus twins.
Gaist G, Piazza G, Galassi E, Cavina C, Salvioli GP. Craniopagus twins: an unsuccessful separation and a clinical review of the entity.
Georges LS, Smith KW, Wong KC. Anesthetic challenges in separation of craniopagus twins.
Goh KYC. Separation surgery for total vertical craniopagus twins.
Goodrich JT, Staffenberg DA. Craniopagus twins: clinical and surgical management.
Grossman HJ, Sugar O, Greeley PW, Sadove MS. Surgical separation in craniopagus.
Guttmacher AF, Nichols BL. Teratology of conjoined twins.
Harding BN, Copp AJ. Pathology of malformations. In: Graham DI and Lantos PL, editors. Greenfield's Neuropathology, 7th edn., Vol 1. London: Arnold;
Ho YL, Goh KY, Golay X, Hong W, Lim S, Pan AB, et al. Functional magnetic resonance imaging in adult craniopagus for presurgical evaluation.
Huang WQ, Fang JY, Xiao LC, Jiang XP, Xia JH, Feng X, et al. Anesthetic management for separation of craniopagus twins.
Jansen O, Mehrabi VA, Sartor K. Neuroradiological findings in adult cranially conjoined twins. Case report.
Khan ZH, Tabatabai SA, Saberi H. Anesthetic and surgical experience in a case of total vertical craniopagus.
Kohama A, Watanabe Y, Okatani K, Horibe K, Takasugi Y, Ohta M. Experience of anesthesia for separation of parietal conjugated twins (craniopagus).
Konovalov AN, Vaichis CM. The successful separation of a craniopagus.
Lansdell H. Intelligence test scores from infancy to adulthood for a craniopagus twin pair neurosurgically separated at 4 months of age.
Lemire RJ, Loeser JD, Leech RW, Alvord EC. Normal and abnormal development of the human nervous system. New York: Harper & Row;
Lenard HG, Schulte FL. Polygraphic sleep study in craniopagus twins.
Levin M, Roberts DJ, Holmes LB, Tabin C. Laterality defects in conjoined twins.
Loverro G, Occhiogrosso M, Caruso G, Greco P, Selvaggi L. Prenatal diagnosis of craniopagus: case report.
Marcinski A, Urbanik Lopatec H, Wermenski K, Wocjan J, Gajewski Z, Kaminski W, et al. Angiographic evaluation of conjoined twins.
O'Connell JEA. Surgical problems in the separation of craniopagus twins.
O'Connell JEA. Craniopagus twins: surgical anatomy and embryology and their implications.
O'Neill JA Jr, Holcomb GW III, Schnaufer L, Templeton JM, Bishop HC, Ross AJ, et al. Surgical experience with 13 conjoined twins.
Pertuiset B, Lafourcade J, Viars P, Nicoletis C, Metzger J, Ancri D, et al. Separation reussie en 1974 de jumelles craniopages unies par le vertrex—indications, techniques, evolution sur 14 ans.
Piza-Katzer H. Free allogeneic muscle transfer for cranial reconstruction.
Potter EL. Pathology of the fetus and the newborn. Chicago: Yearbook Medical Publishing;
Roberts TS. Cerebral venous abnormalities in craniopagus twins. In: Kapp JP and Schmidek HH, editors. The cerebral venous system and its disorders. Orlando, FL: Grune & Stratton;
Roberts TS. Craniopagus twins. In: Wilkins RH and Rengachary SS, editors. Neurosurgery. Vol. 3. New York: McGraw-Hill;
Roberts TS. Cephalopagus twins. In: Hoffman HJ and Epstein F, editors. Disorders of the developing nervous system: diagnosis and treatment. Boston: Blackwell Scientific;
Sathekge MM, Venkannagari RR, Clauss RP. Scintigraphic evaluation of craniopagus twins.
Schindler E, Hajek P. Craniopagus twins: neuroradiological findings.
Schultz RC, Danielson JR, Habakuk S. The use of uniform simulated models in the reconstruction of craniopagus twins.
Spencer R. Conjoined twins. Developmental malformations and clinical implications. Baltimore: Johns Hopkins University Press;
Staffenberg DA, Goodrich JT. Separation of craniopagus conjoined twins: an evolution in thought.
Stanley P, Anderson FM, Segall HD. Radiologic investigation of craniopagus twins (partial type).
Sugar O, Grossman H, Greeley P, Destro V. The Brodie craniopagus twins.
Swift DM, Weprin B, Sklar F, Sacco D, Salyer K, Genecov D, et al. Total vertex craniopagus with crossed venous drainage: case report of successful surgical separation.
Todorov AB, Cohen KL, Spilotro V, Landau E. Craniopagus twins.
van Ouwerkerk WJR, van den Berg R, Allison CE, Sibarani R, van Wijl JAE, Smit LME, et al. Craniopagus: the Suriname-Amsterdam conjunction.
Villarejo F, Soto M, Amaya C, Pascal-Castroviejo I, Morales C. Total craniopagus twins.
Volpe JJ. Neural tube formation and prosencephalic development; neuronal proliferation, migration, organization, and myelination. In: Volpe JJ, editor. Neurology of the newborn. 4th edn. Philadelphia: W.B. Saunders;
Voris HC, Slaughter WB, Christian JR, Cayia ER. Successful separation of craniopagus twins.
Walker M, Browd SR. Craniopagus twins: embryology, classification, surgical anatomy, and separation.
Willis RA. The borderland of embryology and pathology. 2nd edn. London: Butterworth's;
Wilson H, Storer EH. Surgery in Siamese twins: a report of three sets of conjoined twins treated surgically.
Winston KR. Craniopagi: anatomical characteristics and classification.
Winston KR, Rockoff MA, Mulliken JB, Strand RD, Murray JE. Surgical division of craniopagi.
Wolfowitz J, Kerr EM, Levin SE, Walker DH, Vetten KB. Separation of craniopagus.
Wong KC, Ohmura A, Roberts TS, Webster LR, Cook GL. Anesthetic management for separation of craniopagus twins.
Wong TG, Ong BC, Ang C, Chee HL. Anesthetic management for a five-day separation of craniopagus twins.
Author notes
1Departments of Neurological Surgery and Neurology, University of Illinois at Chicago, and Division of Neurosurgery, Cook County Hospital, Chicago, IL, USA and 2Division of Pediatric Neurological Surgery, Montefiore Children's Hospital, Albert Einstein College of Medicine, Bronx, New York, NY, USA


