-
PDF
- Split View
-
Views
-
Cite
Cite
Austin Jin Xian See, Abhishekh Hulegar Ashok, Yogish Joshi, Mathew Guilfoyle, Teik Choon See, Endovascular intervention to treat spontaneous carotid-cavernous fistula in a patient with Ehlers-Danlos Syndrome with an access site anatomical variant, BJR|Case Reports, Volume 10, Issue 2, March 2024, uaae006, https://doi.org/10.1093/bjrcr/uaae006
Close - Share Icon Share
Abstract
Vascular Ehlers-Danlos Syndrome (vEDS) is a rare and potentially life-threatening inherited connective tissue disorder. Patients with vEDS can present with spontaneous arterial dissections and ruptured aneurysms. There are previous reports of large artery dissections and vessel rupture following conventional catheter diagnostic angiography. We present the case of a patient with vEDS who had a spontaneous carotid-cavernous fistula (CCF) and visceral aneurysms, associated with a normal variant of corona mortis. A CCF was successfully treated with a transvenous approach with detachable coils.
Introduction
Vascular Ehlers-Danlos Syndrome (vEDS), a subtype of Ehlers-Danlos Syndrome, is a rare and potentially life-threatening inherited connective tissue disorder primarily caused by COL3A1 gene mutations, resulting in abnormal type III collagen production.1 This leads to thin and translucent skin, visible veins, spontaneous ecchymosis, fragile blood vessels, and hollow organs. The common sites involved are abdominal vessels, particularly the mesenteric and renal arteries. The vascular complications, including arterial dissections, aneurysms, and ruptures, may have significant morbidity and fatal consequences.
A spontaneous carotid-cavernous fistula (CCF) is pathognomonic of vEDS and occurs in approximately 9.8% of individuals with vEDS.2 A direct CCF causes high-pressure arterial blood to flow directly into the low-pressure cavernous system. This leads to symptoms including headache, blurred vision, bruit, diplopia, and ocular foreign body sensation.3 We present the case of a vEDS patient with a spontaneous CCF and visceral aneurysms associated with a normal variant of corona mortis.
Clinical presentation
A 48-year-old lady with a history of vEDS presented with severe worsening left-sided headache associated with a whooshing sound, subconjunctival haemorrhage, proptosis, anisocoria (left pupil dilated), and restricted left eye movements.
Her EDS was diagnosed following an investigation of her left-sided chest pain 11 years ago. She underwent genetic testing which confirmed the diagnosis. She attended yearly cardiology clinics and previously dislocated her left shoulder. She has a superior mesenteric artery dissection, a 34 mm abdominal aortic aneurysm (AAA), multiple splenic artery aneurysms (up to 11 mm), left renal artery aneurysm (7 mm), and inferior gluteal artery aneurysm (8 mm). She is being managed conservatively with three monthly ultrasounds for her AAA and yearly CT, as per the local multidisciplinary team's recommendation.
Investigations
A CT angiography revealed a 6 mm left intra-cavernous aneurysm arising from the medial wall of the cavernous sinus consistent with a CCF (Figure 1A).
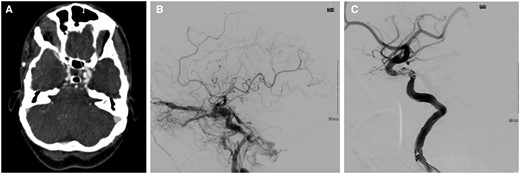
(A) CT angiography showed left carotid-cavernous fistula (CCF) with a 6 mm aneurysm (white arrow). (B) Angiography of CCF pre-embolization. (C) Post-embolization of CCF.
Treatment, outcome, and follow-up
Following an informed discussion with the patient, neuro-interventional radiology, neurology, and neurosurgery, she underwent endovascular intervention of her CCF under general anaesthesia. The right common femoral artery (CFA) was accessed using a micropuncture kit (Cook, United States) under ultrasound guidance. An 8-French (Fr) sheath (Cordis, United States) was inserted. Fluoroscopy screening showed the sheath to be in an abnormal position pointing laterally. The sheath was partially withdrawn until it re-entered the right CFA with the tip positioned more proximally into the right external iliac artery (EIA), guided by a wire. Angiography via the sheath showed significant acute extravasation over the right lateral lower abdomen from the right superficial circumflex iliac artery, consistent with rupture and haemorrhage (Figure 2A). Due to the patient's haemodynamic instability, there was no time for selective embolization. An 8 mm × 5 cm Viabhan stent graft (Gore, United States) was deployed in the distal right EIA, covering the origin of the right superficial circumflex iliac artery. Check angiography showed cessation of extravasation (Figure 2B) and the patient stabilized. A decision was made to continue with CCF embolization.
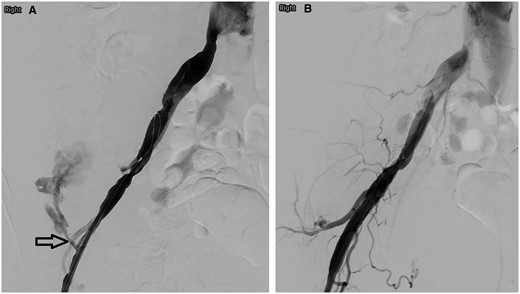
(A) Right iliac artery angiography showed contrast medium extravasation from the right superficial circumflex iliac artery (arrow). (B) No residual extravasation following right external iliac artery stenting.
Cerebral angiography confirmed a left CCF with anterior and posterior venous drainage, as well as midline drainage into the contralateral cavernous sinus (Figure 1B). A 6 French guide catheter (Penumbra, Germany) was exchanged into the proximal descending aorta. A hydrophilic guidewire (Terumo, Belgium) positioned the guide catheter into the petrous segment of the left internal carotid artery (ICA). Initially, attempts were made to float a Goldbal2 detachable balloon (Balt, France) from the left ICA into the cavernous sinus. This did not prove successful, and the balloon was withdrawn after several attempts.
Leaving the guide catheter within the left ICA on a heparinized saline flush for control angiography, venous access to the fistula was obtained. The right common femoral vein was punctured, and a 6-Fr sheath (Cordis, United States) was inserted to position a 6-French guide catheter (Envoy, Mexico) into the superior left internal jugular vein. This was used to direct a headway duo microcatheter (Microvention, United States) over a traxcess-14 microwire (Microvention, United States) into the left cavernous sinus, and beyond this into the left superior ophthalmic vein. Following the placement of an additional headway duo microcatheter into the same position, coils were deployed from the left superior ophthalmic vein backwards into the cavernous sinus and left inferior petrosal sinus. A sceptre XC 4 mm × 11 mm occlusion balloon catheter (Microvention, United States) was positioned in the cavernous segment of the left ICA to delineate the vessel and prevent coil migration into this vessel across the fistulous point.
Following the deployment of multiple coils, cessation of flow through the left CCF was noted (Figure 1C). No further flow into the superior ophthalmic vein was noted.
At the end of the procedure, the anaesthetist raised concern regarding ongoing bleeding due to difficulty in maintaining blood pressure despite fluid replacement, blood products, and vasopressors. Right internal iliac artery (IIA) angiography showed slow extravasation near the previous haemorrhage site and appeared to be fed by a convoluted branch of the right IIA which crosses the distal right EIA (Figure 3A). Balloon occlusion of the right IIA with angiography of the right common iliac artery (CIA) did not reveal extravasation, hence excluding endoleak from the stent graft. The right IIA branch was catheterized by a 4-Fr cobra catheter (Cordis, United States) followed by a 2.7-Fr Progreat microcatheter (Terumo, Japan) to superselect the feeding IIA branch. Embolization was performed with approximately 2 mL of glubran (GEM, Italy):lipiodol (Guerbet, France) mixture (ratio 1:2) to close backfilling of the bleeding point. Multiple check angiography in various right IIA, CIA, and EIA positions did not reveal any residual extravasation (Figure 3B).
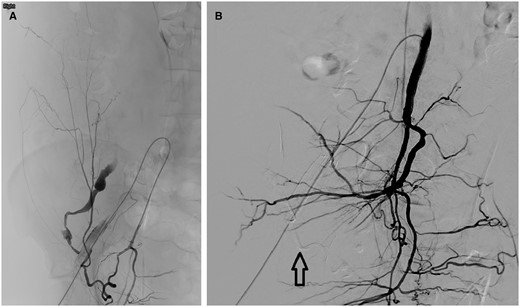
(A) Right obturator artery angiography showed contrast medium extravasation from the previous haemorrhage site. (B) Right internal iliac angiography showed no residual extravasation. Arrow points to glubran and lipiodol mixture used for embolization.
An 8 French Angio-Seal closure was performed in the right CFA. Haemostasis was achieved in the right CFV with manual compression. A venous central line was inserted in the left CFV during the procedure to help with volume replacement using ultrasound guidance. A right radial arterial monitoring line was inserted under ultrasound guidance at the end of the procedure to allow continuous arterial monitoring.
A post-procedure CT revealed an anatomical variant of corona mortis consisting of an anastomosis between the right obturator artery, inferior epigastric artery, and the distal EIA (Figure 4). This variant refilled the distal right EIA and right superficial circumflex iliac artery post-stenting and explains the recanalization of flow and ongoing haemorrhage post-stenting. Embolization using glubran/lipiodol mixture with the microcatheter in the right obturator artery occluded the right obturator artery, the proximal segment of the right inferior epigastric artery, and the part of the distal right EIA at the arterial anastomosis subjacent to the stent.
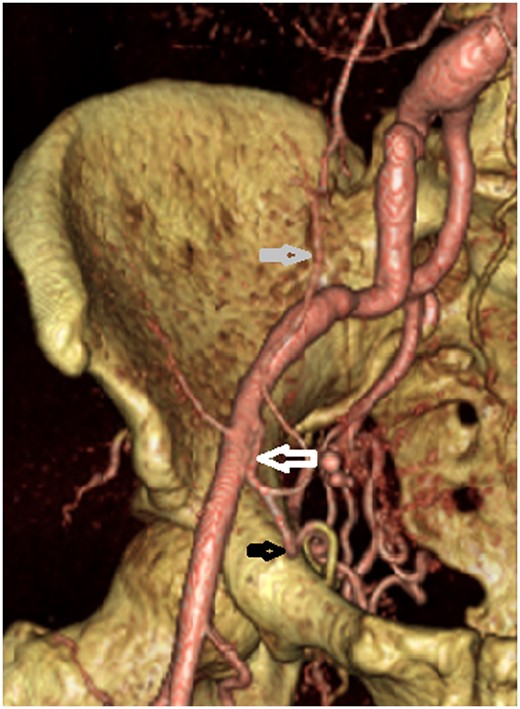
CT reconstruction showing corona mortis (white arrow) which connects the obturator artery (black arrow) and the inferior epigastric artery (grey arrow) with the external iliac artery.
A CT the following day showed no further haemorrhage. Bilateral segmental and subsegmental pulmonary emboli were found incidentally and anticoagulation with low molecular weight heparin commenced.
One week after anticoagulation, she developed 2 new rectus sheath haematomas measuring up to 19 cm and a left pararenal haematoma. These were managed conservatively.
A follow-up CT 10 days later found a new 9 mm common hepatic artery (CHA) aneurysm. The patient has a replaced right hepatic artery from the superior mesenteric artery. The coeliac artery gives rise to CHA, left HA and gastroduodenal artery (GDA). The aneurysm increased to 22 mm 2 weeks later (Figure 5A). Given the short interval size increment, a multidisciplinary decision was made to embolize the aneurysm under general anaesthesia.
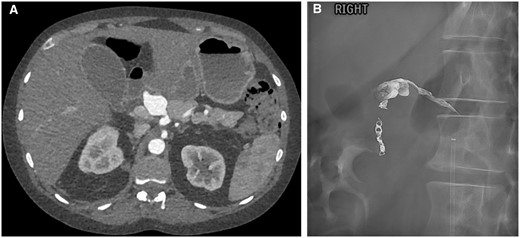
(A) Common hepatic artery (CHA) aneurysm. (B) Post-embolization showing coils in the gastroduodenal artery and onyx within the CHA aneurysm with some reflux proximally.
The right CFA was accessed across the right EIA stent. Coeliac angiography showed CHA aneurysmal sac was slightly smaller than on CT due to a thrombus. However, the CHA is more fusiform and dilated. The plan was to embolize the GDA and left HA (back door), followed by embolization of the CHA aneurysm.
Cannulation of the CHA proved challenging due to the angulation of the coeliac artery. Vasospasm of the CHA was relieved with 200 µg glyceryl trinitrate. GDA was eventually cannulated and embolized with microcoils. Despite multiple attempts, catheterizing the left HA was not possible due to a kink and stenosis at the origin. A 2.4 Fr Rebar microcatheter (Micro Therapeutics, United States) was positioned within CHA aneurysmal sac. The catheter was flushed with dimethylsulfoxide (DMSO) solution. One vial of Onyx-34 liquid embolic (Medtronic, United States) was delivered into the sac to good effect. Some reflux of onyx was noted along the CHA (Figure 5B). Post-embolization angiography showed minimal residual filling of the sac and left HA. Subsequent CT examinations 3 days and 4 weeks later showed complete exclusion of the CHA aneurysm.
The patient developed sudden left iliac fossa swelling and pain five weeks post-embolization. CT showed interval size increment of the left rectus sheath haematoma, with active bleeding, likely from a distal branch of the left inferior epigastric artery. INR level was raised to 3.17 (reference range 1-1.15). As she was haemodynamically stable, this was managed conservatively.
The anticoagulation issue was discussed with her. Her pulmonary embolism occurred when she was unwell with reduced mobility during previous hospital admission. Treating future bleeds whilst on anticoagulation would be complex in the context of her vEDS. Her preference to stop anticoagulation was agreed upon. She was discharged 10 weeks following CCF embolization. At discharge, clarity of left eye vision was subjectively improved.
The multiple hospital admissions, interventions, potentially life-threatening bleeds, and frequent hospital follow-ups have had a significant psychological impact on her. Additionally, both her children have confirmed EDS, adding emotional strain and anxiety. She is seeing a psychologist to help alleviate the stress.
Discussion
To our knowledge, this is the first reported vEDS case involving a CCF, a CHA aneurysm, and corona mortis. Literature on vEDS and endovascular treatment of CCF is extensively described elsewhere.2,4 Patients with vEDS have abnormal collagen, predisposing them to arterial aneurysms and ruptures at a young age, with a median life expectancy of 51 years.5 While the most common cause of death is arterial rupture, the fragility of vessels and tissues makes invasive interventions particularly high risk, especially in the emergency setting. It is well-recognized that open surgery and endovascular treatment may be associated with high morbidity and mortality rates. Freeman et al reported major complications in 22% and a mortality rate of 5.6% among 18 vEDS patients who underwent angiography.6 Bergqvist et al reported a higher mortality rate with open surgical repair of arterial complications (30%) than with endovascular procedures (24%).7 Currently, there are no evidence-based guidelines on managing vascular events in vEDS. The uncertain outcome either with conservative or invasive management makes counselling and decision-making challenging.
Carotid-cavernous fistulas are one of the most common intracranial complications of vEDS. Endovascular interventions for CCF offer a 90%-100% cure rate with a low complication rate and an acceptably low mortality rate of <1%.3 However, vEDS patients have much higher complication rates due to underlying vascular fragility.
Our case was compounded by the anatomical variant of corona mortis, identified only on post-procedure CT. Corona mortis is Latin for “Crown of death” as damage to these blood vessels can result in unexpected, significant bleeding and complications.8 Considering these anatomical variations when planning and performing procedures is essential as it enables modification of the approach to minimize complications. The ultrasound guided puncture was performed and a sheath was introduced. By screening the guidewire prior to insertion of the sheath, the initial rupture could have been avoided.
A multi-institutional experience in the aortic and arterial pathology in 86 individuals with genetically confirmed vEDS identified 139 aortic/arterial pathologies in 53 individuals (61.6%). The aortic/arterial events presented as an emergency in 52 cases (37.4%). The most commonly affected arteries were the mesenteric arteries (31.7%), followed by cerebrovascular (16.5%), iliac (16.5%), and renal arteries (12.2%). The most common management was medical management. When undertaken, the predominant endovascular interventions were arterial embolization of medium-sized arteries (13.4%), followed by stenting (2.5%).9
We agree with Alqahtani et al that the decision to treat aneurysms in vEDS should be made before vascular rupture and based on the speed of vascular growth and the ratio between the size of the native artery that holds the aneurysm and the aneurysmal diameter itself.10 The decision to treat should not be postponed because the patient is suspected to have vEDS. Our case illustrated the importance of case planning, awareness of access site and other vascular complications, and availability of emergency support should acute haemorrhage occur. The procedure should ideally be supported by the anaesthetic team, and the use of liquid embolic materials should be prioritized if feasible to reduce the operative time.
Finally, the emotional and psychological stress this condition puts the patient under is immense. Lifestyle advice from a young age can help acceptance, understanding the diagnosis, and inform choices.11 The spontaneity of vEDS can lead to significant anxiety about the future. Regular monitoring requires regular hospital attendance which has a massive impact on patients’ wellbeing. vEDS is an autosomal dominant condition and therefore there is a 50% chance of inheritance. Genetic counselling can help parents understand the inheritance pattern and the risks posed to their children.
Learning points
Vascular Ehlers-Danlos syndrome is a rare, complex, and life-threatening connective tissue disorder that requires multidisciplinary clinical input and patient involvement.
Management options for recurrent aneurysms and haemorrhages on multiple sites require careful consideration of the risks and benefits.
Endovascular complications in vEDS are considerably high, and pre-procedure planning, awareness of potential normal variant, and the use of appropriate devices are essential.
The use of anticoagulation in vEDS should be balanced with the frequent risk of spontaneous haemorrhage.
Frequent hospital visits and interventions, along with the inheritance nature of the condition have an immense emotional and psychological impact on vEDS patients.
Acknowledgements
The authors would like to thank the patient for consenting to this case report. They also would like to thank Radiology, Neuro-surgery, Vascular Surgery, Neurology, Cardiology, Hepatobiliary, and Critical care teams for their care for the patient.
Funding
T.C.S. research time is funded by Cancer Research UK.
Conflicts of interest
None declared.
Informed consent statement
Written informed consent was obtained from the patient for publication of this case report, including accompanying images.



