-
PDF
- Split View
-
Views
-
Cite
Cite
A David Burden, Hervé Bachelez, Siew Eng Choon, Slaheddine Marrakchi, Tsen-Fang Tsai, Hamida Turki, Akimichi Morita, Mark G Lebwohl, Robert Bissonnette, Min Zheng, Milan J Anadkat, Alexander A Navarini, Ming Tang, Christian Thoma, Kristina Callis Duffin, The Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) score: online assessment and validation study of a specific measure of GPP disease activity, British Journal of Dermatology, Volume 189, Issue 1, July 2023, Pages 138–140, https://doi.org/10.1093/bjd/ljad071
Close - Share Icon Share
https://doi.org/10.1093/bjd/ljad071
Dear Editor, Generalized pustular psoriasis (GPP), a rare and potentially life-threatening neutrophilic skin disease, is characterized by recurrent flares of pustulation on nonacral skin with associated erythema, crusting and scaling.1,2 There are no agreed or validated measurements for assessing GPP severity or symptom improvement; clinically validated endpoints that incorporate key manifestations of GPP, and are meaningful and reliable for the evaluation of treatment response, are needed. To date, clinical trials in patients with GPP have used measures developed for plaque psoriasis [Psoriasis Area Severity Index (PASI) or the Physician Global Assessment (PGA)]; in addition, the Japanese Dermatological Association GPP Severity Index (JDA-GPPSI) and Clinical Global Impression (CGI) scores have been used in clinical trials in Japan.3 However, these assessments have limitations in specificity and usability, have not been validated in GPP, and do not all assess the presence (PASI) or severity of pustulation (JDA-GPPSI), the defining cutaneous feature of GPP.
Therefore, the Generalized Pustular Psoriasis Area and Severity Index [GPPASI; scores from 0 (least severe) to 72 (most severe)] and the Generalized Pustular Psoriasis Physician Global Assessment [GPPGA; scores from 0 (clear) to 4 (severe)] were developed to assess the key cutaneous manifestations of GPP, namely erythema, scaling and pustulation, in recent studies of spesolimab in GPP.4 The GPPASI and GPPGA are adaptations of the PASI and PGA, whereby induration has been replaced by pustulation. Further information on the GPPASI and GPPGA scores is available via a download from Figshare (https://doi.org/10.6084/m9.figshare.22664869.v1)4.
The objectives of the current study were to demonstrate the validity of the GPPGA and determine its suitability as an endpoint measure for GPP. To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the International Committee of Medical Journal Editors (ICMJE) criteria, Boehringer Ingelheim grants all external authors access to clinical study data pertinent to the development of the publication. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data when it becomes available on Vivli – Center for Global Clinical Research Data, at the earliest after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete, and other criteria are met. Please visit Medical & Clinical Trials | Clinical Research | MyStudyWindow for further information. This study did not involve intervention or observations of patients; therefore, no Institutional Review Board or Ethics Committee Review was deemed necessary. All patients provided written informed consent prior to use of clinical images for publication, and photographs were de-identified; consent was provided as part of the written informed consent prior to participation in the EffisayilTM 1 study (clinicaltrials.gov identifier NCT03782792; see also Bachelez et al.4).
Representative photographs from patients with GPP participating in the EffisayilTM 1 study (NCT03782792)4 were evaluated by GPP-experienced dermatologists, who were participating in the EffisayilTM 2 trial (NCT04399837) and trained on the GPPGA using an instructional guide (Figure 1). Participants scored 16 images twice, 10–14 days apart, using an online portal. Intra-rater (reproducibility of grading by a single rater over time) and inter-rater (reproducibility between raters at the same time point) reliability were assessed by the intraclass correlation coefficient (ICC). Descriptive statistics of GPPGA total score and component subscores of each image at each time point were summarized separately.
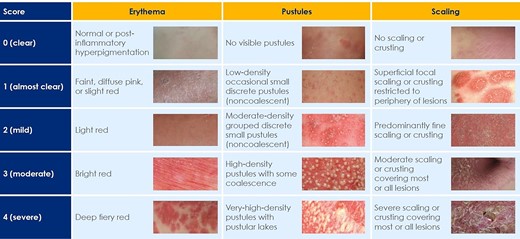
GPPGA severity criteria excerpts from the Generalized Pustular Psoriasis Severity Scoring (GPPASI and GPPGA) pocket guide (please also refer to ‘The GPPGA score’, available on Figshare, https://doi.org/10.6084/m9.figshare.22664869.v1).
GPPASI, Generalized Pustular Psoriasis Area Severity Index; GPPGA, Generalized Pustular Psoriasis Physician Global Assessment.
Composite mean score = (erythema + pustules + scaling)/3; total GPPGA score given is: 0 if mean is 0 for all three components;
1 if 0 < mean < 1.5; 2 if 1.5 ≤ mean < 2.5; 3 if 2.5 ≤ mean < 3.5; 4 if mean ≥ 3.5.
Of 56 dermatologists invited, 26 participated and completed the first assessment; 20 completed both assessments. Intra-rater reliability was generally excellent; mean (SD) ICC absolute agreements were: GPPGA total score 0.9 (0.06); erythema 0.89 (0.07); pustules 0.90 (0.07); scaling 0.87 (0.09). Only one rater recorded an ICC < 0.75, indicating ‘good’ reliability [scaling 0.66 (95% confidence interval, CI) 0.29–0.85]. Inter-rater reliability (first time point) was generally excellent; ICC absolute agreements (95% CI) were: GPPGA total score 0.82 (0.73–0.91); erythema 0.82 (0.73–0.91); pustules 0.78 (0.67–0.88); scaling 0.76 (0.65–0.87). Additional results are available on Figshare (https://doi.org/10.6084/m9.figshare.22664869.v1).
This validation study indicates that the GPPGA is reproducible, with reliability demonstrated among dermatologists with varied experience of managing and treating patients with GPP. Clinical validation of the accuracy and responsiveness of the GPPGA has also been demonstrated.5 Having suitable processes for conducting measurements and effective training, as well as the provision of representative images, improves scoring in dermatological assessments.6 The excellent intra-rater and inter-rater reliability results indicate the effectiveness of training materials created for the GPPGA in enabling dermatologists to consistently assess key cutaneous manifestations of GPP. While photographs may not replicate direct patient examination, overall reliability remained excellent. Considering the rise in telemedicine within dermatology,7,8 the potential to accurately determine disease severity from two-dimensional images could be beneficial.
Study strengths of assessing the GPPGA (an expert-developed, clinical trial-tested endpoint) include a robust image-selection process, the number of participating physicians and appropriate physician training. Study limitations include participant selection from a relatively experienced cohort (perhaps more confident assessing GPP severity than nonspecialists), assessment of severity from 16 photographs rather than direct-person examination, and a lack of variation in skin phototypes among patient images; however, these limitations are difficult to overcome given the rarity of GPP, its episodic nature and the severity of flares. Further experience from clinical studies using the GPPGA, including exploration of the potential effect of skin phototypes, would be valuable.
The GPPGA is a robust, reliable and reproducible assessment of disease severity, specifically designed for GPP. As GPPGA measures GPP-related disease components in a format already well understood by dermatologists, it should be considered a suitable clinical endpoint for future GPP trials and incorporated into clinical practice where possible. GPPGA has the potential to become a standard assessment for GPP severity, along with markers of systemic inflammation and patient reported outcomes.
Acknowledgements
The authors would like to thank Na Hu for support with the development and analysis of the study, Eric Zudak and the team at Trifecta Clinical for online assessment portal support, and the team at QuantifiCare for clinical photography support. Daniel Clarke PhD, Isabella Goldsbrough PhD and Carolyn Bowler PhD, of OPEN Health Communications (London, UK) provided writing, editorial and formatting support in the preparation of this manuscript, which was contracted and funded by Boehringer Ingelheim.
Funding sources
This study was supported and funded by Boehringer Ingelheim. Agreements between Boehringer Ingelheim and the authors included the confidentiality of the study data. The authors met criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of the manuscript. All authors collaborated on the writing of the manuscript and made the decision to submit the manuscript for publication.
References
Author notes
Conflicts of interest The full list is provided in Appendix S1 (see Supporting Information).



