-
PDF
- Split View
-
Views
-
Cite
Cite
A. Wollenberg, A. Blauvelt, E. Guttman‐Yassky, M. Worm, C. Lynde, J.‐P. Lacour, L. Spelman, N. Katoh, H. Saeki, Y. Poulin, A. Lesiak, L. Kircik, S.H. Cho, P. Herranz, M.J. Cork, K. Peris, L.A. Steffensen, B. Bang, A. Kuznetsova, T.N. Jensen, M.L. Østerdal, E.L. Simpson, on behalf of the ECZTRA 1 and ECZTRA 2 study investigators, Tralokinumab for moderate‐to‐severe atopic dermatitis: results from two 52‐week, randomized, double‐blind, multicentre, placebo‐controlled phase III trials (ECZTRA 1 and ECZTRA 2), British Journal of Dermatology, Volume 184, Issue 3, 1 March 2021, Pages 437–449, https://doi.org/10.1111/bjd.19574
Close - Share Icon Share
Summary
Tralokinumab, a fully human monoclonal antibody, specifically neutralizes interleukin‐13, a key cytokine driving peripheral inflammation in atopic dermatitis (AD). In phase II studies, tralokinumab combined with topical corticosteroids provided early and sustained improvements in AD signs and symptoms.
To evaluate the efficacy and safety of tralokinumab monotherapy in adults with moderate‐to‐severe AD who had an inadequate response to topical treatments.
In two 52‐week, randomized, double‐blind, placebo‐controlled, phase III trials, ECZTRA 1 and ECZTRA 2, adults with moderate‐to‐severe AD were randomized (3 : 1) to subcutaneous tralokinumab 300 mg every 2 weeks (Q2W) or placebo. Primary endpoints were Investigator’s Global Assessment (IGA) score of 0 or 1 at week 16 and ≥ 75% improvement in Eczema Area and Severity Index (EASI 75) at week 16. Patients achieving an IGA score of 0 or 1 and/or EASI 75 with tralokinumab at week 16 were rerandomized to tralokinumab Q2W or every 4 weeks or placebo, for 36 weeks. The trials were registered with ClinicalTrials.gov: NCT03131648 and NCT03160885.
At week 16, more patients who received tralokinumab vs. placebo achieved an IGA score of 0 or 1: 15·8% vs. 7·1% in ECZTRA 1 [difference 8·6%, 95% confidence interval (CI) 4·1–13·1; P = 0·002] and 22·2% vs. 10·9% in ECZTRA 2 (11·1%, 95% CI 5·8–16·4; P < 0·001) and EASI 75: 25·0% vs. 12·7% (12·1%, 95% CI 6·5–17·7; P < 0·001) and 33·2% vs. 11·4% (21·6%, 95% CI 15·8–27·3; P < 0·001). Early improvements in pruritus, sleep interference, Dermatology Life Quality Index, SCORing Atopic Dermatitis and Patient‐Oriented Eczema Measure were observed from the first postbaseline measurements. The majority of week 16 tralokinumab responders maintained response at week 52 with continued tralokinumab treatment without any rescue medication (including topical corticosteroids). Adverse events were reported in 76·4% and 61·5% of patients receiving tralokinumab in ECZTRA 1 and ECZTRA 2, respectively, and in 77·0% and 66·0% of patients receiving placebo in ECZTRA 1 and ECZTRA 2, respectively, in the 16‐week initial period.
Tralokinumab monotherapy was superior to placebo at 16 weeks of treatment and was well tolerated up to 52 weeks of treatment.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder with an estimated prevalence in adults of between 2·1% and 4·9% across North America, Europe and Japan.1 As we gain a better understanding of the inflammatory pathways driving AD, targeted treatment options are being developed to improve long‐term therapeutic options for patients with moderate‐to‐severe AD.2–4 Recent evidence identified the type 2 cytokine interleukin (IL)‐13 as a key driver of the underlying inflammation in AD.5–7 IL‐13 is implicated in skin barrier disruption, skin inflammation, increased risk of skin infections, itch signalling and epidermal hyperplasia,6 with levels of IL‐13 in lesional skin correlating with AD severity.8–13 Currently, dupilumab (an IL‐4 receptor α antagonist) is the only biologic available for the treatment of moderate‐to‐severe AD.
Tralokinumab, a fully human IgG4 monoclonal antibody, specifically binds with high affinity to IL‐13 alone, preventing its interaction with the receptor and subsequent downstream signalling.14 In a phase II trial, tralokinumab provided early and sustained improvement in disease signs and symptoms in adult patients with moderate‐to‐severe AD.15 Here we report the results of the first phase III trials to investigate the long‐term efficacy and safety of IL‐13‐specific inhibition with tralokinumab monotherapy compared with placebo in adult patients with moderate‐to‐severe AD for up to 1 year, as assessed by the severity and extent of AD, itch, Staphylococcus aureus colonization and health‐related quality of life.
Patients and methods
Study design and oversight
ECZTRA 1 (NCT03131648) and ECZTRA 2 (NCT03160885) were identically designed 52‐week, multinational, randomized, double‐blind, placebo‐controlled trials. Patients were randomized 3 : 1 to subcutaneous tralokinumab 300 mg, after a 600‐mg loading dose on day 0, or placebo every other week for 16 weeks (Figure S1; see Supporting Information). Randomization was performed using a computer‐generated randomization schedule stratified by region and baseline disease severity. Treatment allocation was blinded to patients and investigators (Appendix S2; see Supporting Information). After a 16‐week initial treatment period, tralokinumab‐treated patients who achieved the prespecified criteria for clinical response of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear), or ≥ 75% improvement in Eczema Area and Severity Index (EASI 75) were rerandomized 2 : 2 : 1 to tralokinumab 300 mg every 2 weeks (Q2W) or every 4 weeks (Q4W), or placebo for a 36‐week maintenance treatment period.
Patients who achieved the clinical response criteria with placebo continued to receive placebo Q2W to maintain blinding of the study and were not included in analyses after week 16. Patients not achieving the clinical response criteria at week 16 were transferred to open‐label tralokinumab 300 mg Q2W with optional topical corticosteroids (TCS). Additionally, patients who, during maintenance treatment, experienced decline in effect by meeting specific protocol‐defined transfer criteria over a 4‐week period (Appendix S2) were transferred to open‐label tralokinumab. All patients had a final safety follow‐up 16 weeks after the last dose of study medication, unless transferred to the long‐term ECZTEND trial (NCT03587805).
Prior to randomization, AD treatments were washed out: 4 weeks for systemic treatments and 2 weeks for TCS and other topical treatments. Rescue treatment (Appendix S2) could be used at the discretion of the investigator to control intolerable symptoms and did not disqualify patients from continuing to randomized or open‐label treatment. However, patients who received rescue treatment (including TCS) were considered nonresponders in the primary analyses (see Statistical analysis). Patients were instructed to use an emollient twice daily throughout the trials.
The trials were sponsored by LEO Pharma and conducted in accordance with the ethical principles derived from the Declaration of Helsinki and Good Clinical Practice guidelines and were approved by the local institutional review board or ethics committee of each institution. All patients provided written informed consent. Patients were enrolled from 30 May 2017 to 5 March 2018 in ECZTRA 1 and from 29 June 2017 to 26 April 2018 in ECZTRA 2.
Study population
Patients were ≥ 18 years of age, with a diagnosis of AD for ≥ 1 year, who were candidates for systemic therapy due to a recent (within 1 year) history of inadequate response to treatment with topical treatments or for whom topical treatments were medically inadvisable. Patients were required to have EASI ≥ 12 at screening and ≥ 16 at baseline, an IGA score of ≥ 3 and AD involvement of ≥ 10% of body surface area at screening and baseline, and worst daily pruritus numerical rating scale (NRS) average score ≥ 4 during the week prior to baseline (Appendix S2; see Supporting Information).
Efficacy outcomes
Outcomes were analysed according to a prespecified hierarchy (Figure S2; see Supporting Information). Primary endpoints were IGA score of 0 (clear skin) or 1 (almost clear skin) at week 16 and EASI 75 at week 16.
Key secondary endpoints at week 16 included in the hierarchy were reduction of weekly average worst daily pruritus NRS of ≥ 4 points, change in SCORing Atopic Dermatitis (SCORAD)16 and change in Dermatology Life Quality Index (DLQI),17 all from baseline to week 16.
Maintenance endpoints assessed at week 52 and included in the hierarchy were IGA score of 0 or 1 in patients initially randomized to tralokinumab, with IGA score of 0 or 1 at week 16 achieved without rescue medication (including TCS), and EASI 75 in patients initially randomized to tralokinumab, with EASI 75 at week 16 achieved without rescue medication (including TCS).
Additional secondary endpoints included the proportions of patients achieving ≥ 50% and ≥ 90% improvement in EASI (EASI 50 and EASI 90), change in EASI, change in worst daily pruritus NRS, reduction in worst daily pruritus NRS by ≥ 3 points, change in Patient‐Oriented Eczema Measure (POEM) and reduction in DLQI by ≥ 4 points at week 16.
S. aureus colonization on lesional skin was assessed in ECZTRA 1 and antidrug antibodies were assessed in both studies (Appendix S2; see Supporting Information).
Safety assessments
All adverse events (AEs) were recorded and classified by severity, causality and outcome. Clinical laboratory tests, vital signs and other safety assessments were performed at scheduled visits. A data‐monitoring committee independent of the trial and LEO Pharma reviewed safety data during the study.
Statistical analysis
For the primary endpoint of IGA score of 0 or 1 at week 16, a sample size of 780 patients randomized 3 : 1 would provide > 99% power to detect a target difference between the two arms with a response rate of 30% in the tralokinumab arm and 10% in the placebo arm, with a 5% two‐sided level of significance. For EASI 75 at week 16, a sample size of 780 would provide > 99% power to detect a target difference between tralokinumab and placebo at week 16, assuming EASI 75 response rates of 40% and 15% for tralokinumab and placebo, respectively (Appendix S2; see Supporting Information). To control the overall type 1 error rate at a 5% significance level, a prespecified testing hierarchy was used for assessment of the primary, key secondary and maintenance endpoints (Figure S2; see Supporting Information).
A number of prespecified statistical analyses were conducted for binary and continuous endpoints applying different analytical approaches to handle missing data, rescue medication use and permanent discontinuation (Appendix S2). The primary analytical approach for the binary endpoints considered patients who received rescue medication (including TCS) and patients with missing data to be nonresponders. The difference in response rates between treatment groups was analysed using the Cochran–Mantel–Haenszel test stratified by region and baseline disease severity (IGA score of 3 or 4). An alternative analysis was also applied, where all observed data were used irrespectively of rescue medication use, with missing data imputed as nonresponse.
For the primary analyses of the continuous endpoints, a repeated‐measurements model was used, where data collected after permanent discontinuation or initiation of rescue medication were excluded from the analysis, and the model included all data up to the timepoint of discontinuation or rescue medication use. The model included baseline IGA, region and treatment‐by‐week interaction as factors, and interaction between week and baseline value as a covariate. An unstructured covariance matrix was used to model the within‐patient errors.
For the maintenance endpoints of IGA score of 0 or 1 and EASI 75 at week 52, patients who, prior to week 52, received rescue medication and/or were transferred to open‐label treatment were considered nonresponders. The differences in response rates were analysed using the Cochran–Mantel–Haenszel test stratified by region.
Results
Patients
In total, 802 patients were enrolled in ECZTRA 1 and 794 patients were enrolled in ECZTRA 2 (Figure S3; see Supporting Information). Baseline demographics and disease characteristics were well balanced across treatment groups (Table 1). About half of all patients had severe AD (IGA score of 4). The median EASI at baseline ranged from 28·2 to 30·3, the median disease duration was 25–28 years and the median affected body surface area was approximately 50% in all randomized groups. Almost all patients (98–100%) had a history of TCS use prior to enrolment; systemic corticosteroids had been used by 59·2–69·1% and ciclosporin by 32·3–37·6% (Table S1; see Supporting Information). Discontinuation rates prior to week 16 were similar between treatment groups in ECZTRA 1 but greater in the placebo group in ECZTRA 2 (Figure S4; see Supporting Information). Rescue medication was used in the first 16 weeks by 35·8% and 22·8% of patients receiving tralokinumab in ECZTRA 1 and ECZTRA 2, respectively, and was used at a greater rate in the placebo groups (46·2% and 44·3%, respectively). The majority of patients using rescue medication used TCS, and use of systemic rescue treatment was greater in the placebo groups (Table S2; see Supporting Information). The average time to initiation of rescue medication was shorter in the placebo groups (Figure S4).
Demographics and clinical characteristics of randomized patients at baseline
| ECZTRA 1a | ECZTRA 2b | |||
| Placebo, n = 199 | Tralokinumab Q2W, n = 603 | Placebo, n = 201 | Tralokinumab Q2W, n = 593 | |
| Age (years), median (IQR) | 37·0 (26·0–49·0) | 37·0 (27·0–48·0) | 30·0 (23·0–46·0) | 34·0 (25·0–48·0) |
| Male sex, n (%) | 123 (61·8) | 351 (58·2) | 114 (56·7) | 359 (60·5) |
| Race, n (%) | ||||
| White | 138 (69·3) | 426 (70·6) | 123 (61·2) | 374 (63·1) |
| Black | 18 (9·0) | 41 (6·8) | 17 (8·5) | 43 (7·3) |
| Asian | 40 (20·1) | 120 (19·9) | 52 (25·9) | 154 (26·0) |
| Other or missing data | 3 (1·5) | 16 (2·6) | 9 (4·5) | 22 (3·7) |
| Disease duration (years), median (IQR) | 28·0 (18·0–41·0) | 27·0 (19·0–38·0) | 25·0 (18·0–36·0) | 25·5 (17·0–39·0) |
| Affected BSA (%), median (IQR) | 52·5 (31·0–77·0) | 50·0 (33·0–70·0) | 50·0 (31·0–74·0) | 50·0 (31·0–74·0) |
| EASI, median (IQR) | 30·3 (22·0–41·5) | 28·2 (21·3–40·0) | 29·6 (20·6–41·4) | 28·2 (19·8–40·8) |
| IGA score of 4, n (%) | 102 (51·3) | 305 (50·6) | 101 (50·2) | 286 (48·2) |
| SCORAD score, median (IQR) | 70·8 (63·8–81·0) | 69·2 (61·5–79·1) | 69·9 (61·9–79·1) | 69·5 (60·5–79·1) |
| Weekly average worst daily pruritus NRS, median (IQR) | 7·9 (6·9–8·7) | 7·9 (6·7–8·9) | 8·1 (7·1–9·0) | 8·0 (7·0–9·0) |
| DLQI score, median (IQR) | 16·0 (13·0–22·0) | 17·0 (12·0–22·0) | 18·0 (12·5–24·0) | 18·0 (13·0–23·0) |
| Weekly average eczema‐related sleep interference NRS, median (IQR) | 7·0 (5·7–8·0) | 7·1 (5·7–8·4) | 7·9 (6·4–8·6) | 7·4 (6·2–8·7) |
| POEM score, median (IQR) | 24·0 (20·0–27·0) | 24·0 (20·0–27·0) | 24·0 (20·0–27·5) | 24·0 (20·0–27·0) |
| ECZTRA 1a | ECZTRA 2b | |||
| Placebo, n = 199 | Tralokinumab Q2W, n = 603 | Placebo, n = 201 | Tralokinumab Q2W, n = 593 | |
| Age (years), median (IQR) | 37·0 (26·0–49·0) | 37·0 (27·0–48·0) | 30·0 (23·0–46·0) | 34·0 (25·0–48·0) |
| Male sex, n (%) | 123 (61·8) | 351 (58·2) | 114 (56·7) | 359 (60·5) |
| Race, n (%) | ||||
| White | 138 (69·3) | 426 (70·6) | 123 (61·2) | 374 (63·1) |
| Black | 18 (9·0) | 41 (6·8) | 17 (8·5) | 43 (7·3) |
| Asian | 40 (20·1) | 120 (19·9) | 52 (25·9) | 154 (26·0) |
| Other or missing data | 3 (1·5) | 16 (2·6) | 9 (4·5) | 22 (3·7) |
| Disease duration (years), median (IQR) | 28·0 (18·0–41·0) | 27·0 (19·0–38·0) | 25·0 (18·0–36·0) | 25·5 (17·0–39·0) |
| Affected BSA (%), median (IQR) | 52·5 (31·0–77·0) | 50·0 (33·0–70·0) | 50·0 (31·0–74·0) | 50·0 (31·0–74·0) |
| EASI, median (IQR) | 30·3 (22·0–41·5) | 28·2 (21·3–40·0) | 29·6 (20·6–41·4) | 28·2 (19·8–40·8) |
| IGA score of 4, n (%) | 102 (51·3) | 305 (50·6) | 101 (50·2) | 286 (48·2) |
| SCORAD score, median (IQR) | 70·8 (63·8–81·0) | 69·2 (61·5–79·1) | 69·9 (61·9–79·1) | 69·5 (60·5–79·1) |
| Weekly average worst daily pruritus NRS, median (IQR) | 7·9 (6·9–8·7) | 7·9 (6·7–8·9) | 8·1 (7·1–9·0) | 8·0 (7·0–9·0) |
| DLQI score, median (IQR) | 16·0 (13·0–22·0) | 17·0 (12·0–22·0) | 18·0 (12·5–24·0) | 18·0 (13·0–23·0) |
| Weekly average eczema‐related sleep interference NRS, median (IQR) | 7·0 (5·7–8·0) | 7·1 (5·7–8·4) | 7·9 (6·4–8·6) | 7·4 (6·2–8·7) |
| POEM score, median (IQR) | 24·0 (20·0–27·0) | 24·0 (20·0–27·0) | 24·0 (20·0–27·5) | 24·0 (20·0–27·0) |
Demographics and clinical characteristics of randomized patients at baseline
| ECZTRA 1a | ECZTRA 2b | |||
| Placebo, n = 199 | Tralokinumab Q2W, n = 603 | Placebo, n = 201 | Tralokinumab Q2W, n = 593 | |
| Age (years), median (IQR) | 37·0 (26·0–49·0) | 37·0 (27·0–48·0) | 30·0 (23·0–46·0) | 34·0 (25·0–48·0) |
| Male sex, n (%) | 123 (61·8) | 351 (58·2) | 114 (56·7) | 359 (60·5) |
| Race, n (%) | ||||
| White | 138 (69·3) | 426 (70·6) | 123 (61·2) | 374 (63·1) |
| Black | 18 (9·0) | 41 (6·8) | 17 (8·5) | 43 (7·3) |
| Asian | 40 (20·1) | 120 (19·9) | 52 (25·9) | 154 (26·0) |
| Other or missing data | 3 (1·5) | 16 (2·6) | 9 (4·5) | 22 (3·7) |
| Disease duration (years), median (IQR) | 28·0 (18·0–41·0) | 27·0 (19·0–38·0) | 25·0 (18·0–36·0) | 25·5 (17·0–39·0) |
| Affected BSA (%), median (IQR) | 52·5 (31·0–77·0) | 50·0 (33·0–70·0) | 50·0 (31·0–74·0) | 50·0 (31·0–74·0) |
| EASI, median (IQR) | 30·3 (22·0–41·5) | 28·2 (21·3–40·0) | 29·6 (20·6–41·4) | 28·2 (19·8–40·8) |
| IGA score of 4, n (%) | 102 (51·3) | 305 (50·6) | 101 (50·2) | 286 (48·2) |
| SCORAD score, median (IQR) | 70·8 (63·8–81·0) | 69·2 (61·5–79·1) | 69·9 (61·9–79·1) | 69·5 (60·5–79·1) |
| Weekly average worst daily pruritus NRS, median (IQR) | 7·9 (6·9–8·7) | 7·9 (6·7–8·9) | 8·1 (7·1–9·0) | 8·0 (7·0–9·0) |
| DLQI score, median (IQR) | 16·0 (13·0–22·0) | 17·0 (12·0–22·0) | 18·0 (12·5–24·0) | 18·0 (13·0–23·0) |
| Weekly average eczema‐related sleep interference NRS, median (IQR) | 7·0 (5·7–8·0) | 7·1 (5·7–8·4) | 7·9 (6·4–8·6) | 7·4 (6·2–8·7) |
| POEM score, median (IQR) | 24·0 (20·0–27·0) | 24·0 (20·0–27·0) | 24·0 (20·0–27·5) | 24·0 (20·0–27·0) |
| ECZTRA 1a | ECZTRA 2b | |||
| Placebo, n = 199 | Tralokinumab Q2W, n = 603 | Placebo, n = 201 | Tralokinumab Q2W, n = 593 | |
| Age (years), median (IQR) | 37·0 (26·0–49·0) | 37·0 (27·0–48·0) | 30·0 (23·0–46·0) | 34·0 (25·0–48·0) |
| Male sex, n (%) | 123 (61·8) | 351 (58·2) | 114 (56·7) | 359 (60·5) |
| Race, n (%) | ||||
| White | 138 (69·3) | 426 (70·6) | 123 (61·2) | 374 (63·1) |
| Black | 18 (9·0) | 41 (6·8) | 17 (8·5) | 43 (7·3) |
| Asian | 40 (20·1) | 120 (19·9) | 52 (25·9) | 154 (26·0) |
| Other or missing data | 3 (1·5) | 16 (2·6) | 9 (4·5) | 22 (3·7) |
| Disease duration (years), median (IQR) | 28·0 (18·0–41·0) | 27·0 (19·0–38·0) | 25·0 (18·0–36·0) | 25·5 (17·0–39·0) |
| Affected BSA (%), median (IQR) | 52·5 (31·0–77·0) | 50·0 (33·0–70·0) | 50·0 (31·0–74·0) | 50·0 (31·0–74·0) |
| EASI, median (IQR) | 30·3 (22·0–41·5) | 28·2 (21·3–40·0) | 29·6 (20·6–41·4) | 28·2 (19·8–40·8) |
| IGA score of 4, n (%) | 102 (51·3) | 305 (50·6) | 101 (50·2) | 286 (48·2) |
| SCORAD score, median (IQR) | 70·8 (63·8–81·0) | 69·2 (61·5–79·1) | 69·9 (61·9–79·1) | 69·5 (60·5–79·1) |
| Weekly average worst daily pruritus NRS, median (IQR) | 7·9 (6·9–8·7) | 7·9 (6·7–8·9) | 8·1 (7·1–9·0) | 8·0 (7·0–9·0) |
| DLQI score, median (IQR) | 16·0 (13·0–22·0) | 17·0 (12·0–22·0) | 18·0 (12·5–24·0) | 18·0 (13·0–23·0) |
| Weekly average eczema‐related sleep interference NRS, median (IQR) | 7·0 (5·7–8·0) | 7·1 (5·7–8·4) | 7·9 (6·4–8·6) | 7·4 (6·2–8·7) |
| POEM score, median (IQR) | 24·0 (20·0–27·0) | 24·0 (20·0–27·0) | 24·0 (20·0–27·5) | 24·0 (20·0–27·0) |
Primary outcomes
In both studies, achievement of an IGA score of 0 or 1 and EASI 75 at week 16 was significantly higher with tralokinumab vs. placebo (Table 2). IGA 0 or 1 was achieved by 15·8% with tralokinumab vs. 7·1% with placebo in ECZTRA 1 [difference 8·6%, 95% confidence interval (CI) 4·1–13·1, P = 0·002] and by 22·2% with tralokinumab vs. 10·9% with placebo in ECZTRA 2 (difference 11·1%, 95% CI 5·8–16·4; P < 0·001). These results are depicted in Figure 1(a), Table 2 and Table S3 (see Supporting Information).
Efficacy outcomes for the initial treatment period: primary and key secondary endpoints, full analysis set
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Primary endpoints | ||||||
| IGA score of 0 or 1 at week 16, n (%)a | 14/197 (7·1) | 95/601 (15·8) | 8·6 (4·1 to 13·1);bP = 0·002c | 22/201 (10·9) | 131/591 (22·2) | 11·1 (5·8 to 16·4);bP < 0·001c |
| EASI 75 at week 16, n (%)a | 25/197 (12·7) | 150/601 (25·0) | 12·1 (6·5 to 17·7);bP < 0·001c | 23/201 (11·4) | 196/591 (33·2) | 21·6 (15·8 to 27·3);bP < 0·001c |
| Key secondary endpoints | ||||||
| Improvement in weekly average of worst daily pruritus NRS ≥ 4 points from baseline to week 16, n/N (%)a,d | 20/194 (10·3) | 119/594 (20·0) | 9·7 (4·4 to 15·0);bP = 0·002c | 19/200 (9·5) | 144/575 (25·0) | 15·6 (10·3 to 20·9);bP < 0·001c |
| Adjusted mean change (SE) from baseline in SCORAD score at week 16e | –14·7 (1·80) | –25·2 (0·94) | −10·4 (−14·4 to −6·5); P < 0·001 | −14·0 (1·79) | −28·1 (0·92) | −14·0 (−18·0 to −10·1); P < 0·001 |
| Adjusted mean change (SE) from baseline in DLQI score at week 16e | –5·0 (0·59) | –7·1 (0·31) | −2·1 (−3·4 to −0·8); P = 0·002 | −4·9 (0·60) | −8·8 (0·30) | −3·9 (−5·2 to −2·6); P < 0·001 |
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Primary endpoints | ||||||
| IGA score of 0 or 1 at week 16, n (%)a | 14/197 (7·1) | 95/601 (15·8) | 8·6 (4·1 to 13·1);bP = 0·002c | 22/201 (10·9) | 131/591 (22·2) | 11·1 (5·8 to 16·4);bP < 0·001c |
| EASI 75 at week 16, n (%)a | 25/197 (12·7) | 150/601 (25·0) | 12·1 (6·5 to 17·7);bP < 0·001c | 23/201 (11·4) | 196/591 (33·2) | 21·6 (15·8 to 27·3);bP < 0·001c |
| Key secondary endpoints | ||||||
| Improvement in weekly average of worst daily pruritus NRS ≥ 4 points from baseline to week 16, n/N (%)a,d | 20/194 (10·3) | 119/594 (20·0) | 9·7 (4·4 to 15·0);bP = 0·002c | 19/200 (9·5) | 144/575 (25·0) | 15·6 (10·3 to 20·9);bP < 0·001c |
| Adjusted mean change (SE) from baseline in SCORAD score at week 16e | –14·7 (1·80) | –25·2 (0·94) | −10·4 (−14·4 to −6·5); P < 0·001 | −14·0 (1·79) | −28·1 (0·92) | −14·0 (−18·0 to −10·1); P < 0·001 |
| Adjusted mean change (SE) from baseline in DLQI score at week 16e | –5·0 (0·59) | –7·1 (0·31) | −2·1 (−3·4 to −0·8); P = 0·002 | −4·9 (0·60) | −8·8 (0·30) | −3·9 (−5·2 to −2·6); P < 0·001 |
Efficacy outcomes for the initial treatment period: primary and key secondary endpoints, full analysis set
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Primary endpoints | ||||||
| IGA score of 0 or 1 at week 16, n (%)a | 14/197 (7·1) | 95/601 (15·8) | 8·6 (4·1 to 13·1);bP = 0·002c | 22/201 (10·9) | 131/591 (22·2) | 11·1 (5·8 to 16·4);bP < 0·001c |
| EASI 75 at week 16, n (%)a | 25/197 (12·7) | 150/601 (25·0) | 12·1 (6·5 to 17·7);bP < 0·001c | 23/201 (11·4) | 196/591 (33·2) | 21·6 (15·8 to 27·3);bP < 0·001c |
| Key secondary endpoints | ||||||
| Improvement in weekly average of worst daily pruritus NRS ≥ 4 points from baseline to week 16, n/N (%)a,d | 20/194 (10·3) | 119/594 (20·0) | 9·7 (4·4 to 15·0);bP = 0·002c | 19/200 (9·5) | 144/575 (25·0) | 15·6 (10·3 to 20·9);bP < 0·001c |
| Adjusted mean change (SE) from baseline in SCORAD score at week 16e | –14·7 (1·80) | –25·2 (0·94) | −10·4 (−14·4 to −6·5); P < 0·001 | −14·0 (1·79) | −28·1 (0·92) | −14·0 (−18·0 to −10·1); P < 0·001 |
| Adjusted mean change (SE) from baseline in DLQI score at week 16e | –5·0 (0·59) | –7·1 (0·31) | −2·1 (−3·4 to −0·8); P = 0·002 | −4·9 (0·60) | −8·8 (0·30) | −3·9 (−5·2 to −2·6); P < 0·001 |
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Primary endpoints | ||||||
| IGA score of 0 or 1 at week 16, n (%)a | 14/197 (7·1) | 95/601 (15·8) | 8·6 (4·1 to 13·1);bP = 0·002c | 22/201 (10·9) | 131/591 (22·2) | 11·1 (5·8 to 16·4);bP < 0·001c |
| EASI 75 at week 16, n (%)a | 25/197 (12·7) | 150/601 (25·0) | 12·1 (6·5 to 17·7);bP < 0·001c | 23/201 (11·4) | 196/591 (33·2) | 21·6 (15·8 to 27·3);bP < 0·001c |
| Key secondary endpoints | ||||||
| Improvement in weekly average of worst daily pruritus NRS ≥ 4 points from baseline to week 16, n/N (%)a,d | 20/194 (10·3) | 119/594 (20·0) | 9·7 (4·4 to 15·0);bP = 0·002c | 19/200 (9·5) | 144/575 (25·0) | 15·6 (10·3 to 20·9);bP < 0·001c |
| Adjusted mean change (SE) from baseline in SCORAD score at week 16e | –14·7 (1·80) | –25·2 (0·94) | −10·4 (−14·4 to −6·5); P < 0·001 | −14·0 (1·79) | −28·1 (0·92) | −14·0 (−18·0 to −10·1); P < 0·001 |
| Adjusted mean change (SE) from baseline in DLQI score at week 16e | –5·0 (0·59) | –7·1 (0·31) | −2·1 (−3·4 to −0·8); P = 0·002 | −4·9 (0·60) | −8·8 (0·30) | −3·9 (−5·2 to −2·6); P < 0·001 |
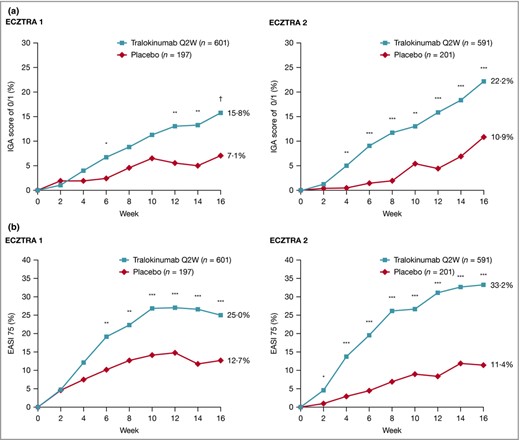
Achievement of (a) Investigator’s Global Assessment (IGA) score of 0 or 1 and (b) ≥ 75% improvement in Eczema Area and Severity Index (EASI 75) in the 16‐week initial treatment period in ECZTRA 1 and ECZTRA 2. Patients who received rescue medication were considered nonresponders. Patients with missing data at week 16 were imputed as nonresponders. The Cochran–Mantel–Haenszel test was used, stratified by region and baseline IGA. *P < 0·05 vs. placebo, **P < 0·01 vs. placebo, ***P < 0·001 vs. placebo. †P = 0.002 vs. placebo. Q2W, every 2 weeks.
EASI 75 was achieved by 25·0% with tralokinumab vs. 12·7% with placebo in ECZTRA 1 (difference 12·1%, 95% CI 6·5–17·7; P < 0·001) and by 33·2% with tralokinumab vs. 11·4% with placebo in ECZTRA 2 (difference 21·6%, 95% CI 15·8–27·3; P < 0·001), as shown in Figure 1(b), Table 2 and Table S4 (see Supporting Information). Following an alternative analysis, irrespectively of rescue medication use, response rates were improved for all treatment arms in both studies (Table S5; see Supporting Information).
Key secondary outcomes at week 16
Tralokinumab showed a significant improvement vs. placebo in all key secondary endpoints. Reduction of weekly average worst daily pruritus NRS by ≥ 4 points from baseline to week 16 was achieved by 20·0% with tralokinumab vs. 10·3% with placebo in ECZTRA 1 (difference 9·7%, 95% CI 4·4–15·0; P = 0·002; Table 2) and by 25·0% vs. 9·5% in ECZTRA 2 (difference 15·6%, 95% CI 10·3–20·9; P < 0·001; Table 2). The adjusted mean changes in SCORAD from baseline to week 16 were −25·2 vs. −14·7 in ECZTRA 1 (difference −10·4%, 95% CI −14·4 to −6·5; P < 0·001; Table 2; and Tables S6 and S7; see Supporting Information) and −28·1 vs. −14·0 in ECZTRA 2 (difference −14·0%, 95% CI −18·0 to −10·1; P < 0·001; Table 2; and Tables S6 and S7; see Supporting Information)
The adjusted mean changes in DLQI from baseline to week 16 were −7·1 for tralokinumab vs. −5·0 for placebo in ECZTRA 1 (difference −2·1%, 95% CI −3·4 to −0·8; P = 0·002; Table 2; and Tables S8 and S9; see Supporting Information) and −8·8 vs. −4·9 in ECZTRA 2 (difference −3·9%, 95% CI −5·2 to −2·6; P < 0·001; Table 2; and Tables S8 and S9; see Supporting Information).
Significant improvements in favour of tralokinumab were observed using the primary and alternative analytical approaches (Tables S10 and S11; see Supporting Information).
Maintenance outcomes at week 52
In ECZTRA 1 and ECZTRA 2, 185 and 227 patients were rerandomized 2 : 2 : 1 to continue tralokinumab Q2W, to reduce the dosing frequency of tralokinumab to Q4W or to switch to placebo Q2W (Figure S3; see Supporting Information). In patients who achieved IGA 0 or 1 with tralokinumab at week 16, IGA 0 or 1 was maintained at week 52 without rescue medication (including TCS) in 51% with continued tralokinumab Q2W vs. 47% with tralokinumab Q2W to placebo (difference 6·0%, 95% CI −21·8 to 33·7; P = 0·68) in ECZTRA 1 and in 59% with continued tralokinumab Q2W vs. 25% with tralokinumab Q2W to placebo (difference 34·1%, 95% CI 13·4–54·9; P = 0·004) in ECZTRA 2 (Figure 2a and Table 3).
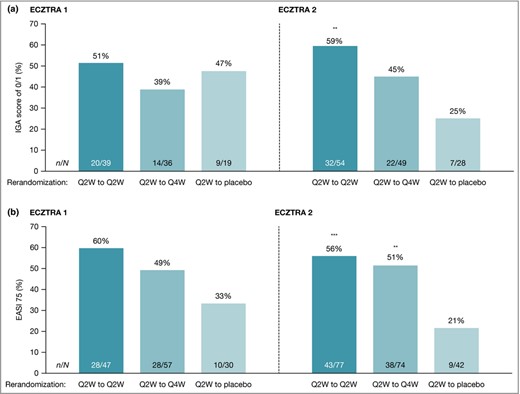
Maintenance of (a) Investigator’s Global Assessment (IGA) score of 0 or 1 and (b) ≥ 75% improvement in Eczema Area and Severity Index (EASI 75) clinical response at week 52 in ECZTRA 1 and ECZTRA 2. Patients who, after week 16, received rescue medication or were transferred to open‐label treatment were considered nonresponders at week 52. Missing values were imputed as nonresponse. The Cochran–Mantel–Haenszel test was used, stratified by region. Maintenance of IGA 0 or 1 was assessed in patients achieving the week 16 primary outcome of IGA score of 0 or 1 without use of rescue medication after initial randomization to tralokinumab. EASI 75 was assessed in patients achieving the week 16 primary outcome of EASI 75 without use of rescue medication after initial randomization to tralokinumab. **P < 0·01, vs. placebo ***P < 0·001 vs. placebo. Q2W, every 2 weeks; Q4W, every 4 weeks.
Efficacy outcomes at week 52 following the maintenance treatment period: maintenance analysis set
| Rerandomization at week 16 | ECZTRA 1 | ECZTRA 2 | ||||
| Tralokinumab Q2W to placebo, n = 35 | Tralokinumab Q2W to Q2W, n = 68 | Tralokinumab Q2W to Q4W, n = 76 | Tralokinumab Q2W to placebo, n = 46 | Tralokinumab Q2W to Q2W, n = 91 | Tralokinumab Q2W to Q4W, n = 89 | |
| IGA score of 0 or 1 at week 52, n/N (%)a,b | 9/19 (47) | 20/39 (51) | 14/36 (39) | 7/28 (25) | 32/54 (59) | 22/49 (45) |
| Difference in percentage vs. placebo (95% CI)c | 6·0 (−21·8 to 33·7); P = 0·68d | −9·5 (−37·1 to 18·0); P = 0·50d | 34·1 (13·4 to 54·9); P = 0·004d | 19·9 (−1·2 to 40·9); P = 0·084d | ||
| EASI 75 at week 52, n/N (%)b,e | 10/30 (33) | 28/47 (60) | 28/57 (49) | 9/42 (21) | 43/77 (56) | 38/74 (51) |
| Difference in percentage vs. placebo (95% CI)c | 21·2 (−0·2 to 42·6); P = 0·056d | 11·7 (−8·7 to 32·0); P = 0·27d | 33·7 (17·3 to 50·0); P < 0·001d | 30·0 (13·7 to 46·4); P = 0·001d | ||
| Rerandomization at week 16 | ECZTRA 1 | ECZTRA 2 | ||||
| Tralokinumab Q2W to placebo, n = 35 | Tralokinumab Q2W to Q2W, n = 68 | Tralokinumab Q2W to Q4W, n = 76 | Tralokinumab Q2W to placebo, n = 46 | Tralokinumab Q2W to Q2W, n = 91 | Tralokinumab Q2W to Q4W, n = 89 | |
| IGA score of 0 or 1 at week 52, n/N (%)a,b | 9/19 (47) | 20/39 (51) | 14/36 (39) | 7/28 (25) | 32/54 (59) | 22/49 (45) |
| Difference in percentage vs. placebo (95% CI)c | 6·0 (−21·8 to 33·7); P = 0·68d | −9·5 (−37·1 to 18·0); P = 0·50d | 34·1 (13·4 to 54·9); P = 0·004d | 19·9 (−1·2 to 40·9); P = 0·084d | ||
| EASI 75 at week 52, n/N (%)b,e | 10/30 (33) | 28/47 (60) | 28/57 (49) | 9/42 (21) | 43/77 (56) | 38/74 (51) |
| Difference in percentage vs. placebo (95% CI)c | 21·2 (−0·2 to 42·6); P = 0·056d | 11·7 (−8·7 to 32·0); P = 0·27d | 33·7 (17·3 to 50·0); P < 0·001d | 30·0 (13·7 to 46·4); P = 0·001d | ||
Efficacy outcomes at week 52 following the maintenance treatment period: maintenance analysis set
| Rerandomization at week 16 | ECZTRA 1 | ECZTRA 2 | ||||
| Tralokinumab Q2W to placebo, n = 35 | Tralokinumab Q2W to Q2W, n = 68 | Tralokinumab Q2W to Q4W, n = 76 | Tralokinumab Q2W to placebo, n = 46 | Tralokinumab Q2W to Q2W, n = 91 | Tralokinumab Q2W to Q4W, n = 89 | |
| IGA score of 0 or 1 at week 52, n/N (%)a,b | 9/19 (47) | 20/39 (51) | 14/36 (39) | 7/28 (25) | 32/54 (59) | 22/49 (45) |
| Difference in percentage vs. placebo (95% CI)c | 6·0 (−21·8 to 33·7); P = 0·68d | −9·5 (−37·1 to 18·0); P = 0·50d | 34·1 (13·4 to 54·9); P = 0·004d | 19·9 (−1·2 to 40·9); P = 0·084d | ||
| EASI 75 at week 52, n/N (%)b,e | 10/30 (33) | 28/47 (60) | 28/57 (49) | 9/42 (21) | 43/77 (56) | 38/74 (51) |
| Difference in percentage vs. placebo (95% CI)c | 21·2 (−0·2 to 42·6); P = 0·056d | 11·7 (−8·7 to 32·0); P = 0·27d | 33·7 (17·3 to 50·0); P < 0·001d | 30·0 (13·7 to 46·4); P = 0·001d | ||
| Rerandomization at week 16 | ECZTRA 1 | ECZTRA 2 | ||||
| Tralokinumab Q2W to placebo, n = 35 | Tralokinumab Q2W to Q2W, n = 68 | Tralokinumab Q2W to Q4W, n = 76 | Tralokinumab Q2W to placebo, n = 46 | Tralokinumab Q2W to Q2W, n = 91 | Tralokinumab Q2W to Q4W, n = 89 | |
| IGA score of 0 or 1 at week 52, n/N (%)a,b | 9/19 (47) | 20/39 (51) | 14/36 (39) | 7/28 (25) | 32/54 (59) | 22/49 (45) |
| Difference in percentage vs. placebo (95% CI)c | 6·0 (−21·8 to 33·7); P = 0·68d | −9·5 (−37·1 to 18·0); P = 0·50d | 34·1 (13·4 to 54·9); P = 0·004d | 19·9 (−1·2 to 40·9); P = 0·084d | ||
| EASI 75 at week 52, n/N (%)b,e | 10/30 (33) | 28/47 (60) | 28/57 (49) | 9/42 (21) | 43/77 (56) | 38/74 (51) |
| Difference in percentage vs. placebo (95% CI)c | 21·2 (−0·2 to 42·6); P = 0·056d | 11·7 (−8·7 to 32·0); P = 0·27d | 33·7 (17·3 to 50·0); P < 0·001d | 30·0 (13·7 to 46·4); P = 0·001d | ||
For the other maintenance‐phase endpoints in the testing hierarchy, EASI 75 at week 52 was maintained by 60% with continued tralokinumab Q2W vs. 33% with tralokinumab Q2W to placebo (difference 21·2%, 95% CI −0·2 to 42·6; P = 0·056) in ECZTRA 1 and 56% with continued tralokinumab Q2W vs. 21% with tralokinumab Q2W to placebo (difference 33·7%, 95% CI 17·3–50·0; P < 0·001) in ECZTRA 2 (Figure 2b and Table 3).
The percentage of patients who maintained IGA 0 or 1 at week 52 when rerandomized to tralokinumab Q4W was 39% in ECZTRA 1 (difference vs. tralokinumab Q2W to placebo −9·5%, 95% CI −37·1 to 18·0; P = 0·50) and 45% in ECZTRA 2 (difference 19·9%, 95% CI –1·2 to 40·9; P = 0·084) (Figure 2a and Table 3). For EASI 75 the proportion with tralokinumab Q2W to Q4W was 49% (difference vs. tralokinumab Q2W to placebo 11·7%, 95% CI −8·7 to 32·0; P = 0·27) in ECZTRA 1 and 51% (difference 30·0%, 95% CI 13·7–46·4; P = 0·001) in ECZTRA 2 (Figure 2b and Table 3).
Additional efficacy analyses
Changes from baseline in SCORAD, worst daily pruritus NRS, POEM and DLQI were greater with tralokinumab vs. placebo throughout the initial 16 weeks, and separation between tralokinumab and placebo was observed from week 1 onwards for weekly average worst daily pruritus NRS (P < 0·001) and from week 2 onwards for SCORAD (P < 0·001) and DLQI (P < 0·01) (Table 4; and Figure S5; see Supporting Information).
Efficacy outcomes for the initial treatment period: other endpoints, full analysis set
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Adjusted mean change (SE) from baseline in SCORAD score at week 2a | −5·0 (0·92) | −10·6 (0·53) | −5·6 (−7·7 to − 3·5); P < 0·001 | −3·9 (0·84) | −10·8 (0·49) | −6·9 (−8·8 to − 5·0); P < 0·001 |
| Adjusted mean change (SE) from baseline in weekly average of worst daily pruritus NRS at week 1a | −0·2 (0·07) | −0·7 (0·04) | −0·4 (−0·6 to − 0·3); P < 0·001) | −0·3 (0·08) | −0·7 (0·05) | −0·4 (−0·6 to −0·2); P < 0·001 |
| Adjusted mean change (SE) from baseline in DLQI at week 2a | −2·5 (0·39) | −4·4 (0·22) | −2·0 (−2·8 to − 1·1); P < 0·001 | −2·2 (0·39) | −4·7 (0·23) | −2·5 (−3·4 to −1·7); P < 0·001 |
| Adjusted mean change (SE) from baseline in weekly average of eczema‐related sleep interference NRS at week 16a | −1·9 (0·23) | −2·6 (0·12) | −0·7 (−1·2 to −0·2); P = 0·007 | −1·5 (0·22) | −2·9 (0·12) | −1·4 (−1·9 to −0·9); P < 0·001 |
| Adjusted mean change (SE) from baseline in POEM score at week 16a | −3·0 (0·66) | −7·6 (0·35) | −4·6 (−6·0 to −3·1); P < 0·001 | −3·7 (0·66) | −8·8 (0·33) | −5·1 (−6·5 to −3·6); P < 0·001 |
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Adjusted mean change (SE) from baseline in SCORAD score at week 2a | −5·0 (0·92) | −10·6 (0·53) | −5·6 (−7·7 to − 3·5); P < 0·001 | −3·9 (0·84) | −10·8 (0·49) | −6·9 (−8·8 to − 5·0); P < 0·001 |
| Adjusted mean change (SE) from baseline in weekly average of worst daily pruritus NRS at week 1a | −0·2 (0·07) | −0·7 (0·04) | −0·4 (−0·6 to − 0·3); P < 0·001) | −0·3 (0·08) | −0·7 (0·05) | −0·4 (−0·6 to −0·2); P < 0·001 |
| Adjusted mean change (SE) from baseline in DLQI at week 2a | −2·5 (0·39) | −4·4 (0·22) | −2·0 (−2·8 to − 1·1); P < 0·001 | −2·2 (0·39) | −4·7 (0·23) | −2·5 (−3·4 to −1·7); P < 0·001 |
| Adjusted mean change (SE) from baseline in weekly average of eczema‐related sleep interference NRS at week 16a | −1·9 (0·23) | −2·6 (0·12) | −0·7 (−1·2 to −0·2); P = 0·007 | −1·5 (0·22) | −2·9 (0·12) | −1·4 (−1·9 to −0·9); P < 0·001 |
| Adjusted mean change (SE) from baseline in POEM score at week 16a | −3·0 (0·66) | −7·6 (0·35) | −4·6 (−6·0 to −3·1); P < 0·001 | −3·7 (0·66) | −8·8 (0·33) | −5·1 (−6·5 to −3·6); P < 0·001 |
Efficacy outcomes for the initial treatment period: other endpoints, full analysis set
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Adjusted mean change (SE) from baseline in SCORAD score at week 2a | −5·0 (0·92) | −10·6 (0·53) | −5·6 (−7·7 to − 3·5); P < 0·001 | −3·9 (0·84) | −10·8 (0·49) | −6·9 (−8·8 to − 5·0); P < 0·001 |
| Adjusted mean change (SE) from baseline in weekly average of worst daily pruritus NRS at week 1a | −0·2 (0·07) | −0·7 (0·04) | −0·4 (−0·6 to − 0·3); P < 0·001) | −0·3 (0·08) | −0·7 (0·05) | −0·4 (−0·6 to −0·2); P < 0·001 |
| Adjusted mean change (SE) from baseline in DLQI at week 2a | −2·5 (0·39) | −4·4 (0·22) | −2·0 (−2·8 to − 1·1); P < 0·001 | −2·2 (0·39) | −4·7 (0·23) | −2·5 (−3·4 to −1·7); P < 0·001 |
| Adjusted mean change (SE) from baseline in weekly average of eczema‐related sleep interference NRS at week 16a | −1·9 (0·23) | −2·6 (0·12) | −0·7 (−1·2 to −0·2); P = 0·007 | −1·5 (0·22) | −2·9 (0·12) | −1·4 (−1·9 to −0·9); P < 0·001 |
| Adjusted mean change (SE) from baseline in POEM score at week 16a | −3·0 (0·66) | −7·6 (0·35) | −4·6 (−6·0 to −3·1); P < 0·001 | −3·7 (0·66) | −8·8 (0·33) | −5·1 (−6·5 to −3·6); P < 0·001 |
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Adjusted mean change (SE) from baseline in SCORAD score at week 2a | −5·0 (0·92) | −10·6 (0·53) | −5·6 (−7·7 to − 3·5); P < 0·001 | −3·9 (0·84) | −10·8 (0·49) | −6·9 (−8·8 to − 5·0); P < 0·001 |
| Adjusted mean change (SE) from baseline in weekly average of worst daily pruritus NRS at week 1a | −0·2 (0·07) | −0·7 (0·04) | −0·4 (−0·6 to − 0·3); P < 0·001) | −0·3 (0·08) | −0·7 (0·05) | −0·4 (−0·6 to −0·2); P < 0·001 |
| Adjusted mean change (SE) from baseline in DLQI at week 2a | −2·5 (0·39) | −4·4 (0·22) | −2·0 (−2·8 to − 1·1); P < 0·001 | −2·2 (0·39) | −4·7 (0·23) | −2·5 (−3·4 to −1·7); P < 0·001 |
| Adjusted mean change (SE) from baseline in weekly average of eczema‐related sleep interference NRS at week 16a | −1·9 (0·23) | −2·6 (0·12) | −0·7 (−1·2 to −0·2); P = 0·007 | −1·5 (0·22) | −2·9 (0·12) | −1·4 (−1·9 to −0·9); P < 0·001 |
| Adjusted mean change (SE) from baseline in POEM score at week 16a | −3·0 (0·66) | −7·6 (0·35) | −4·6 (−6·0 to −3·1); P < 0·001 | −3·7 (0·66) | −8·8 (0·33) | −5·1 (−6·5 to −3·6); P < 0·001 |
A greater reduction in weekly average eczema‐related sleep interference was observed with tralokinumab vs. placebo from week 1 onwards (P < 0·01) in both studies (Figure S5; see Supporting Information). The difference between tralokinumab and placebo in the adjusted mean change from baseline in eczema‐related sleep interference at week 16 was −0·7 in ECZTRA 1 and −1·4 in ECZTRA 2 (Table 4).
In both studies, more patients achieved EASI 50 and EASI 90 with tralokinumab vs. placebo at week 16 (Table 5; and Figure S6; see Supporting Information) and EASI 50 at each scheduled assessment (P < 0·01) from week 2. EASI 90 response was greater than with placebo from week 4 to week 16, with a separation between arms (P < 0·01) from week 6. A greater percentage change in EASI was observed with tralokinumab vs. placebo at week 16, with a separation between arms (P < 0·001) from week 2 onwards (Table 5, Figure 3; and Figure S7; see Supporting Information).
Efficacy outcomes for the initial treatment period: additional secondary endpoints, full analysis set
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Reduction of weekly average of worst daily pruritus NRS ≥ 3 at week 16, n/N (%)a,b | 28/195 (14·4) | 177/597 (29·6) | 15·2 (9·2 to 21·3);cP < 0·001d | 28/200 (14·0) | 199/583 (34·1) | 20·1 (13·9 to 26·2);cP < 0·001d |
| Adjusted mean change (SE) from baseline in weekly average of worst daily pruritus NRS at week 16e | –1·7 (0·21) | –2·6 (0·11) | −0·9 (−1·4 to − 0·4); P < 0·001 | −1·6 (0·21) | −2·9 (0·11) | −1·3 (−1·7 to −0·8); P < 0·001 |
| SCORAD score of 75 at week 16, n/N (%)a | 6/197 (3·0) | 53/601 (8·8) | 5·7 (2·5 to 8·9);cP = 0·007d | 7/201 (3·5) | 68/591 (11·5) | 8·0 (4·4 to 11·6);cP < 0·001d |
| SCORAD score of 50 at week 16, n/N (%)a | 23/197 (11·7) | 156/601 (26·0) | 14·1 (8·6 to 19·6);cP < 0·001d | 29/201 (14·4) | 198/591 (33·5) | 18·9 (12·8 to 25·1);cP < 0·001d |
| DLQI score reduction ≥ 4 at week 16, n/N (%)a,f | 60/190 (31·6) | 258/578 (44·6) | 13·0 (5·4 to 20·5);cP = 0·001d | 54/198 (27·3) | 325/577 (56·3) | 28·9 (21·4 to 36·3);cP < 0·001d |
| Adjusted mean change (SE) from baseline in EASI at week 16e | –9·0 (1·05) | –15·5 (0·55) | −6·4 (−8·8 to − 4·1); P < 0·001 | −7·0 (1·06) | −16·9 (0·55) | −9·9 (−12·2 to −7·5); P < 0·001 |
| EASI 50 at week 16, n/N (%)a | 42/197 (21·3) | 250/601 (41·6) | 20·1 (13·3 to 26·8);cP < 0·001d | 41/201 (20·4) | 295/591 (49·9) | 29·3 (22·5 to 36·1);cP < 0·001d |
| EASI 90 at week 16, n/N (%)a | 8/197 (4·1) | 87/601 (14·5) | 10·3 (6·4 to 14·1);cP < 0·001d | 11/201 (5·5) | 108/591 (18·3) | 12·7 (8·3 to 17·0);cP < 0·001d |
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Reduction of weekly average of worst daily pruritus NRS ≥ 3 at week 16, n/N (%)a,b | 28/195 (14·4) | 177/597 (29·6) | 15·2 (9·2 to 21·3);cP < 0·001d | 28/200 (14·0) | 199/583 (34·1) | 20·1 (13·9 to 26·2);cP < 0·001d |
| Adjusted mean change (SE) from baseline in weekly average of worst daily pruritus NRS at week 16e | –1·7 (0·21) | –2·6 (0·11) | −0·9 (−1·4 to − 0·4); P < 0·001 | −1·6 (0·21) | −2·9 (0·11) | −1·3 (−1·7 to −0·8); P < 0·001 |
| SCORAD score of 75 at week 16, n/N (%)a | 6/197 (3·0) | 53/601 (8·8) | 5·7 (2·5 to 8·9);cP = 0·007d | 7/201 (3·5) | 68/591 (11·5) | 8·0 (4·4 to 11·6);cP < 0·001d |
| SCORAD score of 50 at week 16, n/N (%)a | 23/197 (11·7) | 156/601 (26·0) | 14·1 (8·6 to 19·6);cP < 0·001d | 29/201 (14·4) | 198/591 (33·5) | 18·9 (12·8 to 25·1);cP < 0·001d |
| DLQI score reduction ≥ 4 at week 16, n/N (%)a,f | 60/190 (31·6) | 258/578 (44·6) | 13·0 (5·4 to 20·5);cP = 0·001d | 54/198 (27·3) | 325/577 (56·3) | 28·9 (21·4 to 36·3);cP < 0·001d |
| Adjusted mean change (SE) from baseline in EASI at week 16e | –9·0 (1·05) | –15·5 (0·55) | −6·4 (−8·8 to − 4·1); P < 0·001 | −7·0 (1·06) | −16·9 (0·55) | −9·9 (−12·2 to −7·5); P < 0·001 |
| EASI 50 at week 16, n/N (%)a | 42/197 (21·3) | 250/601 (41·6) | 20·1 (13·3 to 26·8);cP < 0·001d | 41/201 (20·4) | 295/591 (49·9) | 29·3 (22·5 to 36·1);cP < 0·001d |
| EASI 90 at week 16, n/N (%)a | 8/197 (4·1) | 87/601 (14·5) | 10·3 (6·4 to 14·1);cP < 0·001d | 11/201 (5·5) | 108/591 (18·3) | 12·7 (8·3 to 17·0);cP < 0·001d |
Efficacy outcomes for the initial treatment period: additional secondary endpoints, full analysis set
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Reduction of weekly average of worst daily pruritus NRS ≥ 3 at week 16, n/N (%)a,b | 28/195 (14·4) | 177/597 (29·6) | 15·2 (9·2 to 21·3);cP < 0·001d | 28/200 (14·0) | 199/583 (34·1) | 20·1 (13·9 to 26·2);cP < 0·001d |
| Adjusted mean change (SE) from baseline in weekly average of worst daily pruritus NRS at week 16e | –1·7 (0·21) | –2·6 (0·11) | −0·9 (−1·4 to − 0·4); P < 0·001 | −1·6 (0·21) | −2·9 (0·11) | −1·3 (−1·7 to −0·8); P < 0·001 |
| SCORAD score of 75 at week 16, n/N (%)a | 6/197 (3·0) | 53/601 (8·8) | 5·7 (2·5 to 8·9);cP = 0·007d | 7/201 (3·5) | 68/591 (11·5) | 8·0 (4·4 to 11·6);cP < 0·001d |
| SCORAD score of 50 at week 16, n/N (%)a | 23/197 (11·7) | 156/601 (26·0) | 14·1 (8·6 to 19·6);cP < 0·001d | 29/201 (14·4) | 198/591 (33·5) | 18·9 (12·8 to 25·1);cP < 0·001d |
| DLQI score reduction ≥ 4 at week 16, n/N (%)a,f | 60/190 (31·6) | 258/578 (44·6) | 13·0 (5·4 to 20·5);cP = 0·001d | 54/198 (27·3) | 325/577 (56·3) | 28·9 (21·4 to 36·3);cP < 0·001d |
| Adjusted mean change (SE) from baseline in EASI at week 16e | –9·0 (1·05) | –15·5 (0·55) | −6·4 (−8·8 to − 4·1); P < 0·001 | −7·0 (1·06) | −16·9 (0·55) | −9·9 (−12·2 to −7·5); P < 0·001 |
| EASI 50 at week 16, n/N (%)a | 42/197 (21·3) | 250/601 (41·6) | 20·1 (13·3 to 26·8);cP < 0·001d | 41/201 (20·4) | 295/591 (49·9) | 29·3 (22·5 to 36·1);cP < 0·001d |
| EASI 90 at week 16, n/N (%)a | 8/197 (4·1) | 87/601 (14·5) | 10·3 (6·4 to 14·1);cP < 0·001d | 11/201 (5·5) | 108/591 (18·3) | 12·7 (8·3 to 17·0);cP < 0·001d |
| ECZTRA 1 | ECZTRA 2 | |||||
| Placebo, n = 197 | Tralokinumab Q2W, n = 601 | Difference vs. placebo (95% CI) | Placebo, n = 201 | Tralokinumab Q2W, n = 591 | Difference vs. placebo (95% CI) | |
| Reduction of weekly average of worst daily pruritus NRS ≥ 3 at week 16, n/N (%)a,b | 28/195 (14·4) | 177/597 (29·6) | 15·2 (9·2 to 21·3);cP < 0·001d | 28/200 (14·0) | 199/583 (34·1) | 20·1 (13·9 to 26·2);cP < 0·001d |
| Adjusted mean change (SE) from baseline in weekly average of worst daily pruritus NRS at week 16e | –1·7 (0·21) | –2·6 (0·11) | −0·9 (−1·4 to − 0·4); P < 0·001 | −1·6 (0·21) | −2·9 (0·11) | −1·3 (−1·7 to −0·8); P < 0·001 |
| SCORAD score of 75 at week 16, n/N (%)a | 6/197 (3·0) | 53/601 (8·8) | 5·7 (2·5 to 8·9);cP = 0·007d | 7/201 (3·5) | 68/591 (11·5) | 8·0 (4·4 to 11·6);cP < 0·001d |
| SCORAD score of 50 at week 16, n/N (%)a | 23/197 (11·7) | 156/601 (26·0) | 14·1 (8·6 to 19·6);cP < 0·001d | 29/201 (14·4) | 198/591 (33·5) | 18·9 (12·8 to 25·1);cP < 0·001d |
| DLQI score reduction ≥ 4 at week 16, n/N (%)a,f | 60/190 (31·6) | 258/578 (44·6) | 13·0 (5·4 to 20·5);cP = 0·001d | 54/198 (27·3) | 325/577 (56·3) | 28·9 (21·4 to 36·3);cP < 0·001d |
| Adjusted mean change (SE) from baseline in EASI at week 16e | –9·0 (1·05) | –15·5 (0·55) | −6·4 (−8·8 to − 4·1); P < 0·001 | −7·0 (1·06) | −16·9 (0·55) | −9·9 (−12·2 to −7·5); P < 0·001 |
| EASI 50 at week 16, n/N (%)a | 42/197 (21·3) | 250/601 (41·6) | 20·1 (13·3 to 26·8);cP < 0·001d | 41/201 (20·4) | 295/591 (49·9) | 29·3 (22·5 to 36·1);cP < 0·001d |
| EASI 90 at week 16, n/N (%)a | 8/197 (4·1) | 87/601 (14·5) | 10·3 (6·4 to 14·1);cP < 0·001d | 11/201 (5·5) | 108/591 (18·3) | 12·7 (8·3 to 17·0);cP < 0·001d |
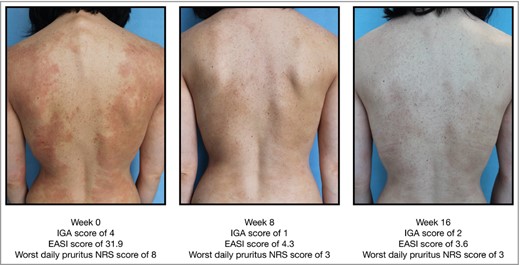
Example of improvement in Eczema Area and Severity Index (EASI) from baseline to week 16 in a patient receiving tralokinumab. IGA, Investigator’s Global Assessment; NRS, numerical rating scale.
Safety
The incidence of AEs was comparable between tralokinumab and placebo in the initial treatment period of both studies (Table 6). The majority of AEs were nonserious and mild or moderate in severity, with most resolved or resolving by the end of the treatment period; few patients had AEs leading to permanent discontinuation. Of the most frequently reported AEs (≥ 5% in any treatment group), upper respiratory tract infection (mainly reported as common cold) and conjunctivitis occurred more frequently with tralokinumab than with placebo, and dermatitis atopic and skin infection occurred more frequently with placebo (Table 6). The frequency of serious AEs (SAEs) was low and comparable between treatment groups in the initial treatment period of both studies; the majority of patients recovered from the events.
Summary of adverse events (AEs) and AEs of special interest (AESIs) in the 16‐week initial treatment period for the safety analysis set
| ECZTRA 1 | ECZTRA 2 | |||
| Placebo, n = 196, PYE = 57·13 | Tralokinumab Q2W, n = 602, PYE = 177·6 | Placebo, n = 200, PYE = 57·35 | Tralokinumab Q2W, n = 592, PYE = 176·9 | |
| AEs | ||||
| Total number of AEs | 491 | 1482 | 408 | 997 |
| Total number of SAEs | 11 | 24 | 6 | 10 |
| Patients with AEs | ||||
| ≥ 1 AE | 151 (77·0) | 460 (76·4) | 132 (66·0) | 364 (61·5) |
| ≥ 1 SAE | 8 (4·1) | 23 (3·8) | 5 (2·5) | 10 (1·7) |
| Severity | ||||
| Mild | 111 (56·6) | 385 (64·0) | 93 (46·5) | 288 (48·6) |
| Moderate | 98 (50·0) | 241 (40·0) | 84 (42·0) | 168 (28·4) |
| Severe | 16 (8·2) | 41 (6·8) | 16 (8·0) | 24 (4·1) |
| Leading to permanent discontinuation of IMP | 8 (4·1) | 20 (3·3) | 3 (1·5) | 9 (1·5) |
| Outcome | ||||
| Not recovered/not resolved | 35 (17·9) | 106 (17·6) | 25 (12·5) | 61 (10·3) |
| Recovering/resolving | 7 (3·6) | 36 (6·0) | 15 (7·5) | 20 (3·4) |
| Recovered/resolved | 139 (70·9) | 429 (71·3) | 125 (62·5) | 340 (57·4) |
| Recovered/resolved with sequelae | 0 | 6 (1·0) | 2 (1·0) | 9 (1·5) |
| Frequent AEsa | ||||
| Dermatitis atopic | 75 (38·3) | 156 (25·9) | 67 (33·5) | 98 (16·6) |
| Viral upper respiratory tract infection | 41 (20·9) | 139 (23·1) | 17 (8·5) | 49 (8·3) |
| Upper respiratory tract infection | 2 (1·0) | 9 (1·5) | 17 (8·5) | 59 (10·0) |
| Conjunctivitis | 4 (2·0) | 43 (7·1) | 3 (1·5) | 18 (3·0) |
| Skin infection | 3 (1·5) | 6 (1·0) | 11 (5·5) | 12 (2·0) |
| Pruritus | 10 (5·1) | 32 (5·3) | 5 (2·5) | 12 (2·0) |
| Headache | 10 (5·1) | 28 (4·7) | 6 (3·0) | 16 (2·7) |
| AESI – eye disorders | 7 (3·6) | 62 (10·3) | 6 (3·0) | 33 (5·6) |
| AESI Conjunctivitisb | 7 (3·6) | 60 (10·0) | 5 (2·5) | 31 (5·2) |
| AESI Keratoconjunctivitis | 0 | 1 (0·2) | 0 | 2 (0·3) |
| AESI Keratitis | 0 | 3 (0·5) | 1 (0·5) | 1 (0·2) |
| AESI – skin infections requiring systemic treatment | 4 (2·0) | 13 (2·2) | 22 (11·0) | 21 (3·5) |
| AESI – eczema herpeticum | 2 (1·0) | 3 (0·5) | 5 (2·5) | 2 (0·3) |
| AESI – malignancies diagnosed after randomization | 0 | 0 | 0 | 1 (0·2) |
| ECZTRA 1 | ECZTRA 2 | |||
| Placebo, n = 196, PYE = 57·13 | Tralokinumab Q2W, n = 602, PYE = 177·6 | Placebo, n = 200, PYE = 57·35 | Tralokinumab Q2W, n = 592, PYE = 176·9 | |
| AEs | ||||
| Total number of AEs | 491 | 1482 | 408 | 997 |
| Total number of SAEs | 11 | 24 | 6 | 10 |
| Patients with AEs | ||||
| ≥ 1 AE | 151 (77·0) | 460 (76·4) | 132 (66·0) | 364 (61·5) |
| ≥ 1 SAE | 8 (4·1) | 23 (3·8) | 5 (2·5) | 10 (1·7) |
| Severity | ||||
| Mild | 111 (56·6) | 385 (64·0) | 93 (46·5) | 288 (48·6) |
| Moderate | 98 (50·0) | 241 (40·0) | 84 (42·0) | 168 (28·4) |
| Severe | 16 (8·2) | 41 (6·8) | 16 (8·0) | 24 (4·1) |
| Leading to permanent discontinuation of IMP | 8 (4·1) | 20 (3·3) | 3 (1·5) | 9 (1·5) |
| Outcome | ||||
| Not recovered/not resolved | 35 (17·9) | 106 (17·6) | 25 (12·5) | 61 (10·3) |
| Recovering/resolving | 7 (3·6) | 36 (6·0) | 15 (7·5) | 20 (3·4) |
| Recovered/resolved | 139 (70·9) | 429 (71·3) | 125 (62·5) | 340 (57·4) |
| Recovered/resolved with sequelae | 0 | 6 (1·0) | 2 (1·0) | 9 (1·5) |
| Frequent AEsa | ||||
| Dermatitis atopic | 75 (38·3) | 156 (25·9) | 67 (33·5) | 98 (16·6) |
| Viral upper respiratory tract infection | 41 (20·9) | 139 (23·1) | 17 (8·5) | 49 (8·3) |
| Upper respiratory tract infection | 2 (1·0) | 9 (1·5) | 17 (8·5) | 59 (10·0) |
| Conjunctivitis | 4 (2·0) | 43 (7·1) | 3 (1·5) | 18 (3·0) |
| Skin infection | 3 (1·5) | 6 (1·0) | 11 (5·5) | 12 (2·0) |
| Pruritus | 10 (5·1) | 32 (5·3) | 5 (2·5) | 12 (2·0) |
| Headache | 10 (5·1) | 28 (4·7) | 6 (3·0) | 16 (2·7) |
| AESI – eye disorders | 7 (3·6) | 62 (10·3) | 6 (3·0) | 33 (5·6) |
| AESI Conjunctivitisb | 7 (3·6) | 60 (10·0) | 5 (2·5) | 31 (5·2) |
| AESI Keratoconjunctivitis | 0 | 1 (0·2) | 0 | 2 (0·3) |
| AESI Keratitis | 0 | 3 (0·5) | 1 (0·5) | 1 (0·2) |
| AESI – skin infections requiring systemic treatment | 4 (2·0) | 13 (2·2) | 22 (11·0) | 21 (3·5) |
| AESI – eczema herpeticum | 2 (1·0) | 3 (0·5) | 5 (2·5) | 2 (0·3) |
| AESI – malignancies diagnosed after randomization | 0 | 0 | 0 | 1 (0·2) |
Summary of adverse events (AEs) and AEs of special interest (AESIs) in the 16‐week initial treatment period for the safety analysis set
| ECZTRA 1 | ECZTRA 2 | |||
| Placebo, n = 196, PYE = 57·13 | Tralokinumab Q2W, n = 602, PYE = 177·6 | Placebo, n = 200, PYE = 57·35 | Tralokinumab Q2W, n = 592, PYE = 176·9 | |
| AEs | ||||
| Total number of AEs | 491 | 1482 | 408 | 997 |
| Total number of SAEs | 11 | 24 | 6 | 10 |
| Patients with AEs | ||||
| ≥ 1 AE | 151 (77·0) | 460 (76·4) | 132 (66·0) | 364 (61·5) |
| ≥ 1 SAE | 8 (4·1) | 23 (3·8) | 5 (2·5) | 10 (1·7) |
| Severity | ||||
| Mild | 111 (56·6) | 385 (64·0) | 93 (46·5) | 288 (48·6) |
| Moderate | 98 (50·0) | 241 (40·0) | 84 (42·0) | 168 (28·4) |
| Severe | 16 (8·2) | 41 (6·8) | 16 (8·0) | 24 (4·1) |
| Leading to permanent discontinuation of IMP | 8 (4·1) | 20 (3·3) | 3 (1·5) | 9 (1·5) |
| Outcome | ||||
| Not recovered/not resolved | 35 (17·9) | 106 (17·6) | 25 (12·5) | 61 (10·3) |
| Recovering/resolving | 7 (3·6) | 36 (6·0) | 15 (7·5) | 20 (3·4) |
| Recovered/resolved | 139 (70·9) | 429 (71·3) | 125 (62·5) | 340 (57·4) |
| Recovered/resolved with sequelae | 0 | 6 (1·0) | 2 (1·0) | 9 (1·5) |
| Frequent AEsa | ||||
| Dermatitis atopic | 75 (38·3) | 156 (25·9) | 67 (33·5) | 98 (16·6) |
| Viral upper respiratory tract infection | 41 (20·9) | 139 (23·1) | 17 (8·5) | 49 (8·3) |
| Upper respiratory tract infection | 2 (1·0) | 9 (1·5) | 17 (8·5) | 59 (10·0) |
| Conjunctivitis | 4 (2·0) | 43 (7·1) | 3 (1·5) | 18 (3·0) |
| Skin infection | 3 (1·5) | 6 (1·0) | 11 (5·5) | 12 (2·0) |
| Pruritus | 10 (5·1) | 32 (5·3) | 5 (2·5) | 12 (2·0) |
| Headache | 10 (5·1) | 28 (4·7) | 6 (3·0) | 16 (2·7) |
| AESI – eye disorders | 7 (3·6) | 62 (10·3) | 6 (3·0) | 33 (5·6) |
| AESI Conjunctivitisb | 7 (3·6) | 60 (10·0) | 5 (2·5) | 31 (5·2) |
| AESI Keratoconjunctivitis | 0 | 1 (0·2) | 0 | 2 (0·3) |
| AESI Keratitis | 0 | 3 (0·5) | 1 (0·5) | 1 (0·2) |
| AESI – skin infections requiring systemic treatment | 4 (2·0) | 13 (2·2) | 22 (11·0) | 21 (3·5) |
| AESI – eczema herpeticum | 2 (1·0) | 3 (0·5) | 5 (2·5) | 2 (0·3) |
| AESI – malignancies diagnosed after randomization | 0 | 0 | 0 | 1 (0·2) |
| ECZTRA 1 | ECZTRA 2 | |||
| Placebo, n = 196, PYE = 57·13 | Tralokinumab Q2W, n = 602, PYE = 177·6 | Placebo, n = 200, PYE = 57·35 | Tralokinumab Q2W, n = 592, PYE = 176·9 | |
| AEs | ||||
| Total number of AEs | 491 | 1482 | 408 | 997 |
| Total number of SAEs | 11 | 24 | 6 | 10 |
| Patients with AEs | ||||
| ≥ 1 AE | 151 (77·0) | 460 (76·4) | 132 (66·0) | 364 (61·5) |
| ≥ 1 SAE | 8 (4·1) | 23 (3·8) | 5 (2·5) | 10 (1·7) |
| Severity | ||||
| Mild | 111 (56·6) | 385 (64·0) | 93 (46·5) | 288 (48·6) |
| Moderate | 98 (50·0) | 241 (40·0) | 84 (42·0) | 168 (28·4) |
| Severe | 16 (8·2) | 41 (6·8) | 16 (8·0) | 24 (4·1) |
| Leading to permanent discontinuation of IMP | 8 (4·1) | 20 (3·3) | 3 (1·5) | 9 (1·5) |
| Outcome | ||||
| Not recovered/not resolved | 35 (17·9) | 106 (17·6) | 25 (12·5) | 61 (10·3) |
| Recovering/resolving | 7 (3·6) | 36 (6·0) | 15 (7·5) | 20 (3·4) |
| Recovered/resolved | 139 (70·9) | 429 (71·3) | 125 (62·5) | 340 (57·4) |
| Recovered/resolved with sequelae | 0 | 6 (1·0) | 2 (1·0) | 9 (1·5) |
| Frequent AEsa | ||||
| Dermatitis atopic | 75 (38·3) | 156 (25·9) | 67 (33·5) | 98 (16·6) |
| Viral upper respiratory tract infection | 41 (20·9) | 139 (23·1) | 17 (8·5) | 49 (8·3) |
| Upper respiratory tract infection | 2 (1·0) | 9 (1·5) | 17 (8·5) | 59 (10·0) |
| Conjunctivitis | 4 (2·0) | 43 (7·1) | 3 (1·5) | 18 (3·0) |
| Skin infection | 3 (1·5) | 6 (1·0) | 11 (5·5) | 12 (2·0) |
| Pruritus | 10 (5·1) | 32 (5·3) | 5 (2·5) | 12 (2·0) |
| Headache | 10 (5·1) | 28 (4·7) | 6 (3·0) | 16 (2·7) |
| AESI – eye disorders | 7 (3·6) | 62 (10·3) | 6 (3·0) | 33 (5·6) |
| AESI Conjunctivitisb | 7 (3·6) | 60 (10·0) | 5 (2·5) | 31 (5·2) |
| AESI Keratoconjunctivitis | 0 | 1 (0·2) | 0 | 2 (0·3) |
| AESI Keratitis | 0 | 3 (0·5) | 1 (0·5) | 1 (0·2) |
| AESI – skin infections requiring systemic treatment | 4 (2·0) | 13 (2·2) | 22 (11·0) | 21 (3·5) |
| AESI – eczema herpeticum | 2 (1·0) | 3 (0·5) | 5 (2·5) | 2 (0·3) |
| AESI – malignancies diagnosed after randomization | 0 | 0 | 0 | 1 (0·2) |
Conjunctivitis as an AE of special interest (AESI) occurred with greater frequency with tralokinumab vs. placebo (Table 6). Most cases of conjunctivitis were mild and resolved by the end of the treatment period; one case led to treatment discontinuation. Tralokinumab‐treated patients had lower rates of eczema herpeticum vs. placebo in both studies, and lower rates of skin infections requiring systemic treatment were reported as an AESI for tralokinumab vs. placebo in ECZTRA 2 (Table 6).
Overall, in the maintenance treatment period, AEs were detected at a lower rate than with tralokinumab Q2W in the initial treatment period, and the pattern of events was comparable with that in the initial treatment period. AEs were more frequently reported in the continued tralokinumab Q2W group than in the tralokinumab Q2W to Q4W group, and a low number of SAEs and AEs leading to permanent discontinuation of tralokinumab was observed (Table S12; see Supporting Information).
No marked differences in SAEs were observed between the treatment groups within each treatment period and between the treatment periods, and there was no clustering with respect to specific system organ class or event types.
In ECZTRA 1, the reduction in S. aureus colonization on lesional skin, from baseline to week 16, as assessed by quantitative polymerase chain reaction, was more than 10 times greater with tralokinumab vs. placebo: the median reduced from 969 to 22 gene copies per cm2 with tralokinumab vs. 649 to 238 gene copies per cm2 with placebo.
Neutralizing antidrug antibodies were detected in three and eight tralokinumab‐treated patients in ECZTRA 1 and ECZTRA 2, respectively. Based on examination of tralokinumab concentrations, antidrug antibody titre levels, AEs, and IGA and EASI scores across the trials, it was considered that the presence of neutralizing antibodies did not have an impact on the efficacy and safety of tralokinumab for any of the patients.
There were no noteworthy differences between treatment groups in laboratory values, vital signs or electrocardiographic assessments. More patients treated with tralokinumab experienced eosinophilia during the initial treatment period, but the mean eosinophil levels returned to baseline values during the maintenance period, and the safety profile of patients with eosinophilia (> 1·5 × 109 L) was comparable with that in the total trial population.
Discussion
The efficacy and safety of tralokinumab monotherapy for the treatment of moderate‐to‐severe AD were examined over 1 year in two large, multinational phase III studies of identical design. Tralokinumab 300 mg Q2W demonstrated superiority over placebo during 16 weeks of treatment across multiple outcome measures reflecting the signs and symptoms of AD. Improvements in itch and sleep scores were apparent as early as week 1 for patients treated with tralokinumab vs. placebo, and the majority of patients who achieved response criteria for improvement in the extent and severity of AD at week 16 maintained response at week 52 without need for rescue medication, including TCS.
The studies were consistent in demonstrating the effectiveness of tralokinumab monotherapy, but there were some differences in the level of response vs. placebo for some endpoints. In particular, there was a greater difference between tralokinumab and placebo for the primary endpoints in ECZTRA 2 compared with ECZTRA 1. One potential explanation is the greater use of rescue medication in tralokinumab‐treated patients in ECZTRA 1 (35·8%) compared with ECZTRA 2 (22·8%). The criteria surrounding use of rescue medication were strict; a single use of TCS was registered as treatment failure following the primary analysis approach. This does not reflect real‐world use of biologic agents, which are initiated as add‐on therapy to TCS for active lesions in 80–90% of patients,18, 19 and therefore the utility of these findings in daily practice is difficult to assess. The ECZTRA 3 study (NCT03363854), of tralokinumab in combination with TCS, is more reflective of the likely clinical use in daily practice.20
More than 50% of patients who achieved clinical responses at week 16 with tralokinumab Q2W maintained that response through to week 52 without any rescue medication, including TCS, and 39–51% of patients maintained response when receiving tralokinumab Q4W. Unexpectedly, a proportion (21–47%) of tralokinumab responders at week 16 who were rerandomized to placebo maintained responses at week 52. Retained response over 36 weeks without active maintenance treatment or TCS suggests that tralokinumab could induce remission of AD and skin normalization for some patients. It has previously been shown that IL‐13 expression is much lower in nonlesional than lesional AD skin.9, 10, 21 It is therefore possible that following a period of clear or almost clear skin achieved with tralokinumab Q2W, IL‐13‐mediated inflammation in the skin may have been extinguished, altering the natural disease course. Further studies are needed to identify whether some patients can experience a true remission or altered natural course upon stopping therapy. There were also patients who could maintain good disease control with Q4W dosing of tralokinumab. These observations suggest the possibility of less frequent maintenance dosing in some patients initially treated with tralokinumab Q2W.
Support for reversal of IL‐13‐associated skin abnormalities with tralokinumab was provided by the observed greater reduction in skin colonization with S. aureus, which was consistent with observations in the phase II study.15 In addition to decreasing S. aureus colonization, fewer skin infections requiring systemic treatment and a lower frequency of eczema herpeticum were seen with tralokinumab. These findings may be due to tralokinumab’s effects on improving skin barrier integrity. Previous studies have also linked upregulation of IL‐13 to decreased expression of antimicrobial genes;22, 23 thus, tralokinumab could be enhancing skin antimicrobial responses in this manner. Analysis of biomarkers from skin biopsies and serum biomarkers collected in ECZTRA 1 may provide further insights into the effects of tralokinumab on the skin microbiome and epidermal changes at a molecular level.
Tralokinumab was well tolerated, with an overall frequency and severity of AEs comparable with placebo over 52 weeks, consistently with those observed in the phase II trial.15 Incidences of eye disorders were collected as AESIs. Conjunctivitis occurred in ≤ 10% of patients receiving tralokinumab in the initial treatment period and in < 9% in any tralokinumab treatment arm in the maintenance periods. Almost all cases of conjunctivitis were mild or moderate, the majority recovered, and one case led to withdrawal. This side‐effect has been observed in other studies of IL‐4 or IL‐13 inhibitors in AD and the aetiology remains unknown.
Consistently with other trials in AD, a limitation of these studies was the requirement to ensure adequate washout of existing treatments, particularly in a patient population with significant disease and high levels of prior medication use. The protocol‐required 2‐week washout period, during which no TCS use was allowed, may have been long enough to exacerbate AD in some patients, leading to use of rescue TCS early in the studies. In fact, approximately half of the patients who used rescue medications did so within the first 4 weeks of the trials.
Most currently available treatment options for AD were not designed to selectively target disease‐specific pathways that have been established in the pathogenesis of the disease, and there is a need for additional long‐term treatment options for patients with moderate‐to‐severe AD. These are the first phase III studies to demonstrate that specific IL‐13 neutralization with tralokinumab monotherapy is efficacious for the treatment of AD, providing further evidence that IL‐13 is a key cytokine in the pathogenesis of this disease.
In conclusion, tralokinumab monotherapy was superior to placebo at 16 weeks of treatment and was well tolerated up to 52 weeks of treatment in a large cohort of patients with moderate‐to‐severe AD.
Acknowledgments
We thank the ECZTRA 1 and ECZTRA 2 investigators and the patients who participated in the studies.
Author Contribution
Andreas Wollenberg: Conceptualization (equal); Data curation (equal); Formal analysis (supporting); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Andrew Blauvelt: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Emma Guttman‐Yassky: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Margitta Worm: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Charles Lynde: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Jean‐Philippe Lacour: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Lynda Spelman: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Norito Katoh: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Hidehisa Saeki: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Yves Poulin: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Aleksandra Lesiak: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Leon Kircik: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Sang Hyun Cho: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Pedro Herranz Pinto: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Michael Cork: Data curation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Ketty Peris: Software (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Louise Abildgaard Steffensen: Data curation (equal); Formal analysis (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Bo Bang: Conceptualization (equal); Formal analysis (equal); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Alexandra Kuznetsova: Data curation (equal); Formal analysis (lead); Writing‐original draft (equal); Writing‐review & editing (equal). Thomas Nedergaard Jensen: Conceptualization (equal); Formal analysis (equal); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Marie Louise Østerdal: Data curation (equal); Formal analysis (supporting); Writing‐original draft (equal); Writing‐review & editing (equal). Eric Lawrence Simpson: Conceptualization (equal); Data curation (equal); Formal analysis (supporting); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
References
Appendix 1 Conflicts of interest
A.W. reports honoraria for conduct of the ECZTRA 1 and ECZTRA 2 trials to Ludwig Maximilian University of Munich from LEO Pharma A/S during the conduct of the study; personal fees from AbbVie, Chugai, Galderma, LEO Pharma, Lilly, MedImmune, Novartis, Pfizer, Regeneron and Sanofi‐Aventis; and grants from LEO Pharma outside the submitted work. A.B. reports personal fees for consulting and clinical study fees to Oregon Medical Research Center from LEO Pharma during the conduct of the study; personal fees for consulting and clinical study fees from AbbVie, Dermira, Incyte, Lilly, Pfizer and Regeneron; and personal fees for consulting from Sanofi outside the submitted work. E.G.‐Y. declares they have no conflicts of interest. M.W. reports honoraria for advisory boards and lecture activities from ALK–AbbVie Deutschland GmbH & Co. KG, Abelló Arzneimittel GmbH, Actelion Pharmaceuticals Deutschland GmbH, Aimmune Therapeutics UK Limited, Allergopharma GmbH & Co. KG, Bencard Allergie GmbH, Biotest AG, DBV Technologies S.A., HAL Allergie GmbH, LEO Pharma GmbH, Lilly Deutschland GmbH, Mylan Germany GmbH, Novartis AG, Regeneron, Sanofi‐Aventis Deutschland GmbH and Stallergenes GmbH outside the submitted work. C.L. reports honoraria for speaking and consulting, and investigator fees from AbbVie, Amgen, Bausch, Galderma, LEO Pharma, Pfizer and Sanofi Genzyme outside the submitted work. J.‐P.L. reports grants from LEO Pharma during the conduct of the study; grants from AbbVie, Amgen, Boehringer Ingelheim, Dermira, Janssen and Pfizer; and grants and personal fees from Celgene, Galderma, Lilly, Novartis and Sanofi outside the submitted work. L.S. reports investigator fees from Amgen, Blaze, BMS, Boehringer Ingelheim, LEO Pharma, Pfizer, Sanofi, Sun and UCB; and investigator fees and fees for advisory board participation from AbbVie, Galderma, Janssen, Lilly and Novartis outside the submitted work. N.K. reports personal fees from AbbVie, Janssen, Maruho, Mitsubishi Tanabe and Taiho; and grants and personal fees from Lilly Japan, LEO Pharma and Sanofi outside the submitted work. H.S. reports personal fees from Kyorin, Kyowa Kirin, LEO Pharma, Sanofi, Taiho and Tokiwa; grants and personal fees from Maruho and Mitsubishi Tanabe; and grants from Eisai and Torii outside the submitted work. Y.P. reports grants from LEO Pharma during the conduct of the study; grant funding as an investigator from AnaptysBio, Arcutis, Asana, AstraZeneca, Baxalta, Baxter, Boehringer Ingelheim, Bond Avillion, Bristol Myers Squibb, Celgene, Dermira, Devonian, Galderma, Genentech, GlaxoSmithKline, Incyte, LEO Pharma, MedImmune, Merck, Novartis, Pfizer, Regeneron, Roche, Serono and Takeda; and grants and honoraria for services as an investigator, speaker and member of advisory boards from AbbVie, Amgen, Bausch, Janssen‐Ortho and UCB. A.L. reports investigator fees paid to the Medical University of Łódź from Regeneron and personal fees and investigator fees paid to the Medical University of Łódź from AbbVie, LEO Pharma, Novartis and Pfizer outside the submitted work. L.K. reports personal fees as a speaker from Sanofi, grants from Arena and grants and personal fees as a consultant or speaker from AbbVie, Arcutis, Dermavant, Dermira, Incyte, LEO Pharma, Lilly, Novartis, Ortho Dermatologics, Pfizer and Regeneron outside the submitted work. S.H.C. reports personal fees and clinical study fees to the Catholic University of Korea from LEO Pharma during the conduct of the study and personal fees and clinical study fees to the Catholic University of Korea from AbbVie, Pfizer, Regeneron and Sanofi outside the submitted work. P.H. declares they have no conflicts of interest. M.J.C. has served as an investigator or consultant for AbbVie, Astellas, Boots, Dermavant, Galapagos, Galderma, Hyphens, Johnson & Johnson, LEO Pharma, L’Oréal, Menlo, Novartis, Oxagen, Pfizer, Procter & Gamble, Reckitt Benckiser, Regeneron and Sanofi Genzyme. K.P. reports grants and personal fees for participation in advisory boards from AbbVie and Galderma and personal fees for participation in advisory boards from Almirall, Janssen, LEO Pharma, Lilly, Novartis, Pierre Fabre, Sanofi and Sun Pharma outside the submitted work. L.A.S., B.B., A.K., T.N.J. and M.L.Ø. are employees of LEO Pharma A/S. E.L.S. reports grants from Celgene, Galderma, Merck, Novartis and Tioga; personal fees from Boehringer Ingelheim, Dermavant, Dermira, Forté Bio, Incyte, Menlo, Ortho Dermatologics, Pierre Fabre, Sanofi and Valeant; and grants and personal fees from AbbVie, Kyowa Hakko Kirin, LEO Pharma, Lilly, MedImmune, Pfizer and Regeneron outside the submitted work.
Author notes
Funding sourcesThe tralokinumab ECZTRA 1 and 2 studies were sponsored by LEO Pharma A/S (Ballerup, Denmark). Medical writing and editorial assistance were provided by Kathryn Woods, PhD, and Jane Beck, MA, from Complete HealthVizion, funded by LEO Pharma A/S.
Conflicts of interestConflicts of interest statements are listed inAppendix 1.
A full list of study investigators is provided inAppendix S1 (see Supporting Information).
*Plain language summary available online



