-
PDF
- Split View
-
Views
-
Cite
Cite
D.M. Chen, A. Odueyungbo, E. Csinady, L. Gearhart, P. Lehane, M. Cheu, M. Maho‐Vaillant, C. Prost‐Squarcioni, V. Hebert, E. Houivet, S. Calbo, F. Caillot, M.L. Golinski, B. Labeille, C. Picard‐Dahan, C. Paul, M.A. Richard, J.D. Bouaziz, S. Duvert‐Lehembre, P. Bernard, F. Caux, M. Alexandre, S. Ingen‐Housz‐Oro, P. Vabres, E. Delaporte, G. Quereux, A. Dupuy, S. Debarbieux, M. Avenel‐Audran, M. D'Incan, C. Bedane, N. Bénéton, D. Jullien, N. Dupin, L. Misery, L. Machet, M. Beylot‐Barry, O. Dereure, B. Sassolas, J. Benichou, P. Musette, P. Joly, the French Study Group on Autoimmune Bullous Diseases, Rituximab is an effective treatment in patients with pemphigus vulgaris and demonstrates a steroid‐sparing effect, British Journal of Dermatology, Volume 182, Issue 5, 1 May 2020, Pages 1111–1119, https://doi.org/10.1111/bjd.18482
Close - Share Icon Share
Summary
Corticosteroids (CS) with or without adjuvant immunosuppressant agents are standard treatment for pemphigus vulgaris (PV). The efficacy of adjuvant therapies in minimizing steroid‐related adverse events (AEs) is unproven.
To utilize data collected in a French investigator‐initiated, phase III, open‐label, randomized controlled trial to demonstrate the efficacy and safety of rituximab and seek approval for its use in PV.
This was an independently conducted post hoc analysis of the moderate‐to‐severe PV subset enrolled in the Ritux 3 study. Patients were randomized to rituximab plus 0·5 or 1·0 mg kg−1 per day prednisone tapered over 3 or 6 months, or 1·0 or 1·5 mg kg−1 per day prednisone alone tapered over 12 or 18 months, respectively (according to disease severity). The primary end point was complete remission at month 24 without CS (CRoff) for ≥ 2 months, and 24‐month efficacy and safety results were also reported.
At month 24, 34 of 38 patients (90%) on rituximab plus prednisone achieved CRoff ≥ 2 months vs. 10 of 36 patients (28%) on prednisone alone. Median total cumulative prednisone dose was 5800 mg in the rituximab plus prednisone arm vs. 20 520 mg for prednisone alone. Eight of 36 patients (22%) who received prednisone alone withdrew from treatment owing to AEs; one rituximab‐plus‐prednisone patient withdrew due to pregnancy. Overall, 24 of 36 patients (67%) on prednisone alone experienced a grade 3/4 CS‐related AE vs. 13 of 38 patients (34%) on rituximab plus prednisone.
In patients with moderate‐to‐severe PV, rituximab plus short‐term prednisone was more effective than prednisone alone. Patients treated with rituximab had less CS exposure and were less likely to experience severe or life‐threatening CS‐related AEs.
What's already known about this topic?
Pemphigus vulgaris (PV) is the most common type of pemphigus.
Corticosteroids, a standard first‐line treatment for PV, have significant side‐effects.
Although their effects are unproven, adjuvant corticosteroid‐sparing agents are routinely used to minimize steroid exposure and corticosteroid‐related side‐effects.
There is evidence that the anti‐CD20 antibody rituximab is effective in the treatment of patients with severe recalcitrant pemphigus and in patients with newly diagnosed pemphigus.
What does this study add?
This study provides a more detailed analysis of patients with PV enrolled in an investigator‐initiated trial.
Rituximab plus prednisone had a steroid‐sparing effect and more patients achieved complete remission off prednisone.
Fewer patients experienced grade 3 or grade 4 steroid‐related adverse events than those on prednisone alone.
This collaboration between academia and industry, utilizing independent post hoc analyses, led to regulatory authority approvals of rituximab in moderate‐to‐severe PV.
Pemphigus is a group of autoimmune bullous disorders involving mucous membranes and/or skin.1 The most common type of pemphigus is pemphigus vulgaris (PV), which accounts for approximately 80% of pemphigus diagnoses in the U.S.A. and Europe, followed by pemphigus foliaceus (PF).2 PV is mediated by circulating autoantibodies targeting desmoglein (Dsg)3 and, in some patients, also Dsg1, resulting in the loss of epidermal cell–cell adhesion.1
The use of corticosteroids (CS) in treating PV is well established. Reported rates of remission vary but occur, on average, in approximately 25% of patients treated with CS alone.3 The unsatisfactory safety profile of chronic high‐dose CS therapy (including diabetes, hypertension, gastrointestinal bleeding and ulceration, myopathy, osteoporosis, osteonecrosis, infection and death)2,4,5,6 has led to treatment approaches that combine CS with an immunosuppressive agent, such as azathioprine or mycophenolate mofetil, and minimize steroid exposure.7,8,9,10 Despite widespread use, it is controversial whether these steroid‐sparing agents are beneficial.11
Rituximab is a chimeric, humanized anti‐CD20 monoclonal antibody12 believed to exert its clinical effects in pemphigus through depletion of Dsg‐specific IgG‐positive B lymphocytes.13 The efficacy of rituximab in the treatment of severe, recalcitrant pemphigus has been reported in the literature for the past decade. In patients with newly diagnosed pemphigus, Joly et al. demonstrated that rituximab plus short‐term CS was safe and significantly more effective than CS alone (the Ritux 3 study; ClinicalTrials.gov NCT00784589).14
The U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation and Breakthrough Therapy Designation to Roche for rituximab in the treatment of PV. The lead investigator, Rouen University Hospital and Roche then collaborated, and Roche independently analysed data collected from patients diagnosed with PV (excluding patients with PF) in the Ritux 3 study and submitted applications to seek regulatory approvals for rituximab as a treatment for moderate‐to‐severe PV in the U.S.A. and the European Union. The 24‐month study treatment results of this independent post hoc analysis of patients with PV are reported herein. These results in patients with PV extend the published findings of Joly et al.14
Materials and methods
Study design
The Ritux 3 study was sponsored by Rouen University Hospital, Rouen, France and designed, managed and conducted by the French Study Group on Autoimmune Bullous Diseases.14 Roche provided the rituximab during the 24‐month study treatment period. The ethics committee (CPP Nord‐Ouest1) approved the study.
Patients were stratified according to disease severity (moderate or severe)15 and randomized 1 : 1 to rituximab plus short‐term low‐dose prednisone (rituximab plus prednisone arm) or long‐term, standard‐dose prednisone (prednisone‐alone arm). After disease control was achieved, doses of prednisone were tapered (Table S1; see Supporting Information). Patients in the rituximab plus prednisone arm received an initial intravenous (IV) infusion of 1000 mg rituximab on study day 1 in combination with 0·5 mg kg−1 per day oral prednisone tapered off over 3 months (moderate disease) or 1 mg kg−1 per day oral prednisone tapered off over 6 months (severe disease) and a second IV infusion of 1000 mg rituximab on study day 15. Maintenance infusions of rituximab 500 mg were administered at months 12 and 18. Patients randomized to prednisone received an initial dosage of 1 mg kg−1 per day oral prednisone tapered off over 12 months (moderate disease) or 1·5 mg kg−1 per day oral prednisone tapered off over 18 months (severe disease) (Table S1; see Supporting Information). A CS tapering regimen in the prednisone‐alone arm similar to that used for the rituximab plus prednisone arm was unacceptable, because it was not standard of care and could result in artificially high relapse rates.
Patients treated with rituximab who relapsed could receive an additional infusion of rituximab 1000 mg in combination with reintroduced or escalated prednisone dose. Maintenance and relapse infusions were administered no sooner than 16 weeks following the previous infusion. Patients treated with prednisone who relapsed resumed or escalated their prednisone dose. If relapse occurred during the prednisone taper, the prednisone dosage was increased to a prior dose level that permitted disease control. If relapse occurred after prednisone withdrawal, prednisone was resumed at a dosage of 0·3 mg kg−1 per day or 0·5 mg kg−1 per day depending on the severity of relapse.
Patients
All patients provided written informed consent before study participation. Patients were aged 18–80 years, newly diagnosed, had moderate or severe disease by Harman's criteria15 and had received no prior therapies for pemphigus. Only patients with PV were included in the efficacy and safety analyses conducted for the applications seeking regulatory approval for rituximab use in PV and are presented in the current manuscript (primary analysis results in patients with PF are provided in File S1; see Supporting Information).
Statistical analyses and methods
Trial data and associated documents (such as case report forms and randomization specification) were obtained from Rouen, translated into English and formatted according to regulatory submission requirements [Clinical Data Interchange Standards Consortium Data Exchange Standards and coding of adverse events (AEs) using the Medical Dictionary for Regulatory Activities] and analysed according to an independent statistical analysis plan prepared by Roche.
Descriptive analysis was used to compare the rituximab plus prednisone arm with the prednisone‐alone arm for the proportion of patients who achieved complete remission (CR) off CS therapy (CRoff), the median cumulative dose of prednisone and the proportion of patients who relapsed. Last observation carried forward imputation was used to impute prednisone data for missed visits. Infusion‐related reactions (IRRs) and CS‐related AEs were identified through post hoc Roche medical review.
This study did not have a prospective plan for immunogenicity assessment; therefore, antidrug antibodies (ADAs) were measured from frozen serum samples as a post hoc retrospective evaluation. A validated bridging enzyme‐linked immunosorbent assay was used for detection and characterization of ADA with rituximab‐naive patients with PV acting as controls.
CD19+ B cells and anti‐Dsg results are described in File S1 (Figs S1, S2; see Supporting Information).
Study outcomes
The primary end point was the proportion of patients at month 24 who achieved CR on 0 mg prednisone for at least two consecutive months (CRoff ≥ 2 months). CRoff ≥ 3 months at month 24 was also analysed. CR was defined as complete epithelialization and absence of new and/or established skin and mucosal lesions. Secondary objectives included the proportion of patients who relapsed (‘relapse’ was defined as the appearance of at least three new lesions in 1 month, which did not heal spontaneously within 1 week, or the extension of established lesions in a patient who had previously achieved disease control), median cumulative prednisone dose at month 24, duration off CS therapy in patients who responded at month 24, and incidences of treatment‐related AEs, all serious AEs (SAEs), and CS‐related AEs. For IRRs, symptoms of intolerance to treatment were documented at the first planned visit after the infusion.
Results
Patient disposition and baseline characteristics
In total, 74 patients with PV were randomized to receive rituximab plus prednisone (n = 38) or prednisone alone (n = 36) (Fig. 1). The median cumulative dose of rituximab at month 24 was 3000 mg. The treatment arms were similar with respect to age, body mass index and body surface area (Table 1). The median age was 51·5 years (25–79). Overall, 66 of 74 patients (89%) had moderate‐to‐severe PV determined by Harman's criteria and 59 of 74 patients (80%) had a Pemphigus Disease Area Index score ≥ 15, representing moderate‐to‐severe PV.16 There were more female patients in the rituximab plus prednisone arm than the prednisone‐alone arm [27 of 38 (71%) vs. 15 of 36 (42%)]. One patient (3%) in the rituximab plus prednisone arm withdrew from treatment owing to pregnancy and delivered a full‐term healthy baby. In the prednisone‐alone arm, 12 of 36 patients (33%) withdrew from treatment; eight patients (22%) withdrew owing to AEs (one patient withdrew owing to AE and because treatment was not effective), four patients (11%) owing to other reasons (treatment not effective in three patients and patient decision in one patient) and one patient (3%) owing to loss of follow‐up.
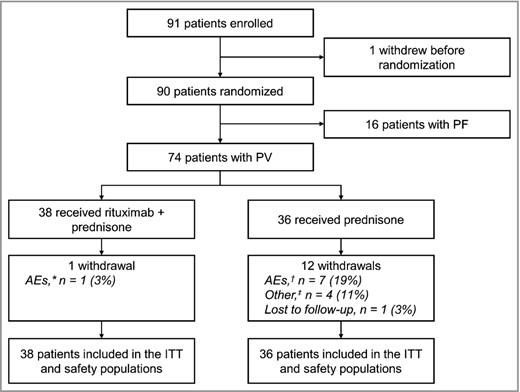
Patient disposition. AE, adverse event; ITT, intention‐to‐treat; PF, pemphigus foliaceus; PV, pemphigus vulgaris.
*Withdrawal from treatment owing to pregnancy. †AEs included corticosteroid‐induced myopathy, necrosis of femoral heads, Cushing syndrome, psychiatric decompensation, chorioretinitis. ‡One patient receiving prednisone discontinued treatment owing to ineffectiveness of treatment (other) and withdrew from the study owing to serious AEs of dyspnoea and oedema.
| Rituximab plus prednisone (n = 38) | Prednisone alone (n = 36) | Total (N = 74) | |
| Sex | |||
| Female, n (%) | 27 (71) | 15 (42) | 42 (57) |
| Male, n (%) | 11 (29) | 21 (58) | 32 (43) |
| Age, years | |||
| Mean (SD) | 54·7 (16·2) | 51·8 (13·4) | 53·3 (14·8) |
| Median (range) | 55·0 (25–79) | 49·0 (30–79) | 51·5 (25–79) |
| Baseline weight, kg | |||
| Mean (SD) | 68·3 (21·4) | 75·2 (18·7) | 71·7 (20·3) |
| Median (range) | 63·0 (43–177) | 74·5 (43–155) | 69·5 (43–177) |
| Baseline body mass index, kg m−2 | |||
| Mean (SD) | 27·0 (15·26) | 25·7 (5·13) | 26·4 (11·45) |
| Median (range) | 25·4 (15–115) | 25·3 (17–46) | 25·3 (15–115) |
| Severity of PV (by Harman's criteria) | |||
| Moderate, n (%) | 5 (13) | 3 (8) | 8 (11) |
| Severe, n (%) | 33 (87) | 33 (92) | 66 (89) |
| PDAI score, 0–250 | |||
| Mean (SD) | 34·1 (29·5) | 46·6 (23·6) | 40·1 (27·4) |
| Median (range) | 26·0 (3–131) | 55·0 (7–106) | 33·0 (3–131) |
| Duration of cutaneous lesions, days | |||
| Mean (SD) | 111·3 (274·3) | 94·4 (134·0) | 103·1 (216·4) |
| Median (range) | 38·5 (0–1590) | 50·0 (0–622) | 44·0 (0–1590) |
| Duration of mucosal lesions, days | |||
| Mean (SD) | 138·7 (131·5) | 115·4 (130·1) | 127·4 (130·5) |
| Median (range) | 109·0 (0–571) | 84·0 (1–391) | 94·0 (0–691) |
| Rituximab plus prednisone (n = 38) | Prednisone alone (n = 36) | Total (N = 74) | |
| Sex | |||
| Female, n (%) | 27 (71) | 15 (42) | 42 (57) |
| Male, n (%) | 11 (29) | 21 (58) | 32 (43) |
| Age, years | |||
| Mean (SD) | 54·7 (16·2) | 51·8 (13·4) | 53·3 (14·8) |
| Median (range) | 55·0 (25–79) | 49·0 (30–79) | 51·5 (25–79) |
| Baseline weight, kg | |||
| Mean (SD) | 68·3 (21·4) | 75·2 (18·7) | 71·7 (20·3) |
| Median (range) | 63·0 (43–177) | 74·5 (43–155) | 69·5 (43–177) |
| Baseline body mass index, kg m−2 | |||
| Mean (SD) | 27·0 (15·26) | 25·7 (5·13) | 26·4 (11·45) |
| Median (range) | 25·4 (15–115) | 25·3 (17–46) | 25·3 (15–115) |
| Severity of PV (by Harman's criteria) | |||
| Moderate, n (%) | 5 (13) | 3 (8) | 8 (11) |
| Severe, n (%) | 33 (87) | 33 (92) | 66 (89) |
| PDAI score, 0–250 | |||
| Mean (SD) | 34·1 (29·5) | 46·6 (23·6) | 40·1 (27·4) |
| Median (range) | 26·0 (3–131) | 55·0 (7–106) | 33·0 (3–131) |
| Duration of cutaneous lesions, days | |||
| Mean (SD) | 111·3 (274·3) | 94·4 (134·0) | 103·1 (216·4) |
| Median (range) | 38·5 (0–1590) | 50·0 (0–622) | 44·0 (0–1590) |
| Duration of mucosal lesions, days | |||
| Mean (SD) | 138·7 (131·5) | 115·4 (130·1) | 127·4 (130·5) |
| Median (range) | 109·0 (0–571) | 84·0 (1–391) | 94·0 (0–691) |
PV, pemphigus vulgaris; PDAI, Pemphigus Disease Area Index.
| Rituximab plus prednisone (n = 38) | Prednisone alone (n = 36) | Total (N = 74) | |
| Sex | |||
| Female, n (%) | 27 (71) | 15 (42) | 42 (57) |
| Male, n (%) | 11 (29) | 21 (58) | 32 (43) |
| Age, years | |||
| Mean (SD) | 54·7 (16·2) | 51·8 (13·4) | 53·3 (14·8) |
| Median (range) | 55·0 (25–79) | 49·0 (30–79) | 51·5 (25–79) |
| Baseline weight, kg | |||
| Mean (SD) | 68·3 (21·4) | 75·2 (18·7) | 71·7 (20·3) |
| Median (range) | 63·0 (43–177) | 74·5 (43–155) | 69·5 (43–177) |
| Baseline body mass index, kg m−2 | |||
| Mean (SD) | 27·0 (15·26) | 25·7 (5·13) | 26·4 (11·45) |
| Median (range) | 25·4 (15–115) | 25·3 (17–46) | 25·3 (15–115) |
| Severity of PV (by Harman's criteria) | |||
| Moderate, n (%) | 5 (13) | 3 (8) | 8 (11) |
| Severe, n (%) | 33 (87) | 33 (92) | 66 (89) |
| PDAI score, 0–250 | |||
| Mean (SD) | 34·1 (29·5) | 46·6 (23·6) | 40·1 (27·4) |
| Median (range) | 26·0 (3–131) | 55·0 (7–106) | 33·0 (3–131) |
| Duration of cutaneous lesions, days | |||
| Mean (SD) | 111·3 (274·3) | 94·4 (134·0) | 103·1 (216·4) |
| Median (range) | 38·5 (0–1590) | 50·0 (0–622) | 44·0 (0–1590) |
| Duration of mucosal lesions, days | |||
| Mean (SD) | 138·7 (131·5) | 115·4 (130·1) | 127·4 (130·5) |
| Median (range) | 109·0 (0–571) | 84·0 (1–391) | 94·0 (0–691) |
| Rituximab plus prednisone (n = 38) | Prednisone alone (n = 36) | Total (N = 74) | |
| Sex | |||
| Female, n (%) | 27 (71) | 15 (42) | 42 (57) |
| Male, n (%) | 11 (29) | 21 (58) | 32 (43) |
| Age, years | |||
| Mean (SD) | 54·7 (16·2) | 51·8 (13·4) | 53·3 (14·8) |
| Median (range) | 55·0 (25–79) | 49·0 (30–79) | 51·5 (25–79) |
| Baseline weight, kg | |||
| Mean (SD) | 68·3 (21·4) | 75·2 (18·7) | 71·7 (20·3) |
| Median (range) | 63·0 (43–177) | 74·5 (43–155) | 69·5 (43–177) |
| Baseline body mass index, kg m−2 | |||
| Mean (SD) | 27·0 (15·26) | 25·7 (5·13) | 26·4 (11·45) |
| Median (range) | 25·4 (15–115) | 25·3 (17–46) | 25·3 (15–115) |
| Severity of PV (by Harman's criteria) | |||
| Moderate, n (%) | 5 (13) | 3 (8) | 8 (11) |
| Severe, n (%) | 33 (87) | 33 (92) | 66 (89) |
| PDAI score, 0–250 | |||
| Mean (SD) | 34·1 (29·5) | 46·6 (23·6) | 40·1 (27·4) |
| Median (range) | 26·0 (3–131) | 55·0 (7–106) | 33·0 (3–131) |
| Duration of cutaneous lesions, days | |||
| Mean (SD) | 111·3 (274·3) | 94·4 (134·0) | 103·1 (216·4) |
| Median (range) | 38·5 (0–1590) | 50·0 (0–622) | 44·0 (0–1590) |
| Duration of mucosal lesions, days | |||
| Mean (SD) | 138·7 (131·5) | 115·4 (130·1) | 127·4 (130·5) |
| Median (range) | 109·0 (0–571) | 84·0 (1–391) | 94·0 (0–691) |
PV, pemphigus vulgaris; PDAI, Pemphigus Disease Area Index.
Clinical efficacy
At month 24, the proportion of patients who achieved CRoff ≥ 2 months was higher in the rituximab plus prednisone arm than in prednisone‐alone arm [34 of 38 (90%) vs. 10 of 36 (28%)] (Table 2). These results were consistent for men [10 of 11 (91%) vs. six of 21 (29%)] and women [24 of 27 (89%) vs. four of 15 (27%)]. The proportion of patients who achieved CRoff ≥ 3 months at month 24 was also higher in the rituximab plus prednisone arm than in the prednisone‐alone arm [34 of 38 (90%) vs. nine of 36 (25%)]. The median duration of CRoff ≥ 2 months at month 24 was longer in patients treated with rituximab plus prednisone than in patients treated with prednisone alone (498·5 days vs. 125·0 days). Fewer patients in the rituximab plus prednisone arm had a severe or moderate relapse at month 24 compared with the prednisone‐alone arm [nine of 38 (24%) vs. 18 of 36 (50%)] (Table 2). In addition, fewer patients in the rituximab plus prednisone arm had at least one relapse (seven patients relapsed once and two patients relapsed twice) than in the prednisone‐alone arm (12 patients relapsed once, five patients relapsed twice and one patient relapsed three times).
| Rituximab + prednisone (n = 38) | Prednisone alone (n = 36) | Difference (95% CI) | P‐valuesa | |
| Primary end point, CRoff ≥ 2 months, n (%) | 34 (90) | 10 (28) | 61·7% (38·4–76·5)b | P < 0·0001c |
| Duration of CRoff ≥ 2 months, median (IQR), days | 498·5 (266·0–527·0) | 125·0 (98·0–189·0) | 373·5 (N/A) | P = 0·0030d |
| Patients with at least one severe or moderate relapse, n (%) | 9 (24) | 18 (50) | −26·3% (−46·7% to −2·5%)b | P = 0·0229c |
| CRmin ≥ 2 months, n (%) | 34 (90) | 12 (33) | P < 0·0001c |
| Rituximab + prednisone (n = 38) | Prednisone alone (n = 36) | Difference (95% CI) | P‐valuesa | |
| Primary end point, CRoff ≥ 2 months, n (%) | 34 (90) | 10 (28) | 61·7% (38·4–76·5)b | P < 0·0001c |
| Duration of CRoff ≥ 2 months, median (IQR), days | 498·5 (266·0–527·0) | 125·0 (98·0–189·0) | 373·5 (N/A) | P = 0·0030d |
| Patients with at least one severe or moderate relapse, n (%) | 9 (24) | 18 (50) | −26·3% (−46·7% to −2·5%)b | P = 0·0229c |
| CRmin ≥ 2 months, n (%) | 34 (90) | 12 (33) | P < 0·0001c |
CI, confidence interval; CRoff, complete remission off prednisone therapy; IQR, interquartile range; N/A, not applicable; CRmin, complete remission on minimal prednisone therapy (prednisone dose ≤ 10 mg per day). aNo adjustment for multiplicity was made for any secondary end points and the P‐values should be interpreted with caution. b95% confidence interval (CI) calculated using the corrected Newcombe interval. cP‐value calculated using Fisher's exact test with mid‐P correction. dP‐value calculated using Mann–Whitney U‐test.
| Rituximab + prednisone (n = 38) | Prednisone alone (n = 36) | Difference (95% CI) | P‐valuesa | |
| Primary end point, CRoff ≥ 2 months, n (%) | 34 (90) | 10 (28) | 61·7% (38·4–76·5)b | P < 0·0001c |
| Duration of CRoff ≥ 2 months, median (IQR), days | 498·5 (266·0–527·0) | 125·0 (98·0–189·0) | 373·5 (N/A) | P = 0·0030d |
| Patients with at least one severe or moderate relapse, n (%) | 9 (24) | 18 (50) | −26·3% (−46·7% to −2·5%)b | P = 0·0229c |
| CRmin ≥ 2 months, n (%) | 34 (90) | 12 (33) | P < 0·0001c |
| Rituximab + prednisone (n = 38) | Prednisone alone (n = 36) | Difference (95% CI) | P‐valuesa | |
| Primary end point, CRoff ≥ 2 months, n (%) | 34 (90) | 10 (28) | 61·7% (38·4–76·5)b | P < 0·0001c |
| Duration of CRoff ≥ 2 months, median (IQR), days | 498·5 (266·0–527·0) | 125·0 (98·0–189·0) | 373·5 (N/A) | P = 0·0030d |
| Patients with at least one severe or moderate relapse, n (%) | 9 (24) | 18 (50) | −26·3% (−46·7% to −2·5%)b | P = 0·0229c |
| CRmin ≥ 2 months, n (%) | 34 (90) | 12 (33) | P < 0·0001c |
CI, confidence interval; CRoff, complete remission off prednisone therapy; IQR, interquartile range; N/A, not applicable; CRmin, complete remission on minimal prednisone therapy (prednisone dose ≤ 10 mg per day). aNo adjustment for multiplicity was made for any secondary end points and the P‐values should be interpreted with caution. b95% confidence interval (CI) calculated using the corrected Newcombe interval. cP‐value calculated using Fisher's exact test with mid‐P correction. dP‐value calculated using Mann–Whitney U‐test.
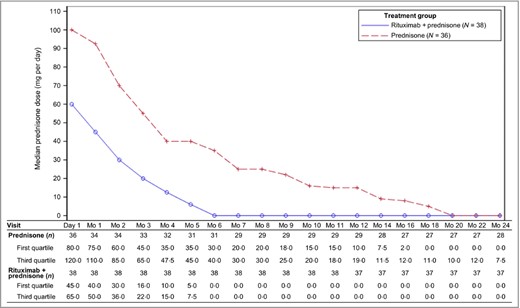
Median prednisone doses decreased over the course of the study in both arms as reflected by the CS tapering regimen; however, patients in the rituximab plus prednisone arm had a median dose of prednisone 0 mg at month 6 and for the remainder of the 24‐month treatment duration (Fig. 2). Median cumulative prednisone exposure at month 24 was 5800 mg (2304–29 303) in the rituximab plus prednisone arm compared with 20 520 mg (2409–60 565) in the prednisone‐alone arm. The number of patients in the rituximab plus prednisone arm off prednisone or on minimal therapy (prednisone dose ≤ 10 mg per day) compared with the prednisone‐alone arm over the 24‐month treatment period showed a steroid‐sparing effect of rituximab (Fig. 3). CRmin (inclusive of prednisone dose 0 mg per day) ≥ 2 months at month 24 was higher in the rituximab plus prednisone arm compared with the prednisone‐alone arm (Table 2). Clinical images of patients treated with rituximab are shown in Figure 4.
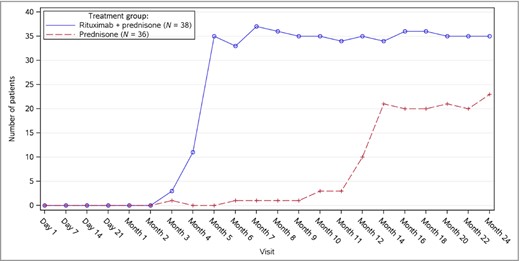
Number of patients who were on or off minimal corticosteroid (≤ 10 mg per day) therapy over time.
![Pemphigus vulgaris (PV). (a) Oral involvement in a patient with severe PV at baseline [Pemphigus Disease Area Index (PDAI) 38] and (b) at month 4 (PDAI 2) after treatment with rituximab plus prednisone. (c) Oral mucosal involvement in a patient with severe PV before and (d) at month 2 after treatment with rituximab plus prednisone.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/bjd/182/5/10.1111_bjd.18482/3/m_bjd18482-fig-0004.jpeg?Expires=1750187551&Signature=grbbQcaEek7Mc0~XY~YQtMvJUCKhcd79wtjBOoVvYXFbkvFn34O5WDh6OELiEP09TErU17p-WpiyrzZ0cSQdGocoHxURtstjMEIiALQ~tnJdPb9htR5e~f66ZqMigkYy6309fvPHQRRR-3GysA0LAYo6QabF55hiUCst7pP-S3lL~HyZROMiefw~L3~2mwSTebUIBY10i7VlBaY9Z9gSjEhFXZ8gaWDId9BOl6gKO3Hoz9MERKhEoWORnsyBlI4VNJOQuW2pVQ9zikIat2-1ovoOL3MHMmC55heJDYSaTI7WgGHCaPV2RQYMLxAyrDgum4vyjKKP4dIZNX7FwnCrJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Pemphigus vulgaris (PV). (a) Oral involvement in a patient with severe PV at baseline [Pemphigus Disease Area Index (PDAI) 38] and (b) at month 4 (PDAI 2) after treatment with rituximab plus prednisone. (c) Oral mucosal involvement in a patient with severe PV before and (d) at month 2 after treatment with rituximab plus prednisone.
Safety
Treatment‐related AEs that occurred at a rate of ≥ 5% among patients in either treatment arm are shown in Table S2 (see Supporting Information). Treatment‐related AEs reported in ≥ 15% of patients in the rituximab plus prednisone arm included IRRs, insomnia, depression and Cushing syndrome; in the prednisone‐alone arm, these AEs included insomnia, weight increase, Cushing syndrome, bronchitis, tremor, arthralgia, asthenia, agitation and muscle disorder. In the rituximab plus prednisone arm, 11 of 38 patients (29%) experienced 28 grade 3 treatment‐related AEs and two of 38 patients (5%) experienced seven grade 4 treatment‐related AEs. In the prednisone‐alone arm, 19 of 36 patients (53%) experienced 53 grade 3 AEs and three of 36 patients (8%) experienced four grade 4 AEs. The grade 3 and 4 treatment‐related AEs that occurred in ≥ 10% of patients in either treatment arm included Cushing syndrome [six of 38 (16%) vs. seven of 36 (19%)], muscle disorder [one of 38 (3%) vs. seven of 36 (19%)], myopathy [0% vs. four of 38 (11%)] and weight increase [0% vs. four of 38 (11%)].
SAEs occurred through month 24 in 11 patients (29%) treated with rituximab plus prednisone and in 16 patients (44%) treated with prednisone alone (Table 3). One patient treated with rituximab plus prednisone withdrew owing to pregnancy. In the prednisone‐alone arm, eight patients (22%) withdrew from treatment owing to 10 SAEs, including necrosis of the femoral heads, CS‐induced myopathy, Cushing syndrome, weight increase, psychiatric decompensation and chorioretinitis. No patients died during the study.
Serious adverse events (SAEs)5 by system organ class and preferred term in patients with pemphigus vulgaris up to month 24
| System organ class, preferred term | Rituximab + prednisone (n = 38) | Prednisone alone (n = 36) |
| Patients with at least one SAE, n (%) | 11 (29) | 16 (44) |
| Total number of SAEs | 26 | 24 |
| Musculoskeletal and connective tissue disorders, patients, n (%) | 5 (13) | 4 (11) |
| Myopathy | 1 (3) | 2 (6) |
| Lumbar spinal stenosis | 0 | 1 (3) |
| Osteonecrosis | 0 | 1 (3) |
| Tendonitis | 0 | 1 (3) |
| Arthralgia | 1 (3) | 0 |
| Myalgia | 1 (3) | 0 |
| Osteoporotic fracture | 1 (3) | 0 |
| Psoriatic arthropathy | 1 (3) | 0 |
| Total number of events | 5 | 5 |
| Respiratory, thoracic and mediastinal disorders, patients n (%) | 3 (8) | 3 (8) |
| Pulmonary embolism | 2 (5) | 2 (6) |
| Dyspnoea | 0 | 1 (3) |
| Nasal septum perforation | 1 (3) | 0 |
| Total number of events | 3 | 3 |
| Infections and infestations, patients, n (%) | 3 (8) | 1 (3) |
| Pneumocystis jirovecii pneumonia | 1 (3) | 1 (3) |
| Infective thrombosis | 1 (3) | 0 |
| Intervertebral discitis | 1 (3) | 0 |
| Lung infection | 1 (3) | 0 |
| Staphylococcal sepsis | 1 (3) | 0 |
| Total number of events | 5 | 1 |
| Nervous system disorders, patients, n (%) | 2 (5) | 3 (8) |
| Cerebrovascular accident | 0 | 1 (3) |
| Motor dysfunction | 0 | 1 (3) |
| Sciatica | 0 | 1 (3) |
| Headache | 1 (3) | 0 |
| Neuropathy peripheral | 1 (3) | 0 |
| Total number of events | 2 | 3 |
| Vascular disorders, patients, n (%) | 2 (5) | 3 (8) |
| Deep vein thrombosis | 0 | 1 (3) |
| Hypertension | 0 | 1 (3) |
| Phlebitis | 0 | 1 (3) |
| Hypotension | 1 (3) | 0 |
| Venous thrombosis limb | 1 (3) | 0 |
| Total number of events | 2 | 3 |
| System organ class, preferred term | Rituximab + prednisone (n = 38) | Prednisone alone (n = 36) |
| Patients with at least one SAE, n (%) | 11 (29) | 16 (44) |
| Total number of SAEs | 26 | 24 |
| Musculoskeletal and connective tissue disorders, patients, n (%) | 5 (13) | 4 (11) |
| Myopathy | 1 (3) | 2 (6) |
| Lumbar spinal stenosis | 0 | 1 (3) |
| Osteonecrosis | 0 | 1 (3) |
| Tendonitis | 0 | 1 (3) |
| Arthralgia | 1 (3) | 0 |
| Myalgia | 1 (3) | 0 |
| Osteoporotic fracture | 1 (3) | 0 |
| Psoriatic arthropathy | 1 (3) | 0 |
| Total number of events | 5 | 5 |
| Respiratory, thoracic and mediastinal disorders, patients n (%) | 3 (8) | 3 (8) |
| Pulmonary embolism | 2 (5) | 2 (6) |
| Dyspnoea | 0 | 1 (3) |
| Nasal septum perforation | 1 (3) | 0 |
| Total number of events | 3 | 3 |
| Infections and infestations, patients, n (%) | 3 (8) | 1 (3) |
| Pneumocystis jirovecii pneumonia | 1 (3) | 1 (3) |
| Infective thrombosis | 1 (3) | 0 |
| Intervertebral discitis | 1 (3) | 0 |
| Lung infection | 1 (3) | 0 |
| Staphylococcal sepsis | 1 (3) | 0 |
| Total number of events | 5 | 1 |
| Nervous system disorders, patients, n (%) | 2 (5) | 3 (8) |
| Cerebrovascular accident | 0 | 1 (3) |
| Motor dysfunction | 0 | 1 (3) |
| Sciatica | 0 | 1 (3) |
| Headache | 1 (3) | 0 |
| Neuropathy peripheral | 1 (3) | 0 |
| Total number of events | 2 | 3 |
| Vascular disorders, patients, n (%) | 2 (5) | 3 (8) |
| Deep vein thrombosis | 0 | 1 (3) |
| Hypertension | 0 | 1 (3) |
| Phlebitis | 0 | 1 (3) |
| Hypotension | 1 (3) | 0 |
| Venous thrombosis limb | 1 (3) | 0 |
| Total number of events | 2 | 3 |
Only those SAEs in a system organ class with at least two patients overall are listed. An SAE is any adverse event that results in the death of a patient, is potentially life‐threatening, requires hospitalization (> 24 h) or a prolongation of initial hospitalization, results in a disability or significant prolonged incapacitation, leads to a congenital abnormality or birth defect, or is any other adverse effect judged to be medically significant by the investigator reporting the event.
Serious adverse events (SAEs)5 by system organ class and preferred term in patients with pemphigus vulgaris up to month 24
| System organ class, preferred term | Rituximab + prednisone (n = 38) | Prednisone alone (n = 36) |
| Patients with at least one SAE, n (%) | 11 (29) | 16 (44) |
| Total number of SAEs | 26 | 24 |
| Musculoskeletal and connective tissue disorders, patients, n (%) | 5 (13) | 4 (11) |
| Myopathy | 1 (3) | 2 (6) |
| Lumbar spinal stenosis | 0 | 1 (3) |
| Osteonecrosis | 0 | 1 (3) |
| Tendonitis | 0 | 1 (3) |
| Arthralgia | 1 (3) | 0 |
| Myalgia | 1 (3) | 0 |
| Osteoporotic fracture | 1 (3) | 0 |
| Psoriatic arthropathy | 1 (3) | 0 |
| Total number of events | 5 | 5 |
| Respiratory, thoracic and mediastinal disorders, patients n (%) | 3 (8) | 3 (8) |
| Pulmonary embolism | 2 (5) | 2 (6) |
| Dyspnoea | 0 | 1 (3) |
| Nasal septum perforation | 1 (3) | 0 |
| Total number of events | 3 | 3 |
| Infections and infestations, patients, n (%) | 3 (8) | 1 (3) |
| Pneumocystis jirovecii pneumonia | 1 (3) | 1 (3) |
| Infective thrombosis | 1 (3) | 0 |
| Intervertebral discitis | 1 (3) | 0 |
| Lung infection | 1 (3) | 0 |
| Staphylococcal sepsis | 1 (3) | 0 |
| Total number of events | 5 | 1 |
| Nervous system disorders, patients, n (%) | 2 (5) | 3 (8) |
| Cerebrovascular accident | 0 | 1 (3) |
| Motor dysfunction | 0 | 1 (3) |
| Sciatica | 0 | 1 (3) |
| Headache | 1 (3) | 0 |
| Neuropathy peripheral | 1 (3) | 0 |
| Total number of events | 2 | 3 |
| Vascular disorders, patients, n (%) | 2 (5) | 3 (8) |
| Deep vein thrombosis | 0 | 1 (3) |
| Hypertension | 0 | 1 (3) |
| Phlebitis | 0 | 1 (3) |
| Hypotension | 1 (3) | 0 |
| Venous thrombosis limb | 1 (3) | 0 |
| Total number of events | 2 | 3 |
| System organ class, preferred term | Rituximab + prednisone (n = 38) | Prednisone alone (n = 36) |
| Patients with at least one SAE, n (%) | 11 (29) | 16 (44) |
| Total number of SAEs | 26 | 24 |
| Musculoskeletal and connective tissue disorders, patients, n (%) | 5 (13) | 4 (11) |
| Myopathy | 1 (3) | 2 (6) |
| Lumbar spinal stenosis | 0 | 1 (3) |
| Osteonecrosis | 0 | 1 (3) |
| Tendonitis | 0 | 1 (3) |
| Arthralgia | 1 (3) | 0 |
| Myalgia | 1 (3) | 0 |
| Osteoporotic fracture | 1 (3) | 0 |
| Psoriatic arthropathy | 1 (3) | 0 |
| Total number of events | 5 | 5 |
| Respiratory, thoracic and mediastinal disorders, patients n (%) | 3 (8) | 3 (8) |
| Pulmonary embolism | 2 (5) | 2 (6) |
| Dyspnoea | 0 | 1 (3) |
| Nasal septum perforation | 1 (3) | 0 |
| Total number of events | 3 | 3 |
| Infections and infestations, patients, n (%) | 3 (8) | 1 (3) |
| Pneumocystis jirovecii pneumonia | 1 (3) | 1 (3) |
| Infective thrombosis | 1 (3) | 0 |
| Intervertebral discitis | 1 (3) | 0 |
| Lung infection | 1 (3) | 0 |
| Staphylococcal sepsis | 1 (3) | 0 |
| Total number of events | 5 | 1 |
| Nervous system disorders, patients, n (%) | 2 (5) | 3 (8) |
| Cerebrovascular accident | 0 | 1 (3) |
| Motor dysfunction | 0 | 1 (3) |
| Sciatica | 0 | 1 (3) |
| Headache | 1 (3) | 0 |
| Neuropathy peripheral | 1 (3) | 0 |
| Total number of events | 2 | 3 |
| Vascular disorders, patients, n (%) | 2 (5) | 3 (8) |
| Deep vein thrombosis | 0 | 1 (3) |
| Hypertension | 0 | 1 (3) |
| Phlebitis | 0 | 1 (3) |
| Hypotension | 1 (3) | 0 |
| Venous thrombosis limb | 1 (3) | 0 |
| Total number of events | 2 | 3 |
Only those SAEs in a system organ class with at least two patients overall are listed. An SAE is any adverse event that results in the death of a patient, is potentially life‐threatening, requires hospitalization (> 24 h) or a prolongation of initial hospitalization, results in a disability or significant prolonged incapacitation, leads to a congenital abnormality or birth defect, or is any other adverse effect judged to be medically significant by the investigator reporting the event.
The onset of grade 4 AEs and most frequent grade 3 events (≥ 5% of patients) are shown in Figure S3 (see Supporting Information).
Infusion‐related reactions
Overall, 22 patients (58%) in the rituximab plus prednisone arm experienced at least one IRR at any time during the study, none of which were fatal or led to discontinuation of rituximab. IRRs occurred primarily at the first [11 patients (29%)] and second infusions [15 patients (40%)], and the incidence decreased to 13% and 11% for the third and fourth infusions, respectively. All IRRs were mild to moderate (grades 1 or 2) except for one grade 3 SAE (arthralgia). No patients withdrew owing to IRRs.
Infections
Treatment‐related infections occurred in 14 patients (37%) in the rituximab plus prednisone arm and 15 patients (42%) in the prednisone‐alone arm. The most frequent infections (≥ 5% in either treatment arm) were bronchitis [three of 38 (8%) vs. seven of 36 (19%)], herpes simplex infection [five of 38 (13%) vs. one of 36 (3%)], urinary tract infection [two of 38 (5%) vs. three of 36 (8%)], fungal infection [two of 38 (5%) vs. two of 36 (6%)], skin bacterial infection [one of 38 (3%) vs. three of 36 (8%)], herpes zoster infection [two of 38 (5%) vs. one of 36 (3%)] and conjunctivitis [two of 38 (5%) vs. 0]. Three patients (8%) in the rituximab plus prednisone arm experienced five serious infections [one patient with Pneumocystis jirovecii pneumonia (no pneumocystis pneumonia prophylaxis was given in the trial); one patient with infective thrombosis, intervertebral discitis and staphylococcal sepsis and one patient with lung infection], and one patient (3%) in the prednisone‐alone arm experienced one serious infection (P. jirovecii pneumonia).
Corticosteroid‐related adverse events
The number of patients with at least one CS‐related AE was similar in both treatment arms [31 of 38 patients (82%) in the rituximab plus prednisone arm and 31 of 36 patients (86%) in the prednisone‐alone arm]; however, fewer patients treated with rituximab plus prednisone experienced grade 3 (severe) or 4 (life‐threatening) CS‐related AEs than patients treated with prednisone alone [13 of 38 (34%) and 24 of 36 (67%), respectively]. The difference between treatment arms was mainly due to more patients who received prednisone experiencing grade 3 or grade 4 myopathy, muscle disorder and weight increase. The majority of grade 3 or grade 4 CS‐related AEs in both treatment arms occurred within the first 9 months of treatment. In the rituximab plus prednisone arm, none of the CS‐related AEs led to study withdrawal. In the prednisone‐alone arm, six patients were withdrawn owing to CS‐related AEs.
Immunogenicity
A total of 34 patients in the rituximab plus prednisone arm had stored serum samples at baseline and at least one time point postbaseline, which were evaluable and analysed retrospectively for ADA status (methods described in File S1; see Supporting Information). Overall, 20 patients (59%) had at least one positive ADA titre at any time before month 24. Of these 20 patients who were ADA positive, 17 patients (85%) achieved the primary end point. Seven of 20 (35%) experienced an IRR after testing positive for ADA compared with six of 14 patients (43%) who were ADA negative. No severe or serious IRRs occurred in patients with a positive ADA titre.
Discussion
This manuscript presents a unique and successful collaboration between academia and industry using investigator‐initiated trial data from patients with PV. The efficacy and safety data in the subset of patients with PV provide more detailed analyses of patient outcomes that extend the published findings of Joly et al.14 These analyses led to the FDA and the European Commission granting approval for the use of rituximab as a treatment in patients with moderate‐to‐severe PV.12
A higher proportion of patients with moderate‐to‐severe PV on rituximab and short‐term low‐dose CS achieved CR and successfully tapered off CS for ≥ 2 months and ≥ 3 months at month 24 compared with patients receiving a standard high‐dose and long‐term CS regimen. Patients treated with rituximab plus prednisone had a longer median duration of CRoff ≥ 2 months. The number and frequency of relapses and the number of patients experiencing severe or moderate relapse were lower in the rituximab plus prednisone arm than in the prednisone‐alone arm. Patient sex had no effect on the proportion of patients achieving CRoff ≥ 2 months at month 24. ADA status appeared to have no impact on the achievement of CRoff ≥ 2 months at month 24.
B‐cell depletion and therefore reduction of anti‐Dsg1 and anti‐Dsg3 autoantibodies were supportive for the mode of action of rituximab in PV (File S1; see Supporting Information).
Rituximab was well tolerated in patients with PV and had a steroid‐sparing effect. Fewer patients in the rituximab plus prednisone arm developed grade 3 or 4 CS‐related AEs than in the standard dose prednisone‐alone arm. The greater frequency and number of grade 3 and grade 4 events related to CS in the prednisone‐alone arm can be explained by the greater prednisone exposure (dose and duration). Treatment discontinuations owing to CS‐related AEs were reported in the prednisone‐alone arm only.
No new safety concerns were identified. The safety profile of rituximab, including occurrence and severity of infection, was consistent with its known profile in the approved autoimmune indications (rheumatoid arthritis, granulomatosis with polyangiitis and microscopic polyangiitis).12 Although more patients in the rituximab plus prednisone arm experienced serious infections than in the prednisone‐alone arm, these infections were manageable and did not result in treatment discontinuation. The nature and severity of IRR symptoms of rituximab were similar to those previously reported for autoimmune indications and occurred primarily at the first and second infusions. Similar proportions of patients who were ADA positive and patients who were ADA negative experienced IRRs. The presence of ADA did not appear to impact the safety and efficacy of rituximab in patients with PV.
This study has its limitations. Firstly, although the study had an open‐label design and the standard dose and duration prednisone regimen could have been favoured over the low‐dose prednisone plus rituximab regimen, the efficacy results showed greater benefit in patients who were treated with rituximab plus prednisone. Furthermore, sensitivity analyses were conducted to assess the robustness of the efficacy results (described in File S1; see Supporting Information) and showed that bias is unlikely to explain the large treatment effect of rituximab compared with prednisone. Secondly, IRRs were derived from retrospective reporting of symptoms of intolerance, and the conservative approach of identifying all symptoms of intolerance and AEs occurring on the day of infusion or 1 day after infusion as IRRs may have led to the overestimation of patients with at least one IRR. More patients experiencing an IRR at the second infusion may have been influenced by the fact that AEs were first documented with the second infusion and were reported retrospectively for the first infusion. A third limitation is that although related and unrelated SAEs were reported, only nonserious AEs considered to be treatment‐related were recorded. Finally, comparisons of the incidence of antibodies to rituximab between our immunogenicity assay and a different assay used in previous trials in different patient populations are not possible because of differences in assay sensitivity and specificity, sample handling, collection time, post hoc analysis of frozen serum samples, and/or other factors.
In conclusion, the treatment regimen of rituximab plus prednisone compared with prednisone alone showed the compelling efficacy of rituximab at achieving CRoff ≥ 2 months in patients with PV. Rituximab had clinically meaningful benefit and allowed for a steroid‐sparing regimen of lower cumulative doses of prednisone over a shorter duration. Fewer patients treated with rituximab experienced severe and life‐threatening CS‐related AEs than patients treated with standard dose and duration prednisone. ADA positivity did not appear to have a negative impact on efficacy or safety outcomes. No new safety concerns with rituximab were identified in patients with moderate‐to‐severe PV.
Acknowledgments
We thank the investigators in the French Study Group on Autoimmune Bullous Diseases who designed the Ritux 3 study and collected data on their study patients, and the patients who participated in the Ritux 3 study. We would like to thank Rouen University who allowed Roche to have access to the study database and supporting documents and we also thank Everest Clinical Research for assisting Roche with the independently conducted data analysis and review of document translations. Support for third‐party writing assistance for this manuscript, furnished by Health Interactions Inc., was provided by F. Hoffmann‐La Roche.
References
Author notes
Funding sources This research was funded by F. Hoffmann‐La Roche, the French Ministry of Health and the French Society of Dermatology.
Conflicts of interest D.M.C. and M.C. are employees of Genentech. A.O., E.C., L.G. and P.L. are employees of Roche. P.J. is a consultant for Roche, GSK, Lilly, Principia Biopharma and Sanofi Aventis. F.C. is a consultant for Pierre Fabre Dermatologie and Principia Biopharma. P.M. is a consultant for Servier and the Singapore Immunology Network. D.J. is a consultant for AbbVie, Celgene, Novartis, Lilly, Janssen Cilag, Pfizer and Merck Sharpe & Dohme.



