-
PDF
- Split View
-
Views
-
Cite
Cite
K. Reich, M. Augustin, D. Thaçi, A. Pinter, A. Leutz, C. Henneges, E. Schneider, A. Schacht, M. Dossenbach, U. Mrowietz, A 24‐week multicentre, randomized, open‐label, parallel‐group study comparing the efficacy and safety of ixekizumab vs. fumaric acid esters and methotrexate in patients with moderate‐to‐severe plaque psoriasis naive to systemic treatment, British Journal of Dermatology, Volume 182, Issue 4, 1 April 2020, Pages 869–879, https://doi.org/10.1111/bjd.18384
Close - Share Icon Share
Summary
Interleukin‐17 antagonists have received a first‐line label for moderate‐to‐severe plaque psoriasis.
We conducted the first head‐to‐head trial between the two most commonly used first‐line therapies in Germany, fumaric acid esters (FAEs) and methotrexate, and the interleukin‐17A antagonist, ixekizumab.
Systemic‐naive patients were randomized in this parallel‐group, active‐comparator, open‐label, rater‐blinded trial (each group n = 54). The primary outcome was the proportion of patients achieving ≥ 75% improvement in Psoriasis Area and Severity Index (PASI 75) at 24 weeks. Key secondary outcomes included 24‐week PASI 90 and 100, static Physician's Global Assessment (sPGA) score of 0 or 1, and Dermatology Life Quality Index (DLQI) score of 0 or 1. Safety events at week 24 were analysed using Fisher's exact test. Missing data were imputed using nonresponder imputation. The trial was registered at ClinicalTrials.gov (NCT02634801) and EudraCT (2015‐002649‐69).
At week 24, more ixekizumab‐treated patients achieved PASI 75 [91% vs. 22% FAEs (P < 0·001) and 70% methotrexate (P = 0·014)], PASI 90 [80% vs. 9% FAEs (P < 0·001) and 39% methotrexate (P < 0·001)] and PASI 100 [41% vs. 4% FAEs (P < 0·001) and 13% methotrexate (P = 0·0041)], as well as sPGA (0,1) and DLQI (0,1).
Ixekizumab was superior in inducing PASI 75/90/100, sPGA (0,1) and DLQI (0,1) responses at week 24 compared with methotrexate and FAEs. Safety profiles for all treatments were consistent with prior studies.
In contrast to the U.S. Food and Drug Administration, the European Medicines Agency (EMA) introduced targeted therapies for the treatment of moderate‐to‐severe plaque psoriasis with a second‐line label. These include the tumour necrosis factor‐antagonizing molecules adalimumab, etanercept and infliximab; the interleukin (IL)‐12/23p40 inhibitor ustekinumab; and the phosphodiesterase 4 inhibitor apremilast. Consequently, European treatment guidelines have traditionally recommended conventional systemic therapies as first‐line and biologics as second‐line treatments,1 and head‐to‐head trials of biologics against conventional therapies were not deemed necessary and were rarely designed. The CHAMPION and RESTORE studies were exceptions and demonstrated that biologic agents such as adalimumab and infliximab provide higher levels of sustained clinical benefit with an acceptable safety profile compared with a conventional systemic therapy.2,3 In 2014 and 2016, the EMA granted first‐line labels to the IL‐17A antagonists secukinumab and ixekizumab, respectively, and, more recently, to the IL‐17A antagonist brodalumab and the IL‐23 receptor antagonist guselkumab, probably reflecting a different view of the risk–benefit profile of these therapies.4,5
Based on these developments, we intended to reassess the value of the recently labelled, targeted therapy ixekizumab, compared with two conventional therapies, methotrexate and fumaric acid esters (FAEs). Here we present data from a direct head‐to‐head trial, which should be informative for the future refinement of systemic treatment algorithms in psoriasis. These two drugs were selected due to their prominent roles among first‐line conventional therapies for the disease. Methotrexate is the most frequently prescribed systemic agent for psoriasis treatment in many European countries and has received renewed interest due to novel data on its clinical2,3 and immunological effects.6 FAEs have been an approved treatment for plaque psoriasis in Germany since 1994 (Fumaderm®), but, due to their attractive long‐term potential and unique mode of action,7 they are also being used as an unlicensed treatment in several European countries.8 Recently, a dimethyl fumarate‐only product (LAS41008) was approved by the European Commission based on a comparator trial with Fumaderm,9 and it is indicated as a first‐line induction and long‐term maintenance treatment for moderate‐to‐severe psoriasis.10
Patients and methods
Study design and participants
This was a phase IIIb, randomized, open‐label, rater‐blinded, active‐comparator, parallel‐group trial conducted at 28 study sites in Germany between January and December 2016. The trial included (i) a screening period lasting ≤ 30 days, (ii) a 24‐week treatment period, (iii) a 36‐week extension period and (iv) a post‐treatment period occurring from the last treatment visit up to a minimum of 12 weeks (Fig. S1; see Supporting Information).
Eligible participants were ≥ 18 years old, had a confirmed diagnosis of chronic moderate‐to‐severe plaque psoriasis for ≥ 6 months before baseline, were naive to systemic treatment (except phototherapy) for psoriasis, and were eligible for systemic treatment according to European guidelines.
Study protocols and informed consent forms were approved by Ethik‐Kommission der Medizinischen Fakultät der Christian‐Albrechts‐Universität zu Kiel, and all patients signed informed consent before undergoing study‐related procedures. The trial was conducted in accordance with the Declaration of Helsinki.11
Randomization and blinding
Patients were randomized 1 : 1 : 1 to FAEs, methotrexate or ixekizumab via an interactive web response system. To ensure that FAE and methotrexate treatments were given according to labels and according to clinical practice (e.g. dose adjustment due to adverse events), the study was conducted open label. Both patients and investigators were unblinded to treatment allocation. A blinded rater assessed all clinical outcome measures to minimize bias for the clinical efficacy assessments of each treatment arm (Appendix 2).
Procedures
All FAE treatments were given according to the summary of product characteristics (SPC).12 Fumaderm® Initial (Biogen Idec GmbH, Ismaning, Germany; one tablet containing 30 mg dimethyl fumarate) was given for 3 weeks according to the SPC stepwise dosing increase (one tablet per day first week, two tablets per day second week, three tablets per day third week). Treatment was then continued with Fumaderm (Biogen Idec GmbH; one tablet containing 120 mg dimethyl fumarate) following a similar stepwise uptitration scheme (one tablet per day first week, two tablets per day second week, until up to six tablets per day in the ninth week). If an adverse event occurred, a dose reduction was considered at the investigator's discretion and according to the SPC, or treatment was ultimately discontinued. If deemed appropriate by the investigator, the daily dosage could be reduced in a stepwise manner to an individual maintenance dose; maintenance therapy was then given according to the SPC.
All methotrexate (Metex®; medac GmbH, Wedel, Germany) treatments were given according to the SPC.13 In addition, the European recommendations14 and the German guidelines1 were considered to reflect the current practice of methotrexate dosing according to efficacy and tolerability. Methotrexate was administered as oral tablets, starting with 7·5 mg per week, with a stepwise increase in dosage of 5–7·5 mg per week until a dose of ≥ 15 mg per week was reached. If ≥ 50% improvement in Psoriasis Area and Severity Index (PASI 50) was not achieved at week 8, the dose was increased to 20 mg per week. If PASI 75 improvement was not achieved at week 16, the dose was increased to 25 mg per week. A maximum dose of 30 mg per week was allowed according to the discretion of the investigator. If an adverse event occurred, a dose reduction was considered at the investigator's discretion and according to the SPC, or treatment was ultimately discontinued. After achievement of the desired clinical response (PASI 75), the dose could then be decreased in a stepwise manner, if considered appropriate by the investigator, until an effective maintenance dose was reached. Patients taking methotrexate were permitted to receive concomitant folic acid orally; the local standard‐of‐care recommendation was 5 mg per week, 24 h after receiving methotrexate.
A 160‐mg starting dose of ixekizumab was given as two subcutaneous (SC) injections, followed by 80 mg given as one SC injection every 2 weeks (weeks 2, 4, 6, 8, 10 and 12) and then 80 mg given as one SC injection every 4 weeks (weeks 16, 20 and 24).
Patients were instructed to provide information regarding prescribed topical treatments (Table 1). In Germany, especially in this early patient population, it is common to use nonprescription topicals.
Baseline patient demographics and clinical characteristics, intention‐to‐treat (ITT) populationa
| FAEs (N = 54) | MTX (N = 54) | IXE (N = 54) | Total (N = 162) | |
| Demographics | ||||
| Age (years), mean ± SD | 43·1 ± 14·2 | 38·7 ± 12·9 | 44·3 ± 13·8 | 42·1 ± 13·8 |
| Patients aged ≥ 65 years, n (%) | 3 (6) | 2 (4) | 3 (6) | 8 (4·9) |
| Weight (kg), mean ± SD | 92·8 ± 21·6 | 90·2 ± 23·8 | 86·3 ± 17·9 | 89·8 ± 21·3 |
| Weight > 100 kg, n (%) | 18 (33) | 18 (33) | 8 (15) | 44 (27·2) |
| BMI (kg m−2), mean ± SD | 29·6 ± 6·1 | 29·3 ± 7·0 | 27·8 ± 5·2 | 28·9 ± 6·1 |
| Male, n (%) | 43 (80) | 36 (67) | 42 (78) | 121 (74·7) |
| White, n (%) | 44 (81) | 42 (78) | 43 (80) | 129 (79·6) |
| Disease characteristics | ||||
| PASI, mean ± SD | 19·8 ± 9·0 | 17·8 ± 7·1 | 18·8 ± 8·3 | 18·8 ± 8·1 |
| Baseline PASI ≥ 20, n (%) | 23 (43) | 19 (35) | 20 (37) | 62 (38·3) |
| sPGA, n (%) | ||||
| 0 or 1 | 0 | 0 | 0 | 0 |
| 2 | 1 (2) | 2 (4) | 2 (4) | 5 (3·1) |
| 3 | 23 (43) | 26 (48) | 27 (50) | 76 (46·9) |
| 4 or 5 | 30 (56) | 26 (48) | 25 (46) | 81 (50·0) |
| % BSA, mean ± SD | 23·8 ± 16·3 | 25·3 ± 15·4 | 25·1 ± 16·2 | 24·7 ± 15·9 |
| DLQI total score, mean ± SD | 16·4 ± 6·2 | 16·6 ± 5·3 | 15·1 ± 4·7 | 16·1 ± 5·4 |
| Age of onset ≥ 40 years, n (%) | 8 (15) | 9 (17) | 14 (26) | 31 (19·2) |
| Duration of psoriasis diagnosis (years), mean ± SD | 14·0 ± 14·0 | 12·9 ± 10·4 | 13·9 ± 13·4 | 13·6 ± 12·6 |
| Pretreatment for psoriasis | ||||
| Topical (prescribed), n (%)b | 39 (72) | 37 (69) | 41 (76) | 117 (72·2) |
| Phototherapy, n (%) | 24 (44) | 13 (24) | 28 (52) | 65 (40·1) |
| Psoralen–ultraviolet A, n (%) | 9 (17) | 5 (9) | 8 (15) | 22 (13·6) |
| Ultraviolet B, n (%) | 15 (28) | 7 (13) | 14 (26) | 36 (22·2) |
| FAEs (N = 54) | MTX (N = 54) | IXE (N = 54) | Total (N = 162) | |
| Demographics | ||||
| Age (years), mean ± SD | 43·1 ± 14·2 | 38·7 ± 12·9 | 44·3 ± 13·8 | 42·1 ± 13·8 |
| Patients aged ≥ 65 years, n (%) | 3 (6) | 2 (4) | 3 (6) | 8 (4·9) |
| Weight (kg), mean ± SD | 92·8 ± 21·6 | 90·2 ± 23·8 | 86·3 ± 17·9 | 89·8 ± 21·3 |
| Weight > 100 kg, n (%) | 18 (33) | 18 (33) | 8 (15) | 44 (27·2) |
| BMI (kg m−2), mean ± SD | 29·6 ± 6·1 | 29·3 ± 7·0 | 27·8 ± 5·2 | 28·9 ± 6·1 |
| Male, n (%) | 43 (80) | 36 (67) | 42 (78) | 121 (74·7) |
| White, n (%) | 44 (81) | 42 (78) | 43 (80) | 129 (79·6) |
| Disease characteristics | ||||
| PASI, mean ± SD | 19·8 ± 9·0 | 17·8 ± 7·1 | 18·8 ± 8·3 | 18·8 ± 8·1 |
| Baseline PASI ≥ 20, n (%) | 23 (43) | 19 (35) | 20 (37) | 62 (38·3) |
| sPGA, n (%) | ||||
| 0 or 1 | 0 | 0 | 0 | 0 |
| 2 | 1 (2) | 2 (4) | 2 (4) | 5 (3·1) |
| 3 | 23 (43) | 26 (48) | 27 (50) | 76 (46·9) |
| 4 or 5 | 30 (56) | 26 (48) | 25 (46) | 81 (50·0) |
| % BSA, mean ± SD | 23·8 ± 16·3 | 25·3 ± 15·4 | 25·1 ± 16·2 | 24·7 ± 15·9 |
| DLQI total score, mean ± SD | 16·4 ± 6·2 | 16·6 ± 5·3 | 15·1 ± 4·7 | 16·1 ± 5·4 |
| Age of onset ≥ 40 years, n (%) | 8 (15) | 9 (17) | 14 (26) | 31 (19·2) |
| Duration of psoriasis diagnosis (years), mean ± SD | 14·0 ± 14·0 | 12·9 ± 10·4 | 13·9 ± 13·4 | 13·6 ± 12·6 |
| Pretreatment for psoriasis | ||||
| Topical (prescribed), n (%)b | 39 (72) | 37 (69) | 41 (76) | 117 (72·2) |
| Phototherapy, n (%) | 24 (44) | 13 (24) | 28 (52) | 65 (40·1) |
| Psoralen–ultraviolet A, n (%) | 9 (17) | 5 (9) | 8 (15) | 22 (13·6) |
| Ultraviolet B, n (%) | 15 (28) | 7 (13) | 14 (26) | 36 (22·2) |
BMI, body mass index; BSA, body surface area (affected by psoriasis); DLQI, Dermatology Life Quality Index; FAEs, fumaric acid esters; IXE, ixekizumab; MTX, methotrexate; PASI, Psoriasis Area and Severity Index; sPGA, static Physician's Global Assessment. aThe denominator for the reported proportions is based on the number of ITT patients. Patients may have missing baseline information. bRefers to whether the patient was ever exposed to prescribed topical therapy.
Baseline patient demographics and clinical characteristics, intention‐to‐treat (ITT) populationa
| FAEs (N = 54) | MTX (N = 54) | IXE (N = 54) | Total (N = 162) | |
| Demographics | ||||
| Age (years), mean ± SD | 43·1 ± 14·2 | 38·7 ± 12·9 | 44·3 ± 13·8 | 42·1 ± 13·8 |
| Patients aged ≥ 65 years, n (%) | 3 (6) | 2 (4) | 3 (6) | 8 (4·9) |
| Weight (kg), mean ± SD | 92·8 ± 21·6 | 90·2 ± 23·8 | 86·3 ± 17·9 | 89·8 ± 21·3 |
| Weight > 100 kg, n (%) | 18 (33) | 18 (33) | 8 (15) | 44 (27·2) |
| BMI (kg m−2), mean ± SD | 29·6 ± 6·1 | 29·3 ± 7·0 | 27·8 ± 5·2 | 28·9 ± 6·1 |
| Male, n (%) | 43 (80) | 36 (67) | 42 (78) | 121 (74·7) |
| White, n (%) | 44 (81) | 42 (78) | 43 (80) | 129 (79·6) |
| Disease characteristics | ||||
| PASI, mean ± SD | 19·8 ± 9·0 | 17·8 ± 7·1 | 18·8 ± 8·3 | 18·8 ± 8·1 |
| Baseline PASI ≥ 20, n (%) | 23 (43) | 19 (35) | 20 (37) | 62 (38·3) |
| sPGA, n (%) | ||||
| 0 or 1 | 0 | 0 | 0 | 0 |
| 2 | 1 (2) | 2 (4) | 2 (4) | 5 (3·1) |
| 3 | 23 (43) | 26 (48) | 27 (50) | 76 (46·9) |
| 4 or 5 | 30 (56) | 26 (48) | 25 (46) | 81 (50·0) |
| % BSA, mean ± SD | 23·8 ± 16·3 | 25·3 ± 15·4 | 25·1 ± 16·2 | 24·7 ± 15·9 |
| DLQI total score, mean ± SD | 16·4 ± 6·2 | 16·6 ± 5·3 | 15·1 ± 4·7 | 16·1 ± 5·4 |
| Age of onset ≥ 40 years, n (%) | 8 (15) | 9 (17) | 14 (26) | 31 (19·2) |
| Duration of psoriasis diagnosis (years), mean ± SD | 14·0 ± 14·0 | 12·9 ± 10·4 | 13·9 ± 13·4 | 13·6 ± 12·6 |
| Pretreatment for psoriasis | ||||
| Topical (prescribed), n (%)b | 39 (72) | 37 (69) | 41 (76) | 117 (72·2) |
| Phototherapy, n (%) | 24 (44) | 13 (24) | 28 (52) | 65 (40·1) |
| Psoralen–ultraviolet A, n (%) | 9 (17) | 5 (9) | 8 (15) | 22 (13·6) |
| Ultraviolet B, n (%) | 15 (28) | 7 (13) | 14 (26) | 36 (22·2) |
| FAEs (N = 54) | MTX (N = 54) | IXE (N = 54) | Total (N = 162) | |
| Demographics | ||||
| Age (years), mean ± SD | 43·1 ± 14·2 | 38·7 ± 12·9 | 44·3 ± 13·8 | 42·1 ± 13·8 |
| Patients aged ≥ 65 years, n (%) | 3 (6) | 2 (4) | 3 (6) | 8 (4·9) |
| Weight (kg), mean ± SD | 92·8 ± 21·6 | 90·2 ± 23·8 | 86·3 ± 17·9 | 89·8 ± 21·3 |
| Weight > 100 kg, n (%) | 18 (33) | 18 (33) | 8 (15) | 44 (27·2) |
| BMI (kg m−2), mean ± SD | 29·6 ± 6·1 | 29·3 ± 7·0 | 27·8 ± 5·2 | 28·9 ± 6·1 |
| Male, n (%) | 43 (80) | 36 (67) | 42 (78) | 121 (74·7) |
| White, n (%) | 44 (81) | 42 (78) | 43 (80) | 129 (79·6) |
| Disease characteristics | ||||
| PASI, mean ± SD | 19·8 ± 9·0 | 17·8 ± 7·1 | 18·8 ± 8·3 | 18·8 ± 8·1 |
| Baseline PASI ≥ 20, n (%) | 23 (43) | 19 (35) | 20 (37) | 62 (38·3) |
| sPGA, n (%) | ||||
| 0 or 1 | 0 | 0 | 0 | 0 |
| 2 | 1 (2) | 2 (4) | 2 (4) | 5 (3·1) |
| 3 | 23 (43) | 26 (48) | 27 (50) | 76 (46·9) |
| 4 or 5 | 30 (56) | 26 (48) | 25 (46) | 81 (50·0) |
| % BSA, mean ± SD | 23·8 ± 16·3 | 25·3 ± 15·4 | 25·1 ± 16·2 | 24·7 ± 15·9 |
| DLQI total score, mean ± SD | 16·4 ± 6·2 | 16·6 ± 5·3 | 15·1 ± 4·7 | 16·1 ± 5·4 |
| Age of onset ≥ 40 years, n (%) | 8 (15) | 9 (17) | 14 (26) | 31 (19·2) |
| Duration of psoriasis diagnosis (years), mean ± SD | 14·0 ± 14·0 | 12·9 ± 10·4 | 13·9 ± 13·4 | 13·6 ± 12·6 |
| Pretreatment for psoriasis | ||||
| Topical (prescribed), n (%)b | 39 (72) | 37 (69) | 41 (76) | 117 (72·2) |
| Phototherapy, n (%) | 24 (44) | 13 (24) | 28 (52) | 65 (40·1) |
| Psoralen–ultraviolet A, n (%) | 9 (17) | 5 (9) | 8 (15) | 22 (13·6) |
| Ultraviolet B, n (%) | 15 (28) | 7 (13) | 14 (26) | 36 (22·2) |
BMI, body mass index; BSA, body surface area (affected by psoriasis); DLQI, Dermatology Life Quality Index; FAEs, fumaric acid esters; IXE, ixekizumab; MTX, methotrexate; PASI, Psoriasis Area and Severity Index; sPGA, static Physician's Global Assessment. aThe denominator for the reported proportions is based on the number of ITT patients. Patients may have missing baseline information. bRefers to whether the patient was ever exposed to prescribed topical therapy.
Outcomes
The primary end point was assessment of the proportion of patients achieving PASI 75 at week 24 compared with baseline, to determine whether ixekizumab was superior to FAEs and to methotrexate. Key secondary end points at week 24 included the proportions of patients achieving PASI 90 and PASI 100 compared with baseline, absolute PASI ≤ 3, static Physician's Global Assessment (sPGA) score of 0 or 1 with ≥ 2‐point improvement from baseline, and Dermatology Life Quality Index (DLQI) score of 0 or 1.
Other secondary end points included firstly, the proportions of patients achieving sPGA 0, PASI ≤ 5, PASI ≤ 1, Itch Numeric Rating Scale (NRS) responder definition (decrease of ≥ 4 points in the Itch NRS in patients with baseline score ≥ 4 points), Itch NRS 0 and DLQI 0. Secondly, they included mean changes from baseline in the percentage of involved body surface area, Itch NRS, skin pain visual analogue scale (range 0–100), DLQI, Quick Inventory of Depressive Symptomatology‐Self Report (16 items) total score, Short Form (36‐item) Health Survey (both the physical and mental component summaries), Work Productivity Activity Impairment Questionnaire – Psoriasis, Nail Assessment in Psoriasis and Psoriatic Arthritis total score, EuroQol 5 Dimensions 5 Levels (EQ‐5D‐5L) ‘bolt on’ index, EuroQol 5D items and visual analogue scale, Palmoplantar Psoriasis Area Severity Index total score, Patient's Global Assessment of Disease Severity, and Psoriasis Scalp Severity Index total score. Thirdly, Psoriasis Skin Appearance Bothersomeness measure, Systemic Therapy Adherence Questionnaire score and Patient Benefit Index were measured.
Statistical analysis
The sample size was determined assuming a PASI 75 response rate of 60% for both FAEs and methotrexate2 and a 92% response rate for ixekizumab at week 24. Using Fisher's exact test for the two‐sided comparison of two proportions, and requiring a power of ≥ 97% and an alpha level of 5%, this required 54 patients per treatment group.
Primary treatment comparisons were analysed according to the assigned treatment regardless of compliance (intention to treat). Safety end points were analysed according to the treatment received in patients who took at least one dose (safety population).
Binary end points were tested using Fisher's exact test. Nonresponder imputation was used to impute patients with missing data. Continuous end points were tested using ancova with terms for treatment and baseline. Modified imputation using the baseline observation carried forward was used to impute missing values: patients who discontinued due to adverse events were imputed with their baseline observation. Patients discontinuing for any other reason were imputed with their last observation carried forward.
Primary comparisons of ixekizumab vs. FAEs and ixekizumab vs. methotrexate at week 24 regarding PASI 75 response were adjusted for multiple comparisons at a global 5% alpha level, using a prespecified Hochberg procedure.15,16 If both primary comparisons were met, a second Hochberg procedure was applied to protect the eight key secondary comparisons for inflated type I error, with two treatment comparisons for each of these end points: PASI 90, PASI 100, sPGA (0,1) and DLQI (0,1).
Safety end points were evaluated in the safety population using Fisher's exact test. A treatment‐emergent adverse event (TEAE) was defined as an event that first occurred or worsened in severity after baseline and on or before the date of the last visit within the treatment period. Rates for treatment discontinuations were compared using the log‐rank test.
Due to the high discontinuation rates observed in the FAE group, post hoc analyses for safety events were deemed necessary. Results include exposure‐adjusted incidence rates and rate ratios from Poisson regression models with a term for treatment and using an offset. A post hoc efficacy analysis was conducted for observed cases, and methotrexate vs. FAE comparisons were also completed post hoc.
Unless noted, P‐values were considered statistically significant at the 5% alpha level, and confidence intervals were two sided at the 95% level. Analyses were programmed using SAS 9·4 (SAS Institute Inc., Cary, NC, U.S.A.).
Results
In total 162 patients were randomized (Fig. 1). Discontinuation rates were significantly higher for FAEs (n = 31, 57%) vs. ixekizumab (n = 5, 9%) (log‐rank test, P < 0·001), but not for methotrexate (n = 5, 9%) vs. ixekizumab (P = 0·99). Kaplan–Meier curves for time to discontinuation in the 24 weeks after initiation of treatment are shown in Figure S2 (see Supporting Information). In general, the patient baseline characteristics were well balanced across treatment groups (Table 1).
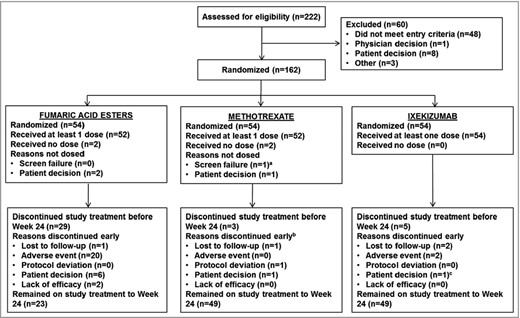
Patient disposition. aPatient was randomized in error, and visit 2 was not performed. bReason for discontinuation is missing for one patient. cReason for discontinuation at visit 11 was documented in error as ‘patient decision’; however, the patient did complete the study up to week 24.
The primary objective and all key secondary efficacy end points of the study were achieved. At week 24, PASI 75 was achieved by 49 patients (91%) treated with ixekizumab, which was significantly higher than the 38 patients (70%) treated with methotrexate and 12 (22%) treated with FAEs (Fig. 2a). Both coprimary comparisons for ixekizumab vs. methotrexate and ixekizumab vs. FAE regarding reduction in PASI 75 at week 24 were statistically significant after multiplicity adjustment (ixekizumab vs. methotrexate: adjusted P = 0·014; ixekizumab vs. FAE: adjusted P < 0·001; methotrexate vs. FAE: unadjusted P < 0·001) (Fig. 2a).
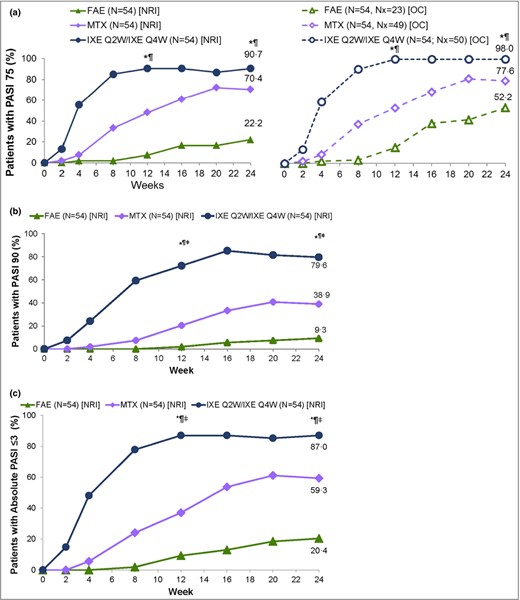
Patients with (a) ≥ 75% improvement in Psoriasis Area and Severity Index (PASI 75), (b) PASI 90 and (c) absolute PASI ≤ 3 responses at week 24 for the intention‐to‐treat (ITT) population with nonresponder imputation (NRI). FAE, fumaric acid ester; IXE, ixekizumab; MTX, methotrexate; Nx, number of patients with nonmissing data at week 24; OC, observed cases; Q2W, every 2 weeks; Q4W, every 4 weeks. *P < 0·05 IXE vs. FAEs, ¶P < 0·05 IXE vs. MTX, ‡ddagger;P < 0·05 MTX vs. FAEs. Prespecified P‐values at 12 and 24 weeks are presented (Hochberg adjusted for PASI 75 and PASI 90).
A summary of the primary and key secondary end points is presented in Table 2. The results for all other secondary outcomes (not shown) were consistent with the overall findings of the study. As both coprimary comparisons were statistically significant, testing continued with the eight key secondary comparisons via the second Hochberg procedure as explained above. PASI 90 was achieved by 43 patients (80%) treated with ixekizumab, compared with 21 (39%) treated with methotrexate (adjusted P = 0·001) and five (9%) treated with FAEs (adjusted P < 0·001). For methotrexate vs. FAE the unadjusted P‐value was < 0·001. The PASI responses observed in the intention‐to‐treat population are displayed in Figure 2(b). PASI 100 was achieved by 22 patients (41%) treated with ixekizumab, compared with seven (13%) treated with methotrexate (adjusted P = 0·0041) and two (4%) treated with FAEs (adjusted P < 0·001). For methotrexate vs. FAE the unadjusted P‐value was 0·16 (Fig. S3; see Supporting Information). Absolute PASI ≤ 3 was achieved by 47 patients (87%) treated with ixekizumab, compared with 32 (59%) treated with methotrexate (P = 0·0020) and 11 (20%) treated with FAEs (P < 0·001) (Fig. 2c).
| Hypothesis | PASI 75 | PASI 90 | PASI 100 | sPGA (0,1) responseb | DLQI (0,1) response |
| FAEs (N = 54), n (%) | 12 (22) | 5 (9) | 2 (4) | 7 (13) [N = 53] | 8 (15) |
| MTX (N = 54), n (%) | 38 (70) | 21 (39) | 7 (13) | 27 (52) [N = 52] | 20 (37) |
| IXE (N = 54), n (%) | 49 (91) | 43 (80) | 22 (41) | 45 (87) [N = 52] | 34 (63) |
| IXE vs. FAE, OR (95% CI) | 34·3 (11·2–105) | 38·3 (12·3–119·0) | 17·9 (3·94–81·2) | 42·2 (13·7–131) | 9·78 (3·85–24·8) |
| Hochberg adjusted P‐value | < 0·001 | < 0·001 | < 0·001 | < 0·001 | < 0·001 |
| IXE vs. MTX, OR (95% CI) | 4·13 (1·39–12·3) | 6·14 (2·60–14·5) | 4·62 (1·76–12·1) | 5·95 (2·27–15·6) | 2·89 (1·32–6·31) |
| Hochberg adjusted P‐value | 0·014 | < 0·001 | 0·0041 | < 0·001 | 0·012 |
| MTX vs. FAE, OR (95% CI) | 8·31 (3·49–19·8) | 6·24 (2·14–18·2) | 3·87 (0·77–19·6) | 7·10 (2·71–18·6) | 3·38 (1·33–8·59) |
| Unadjusted P‐value | < 0·001 | < 0·001 | 0·16 | < 0·001 | 0·015 |
| Hypothesis | PASI 75 | PASI 90 | PASI 100 | sPGA (0,1) responseb | DLQI (0,1) response |
| FAEs (N = 54), n (%) | 12 (22) | 5 (9) | 2 (4) | 7 (13) [N = 53] | 8 (15) |
| MTX (N = 54), n (%) | 38 (70) | 21 (39) | 7 (13) | 27 (52) [N = 52] | 20 (37) |
| IXE (N = 54), n (%) | 49 (91) | 43 (80) | 22 (41) | 45 (87) [N = 52] | 34 (63) |
| IXE vs. FAE, OR (95% CI) | 34·3 (11·2–105) | 38·3 (12·3–119·0) | 17·9 (3·94–81·2) | 42·2 (13·7–131) | 9·78 (3·85–24·8) |
| Hochberg adjusted P‐value | < 0·001 | < 0·001 | < 0·001 | < 0·001 | < 0·001 |
| IXE vs. MTX, OR (95% CI) | 4·13 (1·39–12·3) | 6·14 (2·60–14·5) | 4·62 (1·76–12·1) | 5·95 (2·27–15·6) | 2·89 (1·32–6·31) |
| Hochberg adjusted P‐value | 0·014 | < 0·001 | 0·0041 | < 0·001 | 0·012 |
| MTX vs. FAE, OR (95% CI) | 8·31 (3·49–19·8) | 6·24 (2·14–18·2) | 3·87 (0·77–19·6) | 7·10 (2·71–18·6) | 3·38 (1·33–8·59) |
| Unadjusted P‐value | < 0·001 | < 0·001 | 0·16 | < 0·001 | 0·015 |
CI, confidence interval; DLQI, Dermatology Life Quality Index; FAEs, fumaric acid esters; ITT, intention to treat; IXE, ixekizumab; MTX, methotrexate; NRI, nonresponder imputation; OR, odds ratio; PASI, Psoriasis Area and Severity Index; PASI 75, ≥ 75% improvement in PASI; sPGA, static Physician's Global Assessment. Coprimary PASI 75 comparisons for IXE vs. FAEs and IXE vs. MTX were adjusted via a primary Hochberg procedure at 24 weeks. Key secondary PASI 90, PASI 100, sPGA (0,1) and DLQI (0,1) comparisons for IXE vs. FAEs and IXE vs. MTX were adjusted by a second, separate Hochberg procedure at 24 weeks that was applied when all primary comparisons were statistically significant. aPercentages are based on the ITT population using NRI. bsPGA (0,1) response and ≥ 2‐point improvement from baseline; includes only patients with sPGA ≥ 3 at baseline.
| Hypothesis | PASI 75 | PASI 90 | PASI 100 | sPGA (0,1) responseb | DLQI (0,1) response |
| FAEs (N = 54), n (%) | 12 (22) | 5 (9) | 2 (4) | 7 (13) [N = 53] | 8 (15) |
| MTX (N = 54), n (%) | 38 (70) | 21 (39) | 7 (13) | 27 (52) [N = 52] | 20 (37) |
| IXE (N = 54), n (%) | 49 (91) | 43 (80) | 22 (41) | 45 (87) [N = 52] | 34 (63) |
| IXE vs. FAE, OR (95% CI) | 34·3 (11·2–105) | 38·3 (12·3–119·0) | 17·9 (3·94–81·2) | 42·2 (13·7–131) | 9·78 (3·85–24·8) |
| Hochberg adjusted P‐value | < 0·001 | < 0·001 | < 0·001 | < 0·001 | < 0·001 |
| IXE vs. MTX, OR (95% CI) | 4·13 (1·39–12·3) | 6·14 (2·60–14·5) | 4·62 (1·76–12·1) | 5·95 (2·27–15·6) | 2·89 (1·32–6·31) |
| Hochberg adjusted P‐value | 0·014 | < 0·001 | 0·0041 | < 0·001 | 0·012 |
| MTX vs. FAE, OR (95% CI) | 8·31 (3·49–19·8) | 6·24 (2·14–18·2) | 3·87 (0·77–19·6) | 7·10 (2·71–18·6) | 3·38 (1·33–8·59) |
| Unadjusted P‐value | < 0·001 | < 0·001 | 0·16 | < 0·001 | 0·015 |
| Hypothesis | PASI 75 | PASI 90 | PASI 100 | sPGA (0,1) responseb | DLQI (0,1) response |
| FAEs (N = 54), n (%) | 12 (22) | 5 (9) | 2 (4) | 7 (13) [N = 53] | 8 (15) |
| MTX (N = 54), n (%) | 38 (70) | 21 (39) | 7 (13) | 27 (52) [N = 52] | 20 (37) |
| IXE (N = 54), n (%) | 49 (91) | 43 (80) | 22 (41) | 45 (87) [N = 52] | 34 (63) |
| IXE vs. FAE, OR (95% CI) | 34·3 (11·2–105) | 38·3 (12·3–119·0) | 17·9 (3·94–81·2) | 42·2 (13·7–131) | 9·78 (3·85–24·8) |
| Hochberg adjusted P‐value | < 0·001 | < 0·001 | < 0·001 | < 0·001 | < 0·001 |
| IXE vs. MTX, OR (95% CI) | 4·13 (1·39–12·3) | 6·14 (2·60–14·5) | 4·62 (1·76–12·1) | 5·95 (2·27–15·6) | 2·89 (1·32–6·31) |
| Hochberg adjusted P‐value | 0·014 | < 0·001 | 0·0041 | < 0·001 | 0·012 |
| MTX vs. FAE, OR (95% CI) | 8·31 (3·49–19·8) | 6·24 (2·14–18·2) | 3·87 (0·77–19·6) | 7·10 (2·71–18·6) | 3·38 (1·33–8·59) |
| Unadjusted P‐value | < 0·001 | < 0·001 | 0·16 | < 0·001 | 0·015 |
CI, confidence interval; DLQI, Dermatology Life Quality Index; FAEs, fumaric acid esters; ITT, intention to treat; IXE, ixekizumab; MTX, methotrexate; NRI, nonresponder imputation; OR, odds ratio; PASI, Psoriasis Area and Severity Index; PASI 75, ≥ 75% improvement in PASI; sPGA, static Physician's Global Assessment. Coprimary PASI 75 comparisons for IXE vs. FAEs and IXE vs. MTX were adjusted via a primary Hochberg procedure at 24 weeks. Key secondary PASI 90, PASI 100, sPGA (0,1) and DLQI (0,1) comparisons for IXE vs. FAEs and IXE vs. MTX were adjusted by a second, separate Hochberg procedure at 24 weeks that was applied when all primary comparisons were statistically significant. aPercentages are based on the ITT population using NRI. bsPGA (0,1) response and ≥ 2‐point improvement from baseline; includes only patients with sPGA ≥ 3 at baseline.
An sPGA (0,1) response in patients who had sPGA ≥ 3 at baseline was achieved by 45 patients (87%) treated with ixekizumab, compared with 27 (52%) treated with methotrexate (adjusted P < 0·001) and seven (13%) treated with FAEs (adjusted P < 0·001). For methotrexate vs. FAE the unadjusted P‐value was < 0·001 (Fig. 3a). In addition, DLQI (0,1) was achieved by 34 patients (63%) treated with ixekizumab, compared with 20 (37%) treated with methotrexate (adjusted P = 0·012) and eight (15%) treated with FAEs (adjusted P < 0·001). For methotrexate vs. FAEs the unadjusted P‐value was 0·015 (Fig. 3b).
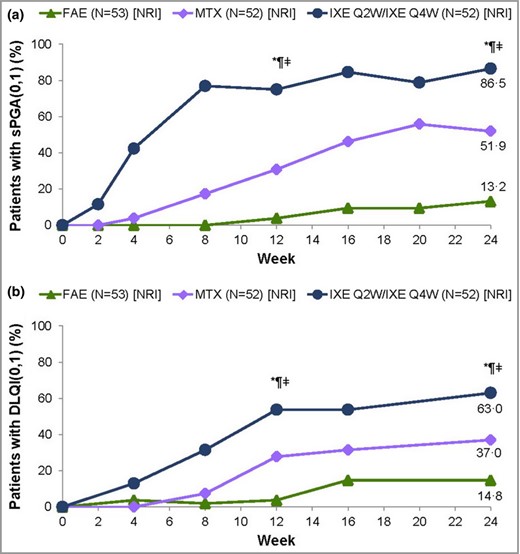
(a) Static Physician's Global Assessment (sPGA) 0 or 1 responses at week 24 for the intention‐to‐treat (ITT) population with baseline sPGA ≥ 3, and (b) Dermatology Life Quality Index (DLQI) 0 or 1 responses at week 24 for the ITT population with nonresponder imputation (NRI). FAE, fumaric acid ester; IXE, ixekizumab; MTX, methotrexate; Q2W, every 2 weeks; Q4W, every 4 weeks. *P < 0·05 IXE vs. FAEs, ¶P < 0·05 IXE vs. MTX, ‡ddagger;P < 0·05 MTX vs. FAEs. Prespecified P‐values at 12 and 24 weeks are presented (Hochberg adjusted).
TEAEs were observed for 46 (85%), 46 (88%) and 43 (83%) patients in the ixekizumab, FAE and methotrexate groups, respectively (Table 3). TEAEs observed in ≥ 2% of the safety population are presented in Table 3 by system organ class and in Table S1 (see Supporting Information) by system organ class and preferred term.
Treatment‐emergent adverse events (TEAEs) by system organ class in ≥ 2% of the safety population
| System organ class | FAEs (N = 52)a | MTX (N = 52) | IXE (N = 54) |
| Patients with at least one TEAE | 46 (88) | 43 (83) | 46 (85) |
| Infections and infestations | 14 (27) | 25 (48) | 30 (56) |
| Gastrointestinal disorders | 35 (67) | 17 (33) | 7 (13) |
| General disorders and administration‐site conditions | 5 (10) | 9 (17) | 16 (30) |
| Nervous system disorders | 7 (13) | 11 (21) | 11 (20) |
| Musculoskeletal and connective tissue disorders | 4 (8) | 12 (23) | 9 (17) |
| Skin and subcutaneous tissue disorders | 6 (12) | 6 (12) | 7 (13) |
| Vascular disorders | 14 (27) | 1 (2) | 2 (4) |
| Investigations | 7 (13) | 3 (6) | 6 (11) |
| Respiratory, thoracic and mediastinal disorders | 3 (6) | 3 (6) | 6 (11) |
| Injury, poisoning and procedural complications | 1 (2) | 6 (12) | 5 (9) |
| Ear and labyrinth disorders | 3 (6) | 3 (6) | 2 (4) |
| Blood and lymphatic system disorders | 5 (10) | 3 (6) | 0 |
| Metabolism and nutrition disorders | 1 (2) | 0 | 3 (6) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 0 | 1 (2) | 2 (4) |
| Renal and urinary disorders | 0 | 0 | 2 (4) |
| Eye disorders | 0 | 2 (4) | 0 |
| System organ class | FAEs (N = 52)a | MTX (N = 52) | IXE (N = 54) |
| Patients with at least one TEAE | 46 (88) | 43 (83) | 46 (85) |
| Infections and infestations | 14 (27) | 25 (48) | 30 (56) |
| Gastrointestinal disorders | 35 (67) | 17 (33) | 7 (13) |
| General disorders and administration‐site conditions | 5 (10) | 9 (17) | 16 (30) |
| Nervous system disorders | 7 (13) | 11 (21) | 11 (20) |
| Musculoskeletal and connective tissue disorders | 4 (8) | 12 (23) | 9 (17) |
| Skin and subcutaneous tissue disorders | 6 (12) | 6 (12) | 7 (13) |
| Vascular disorders | 14 (27) | 1 (2) | 2 (4) |
| Investigations | 7 (13) | 3 (6) | 6 (11) |
| Respiratory, thoracic and mediastinal disorders | 3 (6) | 3 (6) | 6 (11) |
| Injury, poisoning and procedural complications | 1 (2) | 6 (12) | 5 (9) |
| Ear and labyrinth disorders | 3 (6) | 3 (6) | 2 (4) |
| Blood and lymphatic system disorders | 5 (10) | 3 (6) | 0 |
| Metabolism and nutrition disorders | 1 (2) | 0 | 3 (6) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 0 | 1 (2) | 2 (4) |
| Renal and urinary disorders | 0 | 0 | 2 (4) |
| Eye disorders | 0 | 2 (4) | 0 |
The data are presented as n (%). FAEs, fumaric acid esters; IXE, ixekizumab; MTX, methotrexate. aDue to the high discontinuation rates observed in the FAE group, post hoc analyses for safety events (exposure‐adjusted incidence rates and rate ratios from Poisson regression models with a term for treatment and using an offset) were deemed necessary.
Treatment‐emergent adverse events (TEAEs) by system organ class in ≥ 2% of the safety population
| System organ class | FAEs (N = 52)a | MTX (N = 52) | IXE (N = 54) |
| Patients with at least one TEAE | 46 (88) | 43 (83) | 46 (85) |
| Infections and infestations | 14 (27) | 25 (48) | 30 (56) |
| Gastrointestinal disorders | 35 (67) | 17 (33) | 7 (13) |
| General disorders and administration‐site conditions | 5 (10) | 9 (17) | 16 (30) |
| Nervous system disorders | 7 (13) | 11 (21) | 11 (20) |
| Musculoskeletal and connective tissue disorders | 4 (8) | 12 (23) | 9 (17) |
| Skin and subcutaneous tissue disorders | 6 (12) | 6 (12) | 7 (13) |
| Vascular disorders | 14 (27) | 1 (2) | 2 (4) |
| Investigations | 7 (13) | 3 (6) | 6 (11) |
| Respiratory, thoracic and mediastinal disorders | 3 (6) | 3 (6) | 6 (11) |
| Injury, poisoning and procedural complications | 1 (2) | 6 (12) | 5 (9) |
| Ear and labyrinth disorders | 3 (6) | 3 (6) | 2 (4) |
| Blood and lymphatic system disorders | 5 (10) | 3 (6) | 0 |
| Metabolism and nutrition disorders | 1 (2) | 0 | 3 (6) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 0 | 1 (2) | 2 (4) |
| Renal and urinary disorders | 0 | 0 | 2 (4) |
| Eye disorders | 0 | 2 (4) | 0 |
| System organ class | FAEs (N = 52)a | MTX (N = 52) | IXE (N = 54) |
| Patients with at least one TEAE | 46 (88) | 43 (83) | 46 (85) |
| Infections and infestations | 14 (27) | 25 (48) | 30 (56) |
| Gastrointestinal disorders | 35 (67) | 17 (33) | 7 (13) |
| General disorders and administration‐site conditions | 5 (10) | 9 (17) | 16 (30) |
| Nervous system disorders | 7 (13) | 11 (21) | 11 (20) |
| Musculoskeletal and connective tissue disorders | 4 (8) | 12 (23) | 9 (17) |
| Skin and subcutaneous tissue disorders | 6 (12) | 6 (12) | 7 (13) |
| Vascular disorders | 14 (27) | 1 (2) | 2 (4) |
| Investigations | 7 (13) | 3 (6) | 6 (11) |
| Respiratory, thoracic and mediastinal disorders | 3 (6) | 3 (6) | 6 (11) |
| Injury, poisoning and procedural complications | 1 (2) | 6 (12) | 5 (9) |
| Ear and labyrinth disorders | 3 (6) | 3 (6) | 2 (4) |
| Blood and lymphatic system disorders | 5 (10) | 3 (6) | 0 |
| Metabolism and nutrition disorders | 1 (2) | 0 | 3 (6) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 0 | 1 (2) | 2 (4) |
| Renal and urinary disorders | 0 | 0 | 2 (4) |
| Eye disorders | 0 | 2 (4) | 0 |
The data are presented as n (%). FAEs, fumaric acid esters; IXE, ixekizumab; MTX, methotrexate. aDue to the high discontinuation rates observed in the FAE group, post hoc analyses for safety events (exposure‐adjusted incidence rates and rate ratios from Poisson regression models with a term for treatment and using an offset) were deemed necessary.
Overall there were 22 patients who discontinued due to adverse events during the treatment period. Two (4%) were receiving ixekizumab, zero (0%) methotrexate and 20 (38%) FAEs (ixekizumab: P = 0·50 vs. methotrexate and P < 0·001 vs. FAEs).
Adverse events overall and adverse events of special interest (AESI) are summarized in Table S2 (see Supporting Information). Within the AESI classification, statistically significant differences between treatment groups were observed for gastrointestinal adverse events (ixekizumab: P = 0·0026 vs. methotrexate and P < 0·001 vs. FAEs) and infections (P = 0·0033 vs. FAEs).
There was no statistically significant difference in the number of patients who experienced at least one cytopenia classified as an AESI. Data regarding lymphocyte shifts according to grade are reported in Table S3 (see Supporting Information).
Discussion
Considering that the EMA granted a first‐line label to ixekizumab in 2016, we sought to reassess the value of ixekizumab compared with two frequently used conventional therapies, methotrexate and FAEs, in a direct head‐to‐head trial. This study provides a rare comparison of conventional systemic treatment against a biologic in a patient population naive to systemic treatment, and data from it can be applied to future updates of systemic treatment algorithms and treatment guidelines in psoriasis.
In contrast to previous studies,17,18 the data presented here encompass a time course of 24 weeks of treatment to account for the time needed for methotrexate and FAEs to reach full efficacy. Most studies of patients with psoriasis have a primary end point earlier than 24 weeks.2,9 Moreover, dosing is according to the SPC and current guidelines.1,8 Results from this phase IIIb study demonstrate that significantly more patients in the ixekizumab group compared with the FAE and methotrexate groups achieved the primary end point of PASI 75 response at week 24. In addition, this study confirmed the superiority of ixekizumab over FAEs and methotrexate for PASI 90, PASI 100 and sPGA (0,1) in patients with baseline sPGA ≥ 3 and DLQI (0,1). These results are consistent with data from previous trials.17,18 The data from this study lend further support to the rapidly growing body of evidence implicating IL‐17A as a cytokine essential to the pathogenesis of psoriasis.19
Randomly, a lower proportion of patients with a weight > 100 kg were allocated to the ixekizumab group (15%) compared with the methotrexate (33%) and FAE (33%) groups. An earlier analysis of 3855 patients verified no influence of body weight on the efficacy of ixekizumab.20 In contrast, a subanalysis of patients included in the CHAMPION study found a weight‐dependent decrease of methotrexate treatment efficacy for patients corresponding to the third and fourth quartiles of baseline weight,21 while the dosage of FAEs given to a patient is not related to body weight or activity of the disease.22 Thus, it is possible that a more balanced allocation of patients with a weight > 100 kg may have impacted the efficacy of methotrexate.
Four recent studies compared methotrexate with the biologics adalimumab,23 briakinumab24 and infliximab,3 and placebo.6 Dosing schemes varied in these studies; however, all showed similar response rates to induction therapy for methotrexate (36–42%) according to PASI 75 after 16–24 weeks of treatment. Potential explanations for the high efficacy observed in the methotrexate group in our study could be related to the systemic‐naive patient population, or that patients randomized to the methotrexate arm had, by chance, indicators of potentially lower disease severity. For instance, the proportion of patients with baseline PASI ≥ 20 was slightly lower in methotrexate‐treated patients (35%) than in the overall patient population (38%). Methotrexate‐treated patients were also more likely to be naive to phototherapy: only 24% were pretreated with psoralen–ultraviolet A or ultraviolet B, compared with 40·1% in the overall patient population. Lastly, the methotrexate population was younger (by 6 years, on average), the duration of psoriasis was shorter in the methotrexate‐treated population, and there were fewer confounding factors to influence methotrexate tolerability.
Another point to consider is the dosage of methotrexate: starting with week 2, three‐quarters (75%) of methotrexate‐treated patients were prescribed a dose ≥ 15 mg per week until the end of the 24‐week treatment phase. The starting dose schedules of ixekizumab and the two conventional systemic therapies, methotrexate and FAEs, are opposed. The initial starting dose of ixekizumab supports the fast onset of action, in contrast to the slower dosage increase of methotrexate and FAEs to ensure tolerability. Prescribing more aggressive doses of methotrexate and of FAEs may have resulted in faster onset of action, but potentially at the expense of additional earlier discontinuations. This may have, in turn, led to a lower overall treatment success rate in terms of response, combined with treatment retention at week 24.
With respect to FAE treatment, clinical experience has been explored in a few clinical trials focusing on efficacy and safety.8 Despite the known dropout rate at the beginning of therapy (≤ 40%), mainly due to gastrointestinal side‐effects and flushing, and the risk for lymphopenia (including the rare risk for progressive multifocal leucoencephalopathy), the long‐term safety and efficacy are considered favourable. Thus, FAEs are recommended as a long‐term systemic treatment for moderate‐to‐severe plaque psoriasis in the 2015 European evidence‐based S3‐guidelines update.8 Dimethyl fumarate as a single active agent was approved in 2013 and 2014 in the U.S.A. and Europe, respectively, for the therapy of multiple sclerosis. The efficacy and safety of dimethyl fumarate as monotherapy have also been established in psoriasis, and it is now indicated as a first‐line treatment for moderate‐to‐severe psoriasis.
FAEs exhibited surprisingly low efficacy in this study. Previously reported PASI 75 efficacy rates after 16 weeks of treatment ranged from 37·5% to 70·0%.18,25 Thus, to reflect the high dropout rates at the beginning of the therapy, a post hoc efficacy analysis was conducted to show observed cases vs. nonresponder imputation (Fig. 2a). Higher efficacy was observed in patients who remained on FAE treatment. In an open‐label design, the expectations of patients might be higher, and they might be less willing to accept side‐effects. By chance, the patients randomized to the FAE arm had higher disease activity, as the proportion of patients with baseline PASI ≥ 20 was slightly higher (43%, vs. 38·3% in the overall patient population) and slightly more patients had a baseline sPGA of 4 or 5 (56%, vs. 50·0% in the overall patient population) (Table 1).
The high discontinuation rate (38%) for FAEs was mainly brought about by gastrointestinal adverse events. The adverse events leading to discontinuation in the FAE group were upper abdominal pain, diarrhoea, abdominal discomfort, lymphopenia, nausea, colitis, erysipelas, headache and increased hepatic enzymes, all of which are known and expected. Similarly, discontinuation rates have been reported by other studies investigating the safety of FAEs.25,26,27,28 By contrast, markedly fewer discontinuations were found under real‐world conditions in the German psoriasis registry PsoBest (13·5% after 3 months and 21·7% after 6 months). A reason for this might be that in routine care, unlike in clinical trials, an individually optimized approach regarding efficacy and safety is more common, with subjectively less pressure to reach PASI 75 in the updosing period.
The majority of discontinuations occurred 6–8 weeks after randomization to FAEs, and none occurred after 16 weeks of treatment. As early discontinuations from FAEs reduced the time under risk for adverse events, an adjusted analysis for incidence rates was conducted to avoid underestimation of the risk of adverse events in the FAE group. To this end, secondary‐analysis Poisson regression models were applied, and the results suggested that the risks of hepatic adverse events and cytopenias were also statistically significantly increased with FAE treatment (Table S4; see Supporting Information). As a rate ratio from a Poisson model below 1·0 indicates that the rate of events is lower than in the compared treatment (i.e. methotrexate or FAEs), the event rate in this study, adjusted for time under risk, was less with ixekizumab than with methotrexate or FAEs for those safety end points.
In this short‐term trial, methotrexate was well tolerated, and no discontinuations due to adverse events were observed. The favourable safety profile of ixekizumab was similar to that in previously reported trials.17,18
The large differences in efficacy observed in this trial, in terms of both onset and level, as well as safety, may be informative for prescribing rules beyond classical cost‐effectiveness calculations. Specifically, these differences may apply to patients in high demand for rapid relief (i.e. patients with rapidly progressing active disease and very high disease burden).
Some limitations to this study should be considered. As it was an open‐label, real‐world study with an early, systemic‐naive patient population, the study was not placebo controlled. Randomization was 1 : 1 : 1 to ixekizumab, FAEs and methotrexate. No stratification occurred because of the small sample size. The safety data should be considered with caution because this study was not powered for safety. The treatment allocation was open to the patients and investigators, which might have induced bias in response to quality‐of‐life questionnaires and reporting of adverse events, but probably not in response to the clinical outcomes that were assessed by the blinded raters (Appendix 2). Similar studies with a practical blinded‐rater design have been conducted or are ongoing (e.g. NCT02474082 comparing secukinumab vs. FAEs). However, the open‐label design might be closer to the real‐world setting of applying FAEs, methotrexate or ixekizumab than a blinded trial. The duration of the study can be viewed as both a strength and a limitation. For a controlled study, this study was long; however, it included relatively short efficacy data compared with the lifetime duration of the disease.
In conclusion, this study confirmed the benefit of the IL‐17A antagonist ixekizumab over the conventional therapies FAEs and methotrexate. Data from this study may potentially be utilized towards the potential refinement of systemic treatment algorithms and future psoriasis guidelines.
Acknowledgments
The authors would like to thank Birgit Schinzel, Martin Dörr and Anita Eisberg of the Ixekizumab Team of Biostatistics and Statistical Programming, Clinipace Worldwide, Eschborn, Germany, and Lingling Xie of Eli Lilly and Company for providing significant contributions in statistical and programming support. In addition, they would like to thank Brandi Berry, CTM; Bertfried Jost, Clin Ops; and Konstantinos Fotiou, MAP. Lastly, the authors would like to thank Shannon E. Gardell of Syneos Health for providing writing support.
References
1 Appendix
Conflicts of interest. K.R. has served as an advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Amgen, Biogen, Boehringer Ingelheim Pharma, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Eli Lilly and Company, Medac, Merck Sharp & Dohme, Novartis, Ocean Pharma, Pfizer, Regeneron, Sanofi, Takeda, UCB Pharma and Xenoport. M.A. has served as a consultant or paid speaker for clinical trials sponsored by AbbVie, Almirall, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly and Company, GSK, Hexal, Janssen, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB Pharma and Xenoport. D.T. has been an advisor for, received speaker's honoraria and grant support from, and participated in clinical trials for AbbVie, Almirall, Amgen, Biogen Idec, Bioskin, Boehringer Ingelheim, Celgene, Dignity, Dr Reddy's, Eli Lilly and Company, Galapagos, GlaxoSmithKline, LEO Pharma, Janssen‐Cilag, Kymab, Merck Sharp & Dohme, Mundipharma, Morphosis, Novartis, Pfizer, Regeneron, Samsung, Sanofi‐Genzyme, Sandoz and UCB Pharma. A.P. has worked as an investigator, speaker and/or advisor for AbbVie, Almirall‐Hermal, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, GSK, Eli Lilly and Company, Galderma, Hexal, Janssen, LEO Pharma, Medac, Merck Serono, Mitsubishi, MSD, Novartis, Pfizer, Regeneron, Roche, Sandoz Biopharmaceuticals, Schering‐Plough, Tigercat Pharma and UCB Pharma. U.M. has been an advisor for, received speakers honoraria and/or grants from, and/or participated in clinical trials for Abbott/AbbVie, Almirall‐Hermal, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly and Company, Foamix, Forward Pharma, Janssen, LEO Pharma, Medac, Miltenyi Biotech, MSD, Novartis, Pfizer, VBL and Xenoport. A.L., C.H., E.S., A.S. and M.D. were employees of and minor stockholders in Eli Lilly and Company during the conduct of this study.
2 Appendix
Discussion of the open‐label, blinded‐rater study design. An open‐label design was chosen to ensure that treatment with fumaric acid esters and methotrexate was given according to the label and according to clinical practice. We attempted to minimize the inherent limitations of an open‐label design by having a blinded rater assess all clinical outcome measures. Although complete blinding was not possible, the advantages of a blinded‐rater design are that clinical outcomes can be assessed objectively (in a blinded manner) without preventing the participating physicians from treatment decisions as would be done in real clinical practice, when the treatment is known. This minimizes potential bias involved in the evaluation of clinical efficacy. Disadvantages are that patient‐reported outcomes can be biased by the patient's knowledge of treatment. For example, patients may research the new treatment and its side‐effects in publications and may be influenced in their reporting behaviour of these potential side‐effects. In addition, subjective outcomes, such as quality of life, can also be influenced by the patient's knowledge of the study treatment.
Author notes
Funding sources This study was supported by Eli Lilly (Indianapolis, IN, U.S.A.). This study was designed by Lilly Deutschland GmbH. Data were collected by SCIderm GmbH and analysed by Clinipace Worldwide. All authors had full access to the data and participated in manuscript development, with medical writing support paid for by the funder. All authors made the decision to submit the manuscript for publication.
Conflicts of interest Conflicts of interest statements can be found in Appendix 1.



