-
PDF
- Split View
-
Views
-
Cite
Cite
K. Reich, J.L.K. Reich, T.M. Falk, N. Blödorn‐Schlicht, U. Mrowietz, R. von Kiedrowski, C. Pfeiffer, J. Niesmann, Y. Frambach, R.B. Warren, Clinical response of psoriasis to subcutaneous methotrexate correlates with inhibition of cutaneous T helper 1 and 17 inflammatory pathways, British Journal of Dermatology, Volume 181, Issue 4, 1 October 2019, Pages 859–862, https://doi.org/10.1111/bjd.18001
Close - Share Icon Share
Dear Editor, Methotrexate (MTX) is one of the most frequently used conventional systemic therapies for the treatment of moderate‐to‐severe plaque‐type psoriasis. Although it has been available since the 1950s,1 its exact mechanism of action remains to be fully determined. Clinical studies indicate good clinical responses [75% reduction in Psoriasis Area and Severity Index (PASI) score (PASI 75)] in approximately 40% of patients with psoriasis and clinical effects peak after about 16 weeks of therapy.2,3,4 MTX is biologically active as MTX–polyglutamate (MTX‐PG), which is generated by an enzymatic process mainly in erythrocytes. As subcutaneous (SC) administration of MTX may lead to more MTX‐PG with longer PG chains than oral administration, at least in some individuals,5 the former has been postulated to be associated with more stable long‐term clinical responses. A recent study with SC MTX, starting at 17·5 mg weekly and up‐titrated to 22·5 mg weekly in patients not achieving a 50% decrease in PASI score at week 8,4 longevity of high‐level clinical responses over 52 weeks was superior to that previously observed in a similar study with oral MTX,3 with approximately 30% of patients treated with SC MTX achieving a 90% reduction in PASI score after 1 year of therapy. We report further details from a substudy (n = 27/120 patients) of this placebo‐controlled phase III clinical trial (EudraCT number 2012‐002716‐10). Punch biopsies (4 mm) of representative plaques were taken at baseline and after 16 weeks of therapy, and investigated blinded by histology and immunohistochemistry for changes in the epidermal microarchitecture (parakeratosis, epidermal thickness, presence of Munro's microabscesses, Ki‐67) and markers of the inflammatory infiltrate (CD1a, CD3, CD11c). Quantitative real‐time polymerase chain reaction was used to determine cutaneous levels of dominant cytokine circuits [interleukin (IL)‐17A, interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α] before and after treatment.4 6 The study was approved by the ethical committees of the participating centres and informed consent was obtained from all patients. Findings were obtained from three patient subgroups based on their treatment and clinical response. Six of 27 patients (23%) received placebo; seven of 27 (26%) received MTX but did not achieve a PASI 75 response (MTX nonresponders); and 14 of 27 (52%) received MTX and were PASI 75 responders (MTX responders). We found significant reductions in markers of psoriatic epidermal pathology and in infiltrating CD3+ T cells and CD11c+ dendritic cells in patients responding to MTX but not in MTX nonresponders or patients receiving placebo (Fig. 1a–d). This was associated with a significant reduction in cutaneous IL‐17A (to approximately 12% of baseline values) and IFN‐γ mRNA levels (to approximately 26% of baseline values) in MTX PASI 75 responders. Inhibitory effects were weaker in MTX nonresponders, and IL‐17A and IFN‐γ mRNA levels were unchanged or increased in placebo patients (Fig. 1e, f). Numbers of CD1a+ cutaneous dendritic cells and skin levels of TNF‐α mRNA were similar in the three groups and remained unchanged during treatment. Overall, there was a striking correlation between the individual reduction of cutaneous IL‐17A mRNA levels and the clinical response (Fig. 1g). Our findings indicate that the clinical response to MTX correlates with a reduction of T helper (Th)17 and Th1 signature cytokines. Given the central role of IL‐17A in the activation of keratinocytes in psoriasis, we speculate that the marked normalization of microscopic signs of epidermal pathology in MTX responders is a consequence of the inhibitory effects of MTX on the expression of this cytokine. This is further supported by the strong correlation, on an individual basis, between the reduction of cutaneous IL‐17A production and the clinical response as measured by the PASI, which incorporates signs related to keratinocyte hyperproliferation such as scaling and induration. A possible mechanistic explanation is the accumulation of adenosine and activation of the A2A adenosine receptor, the subsequent stimulation of adenylate cyclase and increase of intracellular cyclic adenosine monophosphate levels, and the downregulation of nuclear factor kappa B‐dependent cytokines in immune cells during MTX therapy.7 8 As we did not take sequential biopsies, it remains unclear if the inhibition of cutaneous Th17 and Th1 pathways occurs before or concurrent to the reduction of infiltrating T cells and CD11c+ dendritic cells. The former sequence of events has been identified after administration of the anti‐IL‐17A secukinumab,6 indicating that cytokine inhibition may precede pronounced effects on infiltrating inflammatory cells. The immunomodulatory and clinical effect of MTX in psoriasis seems to involve a reduction in IL‐17A production, a decrease in infiltrating T cells and dendritic cells, and the normalization of keratinocyte pathology.
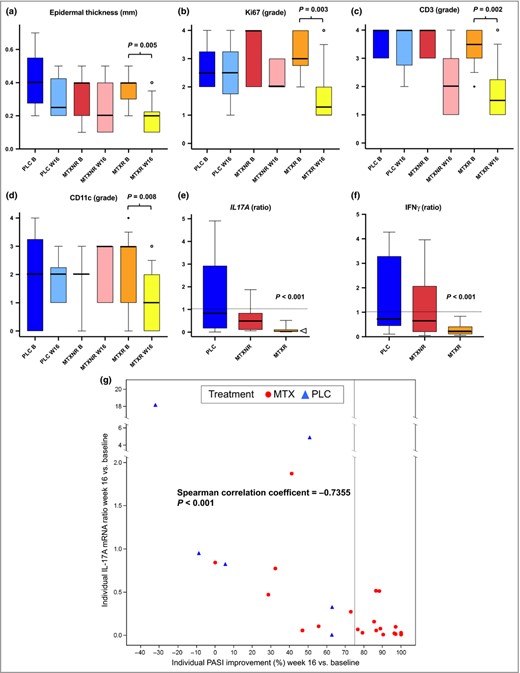
Skin changes during treatment with subcutaneous methotrexate. (a) Quantitative analysis of epidermal thickness and (b) semi‐quantitative analysis (grade 0–4) of proliferating keratinocytes (Ki‐67+), (c) numbers of cutaneous CD3+ T cells and (d) numbers of cutaneous CD11c+ dendritic cells as determined by histology and immunohistochemistry. Grade ≤ 1 corresponds to findings in normal skin. Red bars denote placebo patients (PLC; n = 6), blue bars MTX PASI 75 nonresponders (MTXNR; n = 7) and yellow bars MTX PASI 75 responders (MTXR; n = 14). Dark colours represent baseline (B) and light colours week 16 values (W16). Boxes represent the interquartile range, whiskers the 10–90% range; median values are indicated by thick lines and outliers are shown as circles. All significant differences are shown with two‐sided exact P‐values derived from Wilcoxon matched‐pairs signed rank test. Other markers of psoriatic pathology such as parakeratosis and numbers of Munro's microabscesses were also decreased in MTXR (not shown). (e, f) Cutaneous mRNA levels as fold change of week 16 vs. baseline values with values > 1 (dashed line) indicating fold increase and < 1 indicating fold decrease. Colour codes, legends for treatment groups and boxes and whiskers are as in the upper panels. The median ratio for interleukin (IL)‐17A is indicated by an open triangle. P‐values (median ratio different from 1) in (e) and (f) are from Wilcoxon test. (g) Correlation between individual PASI improvement at week 16 (percentage vs. baseline, x‐axis) and change in cutaneous IL‐17A mRNA levels (ratio of week 16 vs. baseline values, y‐axis). All patients receiving MTX are shown with red circles, patients receiving placebo with blue triangles. The light‐grey line separates PASI 75 responders from individuals not achieving PASI 75 at week 16. PASI 75, 75% reduction in Psoriasis Area and Severity Index (PASI) score; IFN, interferon.
References
Author notes
Funding sources: The clinical trial and the biopsy substudy was supported by a grant by Medac, Germany (K.R.). Medac had no influence on the design and conduct of the study, nor the interpretation of the results or the preparation of the manuscript.
Conflict of interests: K.R. serves on advisory boards, receives research funding, and receives payments for lectures and educational development from AbbVie, Affibody, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Covagen, Forward Pharma, Fresenius Medical Care, GlaxoSmithKline, Janssen‐Cilag, Kyowa Kirin, Leo, Lilly, Medac, Merck Sharp & Dohme, Miltenyi Biotec, Novartis, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant and Xenoport. U.M. has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Almirall, Amgen, Biogen, Boehringer‐Ingelheim, Celgene, Dr. Reddy's, Eli Lilly, Foamix, Formycon, Forward Pharma, Janssen, Leo Pharma, Medac, MSD, Novartis, UCB and Xenoport. R.B.W. has received personal fees from AbbVie, Almirall, Amgen, Boehringer Ingelheim Pharma, Celgene, Janssen‐Cilag, Leo, Lilly, Novartis, Pfizer and Xenoport outside the submitted work. R.v.K. has been an investigator, consultant, advisor or speaker for AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Eli Lilly, GSK, Leo, Janssen‐Cilag, Medac, MSD, Novartis, Pfizer, UCB and VBL.



