-
PDF
- Split View
-
Views
-
Cite
Cite
E. Hagforsen, M. Lampinen, A. Paivandy, S. Weström, H. Velin, S. Öberg, G. Pejler, O. Rollman, Siramesine causes preferential apoptosis of mast cells in skin biopsies from psoriatic lesions, British Journal of Dermatology, Volume 177, Issue 1, 1 July 2017, Pages 179–187, https://doi.org/10.1111/bjd.15336
Close - Share Icon Share
Summary
Skin mast cells are implicated as detrimental effector cells in various inflammatory skin diseases such as contact eczema, atopic dermatitis and psoriasis. Selective reduction of cutaneous mast cells, e.g. by inducing targeted apoptosis, might prove a rational and efficient therapeutic strategy in dermatoses negatively influenced by mast cells.
The objective of the present study was to evaluate whether a lysosomotropic agent such as siramesine can cause apoptosis of mast cells present in psoriatic lesions.
Punch biopsies were obtained from lesional and uninvolved skin in 25 patients with chronic plaque psoriasis. After incubation with siramesine, the number of tryptase‐positive mast cells and their expression of interleukin (IL)‐6 and IL‐17 was analysed. Skin biopsies were digested to allow flow cytometric analysis of the drug's effect on cutaneous fibroblasts and keratinocytes.
Siramesine caused a profound reduction in the total number of mast cells in both lesional and uninvolved psoriatic skin biopsies without affecting the gross morphology of the tissue. The drug reduced the density of IL‐6‐ and IL‐17‐positive mast cells, and showed antiproliferative effects on epidermal keratinocytes but had no apparent cytotoxic effect on keratinocytes or dermal fibroblasts.
Considering the pathophysiology of psoriasis, the effects of siramesine on cutaneous mast cells may prove favourable from the therapeutic aspect. The results encourage further studies to assess the usefulness of siramesine and other lysosomotropic agents in the treatment of cutaneous mastocytoses and inflammatory skin diseases aggravated by dermal mast cells.
Psoriasis is a complex immune‐mediated disease affecting 2–4% of the population in Western countries. Apart from its negative influence on skin and joints, the disease is associated with numerous comorbidities and has a detrimental effect on quality of life. Together, genetic and environmental factors constitute the basis of psoriasis pathogenesis, but the precise disease mechanisms remain unknown. T‐cell subsets, plasmacytoid cells and neutrophils are key immune cells in psoriatic skin lesions, although accumulating evidence suggests that dermal mast cells contribute to both initiation and maintenance of the inflammatory process (reviewed in Harvima et al.1). Mast cells interact intimately with T helper cells, endothelial cells and keratinocytes, all of which are strongly implicated in the pathology of psoriasis.2 In addition, mast cells are in close proximity to sensory axons, and nerve‐derived factors are able to activate residential mast cells.3 In support of a role of mast cells in psoriasis, several studies have shown that the numbers of mast cells are increased substantially in psoriatic lesions.4,5,6,7 For example, early psoriatic lesions contain an increased number of tryptase‐ and chymase‐positive mast cells4 5 filled with cytoplasmatic stores of bioactive substances, including histamine, cytokines, growth factors, proteoglycans and a variety of proteases.8 Besides secreting preformed mediators by degranulation, activated mast cells are major producers of tumour necrosis factor (TNF)‐α, interleukin (IL)‐17, interferon‐γ, vascular endothelial growth factor (VEGF) and other newly synthesized mediators closely influencing psoriasis.9,10,11,12,13,14 However, it should be noted that there is some controversy with regard to the importance of mast cells as a source of IL‐17.
Traditionally, antipsoriatic therapies such as glucocorticoids, ultraviolet (UV) irradiation, synthetic vitamin D, retinoids, methotrexate and ciclosporin are generally directed at T cells, Langerhans cells or epidermal keratinocytes. More recently, systemic administration of anticytokine antibodies against TNF‐α, IL‐17 and IL‐12/23 has emerged as a powerful treatment in more severely affected patients.15 Despite considerable advances in the management of psoriasis, there is still an unmet need for effective, safe and affordable drug alternatives. One possible option may be to target cutaneous mast cells by applying granule stabilizers, e.g. ketotifen or chromoglycate,16 or by inhibiting distinctive mast‐cell‐derived mediators or receptors using, e.g. cyclo‐oxygenase‐2 inhibitors, histamine receptor antagonists or antibodies against the high‐affinity IgE receptor. However, targeted treatment by these means will only partly interfere with the global impact of mast cells, and thus far, none of these strategies has shown antipsoriatic potential.
Given the fact that mast cells contribute to skin inflammation with such a wide variety of mediators and effector mechanisms, it may be a more efficient mode of treatment to reduce the mast‐cell population instead of targeting individual mast‐cell factors.17 In theory, this could be achieved by administering a lysosomotropic drug, i.e. an agent that permeabilizes membranes of lysosomal organelles. Previous studies that we have carried out have shown that siramesine and other lysosomotropic agents cause secretory granule derangement in mast cells, which leads to the release of granule compounds into the cytosol, eventually triggering apoptosis.18,19,20,21,22 Here, we have evaluated the novel concept of targeting mast cells in a clinically relevant setting by assessing whether siramesine is able to induce apoptosis of mast cells populated in psoriatic skin. Indeed, we show that the drug causes efficient apoptosis of resident mast cells in punch biopsies taken from plaque lesions and clinically uninvolved psoriatic skin. Our findings raise the issue of using siramesine or other lysosomotropic agents as therapeutics to reduce detrimental mast‐cell populations in psoriasis.
Materials and methods
Patients
In total, the study included 25 patients (16 men, 9 women) aged 22–79 (mean 56) years with chronic plaque psoriasis. Except for emollients, no topical therapy was allowed for at least 2 weeks prior to sampling. No patient received UV irradiation treatment or systemic antipsoriatic therapy. The overall severity of the disease, according to the Psoriasis Area and Severity Index (PASI), ranged from 3·5 to 18·7 (mean 7·0).23 The severity of the target plaque, according to the Psoriasis Severity Score (PSS; maximum 12), ranged from 2 to 9 (mean 5·4).24 Four punch skin biopsies (3 or 4 mm in diameter) were obtained from each patient after administering local anaesthesia using xylocaine–adrenalin. Two adjacent biopsies spaced 2–5 mm apart were taken from a plaque lesion on the trunk (n = 14), elbow (n = 7) or thigh (n = 4). Another two biopsies of nonlesional skin were taken from the buttock region at least 10 cm from a plaque. The regional ethical review board approved the study (reg. no. 2012/351) and written informed consent was obtained from the patients.
Reagent
A stock solution of 1 mmol L−1 siramesine hydrochloride (kind gift from Lundbeck A/S, Copenhagen, Denmark) in 10% HPBCD [(2‐hydroxypropyl)‐beta‐cyclodextrin] (Sigma‐Aldrich, Stockholm, Sweden) was prepared. This solution was tested to be stable at room temperature for at least 3 months.
Incubation of skin biopsies with siramesine
One of the two adjacent biopsies from each patient and from each region (lesional or nonlesional) was incubated with siramesine (20 μmol L−1) in Dulbecco's modified Eagle's medium (DMEM) with 5% fetal bovine serum (FBS) + 1% penicillin/streptomycin and l‐glutamine (all from Life Technologies, Stockholm, Sweden) at 37 °C in a humidified atmosphere of 5% CO2 in air. The adjacent biopsy was incubated under the same culture conditions except that siramesine was replaced by vehicle. The incubations were performed using 48‐well plates containing 600 μL medium in each well. Every 24 h, the culture medium was replaced with fresh medium containing the same ingredients as above. After 72 h, the submerged biopsies were collected and fixed for 24 h in 4% buffered formalin before dehydration and embedment in paraffin.
Immunohistochemistry
Biopsies were cross‐sectioned (5‐μm thick) and then deparaffinized, hydrated and boiled in a pressure cooker in Reveal Decloaker (Biocare Medical, Concorde, CA, U.S.A.). Background sniper (Biocare Medical) was used to block nonspecific background staining.
Tryptase
The sections were incubated at room temperature for 2 h with a mouse monoclonal tryptase antibody (MAB1222, Chemicon Inc., Temecula, CA, U.S.A.) at 1/2000 dilution. Mouse AP polymer detection kit and Vulcan Fast Red Chromogen kit 2 (both from Biocare Medical) were used for visualization. Counterstaining was made with Mayer's haematoxylin (Histolab, Gothenburg, Sweden). Incubation with mouse IgG was used as negative control.
Ki‐67
Endogenous peroxidase was blocked with Peroxidazed 1 (Biocare Medical) before incubation with the primary antibody mouse antihuman Ki‐67 clone MIB1 (Dako, A/S, Glostrup, Denmark). As a secondary antibody, horse antimouse biotinylated IgG (Vector Labs, Burlingame, CA, U.S.A.) was used. Vectastain ABC‐Elite Kit and DAB kit (both from Vector Labs) were used for visualization. Counterstaining was performed as described above. Incubation with mouse IgG was used as negative control.
Immunofluorescence
Sections (5‐μm thick) were prepared as for the immunohistochemistry, but without treatment for endogenous peroxidase. Omitting the rabbit antibody and mouse IgG were used as negative controls for the double stainings.
Double staining of interleukin‐17 and tryptase
The sections were incubated overnight at 4 °C with rabbit antihuman IL‐17 (LS‐B7397; Lifespan Biosciences Inc., Seattle, WA, U.S.A.) diluted 1/500. Alexa Fluor 568 goat antirabbit IgG (Invitrogen, Stockholm, Sweden) diluted 1/500 was used as a secondary antibody. Thereafter, the sections were incubated with the tryptase antibody MAB1222 diluted 1/1000 followed by Alexa Fluor 488 goat antimouse IgG (Invitrogen). Mounting was done with Vectashield with DAPI (Vector Labs). The ZEN 2009 software (LSM 710; Carl Zeiss, Berlin, Germany) software was used to generate the images.
Double staining of interleukin‐6 and tryptase
The sections were incubated overnight at 4 °C with rabbit antihuman IL‐6 (AHP1040; AbDSerotec, Bio‐Rad Laboratories Inc., Hercules, CA, U.S.A.) diluted 1/1000. Alexa Fluor 568 goat antirabbit IgG (Invitrogen) was used as a secondary antibody. Thereafter, incubation with tryptase antibody and mounting was done as above.
Evaluation of immunostainings
Sections were evaluated under a Zeiss Axiophot Imager‐Z1 microscope with Zen software (Carl Zeiss Microscopy GmbH, Göttingen, Germany). The number of mast cells in the dermis (papillary and reticular) was counted under a 40× objective and 10× ocular lens, in three sections from each biopsy. Pictures were taken to measure the total area of the papillary and reticular dermis and the mean number of mast cells per mm2 was calculated. Cells double‐positive for tryptase and IL‐17 or IL‐6 were evaluated in the same way. The number of Ki‐67‐positive epidermal cells was counted in three sections from each biopsy under a 20× objective lens. The length of the basement membrane in each section was measured and the mean number of Ki‐67‐positive cells per mm2 basement membrane was counted. Calculations and preparations of graphs were done using Graphpad Prism 6 (GraphPad Software Inc., San Diego, CA, U.S.A.). Negative controls (rabbit IgG for IL‐6 staining; omitting the primary antibody for tryptase staining) showed negligible staining (data not shown).
Preparation of skin biopsies for flow cytometry
Two adjacent biopsy samples (4‐mm diameter) from lesional psoriatic skin were incubated with and without siramesine (20 μmol L−1), respectively, for 24 or 48 h in medium (DMEM with 10% FBS + 1% penicillin/streptomycin and l‐glutamine) at 37 °C in a humidified atmosphere of 5% CO2 in air. Incubation times exceeding 48 h were avoided because reduced expression of surface antigens on mast cells was found beyond this time period. After incubation, single‐cell suspensions of biopsy cells were obtained by enzymatic digestion: biopsy samples were washed in fresh medium and then incubated for 3 h at 37 °C with the Whole Skin Dissociation Kit (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany) for preparation of keratinocytes and fibroblasts. Because this kit was found to be too harsh for mast cells we instead used a solution of 1 mg mL−1 collagenase D, 1 mg mL−1 hyaluronidase and 0·1 mg mL−1 DNAse in DMEM supplemented with 10% FBS + 1% penicillin/streptomycin and l‐glutamine for preparation of these cells. After digestion, the samples were triturated, passed through a 70‐μm nylon mesh and collected in a tube. The cells were washed twice with a buffer assigned for fluorescence‐activated cell sorting (FACS) containing 0·05% NaN3, 0·1% bovine serum albumin and 0·4% trisodium citrate dihydrate in phosphate‐buffered saline. The cell suspensions were incubated with fluorochrome‐conjugated monoclonal antibodies for 30 min at 4 °C in the dark. The cells were then washed in Annexin V binding buffer. For the evaluation of apoptosis/necrosis, the cells were stained with Annexin V Alexa Fluor 594 and Draq7 for 10 min, and then immediately analysed by flow cytometry. The flow cytometric assay was performed on a two‐laser FC500 MCL system with CXP software (Beckman Coulter, Fullerton, CA, U.S.A.). Ten thousand cells were counted and analysed in each sample. Kaluza software from Beckman Coulter was used for data analysis. Mast cells were identified by their expression of CD45 and CD117. To ensure that the c‐kithigh cells represented mast cells, we additionally stained for FcεRI (the high‐affinity IgE receptor). Indeed, > 90% of the c‐kithigh cells were double‐positive for FcεRI, verifying that the c‐kithigh population represents mast cells (data not shown). Fibroblasts were identified by the expression of CD90, and keratinocytes by CD49f. Confounding haematopoietic cells were excluded by their expression of CD45. Gates were set guided by FMO (fluorescence minus one) controls. An FMO control contains all the fluorochromes in a panel except for the one that is being measured. This ensures that any spread of the fluorochromes into the channel of interest is properly identified. Unspecific staining was minimized using the Fc receptor blocking solution TruSTain (Biolegend, San Diego, CA, U.S.A.) before staining with fluorochrome‐conjugated antibodies.
Statistical analyses
Statistical differences were calculated using Wilcoxon matched‐pairs signed‐rank test, a nonparametric test, assuming that the material is not normally distributed.
Results
Siramesine reduces mast‐cell density in psoriatic skin biopsies
Siramesine was originally designed as an antidepressant σ‐2 agonist, but showed suboptimal clinical efficacy for the intended purpose.25 Subsequently it was shown that siramesine, in addition to its σ‐2 agonist properties, also exhibits lysosomotropic activity.26 In previous studies, we showed that siramesine can cause permeabilization of mast‐cell granule membranes, leading to the initiation of apoptosis.18,19,20,21,22 Here, we evaluated the concept of inducing mast‐cell apoptosis in a clinical setting by exploring whether siramesine can reduce mast‐cell numbers in psoriatic lesions. To this end, we incubated biopsies of psoriatic plaque lesions with siramesine. As a control, biopsies were taken from nonlesional areas of the same patients. After 72 h, residual mast‐cell numbers were assessed using tryptase staining. As seen in Figure 1 and Figure S1 (see Supporting Information), incubation of biopsies with siramesine caused a profound and significant reduction in the number of viable mast cells present in both lesional and normal skin. Notably, siramesine caused a reduction in mast‐cell numbers in both the papillary and reticular dermis. Moreover, siramesine showed approximately equal efficacy on mast cells from males and females. There was no difference in gross morphology between siramesine‐ and vehicle‐exposed biopsies.
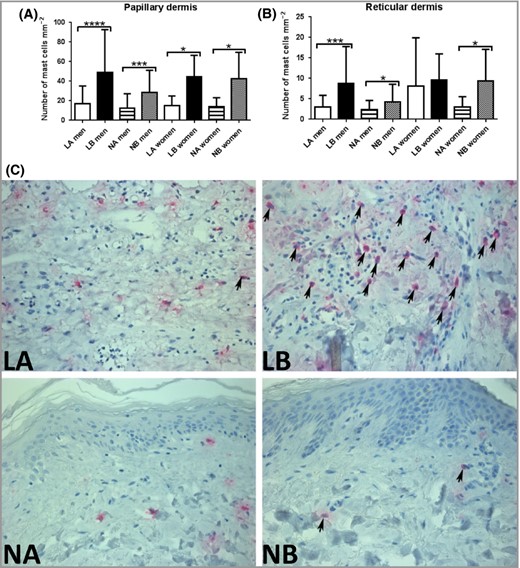
Effect of siramesine on mast‐cell density in biopsies from lesional and normal skin from patients with psoriasis. Skin biopsies (3‐mm) were incubated in medium with or without siramesine (20 μmol L−1) for 72 h (men, n = 18; women, n = 7). Sections from the biopsies were then evaluated for the presence of mast cells by tryptase immunostaining. The graphs show the number of mast cells (mean values ± SD) per mm−2 in papillary dermis (A) and reticular dermis (B). Wilcoxon matched‐pairs signed‐rank test *P < 0·05, ***P < 0·001, ****P < 0·0001. The number of mast cells was higher in lesional skin compared with nonlesional skin in men, both in papillary (P = 0·012) and reticular (P = 0·016) dermis. (C) Representative images showing the effect of siramesine on tryptase‐expressing cells (arrows) in skin biopsies from one patient. LA, treated lesional skin; LB, untreated lesional skin; NA, treated nonlesional skin; NB, untreated nonlesional skin.
Siramesine induces apoptosis of mast cells in psoriatic skin biopsies
As indicated by the data above, siramesine efficiently reduces the number of mast cells in psoriatic lesions. Next, we assessed whether the drug is selective for mast cells or affects other cell types in the skin. To address this issue, skin biopsies were incubated with siramesine and then treated enzymatically to obtain single‐cell suspensions. After staining with markers for mast cells (CD45, CD117), fibroblasts (CD90), keratinocytes (CD49f), and apoptosis/necrosis (Annexin V and Draq7), FACS analysis was performed to determine the effect of siramesine on viability of individual cell types. As depicted in Figure 2, siramesine caused a marked increase in the Annexin V+/Draq7− (apoptotic) mast‐cell population, whereas no change in the proportion of apoptotic fibroblasts was seen. Keratinocytes were not significantly affected by siramesine compared with vehicle exposure, although a small tendency of an increase in apoptotic keratinocytes was observed. Under these conditions, siramesine did not cause any significant increases in the proportion of necrotic (Annexin V+/Draq7+) mast cells, fibroblasts or keratinocytes. Hence, the flow cytometry results concurred with the immunohistochemical findings, suggesting that dermal mast cells undergo apoptotic cell death in response to siramesine. Furthermore, the data indicate that siramesine causes cell death of mast cells preferentially, with minor effects on the major structural cell types of the skin.
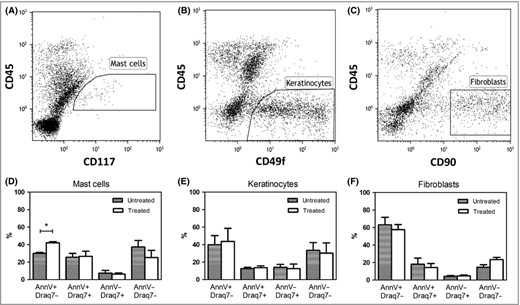
Flow cytometric analyses of skin biopsy cells from lesional psoriatic skin. Identification gates of (A) mast cells (CD45+CD117+); (B) keratinocytes (CD45−CD49f+) and (C) fibroblasts (CD45−CD90+). Viability changes induced by siramesine (20 μmol L−1) treatment of (D) skin mast cells (n = 6 biopsies); (E) keratinocytes (n = 3 biopsies) and (F) fibroblasts (n = 3 biopsies) shown as % expression of Annexin V and Draq7 compared with control (medium without siramesine). Graphs represent means ± SEM.
Siramesine reduces the number of interleukin‐17‐positive mast cells in psoriatic skin biopsies
As key producers of IL‐17, mast cells have been implicated in the pathogenesis of psoriasis.10,11,14 To evaluate if lysosomotropic agents might reduce the number of IL‐17‐positive mast cells, we treated lesional and nonlesional skin biopsies with siramesine, followed by double staining for mast cells (tryptase) and IL‐17. As shown in Figure 3A, the number of IL‐17+ mast cells in biopsies from both lesional and nonlesional skin was significantly reduced by treatment with siramesine. As expected, the total number of IL‐17+ cells was not significantly affected because other IL‐17‐producing cells predominate in human skin.
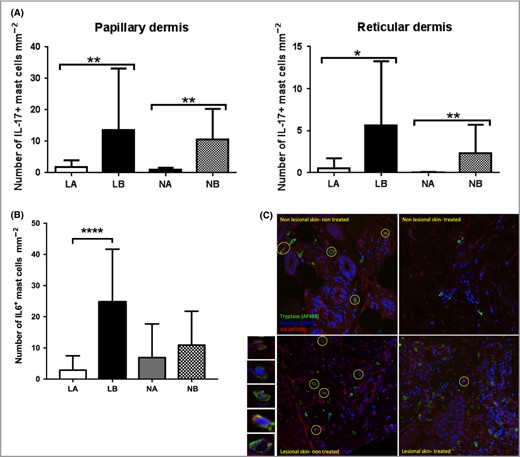
Effect of siramesine on IL‐17 and IL‐6‐positive mast cells in biopsies from patients with psoriasis. Skin biopsies (3‐mm) from 25 patients with psoriasis were incubated for 72 h in medium with or without siramesine (20 μmol L−1). Sections from the biopsies were then stained for the presence of IL‐17+ and IL‐6+ mast cells by double immunofluorescence. (A) The number of IL‐17+ mast cells (mean values ± SD) per mm−2 was calculated in the papillary and reticular dermis, respectively. (B) IL‐6+ mast cells (mean values ± SD) per mm−2 in skin sections. (C) Representative images showing the effect of siramesine on the presence of cells double positive for tryptase (red) and IL‐6 (green). IL‐6+ mast cells (IL‐6+tryptase+) are encircled. Nuclei are visualized by DAPI staining (blue). Representative IL‐6+tryptase+ cells are shown at the left side of the panel at larger magnification. Wilcoxon matched pairs signed rank test *P < 0·05, **P < 0·01. ***P < 0·0001. IL, interleukin; LA, treated lesional skin; LB, untreated lesional skin; NA, treated nonlesional skin; NB, untreated nonlesional skin.
Siramesine reduces the number of interleukin‐6‐positive mast cells in psoriatic skin biopsies
In addition to secreting pathogenic IL‐17, mast cells have been implicated as a source of IL‐6 in psoriasis.27 To determine if siramesine affects the IL‐6 expression by mast cells, we treated biopsies from psoriatic skin with siramesine followed by double staining for IL‐6 and tryptase (Fig. 3B,C). As with IL‐17+ mast cells, the number of IL‐6‐positive mast cells in the lesional skin was profoundly reduced by siramesine exposure. In contrast, the density of IL‐6‐positive mast cells in nonlesional skin was not significantly affected.
Siramesine reduces keratinocyte proliferation in lesional psoriatic skin biopsies
Next, we assessed whether siramesine affects the number of proliferating cells in skin from patients with psoriasis. In lesional skin, Ki‐67‐positive (proliferating) keratinocytes were found in the basal layer and lower layers of the stratum spinosum. In contrast, proliferating cells in clinically normal skin were restricted to the basal epidermal layer. As displayed in Figure 4 and Figure S2 (see Supporting Information), siramesine caused a significant reduction of proliferating keratinocytes in lesional skin (P = 0·0008) as opposed to uninvolved skin. As expected, the number of proliferating epidermal keratinocytes in untreated samples was higher in lesional than in uninvolved biopsies (P < 0·0001).
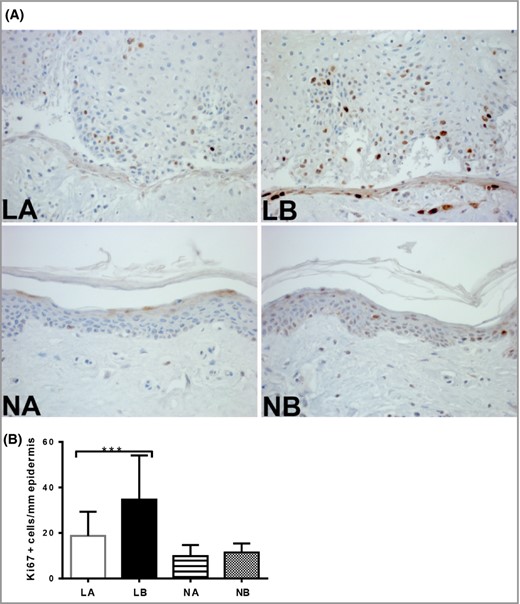
The effect of siramesine on the proliferative activity of epidermal cells in biopsies from patients with psoriasis. Skin biopsies (3‐mm) were incubated in medium with or without siramesine (20 μmol L−1) for 72 h. (A) Sections from the biopsies were then stained with the proliferation marker Ki‐67. (B) Quantification of Ki‐67‐positive cells per mm2 basement membrane. Graphs represent means ± SD (n = 25). Wilcoxon matched‐pairs signed‐rank test ***P < 0·0001. LA, treated lesional skin; LB, untreated lesional skin; NA, treated normal skin; NB, untreated normal skin.
Discussion
Mast cells are generally known for their detrimental impact on various pathologies, most notably allergic conditions, but it is important to emphasize that mast cells also can have activities that are beneficial to their host.28 In the last few decades, increased knowledge on mast‐cell function has led to growing recognition of the driving role of mast cells in chronic inflammatory skin diseases, including psoriasis.1,29,30 However, although there is substantial clinical evidence implicating mast‐cell infiltration as a prominent feature of psoriasis, the functional impact of mast cells on psoriasis remains to be established. A challenging prospect of these research advancements is that targeted mast‐cell apoptosis now appears to be a conceivable therapy to dampen mast‐cell‐mediated effects in various pathological conditions.17 In previous studies, we have highlighted the idea of using lysosomotropic agents for this purpose.18 Mechanistically, these agents induce permeabilization of mast‐cell granule membranes, which results in the release of granule compounds into cytosol and the subsequent triggering of reactive oxygen species and tryptase‐dependent apoptosis (Fig. 5).21 31
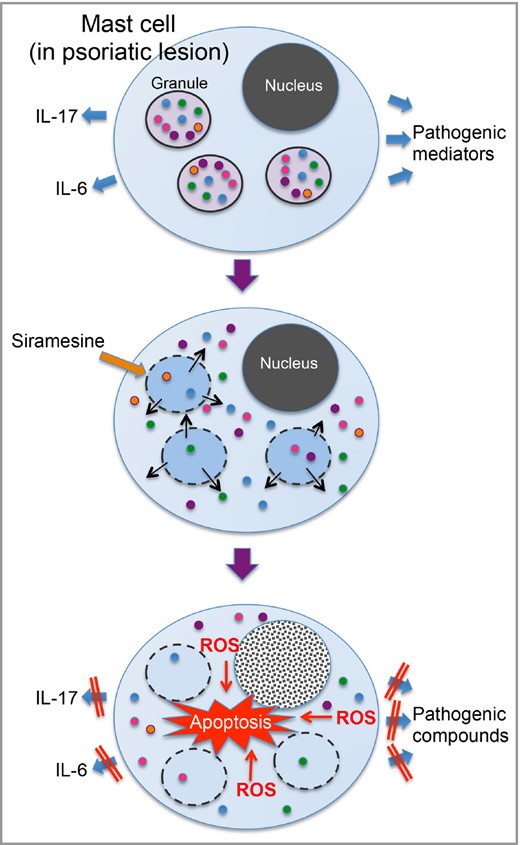
Schematic presentation of the proposed mechanism of mast‐cell apoptosis in response to siramesine. Dermal mast cells in psoriatic lesions are believed to intensify the disease process by releasing pro‐inflammatory interleukins such as IL‐6 and IL‐17 plus other pathogenic mediators. Siramesine has lysosomotropic activity, i.e. it causes permeabilization of lysosome‐like organelles including the secretory granules (secretory lysosomes) of mast cells. Exposure to siramesine will thus lead to the entry of granule contents into the cytosol and subsequent apoptosis due to a reactive oxygen species (ROS)‐dependent mechanism. As a result, the secretion of psoriasis‐driving compounds from mast cells will decline. Note that most cells other than mast cells are less sensitive to siramesine, which probably reflects their lower content of lysosome‐like organelles containing pro‐apoptotic compounds.
So far, the principle of reducing mast‐cell populations by lysosomotropic agents has mainly been adapted to various mast‐cell types in culture and in other preclinical settings.18,19,20,21,22 Here, we have extended the concept into a clinically relevant context, and demonstrate that siramesine clearly reduces the number of mast cells in psoriatic skin ex vivo. Based on these findings, we envision that the principle of using lysosomotropic agents – topically or systemically – may prove clinically useful in order to diminish mast‐cell‐mediated, pro‐inflammatory influences in psoriasis. From a broader perspective, this strategy may well be applicable to other skin diseases associated with mast‐cell infiltration, such as urticaria pigmentosa, cutaneous mastocytoma and, possibly, contact eczema and atopic dermatitis.
It should be emphasized that dermal mast cells may contribute to the pathology of psoriasis in a variety of ways by virtue of their ability to recruit other immune cells, and their capacity to secrete a large number of potentially pathogenic factors from both preformed stores and via de novo synthesis.8 32 Consequently, therapies directed at single mediators, receptors or activities, e.g. histamine receptor antagonism or mast‐cell protease inhibition,16 will only partly interfere with the overall impact of mast cells in psoriasis. The strategy suggested here, i.e. to induce selective apoptosis in dermal mast cells, represents a more comprehensive method to abolish the mast‐cell‐associated effects. As proof‐of‐concept for this notion, we show that siramesine treatment reduces the number of mast cells expressing IL‐17 and IL‐6, which represent key interleukins in psoriasis pathobiology.11,33,34 Most likely, the induction of mast‐cell apoptosis by treatment with siramesine will additionally block the production of a range of de novo‐synthesized mast‐cell mediators, including various cytokines and growth factors.8
A prerequisite for using lysosomotropic agents as mast‐cell‐directed drugs in clinical practice is that the drug does not harm cells other than the target cells. For example, if administered topically in dermatology, the drug should not injure structural skin cells. Under the present study conditions, siramesine was mainly directed at dermal mast cells, whereas the two predominant cell types in human skin, i.e. fibroblasts and keratinocytes, were less disposed to apoptosis. As demonstrated in previous in vitro studies, siramesine also has less of an impact on different leukocyte populations, e.g. neutrophils and eosinophils compared with mast cells.21 22
In addition to inducing apoptosis in cutaneous mast cells in normal18 and psoriatic skin, siramesine is able to reduce the proliferative activity of epidermal keratinocytes in lesional skin. This could potentially be considered a negative property in terms of epidermal turnover. However, in relation to psoriasis, the antiproliferative effect on keratinocytes may represent a positive ‘off‐target’ effect, as keratinocyte hyperproliferation is the root cause of epidermal thickening and scaling of psoriatic plaques. In a previous study we showed that siramesine has direct effects on cultured keratinocytes,18 suggesting that the reduction of skin keratinocyte proliferation represents a direct effect of siramesine on the keratinocytes, rather than representing indirect effects occurring downstream of the induction of mast‐cell apoptosis.
In summary, the principle of eliciting lysosomal membrane leakage offers a promising method to reduce the number of infiltrating mast cells in psoriatic skin. Forthcoming in‐depth experiments in laboratory and clinical settings will explore the applicability of siramesine and other lysosomotropic drug therapies in mast‐cell‐associated dermatoses.
Acknowledgments
This work was supported by grants from the Welander‐Finsen Foundation, the Swedish Psoriasis Association, the Swedish Research Council, the Swedish Cancer Foundation and the Swedish Heart and Lung Foundation.
References
Author notes
Funding sources
This work was supported by grants from the Welander‐Finsen Foundation, the Swedish Psoriasis Association, the Swedish Research Council, the Swedish Cancer Foundation and the Swedish Heart and Lung Foundation.
Conflicts of interest
None declared.
Plain language summary available online



