-
PDF
- Split View
-
Views
-
Cite
Cite
J. Borowczyk‐Michalowska, E. Zimolag, A. Waligorska, J. Dobrucki, Z. Madeja, J. Drukala, Stage‐specific embryonic antigen‐4 as a novel marker of ductal cells of human eccrine sweat glands, British Journal of Dermatology, Volume 176, Issue 6, 1 June 2017, Pages 1541–1548, https://doi.org/10.1111/bjd.15154
Close - Share Icon Share
Summary
Different populations of unipotent or multipotent stem cells have been identified in human epidermis and its appendages. It is well documented that these cells maintain tissue homeostasis and actively participate in epidermal regeneration after injury. However, there is no evidence of the presence of pluripotent stem cells in human epidermis.
In this study we investigated whether cells positive for embryonic stem cell marker stage‐specific embryonic antigen‐4 (SSEA‐4) are present in adult human epidermis and, if so, whether they are pluripotent and correspond to the population of primitive stem cells.
The expressions of SSEA‐4 and pluripotency transcription factors were analysed using flow cytometry. By means of immunohistochemical staining, we studied the exact localization of these cells in sections of human skin.
We show that a population of SSEA‐4+ cells is present in human epidermis. In contrast to the commonly accepted belief, the expression of SSEA‐4 is not connected with the pluripotent character of isolated cells. We found that these SSEA‐4+ cells are localized in the ducts of eccrine sweat glands.
Our results indicate that SSEA‐4 is a novel marker identifying the ductal cells of human sweat glands. The surface character of the antigen provides for a simple method of isolating this cell population and suggests applications of SSEA‐4 for future cell therapy research.
The skin serves as a physiological barrier that separates the internal organs of the body from the external environment and it performs a number of vital functions. Epidermal stem cells play a key role in maintaining epidermis homeostasis by providing a pool of new cells to replace those lost during skin turnover.1 Moreover, the critical functions of the skin are also fulfilled by accessory appendages in mammals, such as hair follicles, nails, and sebaceous and sweat glands. These complex structures consist of cells of both ectodermal and mesodermal origin and to date multiple populations of stem cells that support the cyclic changes of the appendages during homeostasis have been distinguished among them. Additionally, it has been shown that these cells may participate in wound healing in the case of injury.2 The population of nestin‐positive stem cells detected in human hair follicles, sebaceous and sweat glands is one of the fields of interest of regenerative medicine.3,4 However, detailed analysis has revealed that these cells are localized exclusively in the connective‐tissue sheaths of human appendages.5 As cells of intramesenchymal skin compartments, they mainly enhance wound healing by releasing bioactive molecules. Thus, they promote vascularization and dermal regeneration rather than acting through direct differentiation into epidermal cells in vivo.6,7,8 The epithelial cells of hair follicles or sweat glands, on the other hand, not only regenerate and repair appendages but may also migrate to the interfollicular epidermis and support re‐epithelialization during wound healing.2,9,10
The treatment of deep and extensive burns often involves the application of autologous epidermal cells expanded in vitro.11 Although this approach ensures the restoration of the epidermis, the sweat glands and other appendages do not regenerate, which affects the quality of life of burn patients.12,13 Thus, the search for cells with a higher potential for differentiation, such as pluripotent stem cells, is justified. Additionally, it would also be desirable to develop a relatively simple method to isolate such cells for autologous cell‐based regenerative medical strategies.
The globoseries glycosphingolipid stage‐specific embryonic antigen‐4 (SSEA‐4) is a cell surface marker widely used to define human pluripotent stem cells. SSEA‐4 and SSEA‐3 are highly expressed in human embryonic stem cells or embryonal carcinoma cells and are downregulated upon differentiation.14,15,16,17 However, the functions of SSEA‐4 and its role as a pluripotent marker remain controversial.18,19,20,21 Recently, SSEA‐4 has also been described at the surface of cells residing in various adult tissues, including cardiomyocyte progenitors or pancreatic ductal progenitor cells.22,23
In agreement with the latter, in this study we discovered that ductal cells of human eccrine sweat glands also express SSEA‐4 on their surface. SSEA‐4 was detected in all cells of both layers at the full length of the duct, but not in the secretory portion of sweat glands. Thus, the present article is, to the best of our knowledge, the first description of SSEA‐4 as a novel marker exclusively present at the ductal but not secretory cells of eccrine sweat glands.
Materials and methods
Human tissue
Skin biopsies were obtained from healthy patients of both sexes undergoing plastic surgery. The informed consent and prior approval of the local ethics committee were obtained according to Polish law (No. KBET/72/B/2008). In order to carry out flow cytometry analysis we used 28 biopsies obtained from the following body sites: the abdomen, breast, ears, eyelids, foreskin and labia minora. The age range of the included patients was 11–61 years with a median age of 32 years. For immunohistochemical analysis we used 10 biopsies obtained from the abdomen, breast, eyelids, foreskin and palm. The age range of the included patients was 10–41 years with a median age of 39 years.
Flow cytometry analysis
Human epidermal cells were isolated from skin biopsies. Briefly, the skin sample was rinsed in phosphate‐buffered saline (PBS) supplemented with penicillin (5000 U mL−1) and streptomycin (5 mg mL−1) (both from Sigma‐Aldrich, St Louis, MO, U.S.A) and the subcutaneous tissue was removed. The skin biopsy was cut into small pieces and digested in dispase II (12 U mL−1, Gibco, Grand Island, NY, U.S.A) overnight at 4 °C. The following day the epidermis was detached from the dermis and treated with 0·05% trypsin with 2 mmol L−1 ethylenediaminetetraacetic acid (Sigma‐Aldrich) to isolate the epidermal cells. The freshly isolated cells were filtered with a 70‐μm strainer, fixed with 3·7% paraformaldehyde for 20 min at room temperature and then washed with Perm/Wash Buffer (BD Biosciences, Franklin Lakes, NJ, U.S.A.). The cells were labelled with the following monoclonal antibodies: anti‐CD45 Pacific Blue (Beckman Coulter, Brea, CA, U.S.A.), anti‐Lineage‐FITC (CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, CD235a, all from BD Biosciences) and anti‐SSEA‐4‐PE (BioLegend, San Diego, CA, U.S.A.) in concentrations suggested by the manufacturers for 30 min at room temperature. Dead cells were excluded with 7‐amino‐actinomycin‐D (BD Biosciences).
For transcription factors analysis, cells were analysed with the BD Human Pluripotent Stem Cell Transcription Factor Analysis Kit (BD Biosciences) according to the manufacturer's protocol. The kit contains the following mouse monoclonal antibodies: Nanog‐PE, Oct3/4‐PerCP‐Cy5·5 and Sox2‐Alexa Fluor 647. Additionally, anti‐SSEA‐4‐AlexaFluor 488 monoclonal antibody (eBioscience, San Diego, CA, U.S.A) was added. Appropriate isotype controls were included and gates were set according to isotype‐matched controls. Dead cells were excluded with Hoechst 33258 (Sigma‐Aldrich).
The cytometric analysis of the cells was performed on a LSR‐II Flow Cytometer (BD Biosciences) and data were evaluated using the FACSDiva v.6 software (BD Biosciences).
Immunohistochemistry of human skin sections
Skin tissue specimens were fixed in 3·7% formaldehyde for 5 days and embedded in Tissue‐Tek O.C.T (Fisher Scientific, Pittsburgh, PA, U.S.A.) and then 6–8‐μm cryosections were cut for staining. Antigen retrieval was performed in 10 mmol L−1 citrate buffer pH 6·0 for 30 min at 95 °C.
For light microscopy, cryosections were stained using EnVision G|2 System/AP, Rabbit/Mouse (Permanent Red) Kit (Dako, Glostrup, Denmark) according to the manufacturer's protocol. The following dilutions of primary antibodies were used: mouse monoclonal anti‐SSEA‐4 (1 : 800, Merck Millipore, Billerica, MA, U.S.A), rabbit monoclonal anti‐carcinoembryonic antigen (CEA, 1 : 1000, Abcam, Cambridge, U.K.), rabbit monoclonal anti‐keratin 7 (K7, 1 : 400, Abcam), mouse monoclonal anti‐α smooth muscle actin (αSMA, 1 : 3000, BD Biosciences) and mouse monoclonal anti‐keratin 10 (K10, 1 : 2000, Abcam). After having been stained, the cryosections were counterstained with Mayer's haematoxylin solution, mounted with Glycerol Mounting Medium (Dako) and then examined with an Olympus BX51 microscope with a VC50 camera (Olympus Co., Tokyo, Japan).
For immunofluorescence, cryosections were preincubated with 5% normal goat serum (Sigma‐Aldrich) in PBS for 1 h. Next, mouse monoclonal anti‐SSEA‐4 (1 : 100, Merck Millipore) with rabbit monoclonal anti‐CEA (1 : 200, Abcam) or rabbit monoclonal anti‐K7 (1 : 200, Abcam) was added for 2 h and then visualized with goat antimouse‐AlexaFluor 488 (1 : 300) and goat anti‐rabbit‐AlexaFluor 546 (1 : 600, both from Invitrogen, Carlsbad, CA, U.S.A.) secondary antibodies. All dilutions of antibodies were prepared in 3% bovine serum albumin with 0·05% Tween‐20 in PBS. Nuclei were stained with Hoechst 33258 (Sigma‐Aldrich). Cryosections were mounted with Fluorescent Mounting Medium (Dako) and then examined with a Leica TCS SP5 confocal microscope equipped with Leica Las AF software (Leica Microsystems, Wetzlar, Germany).
Statistical analysis
Statistical analyses were carried out from at least three independent samples using the Kruskal–Wallis test in Statistica 10 software (StatSoft, Tulsa, OK, U.S.A.); P < 0·05 was considered statistically significant, n refers to the number of the analysed samples from different patients.
Results
Nonpluripotent stage‐specific embryonic antigen‐4‐positive cells are present in human epidermis
Firstly, we investigated whether cells positive for embryonic stem cell marker SSEA‐4 are present in adult human epidermis. A multicolour flow cytometry analysis of freshly isolated epidermal cells was performed and cells of haematopoietic origin (Lin+/CD45+) were excluded (Fig. 1a). Interestingly, we identified a distinct population of cells expressing SSEA‐4 at their surface. Given that SSEA‐4 is a marker characteristic of embryonic stem cells, we reasoned that SSEA‐4+ cells present in the epidermis might be pluripotent. Oct‐4, Nanog and Sox‐2 are three pluripotent transcription factors that are well known as master regulators of stem cell pluripotency.24 However, the results obtained from the flow cytometry analysis showed that SSEA‐4+ cells did not express any of these transcription factors, so they did not correspond to the population of primitive stem cells in adult human epidermis (Fig. 1b).
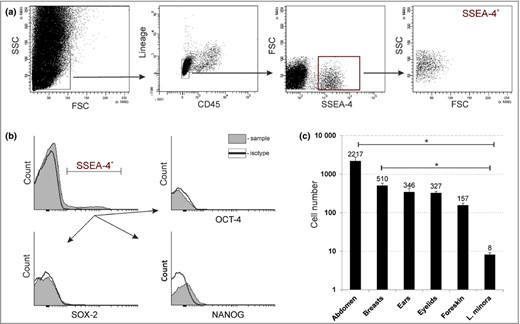
Non‐pluripotent stage‐specific embryonic antigen‐4 (SSEA‐4)+ cells are present in adult human epidermis. (a) Flow cytometric analysis showing the gating strategy of enzymatically isolated cells from human epidermis. The cells were analysed for SSEA‐4 antigen expression after excluding cells of haematopoietic origin (Lineage+, CD45+). (b) Flow cytometric analysis of expression of pluripotency transcription factors; that is Oct‐4, Nanog and Sox‐2, in SSEA‐4+ cells. (c) Quantification of SSEA‐4+ in skin samples collected from specific body sites. Results are normalised per 100 000 analysed events and presented as means ± SEM (n = 5, except for eyelids with n = 3). Significance: P < 0·05 tested by a Kruskal–Wallis test. FSC, forward scatter; SSC, side scatter; L. minora, labia minora.
We estimated that the average number of epidermal SSEA‐4+ cells was 264 ± 115 per 100 000 analysed events (n = 10). We observed significant differences in the number of SSEA‐4+ cells between the analysed samples; however, we did not find any association between the number of these cells and the patients’ age or sex. Surprisingly, the data obtained revealed an unambiguous correlation between the number of SSEA‐4+ cells and the anatomical site of the body, indicating that these cells are most abundant in the abdomen and breast epidermis [2217 ± 539 and 510 ± 72 (n = 5) per 100 000 analysed events, respectively]. We found that the number of SSEA‐4+ cells was lower and amounted to 346 ± 77 (n = 5) and 327 ± 32 (n = 3) in the case of epidermis isolated from the ears or eyelids, respectively. The smallest SSEA‐4+ cell population was observed in epidermis from the genitals, in which we detected 157 ± 20 (n = 5) cells in the foreskin and only 8 ± 1 (n = 5) cells in the labia minora (Fig. 1c).
Epidermal stage‐specific embryonic antigen‐4‐positive cells are localized in orifices of sweat gland ducts
Next, we used immunohistochemical staining of skin tissue cryosections to identify the precise localization of SSEA‐4+ cells in human epidermis. We did not find SSEA‐4+ cells within the rete ridges, a common niche of epidermal stem and progenitor cells, or anywhere along the basal layer, or in any other layer of differentiating cells (Fig. 2a). However, we noticed cells expressing SSEA‐4 within single elongated and spiral structures. The observed structures traversed all layers of epidermis and opened directly onto the skin surface (Fig. 2b). Additionally, the skin samples analysed from different body regions varied in the number of structures in agreement with previously obtained results from flow cytometry (data not shown).
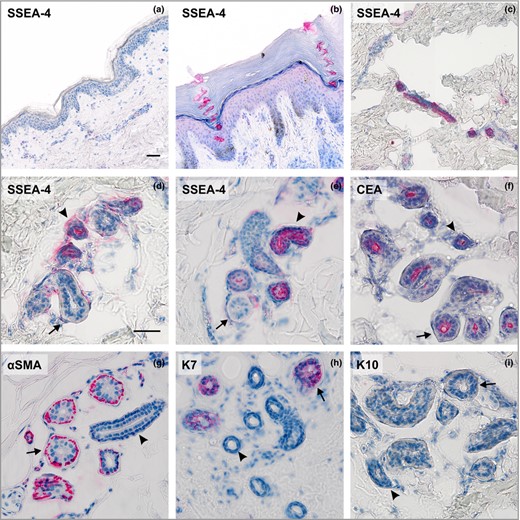
The expression pattern of stage‐specific embryonic antigen‐4 (SSEA‐4) and sweat gland markers in human skin. Analysis of SSEA‐4 antigen expression: (a) the interfollicular epidermis, breast; (b) the acrosyringium, palm; (c) the intradermal straight duct, breast; (d, e), proximal reabsorption and secretory portion of the eccrine sweat glands, breast and abdomen. Staining of sweat gland markers: (f) carcinoembryonic antigen (CEA) present in luminal membranes of both secretory and ductal cells, breast; (g) α smooth muscle actin (αSMA) expressed in myoepithelial cells, abdomen; (h) keratin 7 (K7) localized only in luminal cells in the secretory coil of sweat glands, palm; (i) keratin 10 (K10) absent from sweat glands, breast. Ducts indicated by arrowheads; secretory portion indicated by arrows; (a–c) × 200, (d–i) × 400. Scale bars 50 μm.
Surprisingly, we identified the observed structures as intraepidermal parts of eccrine sweat gland ducts, called the acrosyringia. Thus, we conclude that SSEA‐4 is not present on the surface of cells residing in the interfollicular epidermis itself, but is present in its appendages (i.e. sweat glands). The acrosyringium spirals through the surface epidermis in a corkscrew pattern that is preserved even in cornified layer, such as in the section from a palm (Fig. 2b).
Stage‐specific embryonic antigen‐4 is expressed in ductal but not secretory cells of eccrine sweat glands
Then, we asked whether the SSEA‐4 was present only in the intraepidermal sweat duct unit or whether it was present in the whole structure of the gland. With further immunohistochemical analyses, we confirmed the presence of SSEA‐4 in all cells located along the full length of the sweat gland duct, that is in the spiralled intraepidermal acrosyringium (Fig. 2b) and all the way through the dermis (Fig. 2c) to the coiled section leading to the secretory portion (Fig. 2d, e). It seemed that SSEA‐4 was present at the surface of both basal and suprabasal layers of cuboidal epithelial cells of ducts. A similar staining pattern was observed in all examined eccrine sweat glands both in samples of glabrous and nonglabrous skin (Fig. 2b–e). Intriguingly, we did not observe SSEA‐4 expression in the luminal or myoepithelial cells of the secretory portion (Fig. 2d, e).
To confirm the localization of the glycosphingolipid antigen, we conducted double immunofluorescent staining of the cryosections. CEA is generally considered as a cell surface marker of human sweat glands.25 CEA is present at the luminal surface of the secretory and ductal cells in eccrine sweat glands, with the latter showing stronger labelling.26 K7, on the other hand, is expressed solely in the luminal cells of the secretory coil.25 Our results clearly showed the coexpression of SSEA‐4 and CEA in the luminal membranes of the suprabasal cells of sweat ducts, whereas, as expected, the basal cells were labelled only with SSEA‐4 (Fig. 3a). For the secretory portion, only CEA was detected in the luminal membranes of the secretory cells (Fig. 3a). In addition, the absence of SSEA‐4 coexpression with K7 confirmed that the presence of the glycosphingolipid antigen is restricted to the ductal cells and is not found in the secretory coil (Fig. 3b).
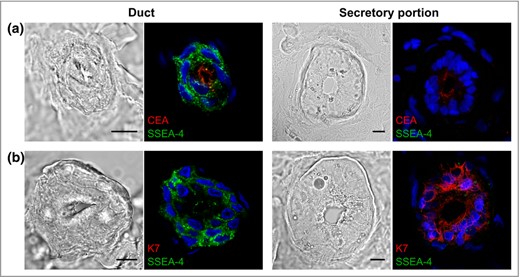
The expression of stage‐specific embryonic antigen‐4 (SSEA‐4) antigen in human sweat glands. (a) Confocal microscopy analysis of tissue cryosections stained with antibodies against SSEA‐4 (green) and carcinoembryonic antigen (CEA) (red) or (b) SSEA‐4 (green) and keratin 7 (K7) (red). Nuclei (blue) were counterstained with Hoechst 33258. Scale bars 10 μm.
Discussion
Standard treatment of deep and extensive burns with in vitro cultured cells mainly focuses on restoring the epidermis as a barrier to prevent loss of body fluids and to protect against the invasion of pathogens. However, this approach does not ensure the regeneration of accessory appendages, leading to the disturbance of some pivotal functions of the skin, that is ultraviolet protection, thermoregulation or sebum production.1 There are high hopes over modifications of cell therapy that would promote both epidermis and appendage regeneration and thus be a solution for burns patients.
In light of hopes for the clinical application of epidermal cells in treating skin lesions, we strove to find a cell population in human epidermis that would meet these expectations. Cytometric analysis revealed the presence of a distinct cell population expressing SSEA‐4 at the surface after standard enzymatic digestion of epidermis with trypsin. Contrary to our assumption, we found that these cells were not localized in the interfollicular epidermis but in its appendages; namely, in the sweat glands. Although in humans eccrine sweat glands are distributed widely on the body surface, there are clear regional differences in their density.27 Our study revealed that SSEA‐4 is generally expressed in the ductal cells and thus, the observed differences in SSEA‐4+ cell number in analysed epidermis samples most likely resulted from the variance in sweat gland density in particular regions of the human body.28 The extremely low number of SSEA‐4+ cells detected in labia minora epidermis compared with other samples is justified, as the presence of eccrine sweat glands in this area is still controversial.29,30 Although the functions of sweat glands have been known since the early 1950s, there is still little information on their regenerative potential.31,32
Several reports have documented the proliferation of dissociated sweat gland cells in culture and have shown the contribution of in vitro cultured sweat gland cells to epidermal repair after skin grafting.33,34 Recently, Lu et al. reported their identification of three types of adult cell progenitors within the sweat gland and its duct in mice.35 Although all progenitors actively participated in gland regeneration, only basal progenitors within sweat gland ducts were found to respond and proliferate after epidermal injury. In addition, they regenerated the sweat duct orifice and did not participate in the interfollicular epidermis repair.35 In contrast, experiments conducted on human skin revealed that eccrine sweat glands contribute significantly to re‐epithelialization after partial thickness wounds and demonstrated the important role of ductal cells of human eccrine sweat glands in epidermal repair.9,10 Thus, these findings provide compelling evidence in support of the notion that the cells located in sweat ducts may be the source of the cells that regenerate not only ductal orifices but also the epidermis after injury.
Generally, the sweat gland is a tiny coiled tubular structure that consists of a secretory unit producing sweat and a duct carrying the sweat away. The reabsorptive duct of the eccrine gland is a straight two‐layered channel opening at the surface of the epidermis.27 The cystic fibrosis transmembrane conductance regulator (CFTR) and a glycoprotein CEA are extracellular markers known to be expressed in the ducts of sweat glands; however, their presence is limited to the luminal cell layer.26,36 Here we show that, in contrast to these markers, SSEA‐4 is also expressed in cells residing in the basal layer. To date, a surface antigen specific for the ductal cells of eccrine sweat glands has not been discovered. In the light of these results and to our best knowledge, SSEA‐4 may be the first described marker for these cells. Our article confirms that the expression of SSEA‐4 is not restricted to pluripotent human cells.18,21,37 Moreover, this is the second demonstration that SSEA‐4 is present in the ducts of glandular structures, that is the pancreas and sweat glands.23 The role played by glycosphingolipid SSEA‐4 at the surface of ductal cells, and whether it is associated with the potential of these cells to proliferate and differentiate into other cell lineages, remains unknown.
The question of whether SSEA‐4 expression is unique for eccrine sweat glands or whether it also occurs in apocrine or mammary glands remains open. Further research is urgently needed to settle this question because the results would be of particular interest for the development of tumour diagnostic markers. Among other considerations, it is still difficult to distinguish primary sweat gland carcinomas from cutaneous metastases of breast carcinomas or apocrine and eccrine metastatic sweat gland carcinomas because of their similar morphology and their origin as epidermal appendages.38,39,40 As the treatment for these carcinomas differs it is of clinical importance to identify more reliable markers to distinguish between them: SSEA‐4 may be useful in this respect.
To summarize, we have identified a population of cells within the adult human epidermis that expresses embryonic antigen SSEA‐4 on its surface. The data obtained enabled us to point to SSEA‐4 as a newly discovered marker of the ductal cells of eccrine sweat glands. Currently, intensive research has been directed at finding effective treatment methods for patients with an irreversible loss of functional eccrine sweat glands. Because the evaporation of sweat is the body's primary mechanism for heat loss, hyperthermia is generally thought to be a problem for most burns patients, affecting the quality of their lives.25,26,41 The surface character of SSEA‐4 may be conducive to the efficient acquisition of ductal cells of sweat glands and may become a useful marker in tumour diagnosis and thus find clinical application in the future.
Acknowledgments
The authors would like to thank Dr Joanna Marczynska and Piotr Konieczny for technical assistance.
References
Author notes
Conflicts of interest
None declared
Funding sources
This work was supported by the European Regional Development Fund within the Operational Programme Innovative Economy (POIG 01.02–00–109/99 ‘Innovative methods of stem cell application in medicine’). The Faculty of Biochemistry, Biophysics and Biotechnology of Jagiellonian University is a partner of the Leading National Research Centre (KNOW) supported by the Ministry of Science and Higher Education.



