-
PDF
- Split View
-
Views
-
Cite
Cite
D.L. Morton, A.J. Cochran, The case for lymphatic mapping and sentinel lymphadenectomy in the management of primary melanoma, British Journal of Dermatology, Volume 151, Issue 2, 1 August 2004, Pages 308–319, https://doi.org/10.1111/j.1365-2133.2004.06133.x
Close - Share Icon Share
We appreciate the opportunity to respond to Dr Medalie's and Dr Ackerman's curious and premature attempt to discredit sentinel node biopsy. For 30 years we have struggled with the problem of managing clinically normal regional lymph nodes in patients with primary cutaneous melanoma. Our efforts have produced a minimally invasive procedure that has been universally adopted by most academic and community‐based surgical oncologists and pathologists—and enthusiastically welcomed by the patients they treat. Our extensive published studies on the evolution and application of the sentinel node concept1–16 have been validated not only by the peer‐review process required for publication in respected scientific journals but also by multiple peer‐reviewed studies subsequently published by other investigators.17–22 Although Drs Medalie and Ackerman are certainly entitled to their opinion and are skilled at its forceful expression, they have made no attempt to refute the scientifically‐based data that underpin the development and conduct of preoperative and intraoperative lymphatic mapping and sentinel lymphadenectomy (LM/SL), and their repetitious conclusions are ex cathedra and presented without factual basis. Repeatedly saying that something is true or false does not make it so, no matter how vehemently it is stated. The nature of their argument suggests that Drs Medalie and Ackerman are either unaware of the extensive literature on LM/SL or unwilling to accept its consensus.
Elective vs. therapeutic vs. sentinel lymph node dissection
The claim that sentinel node biopsy is the same as elective lymph node dissection (ELND) reflects a limited understanding of this area of surgical oncology. ELND is the surgical removal of an entire regional group of clinically tumour‐free lymph nodes; the premise is that the primary cutaneous melanoma has risk factors (e.g. intermediate thickness, ulceration) which increase the likelihood that it will have spread to the regional nodes. For reasons discussed below, ELND is not accurate for nodal staging but was previously justified by its possible therapeutic value. However, unless preoperative lymphoscintigraphy is performed, ELND is a blind procedure that may not remove the correct nodal basin. Moreover, its potential morbidity is substantial especially considering that 80–90% of patients (depending upon the thickness of the primary melanoma) cannot benefit because they have no nodal metastases. This is in stark contrast to the sentinel node procedure, which is associated with almost no morbidity—a fact that seems to have escaped Drs Medalie and Ackerman. As we previously reported,1 and as Drs Medalie and Ackerman point out, prospective controlled studies of ELND have not demonstrated a survival advantage for all primary melanoma patients treated this way. Nonetheless, a subset of individuals with limited tumour burden may benefit.23, 24 We remind Drs Medalie and Ackerman of the results of the Intergroup Melanoma Surgical Trial, a randomized study that demonstrated a survival benefit of ELND for prospectively defined subgroups of melanoma patients.24
Therapeutic lymph node dissection removes the regional nodes from patients who have detectable nodal metastases. This confers some therapeutic advantage and can lead to prolonged survival, to a degree that is inversely related to the number of tumour‐involved nodes.2, 25
Sentinel node biopsy, which we prefer to describe as preoperative and intraoperative lymphatic mapping and sentinel lymphadenectomy, is a staging and diagnostic procedure that samples the sentinel node (SN). The SN is the first regional lymph node on the direct lymphatic drainage pathway from the primary melanoma—the node which is most likely to contain any tumour cells that have metastasized from the primary.3 LM/SL is designed to identify clinically occult metastases. As noted above, this procedure has little or no morbidity when performed by experienced surgical oncologists,1 and its diagnostic accuracy far exceeds that of blind ELND because the SN specimen is small enough for a highly focused examination by histopathology, immunohistology and molecular biology methods.1, 4
LM/SL is NOT the same as selective complete lymph node dissection (CLND), the purpose of which is the therapeutic excision of all nodes within the regional basin of a patient whose nodal metastases have already been identified by LM/SL staging. Remember that LM/SL was developed and is currently accepted as a staging technique that avoids CLND in approximately 80% of patients without metastases in the SN. It is theoretically possible that this procedure might also be therapeutic if regional metastasis is confined to the SN, and this possibility is currently under study in a Phase III multicentre trial sponsored by a National Institutes of Health Program Project grant. However, if metastasis in the regional nodal basin beyond a tumour‐positive SN is suspected or confirmed, regional nodal surgery beyond the SN procedure is, by definition, a therapeutic lymph node dissection. That Drs Medalie and Ackerman fail to grasp this distinction is surprising.
It is claimed that ‘if END is bereft of benefit, so, too, it must be for SNB followed by RND’. This bald and unsupported statement ignores the diagnostic utility of LM/SL and discloses the absence of any real understanding of the scientific method of clinical investigation. The statement may be true but is clearly susceptible to scientific proof. Ongoing trials will answer this question with a degree of certainty that cannot come from flamboyant dialectics.
Any attempt to discredit LM/SL for a lack of possible impact on survival/cure ignores the undisputed potential clinical benefit of early cancer diagnosis. Are Drs Medalie and Ackerman suggesting that we withhold assessment of the regional tumour‐draining lymph nodes until they become clinically suspicious? Do they truly countenance this type of passive therapeutic nihilism? Such an approach would allow disease to advance from a relatively early clinical stage that can be readily diagnosed by LM/SL and is amenable to therapy, to more advanced and potentially untreatable stages. Are we being asked to discount or ignore the peer‐reviewed literature?26, 27
Even though the chance of long‐term survival is inversely related to the tumour burden within regional nodes, Drs Medalie and Ackerman apparently would have us wait until a patient develops multiple palpable (and thus relatively burdensome) metastases with a concomitant decrease in the chance of long‐term survival. Since virtually all patients who have SN micrometastases detectable by LM/SL will eventually develop clinically detectable nodal metastases if managed only by wide excision and observation, what do they propose? Surely they would not leave a patient with grossly involved, melanoma‐filled lymph nodes untreated by surgery. If the patient is going to require therapeutic CLND within 12–31 months after wide excision (the period of latency before clinical evidence of nodal metastases, as indicated by our own published findings as well as the inappropriately maligned assertions of Drs Pack and Balch) (Table 1), why leave that patient untreated and at risk of dissemination from the nodal metastases for such a long period of time? Their premise is that all patients with nodal metastases also have distant metastases and eventually will die of melanoma; therefore, all attempts at surgical therapy of the regional nodes are futile. If we are to believe this (and ignore the available peer‐reviewed studies2, 25, 28), even microscopic metastasis to regional lymph nodes must inevitably be accompanied by fatal haematogenous metastasis. As shown below, this is not the case. In fact, from clinical, epidemiological and autopsy studies it is probable that many tumour cells that spread systemically either fail to survive or form latent or dormant microcolonies that may never grow sufficiently to become clinically detectable or cause death. It is in their treatment of the subject of mechanisms and significance of metastases that the authors seem least informed and their arguments weakest.
Incidence of positive nodes found at LM/SL compared with the incidence of clinically evident nodal metastases after wide excision (WE) alone4
| Breslow thickness of primary melanoma | WE + LM/SL (n = 1599) | P‐valueb | WE only (n = 4590) | ||||
| No. of patients with positive nodes/total (%) | P‐valuea | Mean no. of positive nodes in patients with positive nodes ± SE | No. of patients developing nodal metastases/total (%) | Mean no. of positive nodes in patients with positive nodes ± SE | Median time from WE to nodal metastasis, months | ||
| ≤ 1·0 mm | 34/465 (7·30) | 0·004 | 1·32 ± 0·13 | 0·0005 | 238/1979 (12·0) | 2·15 ± 0·21 | 31·1 |
| 1·01–2·0 mm | 116/588 (19·7) | 0·001 | 1·33 ± 0·8 | 0·0001 | 285/890 (32·0) | 2·68 ± 0·33 | 20·7 |
| 2·01–4·0 mm | 103/310 (33·2) | 0·728 | 2·58 ± 0·68 | 0·367 | 195/567 (34·4) | 2·11 ± 0·17 | 16·7 |
| ≥ 4·0 mm | 60/151 (39·7) | 0·055 | 2·17 ± 0·31 | 0·0087 | 65/216 (30·1) | 4·63 ± 0·80 | 12·7 |
| Not available | 9/85 (10·6) | 0·004 | 1·11 ± 0·33 | 0·0001 | 229/938 (24·4) | 2·3 ± 0·28 | 25·9 |
| Breslow thickness of primary melanoma | WE + LM/SL (n = 1599) | P‐valueb | WE only (n = 4590) | ||||
| No. of patients with positive nodes/total (%) | P‐valuea | Mean no. of positive nodes in patients with positive nodes ± SE | No. of patients developing nodal metastases/total (%) | Mean no. of positive nodes in patients with positive nodes ± SE | Median time from WE to nodal metastasis, months | ||
| ≤ 1·0 mm | 34/465 (7·30) | 0·004 | 1·32 ± 0·13 | 0·0005 | 238/1979 (12·0) | 2·15 ± 0·21 | 31·1 |
| 1·01–2·0 mm | 116/588 (19·7) | 0·001 | 1·33 ± 0·8 | 0·0001 | 285/890 (32·0) | 2·68 ± 0·33 | 20·7 |
| 2·01–4·0 mm | 103/310 (33·2) | 0·728 | 2·58 ± 0·68 | 0·367 | 195/567 (34·4) | 2·11 ± 0·17 | 16·7 |
| ≥ 4·0 mm | 60/151 (39·7) | 0·055 | 2·17 ± 0·31 | 0·0087 | 65/216 (30·1) | 4·63 ± 0·80 | 12·7 |
| Not available | 9/85 (10·6) | 0·004 | 1·11 ± 0·33 | 0·0001 | 229/938 (24·4) | 2·3 ± 0·28 | 25·9 |
a Proportion of patients with nodal metastases found by histopathological assessment of SN vs. proportion of patients developing clinical nodal recurrence after WE.
b Student's t‐test for total number of tumour‐involved nodes in patients undergoing immediate CLND after LM/SL vs. those undergoing delayed CLND for clinical nodal recurrence after WE. LM/SL, Lymphatic mapping and sentinel lymphadenectomy; CLND, complete lymph node dissection.
Incidence of positive nodes found at LM/SL compared with the incidence of clinically evident nodal metastases after wide excision (WE) alone4
| Breslow thickness of primary melanoma | WE + LM/SL (n = 1599) | P‐valueb | WE only (n = 4590) | ||||
| No. of patients with positive nodes/total (%) | P‐valuea | Mean no. of positive nodes in patients with positive nodes ± SE | No. of patients developing nodal metastases/total (%) | Mean no. of positive nodes in patients with positive nodes ± SE | Median time from WE to nodal metastasis, months | ||
| ≤ 1·0 mm | 34/465 (7·30) | 0·004 | 1·32 ± 0·13 | 0·0005 | 238/1979 (12·0) | 2·15 ± 0·21 | 31·1 |
| 1·01–2·0 mm | 116/588 (19·7) | 0·001 | 1·33 ± 0·8 | 0·0001 | 285/890 (32·0) | 2·68 ± 0·33 | 20·7 |
| 2·01–4·0 mm | 103/310 (33·2) | 0·728 | 2·58 ± 0·68 | 0·367 | 195/567 (34·4) | 2·11 ± 0·17 | 16·7 |
| ≥ 4·0 mm | 60/151 (39·7) | 0·055 | 2·17 ± 0·31 | 0·0087 | 65/216 (30·1) | 4·63 ± 0·80 | 12·7 |
| Not available | 9/85 (10·6) | 0·004 | 1·11 ± 0·33 | 0·0001 | 229/938 (24·4) | 2·3 ± 0·28 | 25·9 |
| Breslow thickness of primary melanoma | WE + LM/SL (n = 1599) | P‐valueb | WE only (n = 4590) | ||||
| No. of patients with positive nodes/total (%) | P‐valuea | Mean no. of positive nodes in patients with positive nodes ± SE | No. of patients developing nodal metastases/total (%) | Mean no. of positive nodes in patients with positive nodes ± SE | Median time from WE to nodal metastasis, months | ||
| ≤ 1·0 mm | 34/465 (7·30) | 0·004 | 1·32 ± 0·13 | 0·0005 | 238/1979 (12·0) | 2·15 ± 0·21 | 31·1 |
| 1·01–2·0 mm | 116/588 (19·7) | 0·001 | 1·33 ± 0·8 | 0·0001 | 285/890 (32·0) | 2·68 ± 0·33 | 20·7 |
| 2·01–4·0 mm | 103/310 (33·2) | 0·728 | 2·58 ± 0·68 | 0·367 | 195/567 (34·4) | 2·11 ± 0·17 | 16·7 |
| ≥ 4·0 mm | 60/151 (39·7) | 0·055 | 2·17 ± 0·31 | 0·0087 | 65/216 (30·1) | 4·63 ± 0·80 | 12·7 |
| Not available | 9/85 (10·6) | 0·004 | 1·11 ± 0·33 | 0·0001 | 229/938 (24·4) | 2·3 ± 0·28 | 25·9 |
a Proportion of patients with nodal metastases found by histopathological assessment of SN vs. proportion of patients developing clinical nodal recurrence after WE.
b Student's t‐test for total number of tumour‐involved nodes in patients undergoing immediate CLND after LM/SL vs. those undergoing delayed CLND for clinical nodal recurrence after WE. LM/SL, Lymphatic mapping and sentinel lymphadenectomy; CLND, complete lymph node dissection.
A brief history of lymphatic mapping and sentinel lymphadenectomy
The single most important prognostic factor for most primary solid neoplasms including melanoma is the tumour status of regional lymph nodes draining the primary neoplasm (Table 2).25 Until recently, the only method to identify regional node metastasis in patients without palpable lymph nodes was ELND and pathological examination of all excised nodes, usually amounting to examination of one cross‐section of each node by haematoxylin and eosin (H&E) staining. This pathological technique sampled only a small percentage of the total nodal area and therefore underestimated the true frequency of nodal metastases,5 but there was no cost‐effective alternative. To improve sensitivity it would be necessary to examine every node in the lymphadenectomy specimen by serial sections, an approach that would be prohibitively costly and beyond the workload capacity of pathology departments.
| Variable | Degrees of freedom | χ2 (Wald) | P‐value | Risk ratio | 95% Confidence interval |
| Nodal status | 1 | 100·69 | < 0·00001 | 2·239 | 1·913–2·621 |
| Thickness | 1 | 81·85 | < 0·00001 | 1·583 | 1·433–1·749 |
| Ulceration | 1 | 78·83 | < 0·00001 | 1·938 | 1·674–2·242 |
| Site | 1 | 27·86 | < 0·00001 | 1·483 | 1·281–1·716 |
| Age | 1 | 14·25 | 0·0002 | 1·095 | 1·044–1·147 |
| Sex | 1 | 1·88 | 0·1705 | 0·900 | 0·774–1·046 |
| Level | 1 | 0·01 | 0·9082 | 1·007 | 0·896–1·131 |
| Variable | Degrees of freedom | χ2 (Wald) | P‐value | Risk ratio | 95% Confidence interval |
| Nodal status | 1 | 100·69 | < 0·00001 | 2·239 | 1·913–2·621 |
| Thickness | 1 | 81·85 | < 0·00001 | 1·583 | 1·433–1·749 |
| Ulceration | 1 | 78·83 | < 0·00001 | 1·938 | 1·674–2·242 |
| Site | 1 | 27·86 | < 0·00001 | 1·483 | 1·281–1·716 |
| Age | 1 | 14·25 | 0·0002 | 1·095 | 1·044–1·147 |
| Sex | 1 | 1·88 | 0·1705 | 0·900 | 0·774–1·046 |
| Level | 1 | 0·01 | 0·9082 | 1·007 | 0·896–1·131 |
Reprinted with permission from Balch et al.25
| Variable | Degrees of freedom | χ2 (Wald) | P‐value | Risk ratio | 95% Confidence interval |
| Nodal status | 1 | 100·69 | < 0·00001 | 2·239 | 1·913–2·621 |
| Thickness | 1 | 81·85 | < 0·00001 | 1·583 | 1·433–1·749 |
| Ulceration | 1 | 78·83 | < 0·00001 | 1·938 | 1·674–2·242 |
| Site | 1 | 27·86 | < 0·00001 | 1·483 | 1·281–1·716 |
| Age | 1 | 14·25 | 0·0002 | 1·095 | 1·044–1·147 |
| Sex | 1 | 1·88 | 0·1705 | 0·900 | 0·774–1·046 |
| Level | 1 | 0·01 | 0·9082 | 1·007 | 0·896–1·131 |
| Variable | Degrees of freedom | χ2 (Wald) | P‐value | Risk ratio | 95% Confidence interval |
| Nodal status | 1 | 100·69 | < 0·00001 | 2·239 | 1·913–2·621 |
| Thickness | 1 | 81·85 | < 0·00001 | 1·583 | 1·433–1·749 |
| Ulceration | 1 | 78·83 | < 0·00001 | 1·938 | 1·674–2·242 |
| Site | 1 | 27·86 | < 0·00001 | 1·483 | 1·281–1·716 |
| Age | 1 | 14·25 | 0·0002 | 1·095 | 1·044–1·147 |
| Sex | 1 | 1·88 | 0·1705 | 0·900 | 0·774–1·046 |
| Level | 1 | 0·01 | 0·9082 | 1·007 | 0·896–1·131 |
Reprinted with permission from Balch et al.25
In addition to its inadequacies as a staging procedure, ELND exposes patients to troublesome postoperative morbidity—in most cases without the possibility of therapeutic benefit. Only 20% of patients who have intermediate‐thickness primary melanomas and nonpalpable regional lymph nodes actually have nodal metastasis by histology, including immunohistology. The remaining 80% of patients with intermediate melanomas have tumour‐free regional nodes and therefore will be unnecessarily placed at risk for acute wound problems, chronic oedema, nerve injury and the anaesthetic complications of ELND.
Our lymphatic studies leading to the SN hypothesis and the selective approach to CLND were initiated 30 years ago. At that time, ELND was avoided for truncal melanomas located in ambiguous sites such as the midline of the back or close to the umbilicus or shoulder because these tumours can drain to two or more lymphatic basins. In 1977, we described the use of cutaneous lymphoscintigraphy to identify the lymph basins at risk for metastasis from truncal primary melanomas and the concept of using the tumour status of the node of Cloquet as the indicator node to select patients for deep iliac/obturator LN dissection.6, 7 We found that lymphatic drainage often did not follow the expected anatomical pattern; thus, for example, a melanoma on the right back might drain to the left axilla, or a melanoma of the knee might drain to an ectopic lymph node in the mid‐thigh rather than to the inguinal nodes. Our initial studies of radiopharmaceutical‐based mapping of the lymphatics suggested that lymphatic pathways from the primary tumour to the regional lymphatic basin initially drained to one or two lymph nodes. In animal studies, we found that intradermal injection of isosulfan blue or patent blue dye optimally stained the afferent lymphatic channels and one or two regional lymph nodes. Regional nodes were stained in a sequential and temporal fashion, and thus we identified the SN as the first blue‐stained lymph node within the lymphatic basin reached by a blue‐stained afferent lymphatic channel draining the primary lesion. Although Cabanas29 and others had used the term sentinel to identify nodes in a fixed anatomic location in penile cancer, our concept described the SN by its function rather than its location. Each SN is unique because its location varies with the cutaneous location of the primary melanoma and with the inconsistent lymphatic drainage patterns of different patients. Thus identification of the SN requires a dynamic individualized mapping approach.
In the early 1980s we developed S‐100 protein as the first practical immunohistochemical marker for cells of melanocytic lineage.30, 31 Staining for S‐100 demonstrated the presence of occult melanoma cells in regional nodes at a frequency higher than previously appreciated on the basis of H&E evaluation.5 We also noted that when the absolute amount of melanoma present in the node group was small, tumour was present in only one or two nodes—usually the nodes closest to the primary tumour.8 These proximal, tumour‐containing nodes were also immunosuppressed, as indicated by selective reduction of the area of the nodal paracortex and reductions in the density and dendritic complexity of antigen‐presenting paracortical dendritic leucocytes.9
When we introduced LM/SL in 1990, as a presentation to the Society of Surgical Oncology, this technique was designed to provide a cost‐effective, minimally morbid but highly accurate solution to the controversy surrounding the use of ELND as a staging procedure in malignant melanoma. LM/SL evolved from our hypothesis that a primary melanoma metastasizing via the lymphatics will lodge in the first lymph nodes on the direct lymphatic drainage pathway from the primary cutaneous site, before spreading to other nodes further along the lymphatic chain. Thus, the tumour status of this index node—the SN—will indicate the tumour status of the entire lymphatic basin.
Our first published study of LM/SL in 1992 described the results of dye‐directed LM/SL in 237 lymphatic basins of 223 patients with primary cutaneous melanoma.3 Patients underwent preoperative dynamic lymphoscintigraphy to identify the basin of lymphatic drainage, localize specific SNs within this basin, and avoid missing aberrant draining lymph nodes. At the time of surgery, vital blue dye was injected around the primary melanoma. Intraoperative dye‐directed LM identified a SN in 194 (82%) lymphatic basins. Forty of these basins (21%) had tumour‐positive SNs. Only two lymphatic basins had tumour‐negative SNs in the presence of tumour‐positive non‐SNs, a false‐negative rate of 1·0%.
Although this seminal work was undertaken with vital dyes as the mapping agents, our group and other investigators have since reported the combined use of radiopharmaceuticals and blue dye for mapping the SN. Data from an ongoing Phase III international trial (MSLT‐I) have confirmed and validated that LM/SL with a vital blue dye and radiopharmaceutical identifies and removes the first lymph node in the lymphatic pathway from the primary tumour site in 99% of patients with primary melanoma.1 Focused histological evaluation based on immunohistochemical staining and molecular analysis of multiple sections of this SN is more likely to reveal nodal metastasis than is routine H&E staining of limited sections from the 12–40 bivalved nodes identified in a conventional ELND specimen.
Staging based on lymphatic mapping and sentinel lymphadenectomy: the patient's right to an informed decision
Studies of LM/SL in melanoma,17–22 breast cancer,32 colon cancer,33 and virtually all solid neoplasms that spread to the lymph nodes10 have confirmed the SN concept of an orderly progression of metastatic cells from the primary site through the lymphatics to one or two SNs in the regional nodal basin.
This means that a focused histopathological and molecular assessment of the SN can provide staging information far more accurate than that provided by random nodal biopsy or standard pathology assessment of the ELND specimen. For this reason, the staging information provided by LM/SL recently has been incorporated into the American Joint Committee on Cancer's (AJCC's) staging system for cutaneous melanoma.28
Drs Medalie and Ackerman apparently have little confidence in the AJCC staging system or in the 17 000‐patient database used to validate this system. They stubbornly cling to their notion that melanoma metastatic to the regional lymph nodes is always associated with a fatal outcome. However, although ‘we are all dead in the end’, a newly diagnosed patient still wants an informed estimate of his or her life‐expectancy and risk of dying from melanoma. Unlike Drs Medalie and Ackerman, who apparently expect 100% prognostic accuracy, the patient realizes that the oncologist's evaluation is an estimate and thus cannot be absolutely precise. No diagnostic or prognostic test is completely accurate. The ability of even the most competent dermatopathologist to differentiate between a benign or malignant melanocytic lesion will occasionally be questioned when a patient with a reportedly benign lesion subsequently develops fatal metastases (a fallibility that Drs Medalie and Ackerman accept).
Again, we take issue with the assertion that the tumour status of the SN has no clinical relevance. Based upon 215 patients seen by our group before 1994, if the lymph node is positive by pathology or immunohistopathology, the chance of recurrence is 60%; if the node is positive only by molecular methods,11 the chance of recurrence is at least 50% (Fig. 1).4 By contrast, if this node is negative by both histopathological and molecular techniques, by 8 years the chance of recurrence is only 10% and the chance of survival is 96%. These highly significant differences in prognosis between SN‐positive and SN‐negative survival (P < 0·001) represent valuable information for a patient who is attempting to make an informed decision about the future and explore treatment options. If the SN contains a metastasis, the chance of survival of 15 years or longer will be 40–70% depending upon the number of involved lymph nodes and their tumour burden. However, if the SN is negative by both histopathology and molecular methods,4, 11 the chance of long‐term survival will be 90% or more (Fig. 1).
![Disease‐free and cumulative survival according to histopathological [haematoxylin and eosin (H&E) and immunohistochemistry (IH)] and molecular (mRNA markers for tyrosinase, MART‐1, MITF and TRP‐2) status of the sentinel node. Reprinted with permission from Morton et al.,4 SN, Sentinel node; DFS, disease‐free survival.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/bjd/151/2/10.1111_j.1365-2133.2004.06133.x/3/m_bjd_6133_f1.gif?Expires=1750533317&Signature=RGXXb4Dc7-CdWO0v9RcvlyvHrYgrwdbrx1Vgyqh0jt0cPMz2Yx-yOk5j38kmppTWcJ7CC9qKV1FxxrxLDACGnOMQGqNwRK6gvro11qDv4H61BQvYVppLgcHxz3GDXb655x~YRpb~dz4dA5LAzLTlvhBFaxGshAPpcPGTnr-zUaBN89EhZmPpqN47NpIjB8uzRjt1FIBAjWVoSpkg3G9LU484BGwhYqX-aNOWamK5xDs6TULUUruO7QXvqUt42yyO~waIy6gzUi2~g4-r~TQsG16xiQ3ua0tFxsMB8SMGp-0a8Y40C88mX-7mF6NM3C7Q66alZ6IT6tpVe6N6oQuX5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Disease‐free and cumulative survival according to histopathological [haematoxylin and eosin (H&E) and immunohistochemistry (IH)] and molecular (mRNA markers for tyrosinase, MART‐1, MITF and TRP‐2) status of the sentinel node. Reprinted with permission from Morton et al.,4 SN, Sentinel node; DFS, disease‐free survival.
There are no absolutes in medicine, which is why we have an informed consent process in which the patient can learn about choices and chances. During the preoperative informed‐consent discussion with our patients, we state that the therapeutic utility of LM/SL and selective CLND has not been proven at this time. At this time we certainly do not know whether the SN procedure is therapeutic. This contrasts with Drs Medalie and Ackerman, who claim to know that it is not therapeutic, although they do not choose to share with us the source of that critical information and provide no data to support their assertions. However, LM/SL can indicate with a high degree of accuracy whether the patient has nodal metastases and thereby help establish the risk of recurrence. From our experience, almost all patients choose to undergo LM/SL because they want this information about the extent of their disease and its risk of recurrence. Thus, LM/SL has become a standard staging procedure in cancer centres throughout the world.
The timing of lymph node dissection: incubator vs. marker hypotheses
As advocated more than a century ago by British surgeon Herbert L. Snow,34 routine ELND in patients without clinical evidence of regional metastasis should interrupt the metastatic cascade. Multiple retrospective single‐institution studies lend support to Snow's approach,2, 35, 36 reporting a survival advantage when patients without evidence of nodal metastasis undergo routine ELND. However, if metastatic melanoma does not frequently spread first to the regional nodes before spreading to distant sites as biologically relevant progressive disease, then ELND may not be justified. In fact, randomized prospective clinical trials have failed to show an overall survival benefit for all melanoma patients undergoing ELND, although a subset of individuals with limited tumour burden may benefit.23, 24
We have considered two possible paths of metastasis from a primary melanoma (Fig. 2).4 According to the incubator hypothesis, the primary tumour spreads primarily to sentinel nodes in the regional lymph basin, where the metastatic tumour cells may survive and grow slowly or remain latent before, in some patients, spreading to distant sites. Less frequently melanoma cells may spread directly from the primary to distant sites. However, according to the marker hypothesis, a primary melanoma metastasizes simultaneously via lymphatic and haematogenous routes, so that the presence of nodal metastases becomes a marker rather than a precursor of clinically relevant and progressive systemic disease.
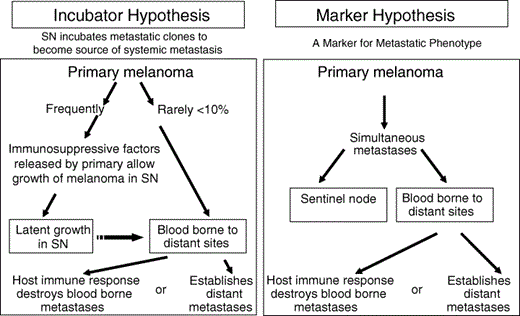
Incubator and marker hypotheses to explain the metastasis of a primary cutaneous melanoma. According to the incubator hypothesis, primary melanoma initially metastasizes via the lymphatics to the sentinel node (SN), which is immunosuppressed by factors released from the primary melanoma. Metastatic foci in the SN may grow but remain latent (incubate) before spreading to distant sites. Thus, finding tumour cells in the SN indicates that the primary melanoma has the ability to metastasize; removal of the tumour‐involved SN before there is further spread should prevent distant metastasis. According to the marker hypothesis, a primary melanoma metastasizes simultaneously via lymphatic and haematogenous routes. Thus, finding tumour cells in the SN is merely a marker of a primary melanoma that can metastasize; removal of the tumour‐involved SN is unlikely to influence the growth of distant metastases and would have no therapeutic effect. The absence of melanoma cells in the SN indicates a primary melanoma that is unlikely to spread to distant sites; this has important prognostic implications. Reprinted with permission from Morton et al.4

Prognostic factors used to match 287 patients undergoing immediate CLND (tumour‐involved SN) with 287 patients undergoing delayed CLND (palpable lymph nodes)
| Factor | WE + LM/SL | WE only |
| AJCC primary T stage | ||
| 1 | 6 | 6 |
| 2 | 44 | 44 |
| 3 | 145 | 145 |
| 4 | 89 | 89 |
| Total number of tumour‐involved nodes | ||
| 1 | 203 | 203 |
| 2–3 | 65 | 65 |
| > 4 | 19 | 19 |
| Ulceration | ||
| No/unknown | 225 | 225 |
| Yes | 62 | 62 |
| Age | ||
| < 50 years | 158 | 158 |
| > 50 years | 129 | 129 |
| Sex | ||
| Female | 120 | 120 |
| Male | 167 | 167 |
| Factor | WE + LM/SL | WE only |
| AJCC primary T stage | ||
| 1 | 6 | 6 |
| 2 | 44 | 44 |
| 3 | 145 | 145 |
| 4 | 89 | 89 |
| Total number of tumour‐involved nodes | ||
| 1 | 203 | 203 |
| 2–3 | 65 | 65 |
| > 4 | 19 | 19 |
| Ulceration | ||
| No/unknown | 225 | 225 |
| Yes | 62 | 62 |
| Age | ||
| < 50 years | 158 | 158 |
| > 50 years | 129 | 129 |
| Sex | ||
| Female | 120 | 120 |
| Male | 167 | 167 |
Reprinted with permission from Morton et al.4 AJCC, American Joint Committee on Cancer; CLND, complete lymph node dissection; LM/SL, lymphatic mapping and sentinel lymphadenectomy; SN, sentinel node; WE, wide excision.
Prognostic factors used to match 287 patients undergoing immediate CLND (tumour‐involved SN) with 287 patients undergoing delayed CLND (palpable lymph nodes)
| Factor | WE + LM/SL | WE only |
| AJCC primary T stage | ||
| 1 | 6 | 6 |
| 2 | 44 | 44 |
| 3 | 145 | 145 |
| 4 | 89 | 89 |
| Total number of tumour‐involved nodes | ||
| 1 | 203 | 203 |
| 2–3 | 65 | 65 |
| > 4 | 19 | 19 |
| Ulceration | ||
| No/unknown | 225 | 225 |
| Yes | 62 | 62 |
| Age | ||
| < 50 years | 158 | 158 |
| > 50 years | 129 | 129 |
| Sex | ||
| Female | 120 | 120 |
| Male | 167 | 167 |
| Factor | WE + LM/SL | WE only |
| AJCC primary T stage | ||
| 1 | 6 | 6 |
| 2 | 44 | 44 |
| 3 | 145 | 145 |
| 4 | 89 | 89 |
| Total number of tumour‐involved nodes | ||
| 1 | 203 | 203 |
| 2–3 | 65 | 65 |
| > 4 | 19 | 19 |
| Ulceration | ||
| No/unknown | 225 | 225 |
| Yes | 62 | 62 |
| Age | ||
| < 50 years | 158 | 158 |
| > 50 years | 129 | 129 |
| Sex | ||
| Female | 120 | 120 |
| Male | 167 | 167 |
Reprinted with permission from Morton et al.4 AJCC, American Joint Committee on Cancer; CLND, complete lymph node dissection; LM/SL, lymphatic mapping and sentinel lymphadenectomy; SN, sentinel node; WE, wide excision.
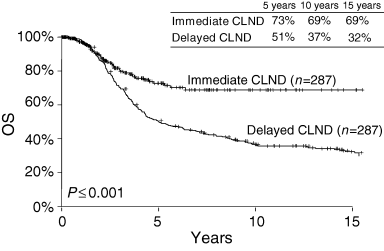
Long‐term survival according to the timing of complete lymph node dissection (CLND) for nodal metastases. Lymph node dissection is performed immediately after detection of tumour cells in the sentinel node (SN), or it is delayed until the regional nodes develop palpable metastases. OS, Overall survival. Reprinted with permission from Morton et al.4
Therapeutic potential of lymph node dissection
We agree with Drs Medalie and Ackerman that no prospective randomized trial has yet demonstrated a conclusive overall benefit for ELND in all patients with early‐stage melanoma. We believe that this reflects a problem of trial design. The question is not whether ELND is beneficial to all patients, but whether a particular patient is likely to benefit because that patient has subclinical regional nodal metastasis without progressive systemic metastasis.
Drs Medalie and Ackerman argue eloquently for the marker hypothesis (on the basis of no discernible data) and state unequivocally that patients with lymph node metastasis also have systemic spread of disease and will die of melanoma (if they don't die of something else first). We rebut their speculation with the data that are conspicuously absent from their presentation. Regarding the inevitability of death due to melanoma, we have consulted the John Wayne Cancer Institute's (JWCI's) computerized melanoma database. This database contains the records of all 11 000 patients seen by our staff since April 1971. Follow‐up is complete to within 30 months of last visit or death for 94% of patients in the database. The database thus represents a 30‐year prospective audit of the results of therapy by JWCI staff. The rate of 25‐year survival (the longest period with adequate numbers of patients on follow‐up) associated with lymph node dissection for palpable nodal metastases (CLND) is 29 ± 1·5%, compared with 42 ± 3% for patients with positive but clinically nonpalpable nodes. These data clearly indicate that not all patients with locoregional metastatic melanoma develop distant metastases and die of melanoma, and thereby refute Dr Medalie's and Dr Ackerman's therapeutic nihilism.
JWCI data are compatible with an interesting report from Meier et al.37 who reviewed their 20‐year database of 3001 patients with primary cutaneous melanoma to identify 466 patients who developed clinical evidence of metastases. Of the 466 patients, 71·9% developed locoregional metastases as the first clinical manifestation of disease spread. However, only 59% of those with regional nodal metastases subsequently developed distant metastases, which suggests that metastatic disease in 41% of patients was not present beyond the regional nodes to a degree that permitted progressive systemic growth at the time of therapeutic lymphadenectomy. These observations are consistent with the incubator hypothesis and confirm that not all patients with regional nodal metastases develop distant metastases. The fact that the median interval before clinical metastasis was longer for directly spread distant disease than for regional nodal disease (25 months vs. 16 months) further suggests that nodal metastasis precedes distant metastasis in many patients. Because the report of Meier's group was based on a clinical staging protocol, there were no data on the incidence of occult (immunohistopathological or molecular) SN metastases in patients with direct distant disease or in‐transit metastatic disease. We have found that many patients with in‐transit metastases have occult nodal metastases detectable by LM/SL.38 For these reasons, we do not agree with the conclusions of Meier et al.;37 instead, we believe that their clinical findings support the SN hypothesis and the use of diagnostic LM/SL followed by therapeutic CLND when the SN contains tumour.
Because our LM/SL investigations began in 1985, we already have 15‐year follow‐up data. The 15‐year rate of survival from wide excision of primary melanoma, LM/SL and immediate CLND for occult metastases in the SN is 69%, compared with only 32% after wide excision and delayed CLND for palpable nodal metastases (Fig. 3).4 This comparison is based on patients matched by the most widely accepted important prognostic factors:25 age and sex, primary T stage and ulceration, and number of tumour‐involved lymph nodes (Table 3). If the marker hypothesis were correct, then there should have been no difference in survival between the two groups; however, we found a 22% survival difference at 5 years (73% vs. 51%), which gradually increased to a 37% difference at 15 years (32% vs. 69%). Thus, if only 20% of the patients had lymph node metastasis, then the possible overall therapeutic benefit of immediate vs. delayed CLND for all patients with primary cutaneous melanoma varies from 4·4% at 5 years (20% of 22%) to 7·4% at 15 years (20% of 37%). These data are fully compatible with the findings of Cascinelli et al. (WHO Melanoma Group),39 whose randomized trial compared survival after immediate vs. delayed lymphadenectomy for nodal metastases (Fig. 4).
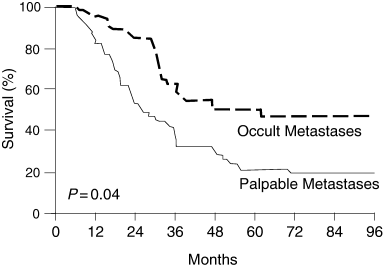
Survival of patients after immediate lymphadenectomy (occult nodal metastases) vs. delayed lymphadenectomy (palpable nodal metastases). Adapted with permission from Cascinelli et al.39
None of the ELND trials thus far reported have been large enough to demonstrate significantly an overall survival difference of only 4–7% between the two treatment arms for the entire study group of melanoma patients. Because patients without nodal metastases cannot benefit from ELND, demonstration of significant differences would require more than 1000 patients in each arm. Even the Multicentre Selective Lymphadenectomy Trial (MSLT), which completed accrual in March of 2002, has only 80% power to detect a statistically significant difference in overall survival between patients who underwent wide excision alone vs. those who underwent wide excision and LM/SL (plus CLND if the SN was positive).
Moreover, as discussed above, the chance of metastasis to the regional nodes varies dramatically with the thickness of the primary lesion (Table 1).4 Because patients with thin primary melanomas have a low risk of nodal metastasis, routine ELND could produce only a small increase in survival rate. Patients with thick primary lesions are more likely to have overtly tumour‐involved regional nodes and a higher risk of distant metastasis. Removal of the regional nodes seems unlikely to affect overall survival in the presence of substantial and progressive distant metastases, although the effect on survival of patients with tiny or latent metastases remains to be determined.
Recognizing these issues, we are about to launch a follow‐up to the MSLT. MSLT‐II will have enough patients to determine whether complete removal of the regional lymph nodes is of therapeutic benefit in patients with lymph node metastases identified by LM/SL. The trial (Fig. 5) will enrol at least 4200 patients at major cancer centres in the United States, Europe and Australia. It is possible that LM/SL alone will prove therapeutic in the vast majority of patients with primary melanomas ≤ 2·01 mm in thickness, since most of these patients will have metastases only in the SN, rendering CLND unnecessary. But what about patients whose SN is positive but who also may have metastases in non‐SNs of the same regional basin? Will early removal of nonpalpable metastases be of benefit? We will have the answer in another 5–10 years if most of the world's major melanoma centres join MSLT‐II. This trial has been awarded funding from the National Cancer Institute. Investigators interested in joining MSLT‐II should contact [email protected].
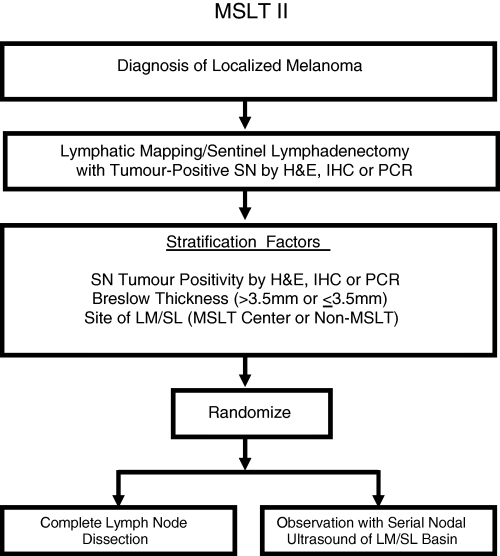
Design of the second Multicentre Selective Lymphadenectomy Trial (MSLT‐II). SN, sentinel node; H&E, haematoxylin and eosin; IHC, immunohistochemistry; PCR, polymerase chain reaction; LM/SL, lymphatic mapping and sentinel lymphadenectomy.
Metastasis, immune suppression, genetic and host factors
During the past few years, we have learned a great deal regarding the pathophysiology of metastasis from the primary melanoma to the regional SNs and to distant sites. The primary melanoma produces immunosuppressive factors,12, 13 as we hypothesized some years ago.13, 40 Thus the nodes anatomically closest to a primary melanoma are immunosuppressed, rendering them vulnerable to metastatic colonization (Fig. 2). This immunosuppression is also evident in the functionally defined SN, i.e. the node most exposed to the tumour's influence via the direct drainage lymphatic pathway from the primary melanoma. Immune downregulation is evidenced in multiple ways but especially in the density and maturity of interdigitating dendritic cells (IDC) and T cells in the SNs (Fig. 6).14 We believe this immunosuppression leads to a reduction in host immunity that contributes to the successful implantation and growth of micrometastases in the SN. Once the volume of metastatic tumour cells in the SN exceeds a critical mass, the production of immunosuppressive factors can lead to further immunosuppression of lymph nodes along the lymphatic chain, and then to micrometastatic involvement of additional lymph nodes, which increases the risk of systemic immunosuppression and the progressive growth of haematogenously spread tumour cells. This interaction could explain the significantly increased number of tumour‐involved nodes and relatively unfavourable survival when CLND is delayed and then performed to remove palpable rather than subclinical metastases (Table 1).2, 4
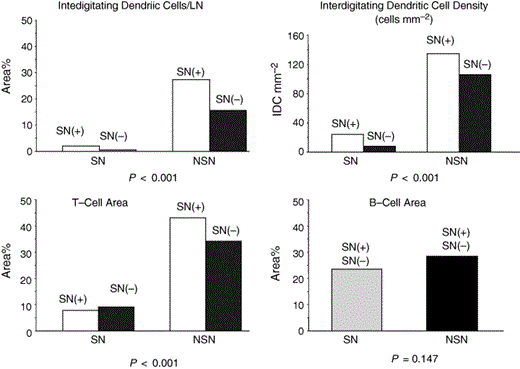
Comparison of the density and area of dendritic cells, T cells and B cells in 21 matched pairs of sentinel nodes (SNs) and nonsentinel nodes (NSNs) from 11 patients with American Joint Committee on Cancer stage I/II melanoma. Each SN was size‐matched with a NSN from the same patient. Reprinted with permission from Morton et al.4
After the development of substantial and progressive systemic metastases, surgical removal of the regional nodes should not influence the growth of distant metastases (unless these are relatively small and composed of tumour cells susceptible to rejection by host immune defences), nor change the long‐term melanoma‐specific survival. Thus, the patients who are most likely to derive benefit from LM/SL are those in whom nodal metastases are still ‘incubating’ in the SN prior to systemic spread. Because the incidence of nodal metastases depends largely on the thickness of the primary melanoma (Table 1),2, 4 lymph node removal should have little effect on melanoma‐specific survival when the primary tumour is very thin. Few of these patients have nodal micrometastases; in the vast majority, the primary is removed before the melanoma has an opportunity to spread to the regional nodes. Conversely, patients with thick (> 4 mm) primary cutaneous melanomas are unlikely to benefit from ELND because the risk of concurrent metastasis beyond the regional lymph nodes is high. However, this leaves a group of patients with intermediate‐thickness primary melanomas, whose metastatic disease is limited to the regional node basin and to the SN within that basin. In these patients, early removal of the SN should abort the metastatic cascade. But how can we identify these patients? We developed LM/SL as a solution to this dilemma.
Although primary tumour size is probably the most important factor in determining the risk of regional and systemic metastases, it is not the sole factor.15, 25, 28 Genetic changes in the primary melanoma associated with activation of various growth‐factor oncogenes are involved, as is the patient's endogenous immune response.16, 41, 42 Even palpable nodal metastases may be confined to the regional nodes by immune defences that inhibit the implantation and growth of blood‐borne circulating tumour cells (Fig. 2), or because genetic characteristics of the metastatic clone make tumour cells unable to adhere to the vascular endothelium and grow at distant sites. These factors could explain why a clinically palpable (2–3 cm) nodal metastasis containing more than 10 billion melanoma cells can exist without additional nodal or systemic involvement, and why therapeutic lymph node dissection can be followed by survival of 25 years or more without recurrence.
Staging with respect to postoperative adjuvant therapy
Although we agree with Drs Medalie and Ackerman with regard to the modest efficacy and high toxicity of high‐dose interferon in the management of patients with nodal metastasis, we hope that current randomized trials with new adjuvant immunotherapeutic agents such as Canvaxin therapeutic polyvalent cancer vaccine will improve the clinical outcome.15 The design of trials of postoperative adjuvant therapies requires entry criteria based on accurate staging of disease. A patient whose disease is accurately staged can benefit by immediate enrolment in a trial of a potentially effective therapy—a therapy that otherwise would not be available until after the trial is completed, which in an adjuvant setting might be many years hence. The repeatedly stated position of Drs Medalie and Ackerman in this regard is thus curious and perhaps unduly pessimistic.
Sentinel node evaluation to aid assessment of the malignant potential of an indeterminate melanocytic lesion
The use of LM/SL to help differentiate a benign from a malignant melanocytic skin lesion has been found useful in rare circumstances when the pathologist cannot give a definitive diagnosis of melanoma or a benign lesion. We do not share the widely broadcast opinion that close inspection of melanocytic lesions by an informed eye will always allow accurate assignment to benign or malignant categories. If the pathologist cannot be 100% confident of the diagnosis, we recommend treating the lesion as a possible malignancy with a wider excision and LM/SL. In these rare instances, the information provided by SN evaluation can assist patient, surgeon and pathologist in determining the appropriate course of management. If the node is negative or contains capsular/trabecular naevus, no additional regional surgery is required and the patient can be reassured. If the node contains true intraparenchymal metastases, CLND is offered and adjuvant therapy discussed. Again, the stated views and rhetoric of Drs Medalie and Ackerman are unnecessarily excessive.
In closing
The use of LM/SL in the management of primary cutaneous melanoma is based on its proven ability to provide more precise staging and prognostic information, on its potential therapeutic benefit in patients whose metastasis is limited to the SN, and—in special and rare circumstances—its value for assisting in the differentiation between benign and malignant melanocytic lesions of the skin. We therefore believe that LM/SL has been shown to have an important role in the staging of primary cutaneous melanoma and in prediction of likely clinical course. Its accuracy for staging and prognosis has been validated in multicentre trials and by multiple investigators in the United States, Europe and Australia.1
Our data indicate that 50–80% of melanoma patients with microscopic nodal metastases detected by LM/SL will be alive and well 15–25 years later and may never manifest recurrent melanoma, the exact percentages varying with the number of tumour‐involved nodes. Therefore, LM/SL may also have therapeutic utility in patients whose primary lesion has spread to the regional lymph nodes; however, the presence and magnitude of a therapeutic benefit cannot be determined until the results of the randomized MSLT‐I and II trials are complete.
Rather than recommend immediate abandonment of the sentinel node procedure, a technique that is justified by its usefulness in staging the regional nodes and determining prognosis (whether or not it proves to be therapeutic), Dr Medalie and Dr Ackerman would be well advised to await outcome analysis of the current Phase III trials and then resume this dialogue, hopefully in more measured terms, on the basis of facts rather than speculation.
Res ipse loquitur



