-
PDF
- Split View
-
Views
-
Cite
Cite
E. Frouin, G. Sebille, S. Freudenberger, B. Menecier, E. Quoix, B. Cribier, D. Lipsker, Asteatotic eczema (‘eczema craquelé’) with histopathological interface dermatitis: a new cutaneous reaction following pemetrexed, British Journal of Dermatology, Volume 166, Issue 6, 1 June 2012, Pages 1359–1360, https://doi.org/10.1111/j.1365-2133.2011.10777.x
Close - Share Icon Share
Madam, Pemetrexed (Alimta®; Lilly, Indianapolis, IN, U.S.A.) is a novel antifolate agent. It is used for the treatment of patients with malignant pleural mesothelioma, or locally advanced or metastatic nonsmall cell lung cancer.
Cutaneous toxicity is an adverse event classified into four grades. In phase III trials leading to its approval, rash was reported in 14% of patients receiving pemetrexed alone or in combination with cisplatin,1 of which 0·8–1·3% were grade 3 or 4.1, 2 Most of these eruptions were macular or papular. More recently, bullous,3 urticarial4 and pustular5 eruptions were reported. We describe three patients with widespread asteatotic eczema (AE) following pemetrexed administration.
Patient 1, a 69‐year‐old man, was hospitalized for stage IV primary adenocarcinoma of the right lung. A fourth‐line chemotherapy with pemetrexed 500 mg m−2 was initiated after a 4‐day premedication (folate and cobalmine). On day 5, he developed a diffuse cutaneous reaction (erythematous and violaceous rhagades) without facial involvement or pruritus. Skin biopsy showed an intense vacuolization of the dermal–epidermal junction with basal necrotic keratinocytes, a sparse lymphocytic inflammatory infiltrate, and a thick granular layer with dotted parakeratosis. Clinically, we made the diagnosis of AE, and suspected pemetrexed as the causative agent. The patient was treated only with dermocorticoids and emollients in the absence of criteria for severe AE. The skin rash disappeared in 10 days. The patient did not receive any other chemotherapy and died rapidly.
Patient 2, a 70‐year‐old man, was hospitalized for a third‐line chemotherapy with pemetrexed for a lung cancer (T3N0M0). Two days after the first injection of pemetrexed, the patient developed a reticulated and violaceous erythema predominantly in the abdomen. All symptoms disappeared in 7 days. He died 1 year later because of his cancer, after a last line of chemotherapy by gemcitabine, without any improvement.
Patient 3, a 57‐year‐old man, was hospitalized for a fifth‐line chemotherapy with pemetrexed for a nonsmall cell lung cancer with contralateral lung metastases (T2N2M1). Five days after pemetrexed was injected, the patient developed a violaceous and reticulated eruption of the trunk, without pruritus (Fig. 1a). Skin biopsy showed a mild spongiotic and dotted parakeratotic epidermis, with few necrotic and vacuolated basal keratinocytes (Fig. 1b). The patient died 2 days later from sepsis.
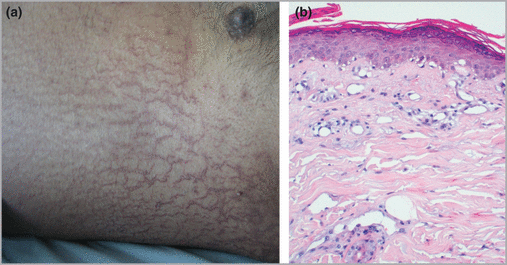
Patient 3. (a) Extensive eruption of the abdomen, with typical appearance of asteatotic eczema. (b) Histology of a skin biopsy shows epidermis covered by dotted parakeratosis with interface dermatitis, vacuolar basal keratinocytes, and a sparse inflammatory infiltrate of the upper dermis (haematoxylin and eosin).
We report three patients with a similar rash occurring 5–7 days after introduction of pemetrexed, with the clinical appearance of AE and the histopathological features of a drug reaction with interface dermatitis. This is the first report of AE following pemetrexed injection. AE, or eczema craquelé, is a frequent condition in elderly patients. The diagnosis is usually clinical. It occurs primarily on the legs, on the shins, and less frequently on the trunk or arms. The skin tends to feel roughened and dry. It commonly appears when the individual is hospitalized or taken into an institution. Extensive or generalized forms are unusual and should raise suspicion of an associated internal malignancy.6 The skin surface is first xerotic and then large polygonal scales delineated by superficial red rhagades appear, leading to a pavement appearance. Low humidity, skin lipid content reduction with age, illness, malnutrition, and skin degreasing by industrial or household cleaners are often quoted as triggering factors.7 Moisturizers are the primary treatment.8 Histopathology usually reveals a mild parakeratotic dermatitis with a varying amount of dermal infiltrate.
The eruption began 5–7 days after the injection, and no patient developed systemic symptoms. The AE disappeared within 7–10 days in two patients and the third died of sepsis before complete regression of the eruption. During a phase II study, skin symptoms decreased after prophylactic treatment with dexamethasone.9 Due to the benign nature of this reaction, no systemic treatment was necessary, and only emollient cream was applied with significant improvement of the symptoms. No reintroduction or patch tests were performed, because of cancer progression.
In two patients, skin biopsy showed typical findings of a lichenoid drug reaction. Differential diagnoses such as dermatomyositis or lupus erythematosus were ruled out by clinicopathological correlation. Dotted parakeratosis without modification of the epidermis is highly evocative of AE, but has rarely been reported. One could argue that AE is usually clinically evident and no biopsy is needed. To our knowledge, interface dermatitis has not been reported with AE.
The physiopathology of AE is still controversial. Decreased epidermal lipids or disturbance of lipid synthesis balance towards increased cholesterol synthesis has been postulated.7 In two cases of cimetidine‐induced AE, the eruption was related to an antiandrogenic effect on sebum production.10 Pemetrexed inhibits thymidylate synthase, and other folate‐requiring enzymes,9 but to our knowledge neither specific action on epidermal lipids nor on sebum production has been reported with pemetrexed.
In conclusion, AE can be a toxicity induced by pemetrexed. Because of its favourable evolution, pemetrexed should not be stopped.
References
Author notes
Funding sources: none.
Conflicts of interest: none declared.



