-
PDF
- Split View
-
Views
-
Cite
Cite
D. Martin, J. Howard, B. Agarwal, Y. Rajalingam, B. Athan, S. Bhagani, I. Cropley, S. Hopkins, S. Mepham, A. Rodger, S. Warren, M. Jacobs, Ebola virus disease: the UK critical care perspective , BJA: British Journal of Anaesthesia, Volume 116, Issue 5, May 2016, Pages 590–596, https://doi.org/10.1093/bja/aew098
Close - Share Icon Share
Abstract
The recent outbreak of Ebola virus disease (EVD) has required the treatment of affected patients in the NHS system within the UK. Managing patients with a confirmed viral haemorrhagic fever requires a thorough understanding of treatment options within the confines of an effective biocontainment setting. The Royal Free Hospital High Level Isolation Unit (HLIU) in London, is a purpose built facility that allows healthcare workers to safely treat patients with highly contagious diseases. This HLIU uses Trexler isolator tents to prevent the spread of infection from patients to healthcare workers. Provision of invasive organ support can be provided in this environment, if considered appropriate, and is achievable without posing additional risk to staff. We report our recent experiences of managing patients with EVD, with particular focus on those aspects of care pertinent to anaesthesia and critical care medicine.
In December 2013 a young child in a village in Guinea died of a febrile illness. This child was the index case for the largest ever outbreak of Ebola Virus disease (EVD). 1 More people were infected and died during this epidemic than all other outbreaks of EVD added together. As of 3rd January 2016, the number of confirmed patients reported during the outbreak was 28 637, with 11 315 deaths. The disease not only devastated three countries in West Africa (Guinea, Sierra Leone and Liberia) but impacted on the rest of the world and resulted in patients needing to be managed in high resource countries. 2–5 The unprecedented spread of a disease of this nature has made every healthcare system in the world, evaluate its ability to safely triage and treat suspected and confirmed patients with highly contagious diseases. In the UK, the Royal Free Hospital (RFH) in London contains a High Level Isolation Unit (HLIU), designed to manage patients with confirmed viral haemorrhagic fever (VHF) and is the country's primary site for this specialist activity. We describe our approach to managing critically ill patients with EVD in this unique and resource rich environment, which differs significantly from how large numbers of patients were managed in the resource limited settings of West Africa. Four confirmed episodes of Ebola virus (EBOV) infection have now been managed at the RFH HLIU during the current outbreak (in three British healthcare workers infected in West Africa), all of whom recovered. The specific details of their treatment and clinical course are reported elsewhere; this article reviews the approach to treatment of patients in the HLIU.
Ebola virus disease
EBOV is an RNA virus of the Filoviridae family (that also includes Marburg virus) and is the causative organism of EVD, previously referred to as Ebola haemorrhagic fever. The route of transmission is via direct mucous membrane or percutaneous exposure to infected body fluids (such as vomit, blood and stool). It is also highly transmissible by needle stick injury from an infected patient. 6 The current EVD outbreak was caused by the Zaire strain of EBOV and the illness has an incubation period of 2–21 days (mean 4–10). The evolution of EVD symptoms can generally be considered in three phases (Table 1 ). Initially the disease begins as a non-descript febrile illness, frequently associated with fatigue, myalgia and anorexia. This is then followed by a predominantly gastrointestinal phase characterized by uncontrolled high volume diarrhoea and vomiting. This commonly leads to significant hypovolaemia, shock, organ failure and, in severe cases, death. Electrolyte disturbance contributes significantly to the morbidity and mortality observed in this phase. Of some surprise was the considerably lower incidence of haemorrhagic manifestations in this outbreak of EVD. Only 18% of patients with EVD exhibited unexplained bleeding and this was associated with a significant increase in the risk of death. 7 This third phase of haemorrhagic symptoms and signs often manifested as gastrointestinal haemorrhage and was frequently fatal. 8 Mortality rates from EVD in West Africa during this outbreak have varied between 38 and 70% but the frequently quoted case fatality rate of 50% may well be a substantial underestimation. 9 Increasing age and a higher viral load are associated with higher fatality in West Africa. 10 With so few patients (27 by May 2015 11 ) having been treated in high-income countries, the mortality rate in the latter setting is hard to gauge.
| Phase 1 | Fever Fatigue Loss of appetite Myalgia Joint pain |
| Phase 2 | Profound weakness Diarrhoea Vomiting Abdominal pain |
| Phase 3 | Bleeding Encephalopathy |
| Phase 1 | Fever Fatigue Loss of appetite Myalgia Joint pain |
| Phase 2 | Profound weakness Diarrhoea Vomiting Abdominal pain |
| Phase 3 | Bleeding Encephalopathy |
| Phase 1 | Fever Fatigue Loss of appetite Myalgia Joint pain |
| Phase 2 | Profound weakness Diarrhoea Vomiting Abdominal pain |
| Phase 3 | Bleeding Encephalopathy |
| Phase 1 | Fever Fatigue Loss of appetite Myalgia Joint pain |
| Phase 2 | Profound weakness Diarrhoea Vomiting Abdominal pain |
| Phase 3 | Bleeding Encephalopathy |
High level isolation unit at the royal free hospital
Coppetts wood hospital
Coppetts Wood hospital (CWH) in North London was founded in 1887 as a hospital for infectious diseases. CWH opened a High Security Infectious Diseases Unit (HSIDU) in 1973 to improve capabilities for managing hazardous infectious diseases, particularly smallpox. The first patient with EVD managed in the UK occurred in 1976, when an investigator at the Microbiological Research Establishment at Porton Down sustained a needle stick injury whilst processing a sample of EBOV. 12 The patient was managed within a Trexler negative-pressure plastic isolator (see below) at the HSIDU in CWH. After administration of convalescent serum from survivors of a recent African outbreak and interferon he slowly improved and eventually made a full recovery. Perhaps the most important aspect of his care was the use of the Trexler isolator system. Healthcare workers are separated by the physical barrier of a flexible plastic tent, which also isolates bodily fluids within the Trexler environment and so limits contamination of the external environment. This prevented any transmission of the disease to the staff caring for the patient at that time and laid the foundations for the UK's approach to managing patients with VHF. Further patients with VHF were subsequently managed in the CWH HSIDU, with critical care support provided by clinicians from the RFH.
Royal free hospital
In 2008 the CWH HSIDU was moved to the RFH site and was subsequently renamed as the HLIU. The current HLIU is a purpose built area of the hospital, occupying a footprint comparable with that required for a standard hospital ward and essentially exists as a hospital within a hospital. This secure unit consists of two negative-pressure isolation rooms, each containing a Trexler isolator tent, a containment level 3+ laboratory, three autoclaves and facilities to accommodate staff and relatives. One-way flow of healthcare workers through the unit is a key component of its safety design. A nursing station between the two isolation rooms permits observation of the patients without the need for any protective precautions being taken. Communication to staff within the isolation rooms is via telephone and fax machine. Key areas of the HLIU (those likely to become contaminated) are subjected to negative pressure and the air passed through a high-efficiency particulate arrestance (HEPA) filter, with additional HEPA filtered units from the Trexler isolator before air extraction from the unit. The design of the unit is focused on the fact that VHFs are primarily spread by direct contact with contaminated bodily fluids, although there is potential risk of aerosolisation of particles from certain medical procedures such as intubation. Guidance for the management of patients within such a unit are provided by NHS England, the Health and Safety Executive (through the Advisory Committee for Dangerous Pathogens). Currently it is recommended that all patients with a VHF in the UK should be managed in a HLIU environment as soon as is safely possible after confirming the diagnosis ( https://www.gov.uk/government/publications/viral-haemorrhagic-fever-algorithm-and-guidance-on-management-of-patients ). Patients are transported to the HLIU either by the Royal Air Force Tactical Medical Wing Air Transportable Isolator Team, using a specially designed patient transport isolator, or a Hazardous Area Response Team of the National Ambulance Resilience Unit, using personal protective equipment (PPE).
The Trexler isolator
The Trexler negative pressure isolator system is a unique but effective way of managing patients with highly contagious diseases (Fig. 1 ). Philip Trexler and his team developed the idea of the patient isolator from devices that were used to contain laboratory animals with microbial infections 13 and reported its success in human patients in the 1970s. 14,15 The same system had also previously been used for isolating immunocompromised patients, but using positive (rather than negative) pressure air flow. 16 The malleable plastic tent surrounds a bed and has five ‘half-suits’ built into the walls of the tent that healthcare workers can insert themselves into, in order to gain access to the patient. Other components within the wall of the tent allow wires and tubing to traverse it safely. The basic design of the tent has changed little since the 1970s, only really being adapted to allow improved access to the patient (particularly at the head end of the bed by addition of an extra ‘half-suit’). The Trexler system has a long history of providing effective protection to healthcare workers managing patients with highly infectious diseases. It is therefore ideally suited for resource-rich countries dealing with low volumes of patients. The alternative to a Trexler isolator is PPE, which is necessary for the management of large numbers of patients but has been associated with transmission of VHFs to healthcare workers. 17 The advantages and disadvantages of each protective approach is summarized in Table 2 . One particular advantage of the Trexler isolator is that is permits non-VHF experts to become involved in direct patient assessment and care, without the long training that is generally required for safe PPE use. This allows the core multidisciplinary team to seek the opinion of external experts in the knowledge that this can be delivered safely. At first glance one might think that the Trexler system precludes the provision of organ support for critically ill patients, but with careful planning, training and experience this can be delivered safely.
The relative advantages and disadvantages of the Trexler isolator system vs personal protective equipment (PPE)
| . | TREXLER . | PPE . |
|---|---|---|
| Risk of staff infection | Low | Moderate |
| Risk of contaminating surrounding environment | Low | High |
| Staff training requirements | Quick, straight forward, can occur when unit operational | Long and potentially complicated |
| Tolerability (working time) | Long | Short |
| Critical care interventions | Constrained working environment requires unique solutions | More straight forward implementation |
| Patient tolerability | Unfamiliar constrained environment can be problematic | Less daunting and permits greater freedom of movement |
| Patient capacity | Low (and not scalable) | High (and scalable) |
| Cost | High during standby | High during use |
| Management of children | Likely to be very challenging, particular in younger children | Potentially less challenging |
| . | TREXLER . | PPE . |
|---|---|---|
| Risk of staff infection | Low | Moderate |
| Risk of contaminating surrounding environment | Low | High |
| Staff training requirements | Quick, straight forward, can occur when unit operational | Long and potentially complicated |
| Tolerability (working time) | Long | Short |
| Critical care interventions | Constrained working environment requires unique solutions | More straight forward implementation |
| Patient tolerability | Unfamiliar constrained environment can be problematic | Less daunting and permits greater freedom of movement |
| Patient capacity | Low (and not scalable) | High (and scalable) |
| Cost | High during standby | High during use |
| Management of children | Likely to be very challenging, particular in younger children | Potentially less challenging |
The relative advantages and disadvantages of the Trexler isolator system vs personal protective equipment (PPE)
| . | TREXLER . | PPE . |
|---|---|---|
| Risk of staff infection | Low | Moderate |
| Risk of contaminating surrounding environment | Low | High |
| Staff training requirements | Quick, straight forward, can occur when unit operational | Long and potentially complicated |
| Tolerability (working time) | Long | Short |
| Critical care interventions | Constrained working environment requires unique solutions | More straight forward implementation |
| Patient tolerability | Unfamiliar constrained environment can be problematic | Less daunting and permits greater freedom of movement |
| Patient capacity | Low (and not scalable) | High (and scalable) |
| Cost | High during standby | High during use |
| Management of children | Likely to be very challenging, particular in younger children | Potentially less challenging |
| . | TREXLER . | PPE . |
|---|---|---|
| Risk of staff infection | Low | Moderate |
| Risk of contaminating surrounding environment | Low | High |
| Staff training requirements | Quick, straight forward, can occur when unit operational | Long and potentially complicated |
| Tolerability (working time) | Long | Short |
| Critical care interventions | Constrained working environment requires unique solutions | More straight forward implementation |
| Patient tolerability | Unfamiliar constrained environment can be problematic | Less daunting and permits greater freedom of movement |
| Patient capacity | Low (and not scalable) | High (and scalable) |
| Cost | High during standby | High during use |
| Management of children | Likely to be very challenging, particular in younger children | Potentially less challenging |
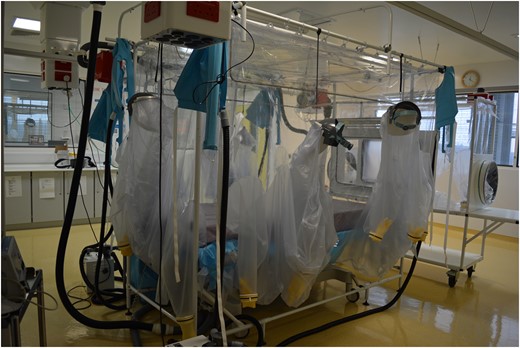
One of the two Trexler isolator tents in the Royal Free Hospital High Level Isolation Unit.
Providing critical care in a HLIU setting
Patients with EVD may require critical care input and invasive organ support if their illness is severe and this can be provided safely in a Trexler isolator environment. The key principle underpinning the care of patients with VHF is primacy of healthcare worker safety, and this must remain the case when considering any form of organ support in this patient group. The need for high level support for a deteriorating patient could be the result of progression of their viral illness, or from a secondary complication. The likely scenarios in which this might arise are summarized in Table 3 . Other commonly required interventions include central venous access, enteral (via a nasogastric tube) and parenteral nutrition, physiotherapy, imaging and sedation. Determining what procedures are appropriate for a patient must prioritize healthcare worker safety at all times. Anaesthetists are a group of clinicians that are arguably at greater risk of infection than other healthcare workers, in view of the fact that their skills can be called upon to perform invasive procedures, such as the insertion of central i.v. catheters. Prior training and experience in this unfamiliar environment are therefore essential for anaesthetists to safely undertake invasive interventions, that would be routine in an intensive care unit.
Possible scenarios that may warrant level 2 or 3 care in a patient with Ebola virus disease
| Situation . | Intervention . |
|---|---|
| Respiratory failure secondary to pneumonia or pulmonary oedema | Non-invasive ventilation Intubation and invasive ventilation |
| Decline in conscious level | Intubation and invasive ventilation |
| Haemodynamic instability (hypovolemia, sepsis) | Vasopressor/inotrope support Invasive bp monitoring |
| Renal failure | Haemofiltration |
| Severe diarrhoea and vomiting | Expert fluid and electrolyte management/central venous access |
| Agitation | Sedation |
| Haemorrhagic complications | Coagulation management and intravascular resuscitation |
| Situation . | Intervention . |
|---|---|
| Respiratory failure secondary to pneumonia or pulmonary oedema | Non-invasive ventilation Intubation and invasive ventilation |
| Decline in conscious level | Intubation and invasive ventilation |
| Haemodynamic instability (hypovolemia, sepsis) | Vasopressor/inotrope support Invasive bp monitoring |
| Renal failure | Haemofiltration |
| Severe diarrhoea and vomiting | Expert fluid and electrolyte management/central venous access |
| Agitation | Sedation |
| Haemorrhagic complications | Coagulation management and intravascular resuscitation |
Possible scenarios that may warrant level 2 or 3 care in a patient with Ebola virus disease
| Situation . | Intervention . |
|---|---|
| Respiratory failure secondary to pneumonia or pulmonary oedema | Non-invasive ventilation Intubation and invasive ventilation |
| Decline in conscious level | Intubation and invasive ventilation |
| Haemodynamic instability (hypovolemia, sepsis) | Vasopressor/inotrope support Invasive bp monitoring |
| Renal failure | Haemofiltration |
| Severe diarrhoea and vomiting | Expert fluid and electrolyte management/central venous access |
| Agitation | Sedation |
| Haemorrhagic complications | Coagulation management and intravascular resuscitation |
| Situation . | Intervention . |
|---|---|
| Respiratory failure secondary to pneumonia or pulmonary oedema | Non-invasive ventilation Intubation and invasive ventilation |
| Decline in conscious level | Intubation and invasive ventilation |
| Haemodynamic instability (hypovolemia, sepsis) | Vasopressor/inotrope support Invasive bp monitoring |
| Renal failure | Haemofiltration |
| Severe diarrhoea and vomiting | Expert fluid and electrolyte management/central venous access |
| Agitation | Sedation |
| Haemorrhagic complications | Coagulation management and intravascular resuscitation |
Point of care tests available in the RFH HLIU containment level 3+ laboratory. Hb, haemoglobin concentration; WBC, white blood cell count; Na + , sodium; K + , potassium; Cl − , chloride; Ca 2+ , calcium; PO 4− , phosphate; Mg 2+ , magnesium; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase, CRP, C-reactive protein; PT, prothrombin time; aPPT, activated partial thromboplastin time; , arterial partial pressure of oxygen; arterial partial pressure of carbon dioxide; BE, base excess; , arterial oxygen saturation
| Available blood tests . |
|---|
| Hb, WBC and differential, platelet count |
| Na + , K + , Cl − , Ca 2+ , PO 43− , Mg 2+ , urea, creatinine |
| Bilirubin, AST, ALT, ALP, |
| Amylase, CK, glucose |
| CRP |
| PT, aPPT, D-dimer |
| pH, , , BE, , lactate |
| Blood cultures HIV testing Malaria antigen testing and blood film microscopy |
| Group & Save/Cross match |
| Thromboelastography |
| Available blood tests . |
|---|
| Hb, WBC and differential, platelet count |
| Na + , K + , Cl − , Ca 2+ , PO 43− , Mg 2+ , urea, creatinine |
| Bilirubin, AST, ALT, ALP, |
| Amylase, CK, glucose |
| CRP |
| PT, aPPT, D-dimer |
| pH, , , BE, , lactate |
| Blood cultures HIV testing Malaria antigen testing and blood film microscopy |
| Group & Save/Cross match |
| Thromboelastography |
Point of care tests available in the RFH HLIU containment level 3+ laboratory. Hb, haemoglobin concentration; WBC, white blood cell count; Na + , sodium; K + , potassium; Cl − , chloride; Ca 2+ , calcium; PO 4− , phosphate; Mg 2+ , magnesium; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase, CRP, C-reactive protein; PT, prothrombin time; aPPT, activated partial thromboplastin time; , arterial partial pressure of oxygen; arterial partial pressure of carbon dioxide; BE, base excess; , arterial oxygen saturation
| Available blood tests . |
|---|
| Hb, WBC and differential, platelet count |
| Na + , K + , Cl − , Ca 2+ , PO 43− , Mg 2+ , urea, creatinine |
| Bilirubin, AST, ALT, ALP, |
| Amylase, CK, glucose |
| CRP |
| PT, aPPT, D-dimer |
| pH, , , BE, , lactate |
| Blood cultures HIV testing Malaria antigen testing and blood film microscopy |
| Group & Save/Cross match |
| Thromboelastography |
| Available blood tests . |
|---|
| Hb, WBC and differential, platelet count |
| Na + , K + , Cl − , Ca 2+ , PO 43− , Mg 2+ , urea, creatinine |
| Bilirubin, AST, ALT, ALP, |
| Amylase, CK, glucose |
| CRP |
| PT, aPPT, D-dimer |
| pH, , , BE, , lactate |
| Blood cultures HIV testing Malaria antigen testing and blood film microscopy |
| Group & Save/Cross match |
| Thromboelastography |
Respiratory support
Both non-invasive (continuous positive airways pressure (CPAP) and bilevel positive airway pressure (BiPAP)) and invasive respiratory support, can be administered safely to a patient in a Trexler isolator. Regardless of modality, the ventilator should be placed entirely inside the isolator so that the expiratory gas is vented within it and then removed via the negative pressure HEPA-filtered airflow system. Whilst EBOV is not thought to spread through the airborne route, this ensures that there is no possibility of generating infected aerosols outside the isolator. All of the devices used to provide ventilation in the RFH HLIU are small portable systems in order to minimize the space they occupy within the isolator. Heat and moisture exchangers that contain a bacterial and viral filter are used at the patient end of breathing circuits and changed every 24 h.
Airway management and intubation is potentially very challenging in this environment and should be rehearsed regularly with a mannequin placed inside the isolator to recreate a live scenario. The Trexler isolator has a suit at the head end to facilitate airway management. All equipment and drugs required for intubation, including for emergencies, must be moved into the isolator before commencing any airway intervention, as this process can be time consuming.
Cardiovascular support
Hypotension unresponsive to i.v. fluid resuscitation may require vasopressor or inotropic support. Central venous access will usually be required to deliver this, along with continuous arterial blood pressure measurement monitoring. Central venous access is relatively straightforward to obtain in an isolated patient; the internal jugular vein is the preferred site as femoral access poses a potential infection risk in the presence of high volume diarrhoea. The risk of pneumothorax is higher with subclavian access 18 and in view of the potential for abnormal coagulation in these patients, should be avoided. We advocate early placement of a central venous catheter as it also avoids the need for frequent venepuncture, hence reducing the risk of needle stick injury. Furthermore, central venous oxygen saturation can be a useful guide to intravascular volume status. This strategy has been shown to be of value when treating patients in Western Africa. 19 Arterial catheterization should be feasible in a Trexler isolator and preferably performed in the radial artery. All fluid containing components of the arterial blood pressure monitoring system must remain within the isolator in case of accidental disconnection. Monitoring of patients in a Trexler isolator is generally provided with the monitor being place on the outside and electrical cables being passed through a port to the patient. Volumetric pumps used to deliver drugs must also be placed entirely within the tent, to remove any possibility of a disconnection leading to spillage of blood outside the confines of the isolator.
Renal support
Fluid and electrolyte imbalance can be life-threatening in patients with EVD. 20 This can be addressed through meticulous clinical management of fluid status, avoiding both hypovolaemia and inadvertent fluid overload, and replacement of deficient electrolytes. Twice daily measurement of blood electrolytes along with urea and creatinine, is therefore an essential part of the management plan during the acute phase of illness. Optimizing i.v. fluid replacement can be extremely challenging, particularly as gastrointestinal losses may be very high. High dose potassium supplementation is usually required, and this is best delivered in a concentrated solution via a central venous catheter. I.V. magnesium and phosphate supplementation is also commonly needed.
Acute kidney injury is common in EVD (up to 50% in one series 17 ) and can lead to a requirement for renal replacement therapy. Different modalities of renal replacement therapy could be considered for patients with EVD. Peritoneal dialysis has been avoided because of gastrointestinal involvement in EVD and the presumed risk of bacterial peritonitis. Intermittent haemodialysis requires haemodynamic stability, whereas continuous veno-venous haemofiltration (CVVHF) is commonly performed in unstable critically ill patients. There are significant logistical issues with CVVHF; any extracorporeal circuit is a risk to healthcare workers as disconnection could result in a significant blood spillage. The filtrate fluid also poses potential risk as it may contain live virus, although one published report showed there to be no detectable EBOV RNA in the CVVHF effluent fluid from an infected patient. 21 At the RFH we have designed a potential solution, which comprises of a stand-alone small isolator that contains a standard CVVHF machine (Fig. 2 ). The satellite isolator could then be connected to the main isolator via a sealed sleeve system, should CVVHF be required. In this way the haemofiltration machine and the extracorporeal circuit are completely contained within the isolator system to avoid any contamination externally. Filtrate would need to be solidified with Vernagel and removed from the isolator in the same way as all other bodily fluids. Low volume filtration (15–20 ml −1 kg −1 h −1 ) will reduce the volume of effluent that needs to be handled by healthcare workers. In the absence of haemorrhagic symptoms, heparin can be used to prolong the life of the filter membrane and therefore reduce unnecessary circuit changes. 22
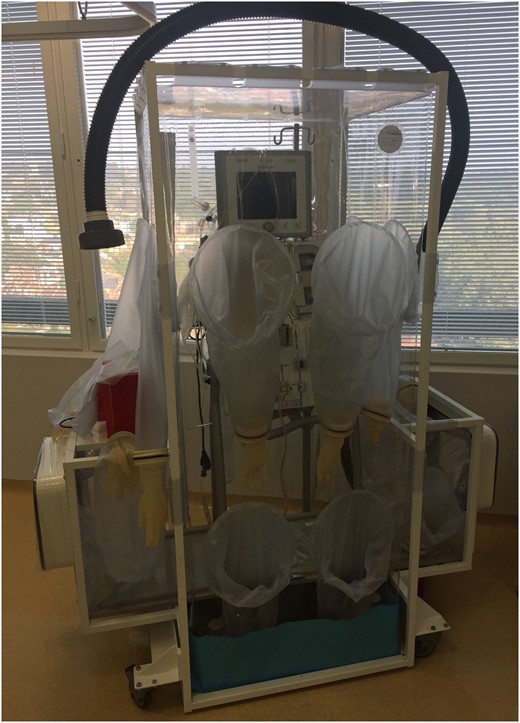
Sedation and analgesia
Patients with EVD can become encephalopathic and/or agitated, which can be challenging to deal with in a Trexler isolator. Patients may become a risk to themselves and the healthcare workers looking after them. Sedation therefore may be required to reduce agitation and provide a more manageable environment. This can be provided by intermittent boluses or infusions. I.M. injections should be avoided unless absolutely necessary because of the risk of bleeding in the haemorrhagic phase of the disease. Haloperidol and opiates are effective at reducing agitation, whilst benzodiazepines risk the development of delirium 23 so avoiding the latter may be advisable. Pain can also be a major component of EVD, particularly sore throat and odynophagia; local analgesia such as cocaine mouthwash can be a useful treatment for this in some patients.
Nutrition
Adequate nutrition is an essential component of the treatment of all critically ill patients and EVD is no exception. Supplementary enteral feeding via a nasogastric tube is usually required in all but the mildest of disease states because of anorexia and odynophagia. If enteral feeding fails as a result of poor gastrointestinal absorption or vomiting, then parenteral nutrition can be commenced through a dedicated central venous catheter, in order not to accrue a substantial calorie deficit.
Point of care blood testing
All routine blood tests from patients with confirmed VHF are processed in the RFH HLIU containment level 3+ laboratory, except for those that are sent to the external containment level 4 reference laboratories for EBOV PCR. In the local HLIU laboratory, a range of point of care tests are available 24 h a day (Table 4 ). Transportation of blood from the isolator to the laboratory within HLIU is carried out under strict conditions to prevent contamination and spillage. The local HLIU laboratory also contains a thromboelastography analyser (Haemonetics Corp, Braintree, MA, USA) to provide detailed information about dynamic blood coagulation. This can be used to guide blood product administration in the haemorrhagic phase of EVD and anticoagulation during post-illness hypercoagulable states. 24
Decontamination of equipment
Equipment such as ventilators and haemofiltration machines are dedicated to the HLIU and can be re-used in that environment. Viricidal wipes are used to remove gross organic material, followed by decontamination with vaporized hydrogen peroxide. 25 It is essential that decontamination is carried out according to well-established protocols, and servicing of equipment is conducted regularly between patients, to ensure that this has not led to any damage.
The UK plan for critical care in patients with EVD
In the early stages of the current outbreak, the provision of level 3 care for patients with EVD in the UK was questioned by some of the critical care community, which led to considerable debate. This situation arose on the grounds of a lack of evidence for the benefit of invasive organ support, in a disease with such a high mortality rate and the potential risk of infection spreading to healthcare workers. In November 2014 a meeting of clinical experts was convened by the Chief Medical Officer at the UK Department of Health to urgently discuss the matter. Whilst it was agreed that little evidence existed for the provision of level 3 care in these patients, there was also nothing to refute its use. Safety of staff caring for patients with EVD was considered to be of the utmost importance and therefore the group concluded that critical care support would be considered on an individual patient basis and only provided at the RFH HLIU. 26 This view was supported by the Faculty of Intensive Care Medicine (FICM), who highlighted that transfer must precede critical care support ( http://www.ficm.ac.uk/news-events/ficm-statement-ebola-clinical-management-guidance ). A further factor to consider was the impact of treating a patient of this nature on NHS resources. With high staffing requirements, the provision of care for a critically ill patient with EVD needed to be in addition to, rather than instead of, other patients at the designated institution. The decision as to whether a patient with EVD should be referred for critical care support should therefore follow the rational of other serious illnesses, with consideration being made of whether there is a reasonable chance of successful outcome, given the individual's current clinical status and trajectory.
Other patients with severe EVD, complicated by multiple organ failure, have been treated in critical care units using PPE and success has been achieved with the implementation of invasive supportive measures. 27 These achievements have led to the suggestion that the provision of critical care should be considered in west Africa, in order to improve survival of patients located there. 11 What has become clear from the patients with EVD in the UK is that supportive therapy is able to provide a window of opportunity during which recovery may be possible, but more experience is needed to define the overall clinical benefit. Careful management of a patient's physiological state creates the optimum environment for the development of an immune response and possibly for novel therapies to take effect. The efficacy of therapies available in a resource rich setting, such as monoclonal antibodies and experimental antivirals is unclear, but given their potential for success, it seems rational to provide organ support during a trial of their use in an individual.
Every hospital in the UK is required to have a clear plan of action of how to safely assess and manage a patient suspected of having EVD or any of the other VHFs. In the past, the capacity for this was haphazard at best in the UK, 28 but hopefully the recent EVD outbreak has led to substantial improvements. Confirmation of infection via one of the UK reference laboratories is necessary before arranging transfer to a designated HLIU. Therefore, difficulties arise in how to manage a rapidly deteriorating patient, in whom a diagnosis has not yet been established because of the inevitable time delay surrounding confirmation from a blood sample. Malaria and other imported diseases should always be considered as an alternative, or additional diagnoses in any febrile traveller from an Ebola affected region of the World. 29 Each patient that presents to the emergency department should be managed on an individual basis and advice sought from an experienced centre if difficulties arise.
Conclusion
The UK experience of providing supportive care for patients with VHF has advanced considerably during the recent EVD outbreak. The successful treatment of four EBOV episodes demonstrates that an experienced multidisciplinary team, that includes critical care and anaesthetic input is imperative to this outcome. The Trexler isolator is a very safe option for primary biocontainment, even if it does provide some challenges when critical care needs to be considered. Whilst the world may never see another Ebola outbreak of this magnitude, other highly infectious diseases and the possibility of bioterrorism using highly infectious organisms are a constant threat. The UK, therefore, needs to be in a state of readiness for the future with regards to high consequence infectious diseases.
Authors’ contributions
Writing paper: D.M., J.H., B.A., Y.R., B.A., S.B., I.C., S.H., S.M., A.R., S.W., M.J.
Revising paper: all authors
Declaration of interest
None declared.
Acknowledgements
We would like to thank every member of staff at the Royal Free Hospital for enabling us to provide a world class setting, in which patients can be cared for in our HLIU. In particular our thanks go to those who were directly involved in caring for the patients that we have admitted and ensuring that the unit is ready to be activated 24 h a day.
References





