-
PDF
- Split View
-
Views
-
Cite
Cite
J.-H. Ryu, M.-H. Kang, K.-S. Park, S.-H. Do, Effects of magnesium sulphate on intraoperative anaesthetic requirements and postoperative analgesia in gynaecology patients receiving total intravenous anaesthesia, BJA: British Journal of Anaesthesia, Volume 100, Issue 3, March 2008, Pages 397–403, https://doi.org/10.1093/bja/aem407
Close - Share Icon Share
Abstract
This randomized, double-blind, prospective study was undertaken to evaluate the effects of magnesium sulphate on anaesthetic requirements and postoperative analgesia in patients undergoing total i.v. anaesthesia (TIVA).
Fifty patients who underwent gynaecological surgery were randomly divided into two groups. Before induction of anaesthesia, the magnesium group (Group M) received magnesium sulphate 50 mg kg−1 i.v. as a bolus and then 15 mg kg−1 h−1 i.v. by continuous infusion. The control group (Group S) received the same amount of isotonic saline. TIVA (propofol+remifentanil) was administered under bispectral index monitoring during anaesthesia induction and maintenance. Rocuronium was administered before orotracheal intubation and during surgery when the train-of-four count was 2 or more. After operation, patient-controlled analgesia with a solution of ketorolac and morphine was used and the consumption of this solution was recorded. Pain scores at rest and upon movement were evaluated 30 min, 4, 24, and 48 h after surgery.
Patients in Group M required less rocuronium than those in Group S [mean (sd) 0.44 (0.09) vs 0.35 (0.07) μg kg−1 min−1, P<0.05]. The total amounts of propofol and remifentanil administered were similar in the two groups. Postoperative pain scores, cumulative analgesic consumption, and shivering incidents were significantly lower in Group M (P<0.05). Mean arterial pressure just after intubation and during the immediate postoperative period was also significantly lower in Group M (P<0.05).
I.V. magnesium sulphate during TIVA reduced rocuronium requirement and improved the quality of postoperative analgesia.
Postoperative respiratory complications associated with wound pain may delay recovery.1 Effective postoperative analgesia may facilitate recovery and reduce morbidity in surgical patients by blunting autonomic, somatic, and endocrine reflexes.2
Magnesium (Mg) is a non-competitive N-methyl-d-aspartate (NMDA) receptor antagonist with antinociceptive effects.3,4 Magnesium sulphate has been previously investigated as a possible adjuvant for intra- and postoperative analgesia. The majority of these studies suggest that perioperative magnesium sulphate reduces anaesthetic requirements and improves postoperative analgesia.5–11 However, some studies have concluded that magnesium sulphate has limited12 or no effect.13,14
Magnesium sulphate infusion has been reported to reduce remifentanil and mivacurium requirements but to have no effect on propofol requirements in patients undergoing vitrectomy.15 Another study found that magnesium sulphate decreased propofol, remifentanil, and vecuronium requirements during spinal surgery.9 In view of the inability of propofol to induce neuromuscular block16 and the need to provide early postoperative analgesia when remifentanil is used,17 we considered that magnesium sulphate might be a near ideal adjunct to propofol–remifentanil-based total i.v. anaesthesia (TIVA). In this placebo-controlled, double-blind study, we investigated the effects of magnesium sulphate administration on intraoperative anaesthetic requirements and postoperative analgesia in patients receiving propofol–remifentanil-based TIVA for gynaecological surgery.
Methods
This study was approved by the Institutional Ethics Committee and written informed consent was obtained from all patients. Fifty ASA I–II female patients aged between 30 and 65 yr undergoing total abdominal hysterectomy were enrolled into the study. Exclusion criteria were allergy to magnesium sulphate or any other study drug, renal, hepatic, or cardiovascular dysfunction, neurological disorders, atrioventricular conductance disturbance, and opioid or analgesic abuse. Patients receiving chronic treatment with calcium channel blockers or magnesium were also excluded.
Patients were randomly (sealed envelope method) assigned to one of the two groups. The magnesium group (Group M, n=25) received 50 mg kg−1 of magnesium sulphate in 100 ml of isotonic saline over 10 min immediately before anaesthesia induction and then 15 mg kg−1 h−1 by continuous i.v. infusion until the end of the operation whereas patients in the saline group (Group S, n=25) received the same volume of isotonic saline over the same period. Infusions were prepared in pharmacy and were delivered to the anaesthesiologist who was blinded to the patient's group assignment. The study data were recorded by an observer who was also blinded to the patient's group.
Upon arrival in the operating room, ECG and non-invasive arterial pressure and pulse oximetry monitoring were established. Electrodes were placed on the forehead to monitor bispectral index (BIS) (A-2000 BIS™ monitor, Aspect® Medical Systems Inc., Natick, MA, USA). Neuromuscular block was monitored at the wrist using a peripheral nerve stimulator (TOF Watch SX®, Organon Ltd, Dublin, Ireland). Anaesthesia was induced by target-controlled infusions (TCI) using an Orchestra® infusion pump system (Fresenius vial, Brezins, France). Patients received propofol 4 µg ml−1 and remifentanil 4 ng ml−1. After loss of consciousness and adequate manual ventilation, rocuronium 0.6 mg kg−1 was administered to facilitate orotracheal intubation. Anaesthesia was maintained using remifentanil and propofol. Patients were ventilated with oxygen and medical air (Fio2=0.5). Propofol effect-site target concentrations were adjusted to maintain the BIS between 40 and 50 and remifentanil effect-site target concentrations were adjusted using clinical signs and haemodynamic measurements. Inadequate analgesia was defined as an increase in mean arterial pressure or heart rate by more than 20% of preanaesthetic values. If inadequate analgesia or hypotension (systolic arterial pressure <90 mm Hg) occurred when BIS was within the recommended range, target remifentanil concentrations were increased or decreased, respectively. Ventilator settings were adjusted to maintain normocapnia (end-tidal carbon dioxide: 4.4–5.1 kPa). During surgery, nasopharyngeal temperature was monitored and a circulating water mattress and air warmer were used to maintain normothermia. Rocuronium (0.15 mg kg−1) was administered when the train-of-four (TOF) count was 2 or more. The TOF was measured every 10 min. Mean arterial pressure and heart rate were measured at the following times: before induction, before intubation, after intubation, and at 5, 15, 30, 60, 90, 120 min thereafter, and at 30 min, 4, 24, 48 h after surgery.
At the end of surgery, pyridostigmine 0.3 mg kg−1 and glycopyrrolate 0.01 mg kg−1 were used to reverse neuromuscular block. The magnesium sulphate and anaesthetic agent infusions were discontinued at skin closure, and ramosetron 0.5 mg and dexamethasone 5 mg were administered. Thereafter, an i.v. patient-controlled analgesia (PCA) device containing morphine 50 mg and ketorolac 120 mg in normal saline in a total volume of 60 ml was connected. This was set to deliver a 1 ml bolus dose with a 15 min lockout period. Times from anaesthetic discontinuation to a BIS value of 70 and to tracheal extubation were noted.
After the operation, the patients were transferred to the recovery room and the consciousness score was evaluated every 5 min using the modified Aldrete score18 until ready for discharge from the recovery room; 0, not responding; 1, arousable with minimal stimulation; and 2, fully awake.
Total amounts of propofol, remifentanil, rocuronium, and magnesium sulphate or placebo infusion administered were recorded. Postoperative PCA analgesic solution consumption at 30 min and at 4, 24, and 48 h after operation were recorded. If necessary, rescue analgesic (ketorolac 30 mg) was administered in the recovery room. Pain scores at rest and during movement were evaluated using a 0–100 mm visual analogue scale (VAS, starting from 0, no pain, to 100, worst pain imaginable). The VAS score was recorded at emergence from anaesthesia and at 30 min, 4, 24, and 48 h after the surgery. In addition, episodes of shivering and of postoperative nausea and vomiting (PONV) were monitored and recorded at emergence and throughout the remainder of the study period. Blood samples for serum magnesium concentration determination were obtained before and immediately after the surgery (the normal range used at our institution is 0.7–1.3 mmol litre−1). Patients’ global satisfaction levels regarding comfort and quality of pain control were assessed using a five-point scale (1, very unsatisfactory; 5, excellent).
The primary outcome of this study was postoperative PCA drug consumption. On the basis of the unpublished pilot data showing a mean (sd) 24 h consumption of PCA solution of 20 (7) ml a sample size of 25 patients per group was calculated based on a minimum clinically significant difference between the groups in postoperative 24 h PCA consumption of 5 ml and taking α=0.05 and β=0.2. Power Analysis and Sample Size software (2005®, NCSS, USA) was used for this calculation. The Wilcoxon rank sum test (PAR score and satisfaction score) and t-test (intraoperative anaesthetic agent consumption) were used for statistical analyses of non-parametric and parametric data. The χ2 (postoperative adverse effects) or Fisher's exact test (consciousness score) was used for comparison of incidence variables categorical data. Repeated measures anova was used to compare measurements over time (haemodynamic variables, PCA volume and postoperative VAS). If there was a statistical difference (P<0.05) between the two groups by repeated measures anova, the t-test (haemodynamic variables) or the Wilcoxon rank sum test (postoperative VAS and cumulative PCA consumption) was used to compare the data at each time point. Values are expressed as counts, percentages, or as means (sd). P-values of <0.05 were considered statistically significant.
Results
The patient characteristics are described in Table 1. Mean intraoperative propofol and remifentanil consumptions were similar in both groups. However, patients in Group M were received significantly less rocuronium [mean (sd) 0.35 (0.07) vs 0.45 (0.09) mg kg−1 h−1, P<0.001] (Table 2). At the end of surgery, patients in Group M had significantly higher serum Mg concentrations [1.5 (0.2) mmol litre−1] than those in Group S [0.9 (0.1) mmol litre−1, P=0.00].
Patients' characteristics and postanaesthetia recovery (PAR) scores. Group S, control group; Group M, magnesium group. Values are expressed as mean (range) for age, mean (sd) or number of the patients (n). Consciousness was scored using the modified Aldrete score:18 0, not responding; 1, rousable with minimal stimulation; and 2, fully awake
| . | Group S (n=25) . | Group M (n=25) . |
|---|---|---|
| Age (yr) | 43.7 (28–49) | 41.1 (28–52) |
| Body weight (kg) | 57.5 (8.0) | 57.4 (5.9) |
| Height (cm) | 158.1 (4.4) | 156.4 (3.8) |
| Duration of surgery (min) | 162.6 (33.7) | 169.2 (32.3) |
| ASA (I/II) | 18/7 | 19/6 |
| Consciousness score immediately after arrival in the recovery room | ||
| 0 (n) | 1 | 3 |
| 1 (n) | 19 | 19 |
| 2 (n) | 5 | 3 |
| . | Group S (n=25) . | Group M (n=25) . |
|---|---|---|
| Age (yr) | 43.7 (28–49) | 41.1 (28–52) |
| Body weight (kg) | 57.5 (8.0) | 57.4 (5.9) |
| Height (cm) | 158.1 (4.4) | 156.4 (3.8) |
| Duration of surgery (min) | 162.6 (33.7) | 169.2 (32.3) |
| ASA (I/II) | 18/7 | 19/6 |
| Consciousness score immediately after arrival in the recovery room | ||
| 0 (n) | 1 | 3 |
| 1 (n) | 19 | 19 |
| 2 (n) | 5 | 3 |
Patients' characteristics and postanaesthetia recovery (PAR) scores. Group S, control group; Group M, magnesium group. Values are expressed as mean (range) for age, mean (sd) or number of the patients (n). Consciousness was scored using the modified Aldrete score:18 0, not responding; 1, rousable with minimal stimulation; and 2, fully awake
| . | Group S (n=25) . | Group M (n=25) . |
|---|---|---|
| Age (yr) | 43.7 (28–49) | 41.1 (28–52) |
| Body weight (kg) | 57.5 (8.0) | 57.4 (5.9) |
| Height (cm) | 158.1 (4.4) | 156.4 (3.8) |
| Duration of surgery (min) | 162.6 (33.7) | 169.2 (32.3) |
| ASA (I/II) | 18/7 | 19/6 |
| Consciousness score immediately after arrival in the recovery room | ||
| 0 (n) | 1 | 3 |
| 1 (n) | 19 | 19 |
| 2 (n) | 5 | 3 |
| . | Group S (n=25) . | Group M (n=25) . |
|---|---|---|
| Age (yr) | 43.7 (28–49) | 41.1 (28–52) |
| Body weight (kg) | 57.5 (8.0) | 57.4 (5.9) |
| Height (cm) | 158.1 (4.4) | 156.4 (3.8) |
| Duration of surgery (min) | 162.6 (33.7) | 169.2 (32.3) |
| ASA (I/II) | 18/7 | 19/6 |
| Consciousness score immediately after arrival in the recovery room | ||
| 0 (n) | 1 | 3 |
| 1 (n) | 19 | 19 |
| 2 (n) | 5 | 3 |
Administered dose of anaesthetic agents. Values are mean (sd). Group S, control group; Group M, magnesium group. *P<0.05 compared with Group S
| . | Group S (n=25) . | Group M (n=25) . |
|---|---|---|
| Propofol (mg kg−1 h−1) | 7.48 (1.17) | 7.67 (0.96) |
| Remifentanil (μg kg−1 min−1) | 0.12 (0.02) | 0.11(0.02) |
| Rocuronium (mg kg−1 h−1) | 0.45 (0.09) | 0.35(0.07)* |
| . | Group S (n=25) . | Group M (n=25) . |
|---|---|---|
| Propofol (mg kg−1 h−1) | 7.48 (1.17) | 7.67 (0.96) |
| Remifentanil (μg kg−1 min−1) | 0.12 (0.02) | 0.11(0.02) |
| Rocuronium (mg kg−1 h−1) | 0.45 (0.09) | 0.35(0.07)* |
Administered dose of anaesthetic agents. Values are mean (sd). Group S, control group; Group M, magnesium group. *P<0.05 compared with Group S
| . | Group S (n=25) . | Group M (n=25) . |
|---|---|---|
| Propofol (mg kg−1 h−1) | 7.48 (1.17) | 7.67 (0.96) |
| Remifentanil (μg kg−1 min−1) | 0.12 (0.02) | 0.11(0.02) |
| Rocuronium (mg kg−1 h−1) | 0.45 (0.09) | 0.35(0.07)* |
| . | Group S (n=25) . | Group M (n=25) . |
|---|---|---|
| Propofol (mg kg−1 h−1) | 7.48 (1.17) | 7.67 (0.96) |
| Remifentanil (μg kg−1 min−1) | 0.12 (0.02) | 0.11(0.02) |
| Rocuronium (mg kg−1 h−1) | 0.45 (0.09) | 0.35(0.07)* |
PONV occurred in significantly fewer patients in Group M (10 patients, 40%) than in Group S (19 patients, 76%, P=0.01) and postoperative shivering also occurred in significantly fewer patients in Group M (1 patient, 4% vs 9 patients, 36%; P=0.005).
Hypotension (systolic arterial pressure <90 mm Hg) or bradycardia (heart rate <60 beat min−1) did not occur during bolus injection of study medication in either group. Repeated measures anova identified for a significant effect of time for the variables mean arterial pressure (P=0.001), cumulative PCA volume (P=0.009), and postoperative VAS (P=0.027 and 0.025, for rest and movement VAS scores, respectively). Mean arterial pressures before (P=0.0034), immediately after intubation (P=0.0146), and 5 min after intubation (P=0.0052) and 30 min after operation (P=0.008) were significantly lower in Group M (Figs 1 and 2).
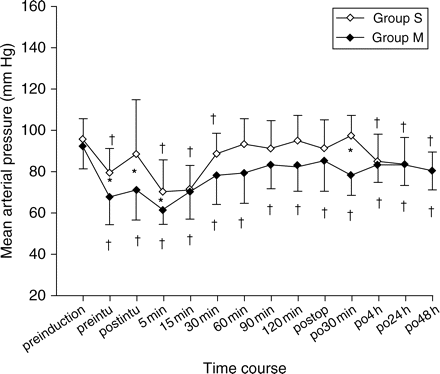
Perioperative changes in mean arterial pressure. Values are mean (sd). Group S, control group; Group M, magnesium group. *P<0.05 compared with Group S; †P<0.05 compared with preinduction value in each group.

Perioperative changes in heart rate. Values are mean (sd). There was no statistically difference in heart rate between the two groups. Group S, control group; Group M, magnesium group. †P<0.05 compared with preinduction value in each group.
Cumulative postoperative analgesic consumption was less in Group M (P=0.026 and 0.005, 24 and 48 h after operation, respectively) (Fig. 3). The postoperative VAS scores were less in Group M (rest VAS scores P=0.011 and P<0.001 at 24 and 48 h after surgery, respectively, and VAS scores on movement at 24 and 48 h after surgery P=0.014 and P<0.001, respectively) (Fig. 4). In addition, fewer patients in Group M required additional analgesic boluses in the recovery room (2 vs 4 patients), although this was not statistically significant.
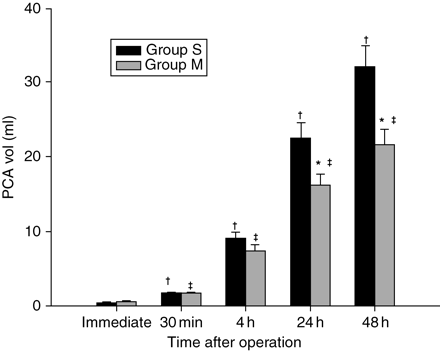
Mean cumulative injected volume of the i.v. patient-controlled analgesia solution in the two groups. The error bars show 1 sd. Group S, control group; Group M, magnesium group. *P<0.05 compared with Group S; †P<0.05 compared with baseline (immediate postoperative) value in Group S; ‡P<0.05 compared with baseline (immediate postoperative) value in Group M.
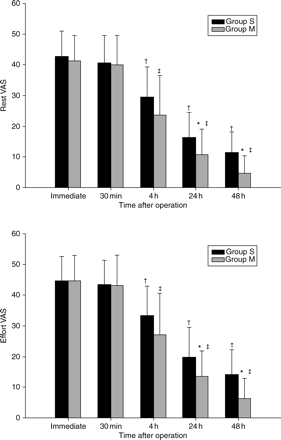
Mean VAS pain scores; rest VAS, pain scores at rest; effortVAS, pain scores during deep breathing. The error bars indicate 1 sd. Group S, control group; Group M, magnesium group. *P<0.05 vs Group S; †P<0.05 compared with baseline (immediate postoperative) value in Group S; ‡P<0.05 compared with baseline (immediate postoperative) value in Group M.
The time from magnesium sulphate infusion discontinuation to BIS 70 [Group S: 5.2 (2.3) min, Group M: 4.6 (3.1) min, P=0.38] and to tracheal extubation [Group S: 9.0 (2.4) min, Group M: 8.7 (3.4) min, P=0.70] were not significantly different between the groups.
There was no statistically significant difference in the consciousness score (Table 1) immediately after recovery room arrival between the two groups. Global satisfaction scores were significantly higher in Group M [4.2 (1.0) vs 3.6 (0.8), P=0.005].
Discussion
This placebo-controlled, double-blind study was designed to assess the effects of magnesium sulphate infusion on perioperative haemodynamics, anaesthetic agent consumption, recovery profiles, and postoperative analgesia in patients undergoing gynaecological surgery. We have shown here that the infusion of magnesium sulphate during TIVA with propofol and remifentanil reduced the need for neuromuscular blocking drugs and postoperative analgesics. Patients receiving magnesium displayed less PONV and shivering.
Magnesium is the second most abundant intracellular cation and is involved in the regulation of many ion channels and enzymatic reactions. Magnesium has applications in anaesthesia because of its actions as a NMDA receptor antagonist and a calcium channel blocker.19 NMDA receptor antagonists are best administered before the generation of noxious stimuli in order to prevent central sensitization.20
Although there have been numerous studies on the clinical efficacy of perioperative magnesium sulphate infusion, this is the first study of magnesium in patients receiving target-controlled i.v. anaesthesia with propofol and remifentanil. Seyhan and colleagues21 compared the effects of magnesium sulphate on i.v. anaesthetic requirements and postoperative analgesia and suggested that magnesium sulphate infusion leads to significant reductions in intraoperative propofol, and neuromuscular blocking agent requirements, and reduces postoperative pain and analgesic consumption. In the present study magnesium sulphate did not reduce propofol requirements. In addition, unlike previous studies in which magnesium was administered during propofol–remifentanil-based TIVA,9,15 the consumption of remifentanil was not changed significantly. This outcome might have been the result of our using target-controlled infusion of propofol. It was recently reported that huge variations between target and serum concentrations of propofol and remifentanil occur during TIVA.22 These variations could have been even greater in the present study because the Schnider and Minto models used by the TCI apparatus for propofol and remifentanil, respectively, were not based on the result of Asians but on that of Caucasians. Trends towards lower mean arterial pressure and heart rate were observed in the magnesium group. These effects of Mg might be explained by the vasodilation due to calcium channel blockade or by its analgesic effect and the consequent inhibition of catecholamine release.19
In the present study, we chose the bolus (50 mg kg−1) and continuous (15 mg kg−1 h−1) infusion doses of magnesium sulphate based on previous investigations.12,13,21 After surgery, patients in the magnesium group showed higher serum Mg concentrations than patients in the saline group, as was expected. Ko and colleagues13 have demonstrated an inverse relation between cerebrospinal fluid (CSF) magnesium concentration and cumulative postoperative analgesic consumption. However, we did not measure CSF magnesium concentration in the present study.
Magnesium sulphate is safe to use. There have been cases of magnesium toxicity leading to cardiac arrest and death.23 However, magnesium toxicity begins at serum concentration of 2.5–5 mmol litre−1,24 which is much higher than the highest level in Group M in this study. Cardiac arrest occurs at 12.5 mmol litre−1.24
This study demonstrates that magnesium sulphate potentiates postoperative analgesia, which concurs with previous studies.7,8,10,11 However, two reports have suggested that magnesium sulphate does not reduce the severity of pain after surgery.13,14 The precise reasons for this discrepancy are unknown, although it is interesting to note that i.v. analgesia was used in the studies which found that magnesium potentiates analgesia, whereas epidural analgesia was used in the latter studies. Thus, it is possible that the superior analgesic efficacy of epidural analgesia might have masked the analgesia-potentiating effect of magnesium sulphate in these latter studies.
It is well known that magnesium prolongs and potentiates neuromuscular block by non-depolarizing neuromuscular blocking agents.19,25,26 However, this effect of magnesium sulphate did not prolong emergence from anaesthesia in the present study probably because neuromuscular transmission was monitored throughout the study and additional doses of rocuronium were administered using a strict criterion, a TOF count of 2 or greater. Unlike inhalation anaesthetics, propofol has no potentiating effect on neuromuscular blocking agents,16 and therefore magnesium sulphate may be a useful adjunct in surgery that requires adequate muscle relaxation.
In the present study, patients in Group M showed less postoperative shivering and PONV. Wadhwa and colleagues27 suggested that magnesium sulphate infusion reduces the shivering threshold in humans, and i.v. magnesium sulphate has been reported previously to suppress post-anaesthetic shivering.28 Shivering causes discomfort and aggravates postoperative pain29 and the prevention of shivering may attenuate postoperative pain and enhance patients' satisfaction.
Some limitations of the present study should be noted. First, we determined serum magnesium concentrations only before and immediately after surgery. The relationship between serum magnesium and postoperative pain levels could not be evaluated. Secondly, we did not determine the CSF magnesium concentration as this was considered to be invasive. A previous investigation demonstrated that postoperative CSF magnesium concentrations in magnesium-treated and control patients were similar, although serum Mg concentrations were different between the two groups.13 On the other hand, a correlation was found between serum and CSF Mg concentration in patients with preeclampsia.30 Therefore, further experiments on the pharmacokinetics of i.v. magnesium sulphate infusion are needed.
In conclusion, pre- and intraoperative administration of magnesium sulphate (50 mg kg−1 bolus followed by 15 mg kg−1 h−1 continuous infusion) in gynaecology patients receiving TIVA significantly reduced requirements for a neuromuscular blocking agent during the operation and reduced analgesic consumption after operation. In addition, perioperative magnesium sulphate administration attenuated the increase in arterial pressure after intubation and surgery, improved satisfaction scores, and reduced PONV and shivering. Thus, we conclude that i.v. magnesium sulphate may be a useful adjunct for gynaecological surgery under propofol–remifentanil-based TIVA.
Funding
The statistics of this study was supported by the Medical Research Collaborating Center of Seoul National University Hospital; Project number: 2007-0101.





