-
PDF
- Split View
-
Views
-
Cite
Cite
A. Scholz, K. Srinivas, M. R. W. Stacey, P. Clyburn, Subglottic stenosis in pregnancy, BJA: British Journal of Anaesthesia, Volume 100, Issue 3, March 2008, Pages 385–388, https://doi.org/10.1093/bja/aem391
Close - Share Icon Share
Abstract
Subglottic stenosis (SGS) in pregnancy is rare but may cause a potentially life-threatening delivery and is a challenge to the anaesthetist and the obstetrician. Clinical signs of SGS may not be obvious and the diagnosis can be difficult. Patients usually present with shortness of breath rather than stridor. Many patients have been wrongly diagnosed with asthma and recurrent bronchitis before subsequent discovery of a SGS. Early diagnosis of SGS and multidisciplinary input is important in managing these patients. We present a case of a pregnant woman with a history of Wegener's granulomatosis and the successful multidisciplinary management of her SGS.
The pathophysiology of subglottic stenosis (SGS) is unclear but it can be acquired or congenital. Acquired causes include trauma, long-term intubation, and Wegener's granulomatosis (WG). The presenting symptoms of SGS can be confusing. Stridor and other symptoms of upper airway obstruction may be absent, leading to misdiagnosis and a possible life-threatening airway compromise.
We report a case of a pregnant woman who presented with an SGS in her second trimester, further complicated by the development of pre-eclampsia and describe her subsequent multidisciplinary management.
Case report
A 24-yr-old primigravida was admitted as an emergency to the labour ward at 29 weeks gestation. She complained of increasing malaise, fatigue, and shortness of breath over the 2 weeks before admission. She had been treated over the past 5 yr for asthma and took regular symbicort nebulizers and steroids as required. She had had a poor response to her bronchodilator therapy. Ten years previously, she had been admitted to the intensive care unit with newly diagnosed WG for which she required ventilation for 13 days, high doses of steroids, cyclophosphamide, and plasmapheresis. She remained on oxygen therapy for 50 days post-extubation and eventually made a full recovery.
On this admission, she was hypertensive (arterial pressure 200/105 mm Hg) and tachycardic (120 beats min−1). She had no signs or symptoms of pre-eclampsia. The baby's cardiotocogram (CTG) was satisfactory. On examination, she was dyspnoeic with a ventilatory frequency of 20 bpm but was able to hold a conversation. On lung auscultation, there were bilateral audible wheezes. Her Spo2 on air was 96%, and she had a measured peak flow of 160 litre min−1. She was also noted to have a systolic murmur in the left parasternal area.
All her blood results were within normal limits, her ECG showed a sinus tachycardia, and her chest X-ray was normal. Her circulating anti-neutrophil cytoplasmatic antibody (cANCA) levels were no greater than usual, which suggested her WG had not worsened. She was started on oral methyldopa and nifedipine and her arterial pressure slowly improved. Her breathlessness was treated with regular salbutamol nebulizers without demonstrable effect. On day 2, she was referred to her regular respiratory physician whom she had known for 10 yr. He diagnosed an exacerbation of asthma and started her on prednisolone 40 mg per day and regular salbutamol and referred her for pulmonary function studies. These were not done immediately because the patient was not well enough to be moved. Transthoracic echocardiography revealed a normal flow murmur with no signs of cardiac failure.
An obstetric anaesthetic consultant examined the patient and was concerned that she might actually have undiagnosed upper airway obstruction and requested an urgent ENT opinion. Nasendoscopy performed by the ENT consultant demonstrated a 50% SGS and this was confirmed by CT imaging. Lung function tests were performed on day 5 and showed evidence of upper airway obstruction. Over the next few days, her dyspnoea worsened to the extent that she was breathless sitting up and unable to finish sentences. Surgery for the SGS was therefore performed 10 days post-admission. An open airway anaesthetic technique without intubation using total i.v. anaesthesia allowed the surgeon access to widen the subglottic narrowing with laser resection. Intraoperatively, there was no evidence of active vasculitis and the stenosis appeared to be due to a mature scar (Fig. 1a and b). Anaesthesia and surgery were uneventful and immediately after operation, the patient felt well and her breathing was greatly improved. Unfortunately, the patient's respiratory function deteriorated over the next few days with an inspiratory stridor due to post-laser laryngeal oedema. This was treated with steroid nebulizers but her airway remained compromised preventing a safe discharge home. At 32 weeks gestation, she developed definite pre-eclampsia with proteinuria, headache, epigastric pain, generalized oedema, and severe hypertension. Despite treatment with i.v. labetolol and hydralazine, she continued to deteriorate and developed pulmonary oedema with severe breathlessness, unable to complete a sentence and requiring regular adrenaline nebulizers for her stridor. Her deteriorating pre-eclampsia necessitated an emergency Caesarean section under spinal anaesthesia. She was sat up during the operation and a baby girl was delivered, weighing 1540 g with APGAR scores of 6 and 9. Her arterial pressure normalized within a week post-partum and there was considerable improvement in her dyspnoea and stridor. Post-natally she was followed-up regularly by respiratory and ENT specialists and her pulmonary function tests showed a marked improvement (Fig. 2b). Six and 12 months post-delivery, she has had further laser surgery and dilatations of her SGS. She remains well with minimal respiratory problems and no longer takes any asthma medication. While awaiting definitive airway surgery she became pregnant again, during the course of that pregnancy she had further episodes of airway narrowing treated in a similar fashion with laser surgery and balloon dilatation.
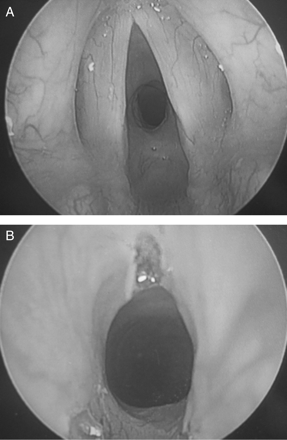
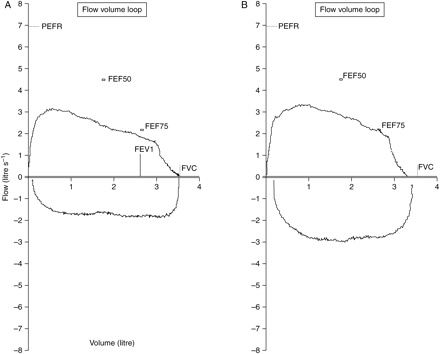
(a) Preoperative flow volume loop; (b) flow volume loop post-laser treatment. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; PEFR, peak expiratory flow rate; FEF75, forced expiratory flow at 75% FVC; FEF50, forced expiratory flow at 50% FVC; FEF50 and 75% maximal expiratory flow measured at the point where 50% of the FVC has expired and after 75% has expired. X-axis, volume in litres; Y-axis, flow in litre s−1.
Discussion
Iatrogenic trauma is the most common cause of SGS in both children and adults. Approximately 90% of all cases of acquired chronic SGS result from prolonged tracheal intubation and tracheostomy. The reported rate of stenosis after intubation ranges from 1% to 9.7%.1,2 Intubation may cause injury at the level of glottis, subglottis, and trachea. The narrowest part of the upper airway is the subglottis and it is therefore most likely to be traumatized. Initial oedema, vascular congestion, and acute inflammation can progress to ulceration and growth of granulation tissue. This may involve chondritis with destruction of the underlying cartilage and loss of framework support.2–4 When the source of irritation is removed, healing occurs with fibroblast proliferation, scar formation, and contracture, leading to stenosis or complete occlusion of the airway.1–5 Our patient had had a prolonged stay on the intensive care unit 10 yr previously during which she was intubated for 13 days and this may have contributed to the development of her SGS. Our patient also suffered from WG, an uncommon systemic vasculitis of uncertain aetiology. It usually affects the upper respiratory tract, lungs, and the kidneys. There is a recognized association between SGS and WG with a reported incidence of SGS of 12–23% in patients with WG.6,7 SGS has been reported to occur more frequently in patients who had the onset of WG at age <20 yr.7 The influence of pregnancy on the course of WG is unknown and reports are rare. From the available data, approximately one-fourth of women with WG who conceived while in remission relapsed during pregnancy.8 However, relapse also occurs in more than 50% of non-pregnant women and male WG patients. Too few case reports exist to determine whether the rate of relapse in the pregnant population is higher than would be expected.8–12 In our patient, there was no obvious relapse of WG during this pregnancy. The patient had been in remission for 10 yr, her cANCA levels had been low throughout her pregnancy and the baby had been growing well.
In SGS, dyspnoea is often the most common presenting symptom rather than stridor and is often misdiagnosed as asthma or recurrent bronchitis.7,13,14
Other symptoms of SGS are hoarseness, brassy cough, and cyanosis.7 Throat discomfort is uncommon and occurs in only a small percentage of patients.7 Adults with mild congenital stenosis are usually asymptomatic and are diagnosed after a difficult intubation or while undergoing endoscopy.7 Patients with acquired SGS are diagnosed from a few days to 10 yr or more following the initial injury.7,13,14 The SGS in our patient was most likely due to her WG and subsequent long-term intubation 10 yr previously. Our patient had no flow–volume curve studies performed until admission to labour ward (Fig. 2a and b). With the benefit of hindsight, the patient had been wrongly diagnosed with asthma and her lack of response to bronchodilator and steroid therapies was due to this. Pulmonary function tests usually give important clues to an upper airway obstruction.14 The severe respiratory compromise and potential airway problems arising through pregnancy itself led to the decision to remove the stenosis at the time rather than waiting until after delivery.
The management of SGS remains one of the most challenging problems facing the ENT surgeon and anaesthetist. The treatment methods available are endoscopic techniques such as dilatation, steroid injections, and laser treatment or surgery.
Surgical intervention includes segmental resection of the stenosed area or expansion of the stenosed segment with bone/cartilage graft. Segmental resection involves substantial risk of damaging vocal cord function; injury to the recurrent laryngeal nerve or to cricoarythenoid joints is possible.15,16 There is debate as to which of these options are the best in a particular case. It depends on subglottic involvement by systemic disease, the stage of any such disease, previous treatments, and the patient's general underlying condition.15,16 Every possible measure should be taken to avoid damage to the vocal cords. In our case, because of the life-threatening airway compromise and the high risk for both mother and baby, the surgeon felt that laser treatment was the safest and quickest option in our patient. We chose a total i.v. anaesthetic technique with propofol and remifentanil, adjusted to such a level that the patient was able to breathe spontaneously and allow the surgeon access to the stenosis with the CO2 laser. Oxygen was insufflated via a nasal airway and the cords sprayed with lidocaine to prevent laryngospasm. Jet ventilation would have been another possible technique, but we were concerned about the risk of pneumothorax. Intubation was avoided to allow the surgeon the best possible access to the subglottic area and to minimize surgical time. Because of the risk of aspiration, the patient was given antacid prophylaxis before operation and was positioned in a head-up position. To avoid aorto-caval compression during the operation, the patient lay tilted to the left on a wedge. A percutanous tracheostomy may have been safer technique for preventing possible aspiration and further airway compromise during her pregnancy. After lengthy discussions with the patient, the surgeon and the respiratory team involved, it was decided to avoid a tracheostomy as it was felt that this would compromise further reconstructive surgery.
Conclusion
Clinical signs of SGS are often subtle and the diagnosis of a developing SGS can be very difficult. Initial symptoms are usually shortness of breath rather than stridor. Many patients are wrongly diagnosed with asthma and recurrent bronchitis before subsequent discovery of a SGS. Our patient had two potential risk factors for developing an SGS: a history of previous long-term intubation and WG. The difficulty with the upper airway was further complicated by pulmonary oedema induced by pre-eclampsia. This case highlights the need for a high index of suspicion of SGS with the onset of respiratory symptoms after prolonged intubation or a history of WG, even if the disease occurred 10–20 yr ago.
Acknowledgements
The authors wish to thank the patient who kindly gave her permission for this report to be published. Thanks also to Dr G. Williams (ENT surgeon) and Prof. D. J. Shale (respiratory physician) for their support.






Comments
Editor- We read the case report by Scholz A (1) on subglottic stenosis (SGS) in pregnancy with great interest. The report states that SGS is often misdiagnosed as asthma or recurrent bronchitis.
We have recently reported a case of a patient with SGS who presented with a year long history of progressive dyspnoea (2). A month after initial onset she presented to her GP and underwent cardio-respiratory investigations which were unremarkable. In view of the absence of any ‘organic’ cause for her dyspnoea, the patient tried to ignore her symptoms and continue with her daily activities. Towards the end of the year her symptoms worsened such that excessive talking would leave her gasping for breath. Additionally she developed hoarseness, prompting the GP to refer her to an ENT specialist.
The patient was medically well otherwise, with no history of tracheal intubation or trauma to the neck and no relevant ENT history. CT scan confirmed the presence of a short segmental focal stenosis in the sub- glottic region, for which she underwent endoscopic resection. Histology of the lesion was non-specific and in the absence of any clear aetiological factor, a diagnosis of idiopathic SGS was made.
It is clear from both our cases and other reports in the literature (3) that in patients presenting with progressive dyspnoea without any clear cardio-respiratory cause, one must consider the diagnosis of SGS.
The report mentions that the patient had unremarkable cANCA levels, which suggested that her Wegeners Granulomatosis (WG) had not worsened. Although elevated cANCA levels are highly specific for WG, 10% of patients may have positive pANCA levels and 20% of patients lack ANCA altogether (4). Thus, knowing the patient’s pANCA levels and ESR may have given further clues on the aetiology of SGS. Furthermore, knowledge of the patient’s ANCA levels at the time of her initial diagnosis would have also helped in the diagnostic process.
The authors felt that the cause of the patient’s SGS was probably due to her initial presentation of WG and subsequent intubation 10 years prior to this presentation. Whilst there are a few reports of isolated involvement of the subglottis in WG in the literature (5), it is a rare phenomenon. In one particular case series examining patients with SGS due to WG, none of them had the disease confined to the subglottic region (3). In the absence of any significant evidence of WG, it is likely that the cause of this patient’s SGS was solely due to the period of prolonged tracheal intubation she experienced 10 years ago.
The aetiology of SGS can be particularly difficult to deal with. Although tracheal intubation is the most common cause, there are no reports in the literature looking at the correlation between the duration of tracheal intubation and the development of subglottic stenosis. Comparison between studies is also difficult, with varying definitions of ‘prolonged tracheal intubation’.
In conclusion, SGS can cause difficulties to the clinician through its ‘misleading’ presentation and the subsequent study to unravel its aetiology.
References
1. Scholz A, Srinivas K, Stacey MRW, Clyburn. Subglottic stenosis in pregnancy. Br J Anaesth 2008; 100 (3): 385-8.
2. Mallick AS, Chaudhry S, Philips S, Al-Shaikh B. Aetiological factors of subglottic stenosis. The Otorhinolaryngologist (in press).
3. Alaani A, Drake Lee AB. Wegener’s granulomatosis and subglottic stenosis: management of the airway. Journal of Laryngology and Otology 2004; 118:786-790
4. Seo P, Stone JH. The antineutrophil cytoplasmic antibody- associated vasculitides. Am J Med 2004; 117:39–50
5. D Hellmann, T Laing, M Petri, D Jacobs, R Crumley, and M Stulbarg. Wegener's granulomatosis: isolated involvement of the trachea and larynx. Ann Rheum Dis. 1987 August; 46(8): 628–631.
Conflict of Interest:
None declared