-
PDF
- Split View
-
Views
-
Cite
Cite
H. Schlotterbeck, R. Schaeffer, W. A. Dow, Y. Touret, S. Bailey, P. Diemunsch, Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia, BJA: British Journal of Anaesthesia, Volume 100, Issue 2, February 2008, Pages 230–234, https://doi.org/10.1093/bja/aem371
Close - Share Icon Share
Abstract
Errors in the judgement of puncture level during neuraxial anaesthesia can lead to significant complications. The aim of this study was to assess, in obstetric anaesthesia, the accuracy of clinical determination of the lumbar spinal interspace level, using surface ultrasound imaging as control.
At the anaesthesia follow-up visit, women who had received lumbar neuraxial anaesthesia during labour were prospectively included. The intervertebral level of needle insertion, located by the needle scar position, was identified by ultrasonography and compared with the clinical level reported on the chart by the anaesthetist who performed the block.
Ninety-nine women were studied. The clinical puncture level was accurate in 36.4% of patients. Ultrasound examination showed the puncture level to be more cephalad than the level noted in the anaesthetic record in almost 50% of patients. In 15% of patients, the puncture level was more caudad than the anaesthetist had assessed. Factors including type of anaesthesia, indication, time period, level of anaesthetic experience, BMI, and spinal pathology did not seem to influence the frequency of errors.
The observed differences between clinical and ultrasonic identification of spinal puncture level highlight the potential for serious complications associated with the performance of neuraxial blocks above the spinous process of L3 in the parturient. With the increase in popularity of techniques involving puncture of the dura mater for labour anaesthesia, we feel that awareness of this risk is important.
Iatrogenic neurological deficits after neuraxial block are rare.1 However, recently, there has been an increase in incidence of severe neurological trauma after spinal anaesthesia, particularly at the level of the conus medullaris. These incidents occurred most often in the obstetric population and direct puncture of the spinal cord by the needle has been suggested as the mechanism.2–4
One of the reasons cited for the cause of these complications is the misjudgement of the intervertebral space by the anaesthetist; this has previously been reported in radiological5 and surgical6 (hip surgery) studies. These studies have shown that the error is usually made in a cephalad direction (i.e. the true puncture level was higher than the predicted). None of these studies have been carried out in the obstetric setting. Furthermore, it has been shown that palpating Tuffier’s line to ascertain the intervertebral level can lead to errors in a cephalad direction, particularly in obese patients.5 The same may apply to the pregnant women.
Another factor that must be considered is the variability in the level of the end of the conus medullaris. This has been shown to reach the upper part of the body of L2 in 43% of women and 27% of men.7,8 Finally, the need to angle the needle in a cranial direction and the increasing use of combined spinal-epidural techniques may present additional risk factors for iatrogenic injury by direct contact of the needle with the spinal cord.
The aim of this prospective study was to assess the reliability of the usual anatomical landmarks in identifying the intervertebral puncture level for neuraxial anaesthesia in the clinical setting of the obstetric patient in labour.
Methods
Local Ethics Committee approval was obtained, and all patients and anaesthetists gave their informed consent to be involved in this non-interventional study. Inclusion criteria were pregnant women having undergone a neuraxial block for labour, the ability to adopt the required position for ultrasonography, and the ability to understand and communicate.
Between 24 and 72 h after delivery, patients were reviewed on the ward by a single independent investigator, unaware of what had been recorded on the chart by the anaesthetist who performed the block. The investigator collected the patient details including age, height, BMI at the time of delivery, and history of any spinal pathology such as lumbar back pain, arthritis, sciatica, scoliosis, and previous lumbar spine surgery.
The patient was examined both clinically and with ultrasound in the same position in which the block had been performed. In cases of multiple punctures and inability to identify the puncture scar, patients were excluded.
A portable ultrasound machine (Tosbee™, Toshiba, Tokyo, Japan) fitted with a soft tissue probe (3.7 MHz) was used to identify the sacrum. The image of the sacrum appeared as a continuous hyperechogenic band. The probe was then moved in a cranial direction to identify the lumbar spinal processes, recognized as hyperechogenic signals with a posterior shadow cone. The intervertebral spaces were seen as areas of hypoechogenicity surrounding zones of hyperechogenicity. If the imaging quality was poor, making identification of the intervertebral spaces uncertain, the patients were excluded from the study.
The intervertebral level of the puncture scar on the skin was established using ultrasound and recorded as the echographic level (EL). The following information was then retrieved from the anaesthetic record: type of anaesthesia (spinal, epidural, or CSE), the indication (analgesia for labour, Caesarean section, manual removal of placenta, or suturing), the time period of the intervention (day—0800 to midnight—or night—midnight to 0800), the level of experience of the anaesthetist [senior staff or junior (trainee)], and the puncture level recorded by the anaesthesiologist performing the anaesthesia, recorded as the clinical level (CL). Patients for whom the anaesthetic chart was inadequately completed were excluded from the study.
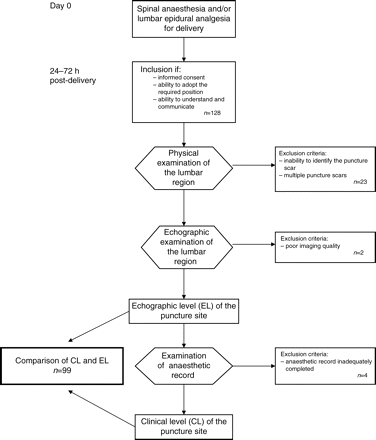
The puncture level recorded on the anaesthesic chart (CL) was compared with the true level as identified by ultrasonography (EL). The study plan is summarized in Figure 1.
Statistical analysis
The intervertebral spaces were attributed the following values: 1 (L1/L2 interspace), 2 (L2/L3), 3 (L3/L4), 4 (L4/L5), and 5 (L5/S1). A new variable (EL–CL) was defined as the arithmetic difference between the EL and the anaesthetic level with potential values ranging from −4 (undervaluation by four levels) to +4 (overvaluation by four levels). The data were arranged in contingency tables allowing statistical analysis of categorical data.
The level of agreement between the CL and EL was evaluated by Cohen’s kappa (K) statistic.9 The agreement is considered good for kappa values >0.75, average for kappa values between 0.45 and 0.75, and poor for kappa values <0.45. Negative values occur when agreement is weaker than that expected by chance. The relation of independence between CL and EL was analysed using the Stuart–Maxwell χ2 test of marginal homogeneity.10 The influence of confounding factors (type of anaesthesia, indication for anaesthesia, time period of the intervention, level of experience of the anaesthetist performing the procedure, pre-existing spinal pathology, and BMI) was analysed and compared with the differences in EL–CL using Pearson's χ2 test. Data were entered into a database (4th dimension 2004™, 4D Inc. San Jose, USA) to allow crossed analysis of the different variables. The calculations were performed by solving Fleiss equations9,10 on an excel spreadsheet (Excel™, Microsoft, Seattle, USA) and by using statistical software (SPSS 10.0™ for Windows, SPSS Inc. Chicago, USA; MH program, Version 1.2; Cohen’s unweighted Kappa, VassarStats). P<0.05 was considered significant.
Results
Twenty anaesthetists treated 128 patients; of whom 29 were excluded because of the following reasons: multiple puncture sites at different intervertebral levels (16 patients), inability to identify the puncture point scar (7 patients), poor echogenicity (2 patients), and inadequately completed anaesthetic record (4 patients). The final study population consisted of 99 subjects.
The mean (sd) age of the studied population was 28 (4.2) yr and the BMI was 28 (4.0) kg/m2. No clinically significant spinal pathology was recorded or observed in this series despite reports of mild backaches and observation of minor scoliosis in 27 patients (Table 1). The spinal interspace levels determined on clinical examination by the anaesthetist and the interspace scar levels identified by ultrasonography are shown in Table 2. Statistical analysis by Kappa test showed the absence of agreement between the clinical and the ultrasound evaluations of the puncture levels (K=−0.027). The Stuart–Maxwell χ2 test of marginal homogeneity showed the existence of a systematic error in clinical determination of the punctured level (P=0.0001).
Potential confounding factors: none of these factors influenced the accuracy of the clinical estimation of the intervertebral space
| Potential confounding factors . | n (%) . |
|---|---|
| Type of block | |
| Epidural anaesthesia | 93 (93.9) |
| Spinal anaesthesia | 6 (6.1) |
| Time of insertion | |
| Day | 54 (54.5) |
| Night | 45 (45.5) |
| Indication of block | |
| Labour analgesia | 92 (92.9) |
| Caesarean section | 7 (7.1) |
| Minor spinal pathology | |
| Yes | 27 (27.3) |
| No | 72 (72.7) |
| Anaesthetist seniority | |
| Junior | 29 (29.3) |
| Senior | 70 (70.7) |
| BMI (kg m−2) | |
| <30 | 72 (72.7) |
| >30 | 27 (27.3) |
| Potential confounding factors . | n (%) . |
|---|---|
| Type of block | |
| Epidural anaesthesia | 93 (93.9) |
| Spinal anaesthesia | 6 (6.1) |
| Time of insertion | |
| Day | 54 (54.5) |
| Night | 45 (45.5) |
| Indication of block | |
| Labour analgesia | 92 (92.9) |
| Caesarean section | 7 (7.1) |
| Minor spinal pathology | |
| Yes | 27 (27.3) |
| No | 72 (72.7) |
| Anaesthetist seniority | |
| Junior | 29 (29.3) |
| Senior | 70 (70.7) |
| BMI (kg m−2) | |
| <30 | 72 (72.7) |
| >30 | 27 (27.3) |
Potential confounding factors: none of these factors influenced the accuracy of the clinical estimation of the intervertebral space
| Potential confounding factors . | n (%) . |
|---|---|
| Type of block | |
| Epidural anaesthesia | 93 (93.9) |
| Spinal anaesthesia | 6 (6.1) |
| Time of insertion | |
| Day | 54 (54.5) |
| Night | 45 (45.5) |
| Indication of block | |
| Labour analgesia | 92 (92.9) |
| Caesarean section | 7 (7.1) |
| Minor spinal pathology | |
| Yes | 27 (27.3) |
| No | 72 (72.7) |
| Anaesthetist seniority | |
| Junior | 29 (29.3) |
| Senior | 70 (70.7) |
| BMI (kg m−2) | |
| <30 | 72 (72.7) |
| >30 | 27 (27.3) |
| Potential confounding factors . | n (%) . |
|---|---|
| Type of block | |
| Epidural anaesthesia | 93 (93.9) |
| Spinal anaesthesia | 6 (6.1) |
| Time of insertion | |
| Day | 54 (54.5) |
| Night | 45 (45.5) |
| Indication of block | |
| Labour analgesia | 92 (92.9) |
| Caesarean section | 7 (7.1) |
| Minor spinal pathology | |
| Yes | 27 (27.3) |
| No | 72 (72.7) |
| Anaesthetist seniority | |
| Junior | 29 (29.3) |
| Senior | 70 (70.7) |
| BMI (kg m−2) | |
| <30 | 72 (72.7) |
| >30 | 27 (27.3) |
Contingency table crossing the clinically evaluated lumbar interspace level with the echographically evaluated level
| Clinical evaluation . | Echographic evaluation . | |||||
|---|---|---|---|---|---|---|
| . | L1L2 . | L2L3 . | L3L4 . | L4L5 . | L5S1 . | Total . |
| L1L2 | 0 | 0 | 0 | 0 | 0 | 0 |
| L2L3 | 1 | 6 | 5 | 2 | 0 | 14 |
| L3L4 | 4 | 28 | 29 | 7 | 1 | 69 |
| L4L5 | 1 | 4 | 10 | 1 | 0 | 16 |
| L5S1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 6 | 38 | 44 | 10 | 1 | 99 |
| Clinical evaluation . | Echographic evaluation . | |||||
|---|---|---|---|---|---|---|
| . | L1L2 . | L2L3 . | L3L4 . | L4L5 . | L5S1 . | Total . |
| L1L2 | 0 | 0 | 0 | 0 | 0 | 0 |
| L2L3 | 1 | 6 | 5 | 2 | 0 | 14 |
| L3L4 | 4 | 28 | 29 | 7 | 1 | 69 |
| L4L5 | 1 | 4 | 10 | 1 | 0 | 16 |
| L5S1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 6 | 38 | 44 | 10 | 1 | 99 |
Contingency table crossing the clinically evaluated lumbar interspace level with the echographically evaluated level
| Clinical evaluation . | Echographic evaluation . | |||||
|---|---|---|---|---|---|---|
| . | L1L2 . | L2L3 . | L3L4 . | L4L5 . | L5S1 . | Total . |
| L1L2 | 0 | 0 | 0 | 0 | 0 | 0 |
| L2L3 | 1 | 6 | 5 | 2 | 0 | 14 |
| L3L4 | 4 | 28 | 29 | 7 | 1 | 69 |
| L4L5 | 1 | 4 | 10 | 1 | 0 | 16 |
| L5S1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 6 | 38 | 44 | 10 | 1 | 99 |
| Clinical evaluation . | Echographic evaluation . | |||||
|---|---|---|---|---|---|---|
| . | L1L2 . | L2L3 . | L3L4 . | L4L5 . | L5S1 . | Total . |
| L1L2 | 0 | 0 | 0 | 0 | 0 | 0 |
| L2L3 | 1 | 6 | 5 | 2 | 0 | 14 |
| L3L4 | 4 | 28 | 29 | 7 | 1 | 69 |
| L4L5 | 1 | 4 | 10 | 1 | 0 | 16 |
| L5S1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 6 | 38 | 44 | 10 | 1 | 99 |
Analysis of confounding factors did not reveal any contribution by these factors (Table 1).
The frequency of choice of intervertebral levels used for performing neuraxial block, the error and the direction of this error is shown in Table 3. There were no serious complications or neurological sequelae in this series.
Intervertebral levels chosen for placement of lumbar blocks and errors in level of localization identified with echography
| Level chosen by the anaesthetist . | n (%) . | Errors identified with echography n (%) . | Errors in a cranial direction n (%) . | Errors in a caudal direction n (%) . |
|---|---|---|---|---|
| L2–L3 | 14 (14.1) | 8 (57.1) | 1 (12.5) | 7 (87.5) |
| L3–L4 | 69 (69.7) | 40 (58) | 32 (80) | 8 (20) |
| L4–L5 | 16 (16.2) | 15 (93.7) | 15 (100) | 0 (0) |
| Level chosen by the anaesthetist . | n (%) . | Errors identified with echography n (%) . | Errors in a cranial direction n (%) . | Errors in a caudal direction n (%) . |
|---|---|---|---|---|
| L2–L3 | 14 (14.1) | 8 (57.1) | 1 (12.5) | 7 (87.5) |
| L3–L4 | 69 (69.7) | 40 (58) | 32 (80) | 8 (20) |
| L4–L5 | 16 (16.2) | 15 (93.7) | 15 (100) | 0 (0) |
Intervertebral levels chosen for placement of lumbar blocks and errors in level of localization identified with echography
| Level chosen by the anaesthetist . | n (%) . | Errors identified with echography n (%) . | Errors in a cranial direction n (%) . | Errors in a caudal direction n (%) . |
|---|---|---|---|---|
| L2–L3 | 14 (14.1) | 8 (57.1) | 1 (12.5) | 7 (87.5) |
| L3–L4 | 69 (69.7) | 40 (58) | 32 (80) | 8 (20) |
| L4–L5 | 16 (16.2) | 15 (93.7) | 15 (100) | 0 (0) |
| Level chosen by the anaesthetist . | n (%) . | Errors identified with echography n (%) . | Errors in a cranial direction n (%) . | Errors in a caudal direction n (%) . |
|---|---|---|---|---|
| L2–L3 | 14 (14.1) | 8 (57.1) | 1 (12.5) | 7 (87.5) |
| L3–L4 | 69 (69.7) | 40 (58) | 32 (80) | 8 (20) |
| L4–L5 | 16 (16.2) | 15 (93.7) | 15 (100) | 0 (0) |
Discussion
To our knowledge, this is the first clinical study carried out in the obstetric population which assesses, with ultrasound imaging, the actual intervertebral level punctured during neuraxial block. We found that the intervertebral level assessed clinically corresponded to the actual level imaged by ultrasound in only 36.4% of patients. This result is consistent with previous smaller studies, all carried out in non-obstetric populations, which showed error rates of 59–71%.5,6,11 An error rate of 60% in identification of the L2/L3 or L3/L4 intervertebral space, equivalent to ours, has been described in the study by Van Gessel and colleagues.6
Our study confirms that the punctured level is often more cephalad than clinically estimated, although our errors were at a lower rate and of lesser magnitude (48.5% cephalad errors with a maximum error of three intervertebral spaces) compared with previous studies in the literature (55–68% cephalad errors with maximum error of four intervertebral spaces).5,6 A concerning result is that in these 99 patients, 6 punctures were actually carried out at the L1/L2 intervertebral space. At this level, there is considerable risk for neurological damage as the conus terminalis may reach the body of L2 in 43% of women.8
Errors in the caudad direction (neuraxial block sited lower than the anaesthetist thought) were more frequent (15.1%, up to two intervertebral spaces) than reported in previous studies (3–4% with a maximum of one intervertebral space).5,6 Another point noted in our study is that the more cephalad the intervertebral space chosen, the greater the error rate in the caudad direction (Table 3).
We found a statistically significant difference between the clinical and ultrasound estimation of the intervertebral level. Possible causes for this include inaccurate landmark palpation, in particular the palpation of the iliac crests, and puncture point level lower than the anaesthetist’s horizontal eye plane leading to tangential vision of the surface landmarks. When aimed at the L4–L5 space, we found that the errors were usually in a cranial direction, which could support this theory. Conversely, if the iliac crest is palpated in front or behind its peak, this may lead to errors in identification of the spinous process of L4 by mistaking it for L5. This will promote puncture errors in a caudal direction.
The anatomical landmark of Tuffier’s line can be more difficult to palpate in pregnant women because of pregnancy-related changes in soft tissues and increase in BMI. Pregnant women have also a more pronounced lumbar lordosis. These elements, added to specific obstetric difficulties (neuraxial blockade is often carried out as an urgent procedure and during the night time), could explain why our results are slightly different from clinical studies performed in non-obstetric anaesthesia populations (errors: 64% in our study vs 59% in orthopaedic hip surgery).6 The fact that the errors we found in our series were more frequently observed in a caudal direction than published in previous studies may be attributed, at least in part, to learn from previous publications showing a tendency to overestimate the actual punctured level.5,6
We have studied the puncture level using ultrasound imaging; a reliable, easy to learn, safe, and acceptable technique, starting with the identification of the sacrum and counting upwards. Other identification methods have been described, counting down the spinous processes from T12 using the 12th rib line as anatomical landmark, or from C7, directly identified as the vertebra prominens.12 Although these methods are applicable, they may be subject to a greater degree of uncertainty because errors could occur in counting a greater number of spinous processes and in the clinical identification of the spinal process of reference (T12 or C7). These methods rely on manual palpation of the spine and have not been shown to be as accurate as ultrasound imaging.
Using the same ultrasound probe (3.7 MHz), using a mixed patient population and with investigators new to ultrasonography briefed for only 5 min, Watson and colleagues13 reported a 76% agreement between ultrasound and MRI identification of the L3/L4 intervertebral space. This shows that ultrasound is an acceptable tool for the identification of spinal levels. Although ultrasonography allows accurate identification of the vertebral level, this technique unfortunately cannot identify the level at which the conus medullaris ends. Hence, because of the variable position of conus medullaris (Table 4),7 ultrasonography is unlikely to exclude completely the risk of its damage.
Morphological distribution of the end of the conus medullaris (in %) on two different population. Adapted from Louis7
| Vertebral level . | European population (%) . | African population (%) . | Total (%) . |
|---|---|---|---|
| Disc T12–L1 | 16 | 8 | 12 |
| Mid-body of L1 | 44 | 8 | 26 |
| Disc L1–L2 | 20 | 52 | 36 |
| Mid-body of L2 | 16 | 24 | 20 |
| Disc L2–L3 | 4 | 8 | 6 |
| Vertebral level . | European population (%) . | African population (%) . | Total (%) . |
|---|---|---|---|
| Disc T12–L1 | 16 | 8 | 12 |
| Mid-body of L1 | 44 | 8 | 26 |
| Disc L1–L2 | 20 | 52 | 36 |
| Mid-body of L2 | 16 | 24 | 20 |
| Disc L2–L3 | 4 | 8 | 6 |
Morphological distribution of the end of the conus medullaris (in %) on two different population. Adapted from Louis7
| Vertebral level . | European population (%) . | African population (%) . | Total (%) . |
|---|---|---|---|
| Disc T12–L1 | 16 | 8 | 12 |
| Mid-body of L1 | 44 | 8 | 26 |
| Disc L1–L2 | 20 | 52 | 36 |
| Mid-body of L2 | 16 | 24 | 20 |
| Disc L2–L3 | 4 | 8 | 6 |
| Vertebral level . | European population (%) . | African population (%) . | Total (%) . |
|---|---|---|---|
| Disc T12–L1 | 16 | 8 | 12 |
| Mid-body of L1 | 44 | 8 | 26 |
| Disc L1–L2 | 20 | 52 | 36 |
| Mid-body of L2 | 16 | 24 | 20 |
| Disc L2–L3 | 4 | 8 | 6 |
None of the potential confounding factors (type of anaesthesia, indication, time period, experience of anaesthetist, spinal pathology, and BMI) were shown to significantly influence our accuracy in clinical identification of the vertebral interspace.
Epidurals performed at night have been shown to have an increased incidence of inadvertent dural puncture independent of the level of experience of the anaesthetist.14 Timing of the procedure did not influence the error rate in our series. Unlike studies looking at other practical procedures (orotracheal intubation or arterial cannulation),15 we found no difference associated with the level of experience of the anaesthetist. This highlights the usefulness of ultrasound imaging for all grades of anaesthetists as an aid to identify difficult intervertebral space.16,17
In our study, we did not find increased BMI to be a significant confounding factor; however, these patients can present a particular challenge and we feel that the use of ultrasound can be very helpful in this situation by showing both the depth of the epidural space and the inclination of the intervertebral space. Reports have already been published describing the role of ultrasound in obese parturients and in facilitating or rescuing difficult epidural needle insertion.18,19 Patients who had multiple needle puncture points were excluded from the study (12.5% of patients recruited). This could represent a bias in our study results because more difficult cases have been excluded. However, showing a high rate of error, even in uncomplicated patients, can only serve to reinforce our study result.
In our study, the same investigator performed all of the ultrasound examinations thereby preventing operator variability. The patients were placed in the same position as they were in at the time of the block, aiming at placing the puncture scar in the same position in relation to deeper structures. Nevertheless, early postpartum changes in cutaneous tissues may have introduced a bias. However, these changes are more noticeable in the anterior abdominal wall than in the lumbar region.
The use of sacrum as a reference point can prove difficult as a result of anatomical variation at the lumbosacral junction. In ∼6% of the population, there is either sacralization of the lumbar vertebra or lumbarization of the sacrum.20 The exact influence of this anatomical variability on our results is unknown, but is unlikely to be major. This anatomical variability and its possible influence on the echographic assessment was not taken into account in the study by Watson and colleagues.13 Nevertheless, the echographic assessment was validated by MRI control in this series with an agreement of 76% between both methods.
In conclusion, using ultrasound imaging, a safe, validated, non-invasive, radiation-free, easy-to-learn technique, we have shown that in obstetric patients the traditional methods of assessing the intervertebral level by palpation of Tuffier’s line is not a reliable technique, with an error rate of >60%. The evidence of puncture points up to three intervertebral levels higher than the expected highlights the potential risk, which is increased with higher target levels, and particularly if the target level chosen is above L3.
Anaesthetists must be aware of this risk, particularly when performing spinal or combined spinal–epidural anaesthesia in obstetric patients.
Funding
This study received no funding and but was supported at an institutional and departmental level by The Department of Anaesthesia, Hautepierre University Hospital, Strasbourg, France.






Comments
We thank Dr Kathirgamanathan for his interesting comments on our article [1]. He raises the fact that the technique used in our study, performed in an overweight population (mean BMI = 28 kg/m²), might minimise the frequency of errors in identification of the intervertebral level punctured.
It is well known that morbidity and mortality increases in correlation with pre-pregnancy BMI [2], and we agree that a BMI of 30 kg/m² does not represent a validated cut-off value for the definition of obesity in the obstetric population. Nevertheless, this value is generally accepted as the threshold value for the definition of obesity in the general population. The definition of obesity in the pregnant patient is not clear and the two factors usually evaluated are the pre-pregnancy BMI and the weight gain during the pregnancy [3].
We did not aim to address the issue of body weight and BMI in terms of being overweight or obese, however we are not convinced that a BMI over 25 kg/m² in pregnant women at term truly represents being overweight. Nevertheless, we looked for possible confounding factors during the determination of the lumbar puncture level and arbitrarily took the cut- off value for the definition of obesity in the general population as a surrogate for an “overweight threshold” in the obstetric population at term.
We completely agree with Dr Kathirgamanathan about the growing place for the use of ultrasound in anaesthesia practice, and in particular for spinal procedures. Our study aimed to use the ultrasound technique to demonstrate that the usual anatomical landmarks are frequently misleading in the obstetric population. This, of course, highlights the possible advantages of using ultrasound for the performance of epidurals and spinals in both easy and difficult cases [4, 5].
1. Schlotterbeck H, Schaeffer R, Dow WA, Touret Y, Bailey S, Diemunsch P. Ultrasonographic control of the puncture level for lumbar neuraxial block on obstetric anaesthesia. Br J Anaesth 2008; 100: 230-4 2. Bhattacharya S, Campbell DM, Liston WA, Bhattacharya S. Effect of Body Mass Index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health 2007; 7: 168 3. DeVader SR, Neeley HL, Myles TD, Leet TL. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet Gynecol 2007; 110: 745-51 4. Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand 2001; 25: 766-71 5. Kawaguchi R, Yamauch M, Sugino S, Tsukigase N, Omote K, Namiki A. Two cases of epidural anesthesia using ultrasound imaging. Masui 2007; 56: 702 -5
Conflict of Interest:
None declared
I have read with interest the article about Ultrasonic control of the puncture level for neuraxial block in obstetric anaesthesia by Schlotterbeck and others(1). They should be congratulated for the timely publication of this article. However I would like to make one comment about the study. In their discussion they mentioned that they did not find increased BMI was not a significant factor to influence the frequency of errors.
To my knowledge the arbitary cut off of more than 30 BMI is not evidence based in terms of anaesthetic morbidity. If the BMI is more than 25, it is classified as overweight. The mean BMI in this study was 28. Therefore it would not be wrong to say that this study was carried out in overweight patients. If the study had been done in patients less than BMI of 25, the frequency of errors might have been even higher than this study. The overweight patients are in the increase in the reproductive age group. The last CEMACH report has made recommendations to have local guidelines in the management of obese pregnant women (2). In this context, the NICE guidelines have recommended to use ultrasound technique during epidural procedure(3). Therefore it is time to consider this reliable easy to use technique to be included in the local guidelines and equipments in the management of obese patients while facilitating the training in the use of this technique in obstetric anaesthesia.
1 Schlotterbeck H, Schaeffer R et al Ultrasonographic control of the puncture level for lumbar neuraxial block on obstetric anaesthesia: Br J Anaesth 2008; 100: 230-234
2 the confidential enquiry into maternal and child health (CEMACH) : Savings Mothers Lives ; reviewing maternal death to make motherhood safe 2003-2005
National Institute for Health and Clinical excellence; Ultrasound guided catheterisation of the epidural space January 2008
Conflict of Interest:
None declared