-
PDF
- Split View
-
Views
-
Cite
Cite
M. R. Rai, T. M. Parry, A. Dombrovskis, O. J. Warner, Remifentanil target-controlled infusion vs propofol target-controlled infusion for conscious sedation for awake fibreoptic intubation: a double-blinded randomized controlled trial, BJA: British Journal of Anaesthesia, Volume 100, Issue 1, January 2008, Pages 125–130, https://doi.org/10.1093/bja/aem279
Close - Share Icon Share
Abstract
Awake fibreoptic intubation (AFOI) is a technique used in patients with difficult airways. This study compares the suitability of remifentanil target-controlled infusion (TCI) to propofol TCI for conscious sedation during AFOI in patients with bona fide difficult airways.
We recruited 24, ASA I–III patients, who were undergoing sedation for elective AFOI. Patients were randomized to one of the two groups, Group P (n=10) received propofol TCI and Group R (n=14) received remifentanil TCI. Primary outcome measures were conditions achieved at endoscopy, intubation, and post-intubation, which were graded using scoring systems. Other parameters measured were the endoscopy time, intubation time, and number of attempts at intubation. A postoperative interview was conducted to determine recall of events and level of patient satisfaction.
Endoscopy scores (0–5) and intubation scores (0–5) were significantly different [Group P 3 (1–4) vs Group R 1 (0–3) P<0.0001, Group P 3 (2–4) vs Group R 1 (0–3) P<0.0001, respectively]; with much better conditions in Group R, endoscopy times and intubation times were also significantly different, being shorter in Group R (P<0.007 and P<0.023, respectively). Patient tolerance of the procedure, judged by the discomfort scores (P<0.004) and the post-intubation scores (P<0.08), was significantly better in Group R. The level of recall for events was higher in Group R. However, there were no significant differences in the patient satisfaction scores.
Remifentanil TCI appears to provide better conditions for AFOI when compared with propofol TCI. The disadvantage of remifentanil in this setting may be a higher incidence of recall.
Awake fibreoptic intubation (AFOI) is a technique used in patients with difficult airways. Ovassapian1 stated that conscious sedation is desirable to make this procedure more tolerable. Moreover, it is essential that patients are cooperative, relaxed, and comfortable, especially when difficult laryngeal anatomy and pathology are encountered. Previous studies have described the use of midazolam, fentanyl, ketamine, propofol, and remifentanil for sedation during this procedure.2–4 However, there are no randomized controlled trials available, and propofol or midazolam appear to be most commonly used.
Controlled sedation and analgesia are paramount to this technique, but deep sedation can result in loss of airway with serious consequences. Remifentanil is of interest for use in this area because of its sedative, analgesic, and anti-tussive properties. In addition, new concepts in target-controlled infusion (TCI) technology such as context detrimental time, time to peak onset, effect-site compartment, and rate constants (Keo) have allowed the introduction of remifentanil TCI to clinical practice. Allowing the target plasma and effect-site concentration to reach equilibrium may produce consistent pharmacodynamic effects; thus, TCI mode allows a safe and predictable sedation level to be maintained. Accurate pharmacokinetic control of sedation is now possible in the clinical setting of difficult airways and this can allow the conditions during instrumentation to be compared.
This study compares the suitability of remifentanil TCI to propofol TCI for conscious sedation during AFOI in patients with bona fide difficult airways.
Methods
Local ethics committee and written informed consent were obtained. Twenty-five, consecutive, ASA grade I–III patients, who were undergoing elective surgery, were offered an AFOI because of their underlying pathology. They were then invited to join this trial and if they agreed, they were randomized into one of the two groups. One patient declined randomization and so 24 patients were recruited to the trial. Group P received propofol TCI sedation, whereas Group R received remifentanil TCI. Exclusion criteria included pregnant patients, patients <18 yr of age, emergency surgery, allergy to any of the drugs used, inability to communicate effectively, patient refusal, and patients on long-term opiods or sedative medication.
Two experienced ‘Head and Neck’ consultant anaesthetists, who routinely performed AFOI, clinically managed the trial. They indicated the clinical reasons for AFOI and conducted the endoscopy and intubation for all recruited patients. The consultants and the patients were blinded as to their group allocation.
On arrival in the anaesthetic room, i.v. access, nasal oxygen (2 litre min−1), and routine monitoring were established in all the patients. Heart rate, arterial pressure, ventilatory frequency, and oxygen saturation were recorded as a baseline and then every 2 min thereafter. I.V. glycopyrrolate 0.2 mg and i.v. midazolam as per body weight (<70 kg, 1 mg; 70–130 kg, 1.5 mg; >130 kg, 2 mg) were administered. A third anaesthetist controlled the drug infusions and recorded the sedation scores, endoscopy scores, intubation scores, patient discomfort scores, and post-intubation score (Appendix 1).
TCI plasma-site concentration (Cp) for propofol or remifentanil was achieved using the Orchestra Base Primea® (Fresenius Vial) infusion system using the Schnider or Minto pharmacokinetic model, respectively. Syringes of 1% propofol and remifentanil (50 µg ml−1) were loaded onto the base primea (Fig. 1) simultaneously and connected to the patient using a Mediplus 2-way TIVA giving set. The drug selected for study was then administered. The initial targets set were 1 µg ml−1 or 3 ng ml−1, respectively. After the target concentration at the effect site (Ce) equilibrated with the plasma concentration (Cp), the TCI was adjusted by 0.5 µg ml−1 or 0.5 ng ml−1, respectively, until the desired level of sedation was reached. This was scored using modified Steward score (0–5, Appendix 1).
While waiting for the desired level of sedation to be reached, the endoscopist applied topical local anaesthesia to the patient's airway (cocaine 100 mg nasally, lidocaine 200 mg to oropharynx via an atomizer). The endoscopist was asked to proceed with endoscopy and intubation once the Cp and Ce had achieved equilibrium. Once the laryngeal structures were identified, an epidural catheter was introduced through the working channel of the fibreoptic scope and the tip was directed under vision to be adjacent to the vocal cords. A further 4 ml of lidocaine 4% was applied via this conduit.
Our primary outcome measures were the scores observed during endoscopy, intubation, and post-intubation. These were graded using standard scoring systems, which were institutionally created for the study. Endoscopy and intubation were scored from 0 to 5, with 0 indicating the best possible condition and higher scores indicating a progressively worse outcome. The post-intubation score was graded from 1 to 3 and again, a low score indicating good conditions. In addition, the endoscopy time (from insertion of the fibreoptic scope in the nostril to visualization of the carina), the intubation time (insertion of tracheal tube into the nose or mouth to confirmation of tracheal intubation with capnography), and the number of attempts at intubation were all recorded. Patient discomfort during the procedure was also graded.
Once intubation was complete and the endotracheal tube secured, general anaesthesia was administered and surgery was allowed to proceed. A postoperative interview was conducted, usually 24 h later, when the patient had recovered from the anaesthetic and was fully awake.
Results
Of the 24 patients recruited, 10 were randomized to Group P and 14 to Group R. One patient declined consent as a result of anxiety related to multiple previous surgical procedures and there were two patients who had exclusion criteria. They were not included in the study. Statistical analysis used the unpaired t-tests for numerical data and the Mann–Whitney test for ordinal data with SPSS 15.0 for Windows software (SPSS Inc). Eight randomized patients were required to show a 20% difference in the outcome scores for a power of 0.9 and a type I error of 0.05. However, we recruited 24 patients to ensure that the results would be clinically relevant.
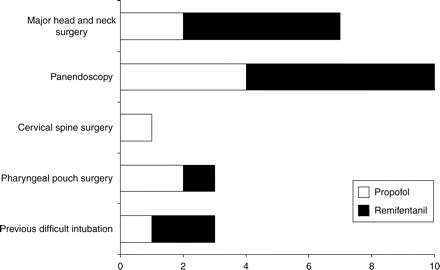
There were no significant differences in the patient characteristics (Table 1). The clinical indications for AFOI in each group are shown in Figure 2.
Patient characteristics in the two groups. Data are given as mean (sd) or numbers
| . | Propofol . | Remifentanil . |
|---|---|---|
| Number | 10 | 14 |
| Sex (M:F) | 8:2 | 9:5 |
| Age (yr) | 71 (50–81) | 62 (41–80) |
| BMI | 26.9 (17–39) | 27 (19–38) |
| ASA | ||
| I | 1 | 1 |
| II | 3 | 5 |
| III | 6 | 8 |
| . | Propofol . | Remifentanil . |
|---|---|---|
| Number | 10 | 14 |
| Sex (M:F) | 8:2 | 9:5 |
| Age (yr) | 71 (50–81) | 62 (41–80) |
| BMI | 26.9 (17–39) | 27 (19–38) |
| ASA | ||
| I | 1 | 1 |
| II | 3 | 5 |
| III | 6 | 8 |
Patient characteristics in the two groups. Data are given as mean (sd) or numbers
| . | Propofol . | Remifentanil . |
|---|---|---|
| Number | 10 | 14 |
| Sex (M:F) | 8:2 | 9:5 |
| Age (yr) | 71 (50–81) | 62 (41–80) |
| BMI | 26.9 (17–39) | 27 (19–38) |
| ASA | ||
| I | 1 | 1 |
| II | 3 | 5 |
| III | 6 | 8 |
| . | Propofol . | Remifentanil . |
|---|---|---|
| Number | 10 | 14 |
| Sex (M:F) | 8:2 | 9:5 |
| Age (yr) | 71 (50–81) | 62 (41–80) |
| BMI | 26.9 (17–39) | 27 (19–38) |
| ASA | ||
| I | 1 | 1 |
| II | 3 | 5 |
| III | 6 | 8 |
Sedation scores were similar in both groups [P 7 (6–9), R 8 (6–9) P<0.1] (Fig. 3). The mean target plasma concentrations for P and R were [1.3 µg ml−1 (1–1.6) sd 0.2] and [3.2 ng ml−1 (2.8–3.5) sd 0.2] (Fig. 4).
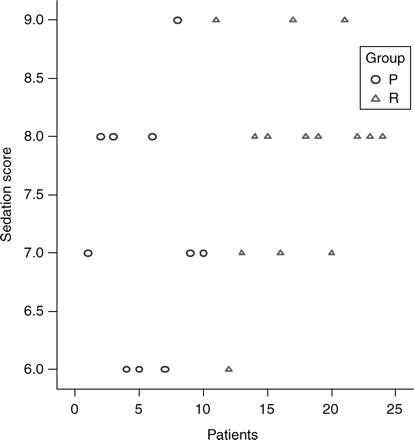
Sedation scores recorded in the two study groups, P (propofol) and R (remifentanil).
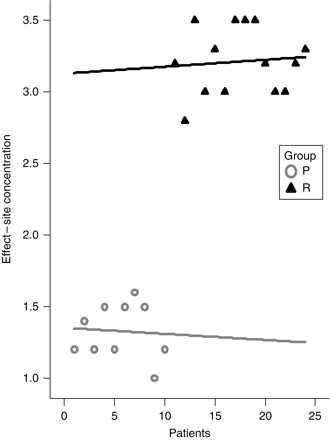
Mean drug effect-site concentrations for propofol (μg ml−1) and remifentanil (ng ml−1).
Endoscopy was more difficult in Group P with higher endoscopy scores [3.0 (1–4)] vs Group R [1 (0–3)] P<0.0001. Intubation was similarly more difficult in Group P [3 (2–4)] vs Group R [1 (0–3)] P<0.0001 (Fig. 5).
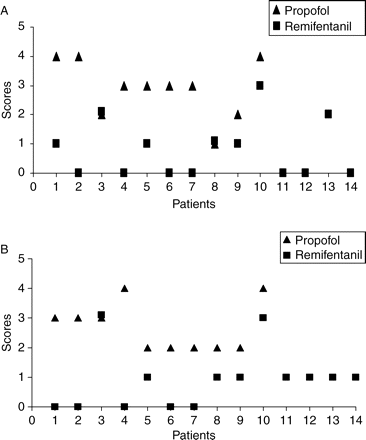
Scatter graph depicting the endoscopy scores (a) and the intubation scores (b) in the two groups.
Endoscopy times and intubation times were also significantly different: Group P [195 s (91–433) 135] vs Group R [126 (65–216) 43] P<0.007 and Group P [101 s (16–240) 76] vs Group R [66 (13–195) 44] P<0.023, respectively. Twenty-three patients were successfully intubated at the first attempt (attempt was defined as the number of times the fibreoptic scope passed the nares) and one patient in Group P was intubated at the second attempt. This patient had a severe bout of coughing, which resulted in the tracheal tube being ejected from the trachea after intubation.
The discomfort scores {Group P [1 (1–3)], Group R [0 (0–2)] P<0.004}, and the post intubation scores {Group P [2(1–2)], Group R [(1–2)] P<0.08} confirmed that the procedure was much better tolerated using remifentanil.
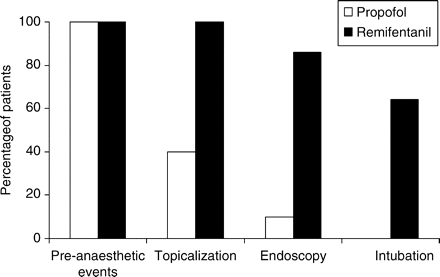
However, the level of recall for pre-anaesthetic events, topicalization, endoscopy, and intubation were higher in Group R (100%, 100%, 86%, and 64%, respectively) compared with Group P (100%, 40%, 10% and 0%, respectively) (Fig. 6). But, despite this higher level of recall in Group R, the satisfaction was the same in both groups [Group P 2 (1–3), Group R 2 (1–3)] P<0.6. There were no adverse events recorded in either of the groups, in particular, no episodes of desaturation (oxygen saturation<94%), profound bradycardia (heart rate <40 beats min−1), hypotension (systolic arterial pressure <90 mm Hg), or chest wall rigidity in either group.
Discussion
There is increasing interest in the use of remifentanil for conscious sedation during an AFOI.5–8 However, there are no randomized or blinded trials showing good evidence to support its use in this clinical setting. Moreover, the use of remifentanil for conscious sedation can be associated with undesirable side-effects.9,10 It may be considered difficult to use because of its narrow therapeutic index and unfamiliarity with its infusion kinetics. In contrast, propofol enjoys ubiquity in anaesthetic practice. Recent advances in infusion technology such as TCI for remifentanil, Schnider pharmacokinetic model for propofol, and more accurate effect-site concentrations for both drugs, can now allow us to achieve reliable, comparable, and safe sedation with both drugs. However, the comparison of two totally different pharmacological agents is always a problem. Even the use of awareness monitoring, such as BIS, to achieve comparable sedation scores involves inaccuracies associated with opioid sedation.
Our primary outcome measures were the endoscopy and intubation scores which quantified the patient tolerance of endoscopy and intubation; both were significantly improved with remifentanil. The sedation scores were comparable in both groups and so any differences observed in the endoscopy and intubation conditions must then be attributed to the pharmacodynamics of the sedation drugs. We would conclude that the improved conditions observed using remifentanil is due to its additional analgesic and anti-tussive action. Moreover, most of the differences in scores between the two groups were due to much less coughing during endoscopy and less localization to the tracheal tube during intubation. In addition, one patient in the propofol group coughed enough to eject the tracheal tube from the trachea after successful intubation.
Only one patient received an oral AFOI because of clinical reasons and they were randomized to the remifentanil group. We believe that this route of intubation is more stimulating and this makes our results more significant. In a group of patients such as ours, with known difficult airways, the importance of excellent endoscopy and intubation conditions cannot be overstated.
Sedation scores and topicalization regimen were the same for the two groups and midazolam was administered to all patients for its amnesic properties; however, in this study, there was a greater recall of events in the remifentanil group. This would suggest that remifentanil has very little inherent amnesic effect at the dose levels used in our study.6 This could be overcome by using larger doses of midazolam but our previous experience would indicate that this can result in respiratory depression and deeper levels of sedation which may be difficult to reverse. However, it is important to note that patients in the remifentanil group were not distressed by recall of events and this is reflected in the similar patient satisfaction score for the two groups.
The mean (range) propofol TCI dosage of in our study was 1.3 (1–1.6) µg ml−1. This is comparable with levels required for conscious sedation by other investigators in previous studies.11,12 There are not many trials investigating remifentanil TCI doses required for conscious sedation.13 Previous studies have also shown that remifentanil concentration >1.7 ng ml−1 is associated with a 50% decrease in minute ventilation.14 The mean effect-site concentration for the remifentanil group in our study was 3.2 (2.8–3.5) ng ml−1, but we did not experience any episodes of desaturation, presumably because of oxygen supplementation during the procedure.
The times required for endoscopy and intubation were also significantly different, with much shorter times in the remifentanil group. We believe that as patients in the remifentanil group were more comfortable, the procedure was smoother and as a result faster than that in the propofol group.
During an AFOI, it is very important that once tracheal intubation has been achieved, the patient is relaxed and comfortable so that tracheal tube position can be confirmed and general anaesthesia then can proceed under controlled conditions. Moreover, in some pathological conditions associated with cervical spine instability, coughing resulting in neck movements must be avoided throughout the procedure. The post-intubation score in our study was significantly different between the two groups. All patients in the remifentanil group scored one, indicating that they were cooperative and comfortable and obeyed commands after tracheal tube placement.
Conclusion
The conditions obtained for AFOI are significantly better when using remifentanil when compared with propofol for conscious sedation. A high incidence of recall is to be expected when using remifentanil, but does not appear to affect patient satisfaction with the procedure.
Funding
No funding was given during the trial or during the interpretation of the results. However, Glaxo Smith Klein did award a medical fellowship grant of £3000 to enable the authors to present the work.
Appendix 1
Patient Sedation Score
Consciousness 0 1 2 3 4
Airway 0 1 2 3
Activity 0 1 2
Modified Steward Score
CONSCIOUSNESS (0–4)
4-fully awake, eyes open, conversive
3-lightly asleep, eyes open intermittently
2-eyes open on command
1-response to ear pinching
0-no response
AIRWAY (0–3)
3-opens mouth, coughs on command
2-opens mouth, clear airway, weak cough
1-airway obstruction relieved by head extension
0-airway obstruction needing jaw retraction/oro-pharyngeal airway
ACTIVITY (0-2)
2-raising arm on command
1-non purposeful movement
0-no movement
Discomfort Score 0 1 2 3Endoscopy Score 0 1 2 3 4 5Intubation Score 0 1 2 3 4 5Post Intubation Conditions 1 2 3
0-no discomfort
1-probable mild discomfort, no patient resistance
2-restless patient, minimal patient resistance
3-restless patient, severe patient resistance
□ Grimacing
□ Localising
□ Coughing on lignocaine via scope
□ Coughing on entering infraglottic space
□ Prolonged coughing
□ Grimacing when tube in nares
□ Localising with one limb at any stage
□ Localising with two limbs at any stage
□ Coughing on entering trachea
□ Prolonged Coughing
1 Cooperative, obeys commands
2 Uncomfortable, GA imminent
3 Other (Specify)
Post operative Interview
Amnesia
Pre anaesthetic events YES NO
Local anaesthesia YES NO
Endoscopy YES NO
Intubation YES NO
Patient Satisfaction Score1 excellent 2 Good3 Reasonable 4 Poor






Comments
Editor We read with interest the paper by Rai and colleagues comparing remifentanil with propofol for sedation in awake fibreoptic intubation (AFOI). 1 We have been using remifentanil for sedation during AFOI for nearly three years and the paper confirms our strongly held belief the intubating conditions afforded by remifentanil are superior to other methods of sedation.
In a recent review of 35 of our own cases all using remifentanil for sedation (plus 1-2 mg of midazolam) and spray-as-you-go local anaesthetic technique we had no significant complications. Passing the fibrescope into the trachea was described as easy in 68% of cases and passing the tube easy in 80%. We also found that recall of the event occurs frequently but 26 out of 28 patients were satisfied with the procedure.
We teach our trainees a standard technique for fibreoptic intubation using remifentanil and run a fibreoptic workshop which all trainees are expected to attend before using the fibrescopes on patients. They then progress through using the fibrescopes on anaesthetised patients before performing awake fibreoptic intubations.
The standard technique includes remifentanil at a dose of 0.3mcg/kg/min with no initial bolus, and topical local anaesthetic to the nose and airway. A small amount of midazolam can be administered which reduces recall of the procedure. Verbal contact is maintained throughout the procedure and monitoring includes the use of a carbon dioxide monitoring device that is placed intranasally We are currently conducting a national survey to evaluate the extent to which remifentanil has been adopted for sedation in awake fibreoptic intubation.
1 Rai MR, Parry TM, Dombrovskis A, Warner OJ. Remifentanil target- controlled infusion vs propofol target-controlled infusion for conscious sedation for awake fibreoptic intubation: a double-blind randomized controlled trial Br J Anaesth 2008; 100:123-130
Dr Glyn Harrison Consultant Anaesthetist Luton and Dunstable Hospital Luton
Dr F Spears Consultant Anaesthetist Luton and Dunstable Hospital Luton
Dr M Osiyemi SpR Anaesthetics Luton and Dunstable Hospital Luton
Conflict of Interest:
None declared
Editor - We read with interest the Randomised Controlled Trial by Rai and colleagues1 on remifentanil target-controlled infusion (TCI) vs propofol TCI for conscious sedation for awake fibreoptic intubation (AFOI). We are not surprised by the conclusions that remifentanil TCI appears to provide better conditions for AFOI when compared to propofol TCI.
Our institution (University Hospital Aintree in Liverpool), is a tertiary referral centre for ENT and maxillofacial surgery and we perform around 400 AFOI a year for bona fide difficult airways. We have used remifentanil as the sole sedation agent for AFOI for the last four years, and we use it for both elective and emergency work. We would not consider using propofol in any circumstances because of remifentanil’s far superior analgesic and anti-tussive properties. Of the twenty or so Consultants, Staff Grades and Associate Specialists who regularly use remifentanil in this setting there is some variation in the use of midazolam and topical anaesthesia of the airway. We have found that cocaine applied nasally is sufficient as the sole topical agent. However some practitioners also use lidocaine spray to the oropharynx and/or 4% lidocaine to the vocal cords. We always have two experienced anaesthetists, one to administer sedation and one to perform the intubation.
A survey we are conducting confirms our impression that patients are satisfied with the technique and have minimal discomfort and recall. Its use has significantly improved confidence through all grades of anaesthetists and senior trainees can expect to undertake approximately 20 AFOI using remifentanil in an average six month attachment.
S. Hargreaves N. Bhuiyan Liverpool, UK *E-mail: [email protected]
1 Rai MR, Parry TM, Dombrovskis A, Warner OJ. Remifentanil target- controlled infusion vs propofol target-controlled infusion for conscious sedation for awake fibreoptic intubation: a double-blinded randomized controlled trial. Br J Anaesth 2008; 100: 125-30
Conflict of Interest:
None declared