-
PDF
- Split View
-
Views
-
Cite
Cite
Y.-F. Chai, J. Yang, J. Liu, H.-B. Song, J.-W. Yang, S.-L. Liu, W.-S. Zhang, Q.-W. Wang, Epidural anaesthetic effect of the 8% emulsified isoflurane: a study in rabbits, BJA: British Journal of Anaesthesia, Volume 100, Issue 1, January 2008, Pages 109–115, https://doi.org/10.1093/bja/aem298
Close - Share Icon Share
Abstract
Studies have shown that local use of volatile anaesthetics produce local anaesthetic effects such as local infiltration anaesthesia (in rats and humans) and spinal anaesthesia (in dogs). However, there is still no report on the epidural anaesthetic effect of volatile anaesthetics. The aim of the present study was to evaluate the epidural anaesthetic effect of the 8% emulsified isoflurane in rabbits.
Forty rabbits chronically instrumented with an epidural catheter were randomly divided into four groups of 10 rabbits each. According to group assignment, rabbits received epidural administration of 8% emulsified isoflurane (v/v) 1 ml in the E-isoflurane group, 1% lidocaine 1 ml in the Lidocaine group, 30% lipid emulsion 1 ml in the Itralipid group, or normal saline 1 ml in the NS group. The sensory and motor functions and the state of consciousness were assessed at baseline and at predetermined regular intervals. Then, the rabbits were continuously observed for 2 weeks to examine the possible long-term neurological complications.
The sensory blockade onset time, motor blockade onset time, and motor blockade duration in the E-isoflurane group [1.4 (0.7), 1.6 (0.7), and 34 (10) min, respectively] were similar to those in the Lidocaine group [1.3 (0.5), 1.7 (0.8), and 38 (8), min, respectively]. The sensory blockade duration in the E-isoflurane group was longer than that in the Lidocaine group [68 (13) vs 49 (13) min, P<0.01]. No epidural anaesthetic effects occurred in the NS group and the Intralipid group. None of the rabbits showed an abnormal consciousness after the epidural drug administration. None of the rabbits showed any long-term neurological deficits during a 2 week observation.
The present study demonstrates that epidural administration of the 8% emulsified isoflurane produces completely a reversible epidural anaesthetic effect that does not affect the level of consciousness in rabbits.
Volatile anaesthetics in the clinical concentration range have little effect on peripheral nerves, with a limited effect on nerve conduction.1 However, in vitro studies have shown that halothane (1 mM) and methoxyflurane (2.5 mM) decreased the amplitude of the action potential and the latency and duration to electric stimulation in peripheral nerves.2,3 Moreover, in vivo studies have demonstrated that intradermal injection of lecithin-coated microdroplets of volatile anaesthetics (0.3–16%, v/v) produced local anaesthesia at injection sites both in rats (methoxyflurane, isoflurane, halothane, enflurane, etc.)4,5 and in humans (methoxyflurane and isoflurane)6 and subarachnoid administration of pure sevoflurane liquid produced spinal anaesthesia in dogs.7 These studies strongly suggest that at a relatively high concentration, volatile anaesthetics are capable of blocking nerve conduction and then producing local anaesthetic effect. On the basis of these findings, we anticipated that epidural administration of volatile anaesthetics (or their injectable preparations) produces epidural anaesthetic effect.
The 8% emulsified isoflurane is an unsaturated lipid emulsion preparation of isoflurane (the vehicle of this preparation is the 30% Intralipid®). Although the 8% emulsified isoflurane was originally developed for i.v. anaesthesia by our laboratory,8,9 the properties of the drug, such as stable in formulation8 and safety for in vivo injection,8–11 also make it suitable for a local injection. With such facilitation, the present study was designed to evaluate the epidural anaesthetic effect of the 8% emulsified isoflurane and compared it with the 1% lidocaine, a commonly used traditional local anaesthetic, in a rabbit model.
Methods
The 8% emulsified isoflurane (v/v) used in the present experiment was manufactured by Huarui Pharmacy, Ltd (Wuxi, Jiangsu, China) according to the preparation methods developed by our laboratory,8 and 20 ml per ampoule of 8% emulsified isoflurane sterile preparation contains pure isoflurane liquid 1.6 ml and 30% Intralipid® (a clinically used lipid emulsion injection) 18.4 ml. The 30% Intralipid® (w/v), the original solvent for the 8% emulsified isoflurane, is provided by the Sino-Swed Pharmaceutical Co. Ltd (China). The 1% lidocaine solution (w/v) was prepared by diluting sterile solution of 2% lidocaine (Shanghai Fortune Zhaohui Pharmaceutical Co. Ltd, China) into an equal volume of normal saline solution. The normal saline solution was produced by QingShanLiKang Pharmaceutical Co. Ltd (Sichuan, China). All experimental drugs were prepared according to the results of the experimental assignment by a person unaware of the objectives of the study just before the drug administration.
With the approval of the Institutional Animal Experimentation Ethics Committee of Sichuan University (PR China), male rabbits of New Zealand White strain weighing 2.0–3.0 kg were used in the present study. The rabbits were housed individually in standard cages, had free access to food and tap water, and were kept on a 12 h light–dark cycle.
All the rabbits were pre-catheterized before the epidural drug administration. Under general anaesthesia with i.v. injection of sodium pentobarbital (35 mg kg−1), the rabbit was fixed in the prone position to facilitate the surgical procedures of epidural catheter implantation. After removing hair, disinfecting, and locally infiltrating with 1% lidocaine 1 ml, a midline skin incision (about 3 cm in length) was made between the sixth and fifth lumbar spinous processes. The muscles between the two spine processes were separated by blunt dissection and the sixth lumbar spine process was removed to expose the ligamentum flavum. Using a 20 gauge needle, a 1 mm slit was carefully made along the midline of the ligamentum flavum until the epidural space was reached. Then a 30 cm long polyethylene catheter (diameter=0.8 mm) was directly inserted through the slit into the epidural space and advanced 7 cm in a cephalic direction. After the stylet was withdrawn carefully to ensure the absence of blood or cerebrospinal fluid (CSF), 1 ml of normal saline solution was injected through the catheter to examine the leakage at the implantation site. If an obvious leakage was found, the rabbit would be abandoned from the study. The catheter was fixed in circles to the s.c. fascia near the fifth lumbar spine process by sutures after the dissected muscles were closed. The rest part of the catheter was s.c. tunnelled along the back to the neck and connected to a capped opaque connector. Then the connector was implanted partially into the skin of the neck only with its cap out to allow epidural injection. Finally, the lumbar skin incision was sutured.
The rabbits were neurologically examined after full recovery from general anaesthesia, and rabbits that showed any signs of abnormal sensory and motor functions were excluded from the study. To confirm the location of the epidural catheter, a test dose of 1% lidocaine 1 ml was injected epidurally and the exhibition of a reversible segmental sensory blockade that does not involve the tail site was considered as the evidence of correct location of the catheter. Only the rabbits with correct location of epidural catheter were allowed for the further experiments.
Twenty-four hours after the epidural catheterization, the rabbits with correct location of epidural catheter were re-examined to ensure no neurological abnormalities and their epidural catheters were re-examined with normal saline 0.2 ml injection to ensure no occlusions. Finally, 40 successfully catheterized rabbits [weight 2.4 (0.3) kg] were selected and randomly assigned to four groups of 10 each. According to group assignment, rabbits received epidural administration of 8% (v/v) emulsified isoflurane 1 ml (E-Isoflurane group), 1% lidocaine 1 ml (Lidocaine group), 30% Intralipid® 1 ml (Intralipid group), or normal saline solution 1 ml (NS group).
In absence of the observer (the same one for the entire study and who was also unaware of the strategies of the study), the experimental drug was injected via the epidural catheter at a rate of 1 ml 10 s−1 and then the catheter was flushed with 0.2 ml of normal saline solution. Since the preparations of the four studied drugs are different in smell and colour, after each injection the connectors of epidural catheters were capped immediately and then several droplets of emulsified isoflurane were dripped deliberately on the surface of the connectors to facilitate the blind.
Sensory and motor functions of the studied rabbit were evaluated 5 min before the epidural drug injection, and at 1, 2, 3, 5, 10, 15, 20, 25, 30 min afterwards, then again at 10 min intervals for at least 2 h (if no blockade observed) or until 1 h after full recovery (if a blockade observed). Sensory function was evaluated by seeking an aversive response to pinprick stimulation with a 23-gauge needle progressing from the sacral to thoracic dermatomes.12 In brief, the needle was successively pierced into the skin of each examined site with a frequency about 2 Hz and the lack of a defensive response (such as jerk, flinch, or evasive movement) within 5 s to this stimulation was considered as loss of sensory function (sensory blockade), otherwise, as normal sensory function. To facilitate testing and understanding, we rated the sensory testing sites as follows: the foot, −2; the knee, −1; the epigastrium, +1; the chest, +2; and the hand, +3.13 Motor function was scored as follows: 0, free movements of the animal using hind limbs needed without limitation or loss of balance; 1, limited or asymmetrical movements of the hind limbs needed to support the body and walk; 2, inability to support the back of the body on the hind limbs, despite existing ability to move the limbs and to respond to a painful stimulus; and 3, total paralysis of the hind limbs.13 Consciousness degree of the studied rabbits was evaluated at the same time sequences as that for the evaluation of sensory and motor functions mentioned earlier, and was scored as follows: 1, spontaneous eye-opening without stimulus (unstimulated eye-opening); 2, rabbit tends to close its eyes spontaneously, but will open them when called or patted on the head; 3, rabbit tends to close its eyes spontaneously, but will open them if there is a painful stimulus (pinprick at nose point); 4, eyes do not open even if there is a painful stimulus (general anaesthesia).14 A score < 2 was considered as a normal consciousness. With these measurements, the following endpoints were determined: onset time of blockade (referred to the time needed between the drug administration and the start of any degree of blockade), duration of blockade (the time during which the rabbit presents any degree of blockade), maximum level of sensory blockade (termed as upper level and lower level of the blocked dermatomes), maximum degree of motor blockade (the highest score during motor blockade period), maximum blockade onset time (time needed between drug administration and maximum sensory blockade level or motor blockade degree achieved), and maximum decrease in consciousness degree (termed as the highest score of consciousness observed during the observation period).
During the experiment period, the ear-artery blood pressures were measured invasively via a pre-inserted 24 gauge i.v. catheter and by an oscillometric method (M1026A, Philips, Germany). A fine pressure monitoring line (2 mm in ID and 1.5 m in length) was used to connect the i.v. catheter and the pressure sensor to avoid disturbing the movements of the animal. The mean arterial pressure (MAP) was recorded at baseline and at 1, 3, 5, 10, 15, 20, 25, 30, and 60 min after the epidural injection.
After these assessments and measurements were accomplished, the epidural catheters of studied rabbits were removed. Then the rabbits were returned and cared for in their original cages for 2 weeks to examine the possible long-term complications (including neurological abnormalities, local tissue necrosis, epidural abscess, and death).
Statistical analysis of the data was performed blindly. Values for the results were expressed as mean (sd). Comparisons of the onset times and durations between the Lidocaine group and the E-isoflurane group were performed by anova. Differences in sensory blockade levels and motor blockade degrees were performed by Kruskal–Wallis test. Comparisons of MAP were assessed by Tukey post hocanova measurement. In all cases, P<0.05 was considered significant.
Results
After the epidural drug administration, epidural anaesthetic effect was observed in both the Lidocaine group and the E-isoflurane group, and all the rabbits in these two groups recovered totally within 3 h thereafter. The onset time and duration of sensory and motor blockade of these two groups are showed in Table 1. The sensory blockade duration of the E-isoflurane group [68 (13) min] is significantly longer than that in the Lidocaine group [49 (13) min] (P<0.01), whereas the other values were not significantly different between the two groups (P>0.05). The sensory and motor blockade observed in both the E-isoflurane group and the Lidocaine group were typically segmental and none of the rabbits in these two groups showed a blockade spreading to its tail and neck. The maximum levels of sensory blockade and the maximum degree of motor blockade in the E-isoflurane group and the Lidocaine group are showed in Figures 1 and 2, respectively, and there are no significant differences between these two groups (P>0.05). No sensory or motor blockade was observed in either the NS group or the Intralipid group during the observation period. After the epidural drug administration, none of the rabbits in the four groups showed a decrease in consciousness level (score was 1 in every case). The MAP of ear artery was decreased by the epidural administration of the lidocaine or emulsified isoflurane but not by the lipid emulsion or normal saline (Fig. 3). The MAP at 3, 5, and 10 min in the Lidocaine group and at 3, 5, 10, and 15 min in the E-isoflurane group is significantly lower than those in the NS group (P<0.05), but there is no significant difference in MAP at each time point between the NS group and the Intralipid group or between the E-isoflurane group and the Lidocaine group (Fig. 3). None of the rabbits in the four groups died or showed any sign of long-term neurological deficits during a 2 week observation period.
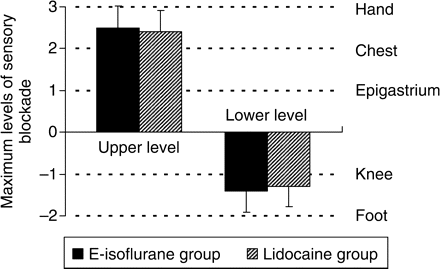
Comparisons of the maximum level of sensory blockade (upper level and lower level of the blocked dermatomes) between the E-isoflurane group and the Lidocaine group. The drug administered is emulsified isoflurane 8%, 1 ml in the E-isoflurane group or lidocaine 1%, 1 ml in the Lidocaine group. Each testing site is rated as follows: hand, +3; chest, +2; epigastrium, +1; knee, −1; foot, −2. Level ‘0’ means that the sensory blockade level cannot be detected. Values are mean (sd) (n=10). There is no significant difference in either upper level or lower level between the two groups (P>0.05).
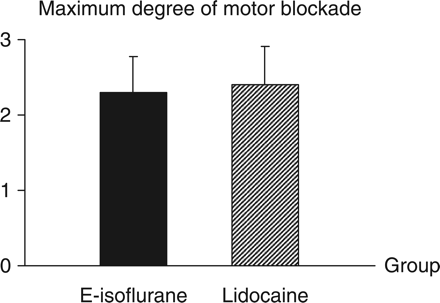
Comparisons of the maximum degree of motor blockade between the E-isoflurane group and the Lidocaine group. The drug administered is 8% emulsified isoflurane 1 ml in the E-isoflurane group or lidocaine 1%, 1 ml in the Lidocaine group. The motor blockade degree is evaluated as follows: 0, free movements of the animal using hind limbs needed without limitation or loss of balance; 1, limited or asymmetrical movements of the hind limbs needed to support the body and walk; 2, inability to support the back of the body on the hind limbs, despite existing ability to move the limbs and to respond to pain stimulus applied by needle; and 3, total paralysis of the hind limbs. Values are mean (sd) (n=10). There is no significant difference between the two groups (P>0.05).
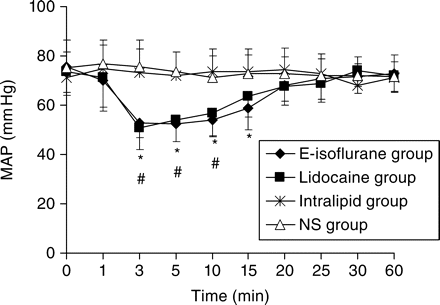
Changes in MAP within the first hour after epidural injection of the drugs. The drug administered is 8% emulsified isoflurane 1 ml in the E-isoflurane group, lidocaine 1%, 1 ml in the Lidocaine group, Intralipid® 30%, 1 ml in the Intralipid group or normal saline 1 ml in the NS group. The time point ‘0’ represents the time point of the base line. Values are mean (sd) (n=10). *P<0.05 vs NS group (for E-isoflurane group), #P<0.05 vs NS group (for Lidocaine group).
The blockade onset times and durations after epidural administration of 8% emulsified isoflurane (v/v) or 1% lidocaine. Values are means (sd) (n=10). *P<0.01 vs Lidocaine group
| Drug administered (1 ml) . | 8% emulsified isoflurane (E-isoflurane group) . | 1% lidocaine (Lidocaine group) . |
|---|---|---|
| Sensory blockade | ||
| Onset (min) | 1.4 (0.7) | 1.3 (0.5) |
| Duration (min) | 68 (13)* | 49 (13) |
| Motor blockade | ||
| Onset (min) | 1.6 (0.7) | 1.7 (0.8) |
| Duration (min) | 38 (8) | 34 (10) |
| Maximum blockade onset | ||
| Sensory (min) | 4.2 (1.6) | 3.9 (1.4) |
| Motor (min) | 3.5 (1.0) | 3.7 (1.4) |
| Drug administered (1 ml) . | 8% emulsified isoflurane (E-isoflurane group) . | 1% lidocaine (Lidocaine group) . |
|---|---|---|
| Sensory blockade | ||
| Onset (min) | 1.4 (0.7) | 1.3 (0.5) |
| Duration (min) | 68 (13)* | 49 (13) |
| Motor blockade | ||
| Onset (min) | 1.6 (0.7) | 1.7 (0.8) |
| Duration (min) | 38 (8) | 34 (10) |
| Maximum blockade onset | ||
| Sensory (min) | 4.2 (1.6) | 3.9 (1.4) |
| Motor (min) | 3.5 (1.0) | 3.7 (1.4) |
The blockade onset times and durations after epidural administration of 8% emulsified isoflurane (v/v) or 1% lidocaine. Values are means (sd) (n=10). *P<0.01 vs Lidocaine group
| Drug administered (1 ml) . | 8% emulsified isoflurane (E-isoflurane group) . | 1% lidocaine (Lidocaine group) . |
|---|---|---|
| Sensory blockade | ||
| Onset (min) | 1.4 (0.7) | 1.3 (0.5) |
| Duration (min) | 68 (13)* | 49 (13) |
| Motor blockade | ||
| Onset (min) | 1.6 (0.7) | 1.7 (0.8) |
| Duration (min) | 38 (8) | 34 (10) |
| Maximum blockade onset | ||
| Sensory (min) | 4.2 (1.6) | 3.9 (1.4) |
| Motor (min) | 3.5 (1.0) | 3.7 (1.4) |
| Drug administered (1 ml) . | 8% emulsified isoflurane (E-isoflurane group) . | 1% lidocaine (Lidocaine group) . |
|---|---|---|
| Sensory blockade | ||
| Onset (min) | 1.4 (0.7) | 1.3 (0.5) |
| Duration (min) | 68 (13)* | 49 (13) |
| Motor blockade | ||
| Onset (min) | 1.6 (0.7) | 1.7 (0.8) |
| Duration (min) | 38 (8) | 34 (10) |
| Maximum blockade onset | ||
| Sensory (min) | 4.2 (1.6) | 3.9 (1.4) |
| Motor (min) | 3.5 (1.0) | 3.7 (1.4) |
Discussion
Our study showed that epidural administration of the 8% emulsified isoflurane (v/v) produced completely reversible sensory and motor blockade without affecting the level of consciousness in rabbits and the sensory and motor blockade observed were typically segmental (no rabbit showed a blockade spreading to its tail and neck areas after epidurally administered 1 ml of the 8% emulsified isoflurane). These anaesthetic characteristics indicate that the sensory and motor blockade produced by the 8% emulsified isoflurane is a typical epidural anaesthetic effect. In the present study, we found that the sensory blockade onset time, motor blockade onset time, and motor blockade duration of the 8% emulsified isoflurane [1.4 (0.7), 1.6 (0.7), and 38 (8) min, respectively] were similar to those of the 1% lidocaine [1.3 (0.5), 1.7 (0.8), and 34 (10) min, respectively], except that the sensory blockade duration of the 8% emulsified isoflurane [68 (13) min] was relatively longer than that of the 1% lidocaine [49 (13) min]. Meanwhile, the spread of analgesia (sensory blockade level) and the intensity (degree) of motor blockade were also similar between the two drugs. These similarities in anaesthetic effect of the two drugs may suggest that, although being used as a local anaesthetic for epidural anaesthesia in rabbits, the 8% emulsified isoflurane (v/v) has similar anaesthetic efficacy and intensity to those of the 1% lidocaine (w/v).
Since the epidural administration of normal saline or 30% Intralipid® (the original solvent for the 8% emulsified isoflurane) produced no anaesthetic effect, we therefore further conclude that the epidural anaesthetic effect of the 8% emulsified isoflurane observed in the present study was induced solely by the isoflurane. Although studies have suggested that volatile anaesthetics in the clinical concentration range have little effect on peripheral nerves and have a limited effect on nerve conduction,1 with relatively high concentrations, in vitro studies have shown that volatile anaesthetics decreased the amplitude of the action potential and the latency and duration to electric stimulation in peripheral nerves,2,3 and in vivo studies have further demonstrated that intradermal injection of lecithin-coated microdroplets of volatile anaesthetics produced local anaesthesia at injection sites both in rats (methoxyflurane, isoflurane, halothane, enflurane, etc.) and in humans (methoxyflurane)4–6 and subarachnoid administration of pure sevoflurane liquid produced spinal anaesthesia in dogs.7 Our finding that epidural administration of the 8% emulsified isoflurane produced epidural anaesthetic effect in rabbits is consistent with these studies, that is, the present study further confirmed that local administration of volatile anaesthetic is capable of blocking the peripheral nerve conduction to produce local anaesthetic effect.
After the onset of epidural anaesthesia, the epidural administration of 8% emulsified isoflurane also induced a significant reduction in arterial blood pressure, similar to the reduction in MAP due to the application of 1% lidocaine. It is accepted that blockade of sympathetic outflow is the predominant cause of haemodynamic alterations induced by epidural block (with traditional local anaesthetics such as lidocaine),15 and studies have shown that the volatile anaesthetics are also capable of reducing sympathetic nervous activity to produce a cardiovascular suppressive effect in vivo.3,16–18 Therefore, the reduction in MAP after epidural administration of the 8% emulsified isoflurane may also be due to its influence on the sympathetic nervous system. Moreover, as the i.m. injection of 8% emulsified isoflurane 1 ml at the hip of the studied rabbits did not significantly affect their MAP in our preliminary experiment, we therefore further speculate that the effect of the epidural emulsified isoflurane on arterial blood pressure may mainly be a result of the influence of the drug on the local rather than systematic sympathetic nervous system.
The present study was not aimed to investigate the underlying mechanisms of the 8% emulsified isoflurane to produce epidural anaesthesia. However, based on the anaesthetic characteristics of the drug observed in our study and the current understanding in mechanisms of epidural anaesthesia, it seems reasonable to speculate here that the nerve roots and peripheral nerves within or nearby the epidural space would be the possible action sites for the 8% emulsified isoflurane to produce its epidural anaesthetic effect. Although studies have shown that the spinal cord is a main action site for isoflurane or other volatile anaesthetics to produce their anti-nociception and immobilization effects during general anaesthesia,19–24 in the present experimental settings, the solubility of the isoflurane in lipid emulsions is much higher than that in water,25–27 which may strongly restrain the isoflurane to diffuse freely through the CSF (an aqueous pathway) to reach the spinal cord while the 8% emulsified isoflurane is administered epidurally. Moreover, the typically ‘segmental’ blockade of sensory/motor functions observed in the present study also indicates that the epidurally administered emulsified isoflurane does not significantly affect the functions of the spinal cord. Therefore, we tend to speculate further that the spinal cord would not be the main or important action site for the 8% emulsified isoflurane to produce its epidural anaesthetic effect. The detailed mechanisms still need further investigations.
The attempts to use volatile anaesthetics or their injectable preparations as local anaesthetics have been made by several investigators.4–7 Haynes and colleagues4–6 have used lecithin-coated microdroplet preparation of volatile anaesthetics and Garcia-Fernandez and colleagues7 have used pure sevoflurane liquid as local anaesthetics in their studies (as mentioned earlier). However, in Haynes and colleagues’ studies, skin ulcers occurred at injection sites even at low concentrations (<4.4%),5 which suggests that the lecithin-coated microdroplets of volatile anaesthetics are not safe enough for a clinical use. In the lecithin-coated microdroplets preparations of volatile anaesthetics, volatile anaesthetics exist in a form of pure liquid microdroplets.4 Studies have shown that direct injection of pure liquid volatile anaesthetic in vivo may result in a severe tissue damage or organ toxicity.28–30 Therefore, the local toxicity observed in the studies of Haynes may be mainly due to the existence of pure volatile anaesthetics liquid in the lecithin-coated microdroplets preparations. Although no toxicity of the pure sevoflurane liquid was mentioned in Garcia-Fernandez and colleagues' paper,7 we tend to believe that using pure volatile anaesthetics liquid as local anaesthetics may not be suitable and safe for clinical use. However, the 8% emulsified isoflurane used in the present study is an unsaturated and stable lipid emulsion preparation of isoflurane in which the isoflurane is dissolved in its solvent (the 30% Intralipid®, a commonly used lipid emulsion for i.v. injection in clinical settings) without gross separation of pure liquid anaesthetic.8 Animal studies have shown that i.v. injection of the 8% emulsified isoflurane is lack of systematic toxicity and local tissue damage (at injection site).8–11 This indicates that the 8% emulsified isoflurane may be a safe and suitable preparation of isoflurane for direct in-vivo injection. From our neurobehavioural observation in this study, epidural administration of the 8% emulsified isoflurane in rabbits produced a reversible sensory/motor blockade without any neurological deficit or any sign of toxic reaction in local tissues and all rabbits recovered rapidly and completely, which further indicates that the 8% emulsified isoflurane is safe and suitable for epidural or local injection. Therefore, with these properties, the 8% emulsified isoflurane would possibly turn out to be a clinical valuable local anaesthetic (at least for epidural anaesthesia).
There are several important implications of the present study. First, using the emulsified isoflurane as a local anaesthetic is a new usage that may provide a great facilitation in studying the local actions or anaesthetic mechanisms of isoflurane or other volatile anaesthetics. Secondly, because the emulsified isoflurane is an i.v. general anaesthetic, an accidental injection of the drug into circulation system or epidural (local) administration of the drug with a large dose would result in less systematic toxicities (such as convulsion) than that of the traditional local anaesthetics (such as lidocaine). In this aspect, using the emulsified isoflurane as local anaesthetic would enhance the safety of epidural (local) anaesthesia in clinical settings.
The limitations of the present study are: first, our results were obtained only from a single dosage protocol. The possible cumulative effect of the lipid emulsion in the epidural space (or local injection site) may become apparent after successive multiple-dose administration of the 8% emulsified isoflurane, which may result in adverse reactions such as spinal compression or local abscess. This is the main disadvantage of using 8% emulsified isoflurane as local anaesthetic when compared with the aqueous solution of lidocaine or the other traditional local anaesthetics, and this issue needs to be resolved before using an emulsified preparation for local anaesthesia in clinical medicine. Secondly, the present study did not include a related histo-pathological study. Although the neurobehavioural studies suggested that the 8% emulsified isoflurane may be safe for epidural administration, neurotoxicity cannot be completely ruled out.
In summary, epidural administration of 8% emulsified isoflurane produces a complete and reversible epidural anaesthetic effect that does not affect the level of consciousness in rabbits.
Funding
973 Program, Beijing, China (2005CB522601); National Research Foundation of Nature Sciences, Beijing, China (30271259).





