-
PDF
- Split View
-
Views
-
Cite
Cite
Zeheng Bai, Yao-zhong Zhang, Yuxuan Pang, Seiya Imoto, PharaCon: a new framework for identifying bacteriophages via conditional representation learning, Bioinformatics, Volume 41, Issue 3, March 2025, btaf085, https://doi.org/10.1093/bioinformatics/btaf085
Close - Share Icon Share
Abstract
Identifying bacteriophages (phages) within metagenomic sequences is essential for understanding microbial community dynamics. Transformer-based foundation models have been successfully employed to address various biological challenges. However, these models are typically pre-trained with self-supervised tasks that do not consider label variance in the pre-training data. This presents a challenge for phage identification as pre-training on mixed bacterial and phage data may lead to information bias due to the imbalance between bacterial and phage samples.
To overcome this limitation, we proposed a novel conditional BERT framework that incorporates label classes as special tokens during pre-training. Specifically, our conditional BERT model attaches labels directly during tokenization, introducing label constraints into the model’s input. Additionally, we introduced a new fine-tuning scheme that enables the conditional BERT to be effectively utilized for classification tasks. This framework allows the BERT model to acquire label-specific contextual representations from mixed sequence data during pre-training and applies the conditional BERT as a classifier during fine-tuning, and we named the fine-tuned model as PharaCon. We evaluated PharaCon against several existing methods on both simulated sequence datasets and real metagenomic contig datasets. The results demonstrate PharaCon’s effectiveness and efficiency in phage identification, highlighting the advantages of incorporating label information during both pre-training and fine-tuning.
The source code and associated data can be accessed at https://github.com/Celestial-Bai/PharaCon.
1 Introduction
Bacteriophages (also known as phages), known as bacterial viruses, play a crucial role in microbial communities. Identifying phages from mixed metagenomic sequences is often the first step in phage analysis. Traditional sequence alignment-based methods have been widely used in real-world applications, such as VIBRANT (Kieft et al. 2020) and VirSorter2 (Guo et al. 2021). These alignment-based methods are relatively computationally intensive as they frequently align target sequences with existing profile hidden Markov models (Eddy 1998).
With the advancement of deep learning technologies, learning-based methods have been proposed to address this limitation. State-of-the-art methods include the CNN-based DeepVirFinder (Ren et al. 2020) and the LSTM-based Seeker (Auslander et al. 2020). Recently, MetaPhaPred (Ma et al. 2023) utilized the attention mechanism and achieved top performance in their proposed experiments.
Leveraging the success of Transformers (Vaswani et al. 2017) in natural language processing (Devlin et al. 2018) and computer vision (Dosovitskiy et al. 2020), Transformer-based foundation models using a pre-train-fine-tune paradigm have recently been applied to tackle biological challenges. Nucleotide sequence encoders such as DNABERT (Ji et al. 2021), DNABERT-2 (Zhou et al. 2023), and Nucleotide Transformer (Dalla-Torre et al. 2025) have demonstrated their ability to address various genomic problems. These foundation models are used to learn generalized genomic features. During pre-training, these methods use self-supervised learning tasks without considering label variance. Even when the label information of pre-training data is known, it is not incorporated into the pre-training process, posing a challenge for pre-training a Transformer-based model for phage identification. Since the amount of known bacteria and phages is imbalanced, pre-training with mixed bacteria and phage data may lead to information bias in the model.
Previously, to address this potential risk, we proposed a method for phage identification that utilized label information in the pre-training data by pre-training bacterial and phage sequences in two separate DNABERT models and fine-tuning them simultaneously within a unified framework. This novel representation learning method, named INHERIT (Bai et al. 2022), achieved a top performance according to our experimental results. INHERIT utilizes label information to some extent by splitting the imbalanced data into two groups to pre-train separately according to the labels. In this paper, we focus on a more efficient way to train a model that can learn label-specific features.
In this study, we proposed a novel architecture that can learn sequence representations with their labels. Previously, in natural language processing, Wu et al. (2019) proposed a conditional BERT for data augmentation. The pre-trained conditional BERT model generates augmented data, which helps re-train traditional sequence classifiers for downstream tasks. Conditional BERT is not incorporated into the downstream tasks.
Different from their work, our proposed architecture shares the same name but introduces different and novel features:
We introduce the label classes as special tokens. Our conditional BERT model attaches the labels during tokenization. While in their work, label information is input as an embedding layer.
Instead of using conditional BERT for generating augmented data without utilizing the learned representations in the downstream tasks, we proposed a novel solution that applies conditional BERT to both pre-training and fine-tuning processes. We introduced a novel fine-tuning scheme that generates “paradox” samples during tokenization, making conditional BERT feasible for classification tasks. Not only do we pre-train the conditional BERT to learn label-specific contextual information, but we also directly fine-tune the conditional BERT as a classifier, predicting the same label classes used in pre-training.
We applied this novel architecture to the phage identification task and named this framework PharaCon. Based on our experimental results, PharaCon achieved top performance compared to selected existing methods in our simulated sequence dataset and was the best learning-based method in our real metagenomic contig dataset. It also proved to be more efficient than alignment-based methods. The source code for PharaCon can be found at https://github.com/Celestial-Bai/PharaCon.
2 Methods
2.1 Conditional BERT
2.1.1 Label attachment and tokenization
To pre-train bacterial and phage samples with their own label constraints, we designed a BERT-style model called conditional BERT. We attached the corresponding label when tokenizing a metagenomic nucleotide sequence. Specifically, we adapted two special label class tokens: [BAC] and [PHA], which are abbreviations for bacteria and phages, respectively. If a tokenized sequence belongs to bacteria, we attach [BAC] at the beginning of the tokens; if a tokenized sequence belongs to phages, we attach [PHA].
To tokenize nucleotide sequences, we utilized the overlapped k-mer tokenizer, following the settings of DNABERT (Ji et al. 2021). Although various tokenizers for nucleotide sequences with different strategies have been proposed recently, there is no definitive conclusion as to whether they are better suited for DNA sequences than the overlapped k-mer tokenizer (Marin et al. 2024). Moreover, to ensure a fair comparison with INHERIT in our experiments, we ultimately decided to use the overlapped k-mer tokenizer.
Figure 1 illustrates two examples of tokenization with DNABERT and our conditional BERT. For DNABERT, the class token [CLS] is attached regardless of whether the nucleotide segment belongs to a bacterium or a phage. Consequently, the model learns contextual information from a mixture of bacterial and phage samples without any constraints, which may cause information bias if bacterial and phage samples are imbalanced. However, conditional BERT attaches the label class tokens [BAC] and [PHA] during tokenization, instead of the traditional class token [CLS].
![Examples of tokenization differences between DNABERT and our designed conditional BERT. DNABERT attaches label token [CLS] to every sample regardless of its label class. In contrast, conditional BERT attaches label tokens according to the label class to apply constraints to the pre-training process, allowing the model to learn the contextual information according to the label classes.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/bioinformatics/41/3/10.1093_bioinformatics_btaf085/1/m_btaf085f1.jpeg?Expires=1750182693&Signature=LNdDgQsLc5RnJ8inpXzlh7l9Zx1Hn6v1nioXMEoTTj7CCvpKv8xXxcgf6sdHwPSYDI0liVVsMWuIBmwEK-lFOjrCo2zuxr~sp2lTaGgYWcGPffxVwfOUzFrP7m--QKm-geKk-ZuHfmY-paRxQ4LJVezg4bSXaMKS3x93lB5Ose20QfSV8RgWXIX0tcUV97Dlw5B9UQYDvIi~TPx7-kGT1ZPJKQQUAcdOCvP-yyANlcyKlxJVpWQD48TM1Otg1N1m35qDDGmkvl0FQpt35rGdDDcabgWHgJC1VF6d9apB9Kiv5KNda79V36gzmeh8Hx0OxwsSJEn-mBKLXqkjFF4SSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Examples of tokenization differences between DNABERT and our designed conditional BERT. DNABERT attaches label token [CLS] to every sample regardless of its label class. In contrast, conditional BERT attaches label tokens according to the label class to apply constraints to the pre-training process, allowing the model to learn the contextual information according to the label classes.
2.1.2 Pre-training
Figure 2A illustrates the pre-training process of conditional BERT. Each 500-bp segment in our pre-training set is tokenized into overlapped k-mers (with in our study). A separator token [SEP] is appended at the end, and a label token is attached at the beginning simultaneously. Following the settings of the traditional masked language modeling task (Devlin et al. 2018, Ji et al. 2021), during pre-training, we randomly masked 15% of the non-special tokens (label tokens and the separator token are considered special tokens). Among the masked tokens, 80% is replaced with the mask token [MASK], 10% are replaced with a randomly selected token from the vocabulary, and the remaining 10% are left unchanged. The model is trained to predict these masked tokens in each input sequence.
![The pipeline of PharaCon. (A) Conditional BERT is pre-trained using labeled data with label tokens (e.g. [BAC], [PHA]) attached during tokenization. This allows learning label-specific contextual representations via masked language modeling. (B) During fine-tuning, sequences are tokenized twice with different label tokens, creating “paradox” samples with incorrect labels. The model is trained on these augmented samples to predict the correct label and identify paradoxes. (C) For prediction, metagenomic sequences are split into 500-bp segments, each tokenized with [BAC] and [PHA] labels. PharaCon generates label class scores, using a label aggregation strategy to convert three-label scores (bacteria, phage, paradox) into binary scores. These scores are averaged across segments for final classification. (D) The label aggregation strategy combines the scores. Scores for bacteria and paradox are combined when tokenized with the [PHA] label, and scores for phage and paradox are combined when tokenized with the [BAC] label.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/bioinformatics/41/3/10.1093_bioinformatics_btaf085/1/m_btaf085f2.jpeg?Expires=1750182693&Signature=Ow14nICUr01MLvmV6GQPivzuWeF3yIBwwIItr57gs7yJbetXcsuyX5VoHxAZoryu19Lc8n4ur6NudyUu3PmBhhQhWsflt3vJ~QiLFQ9C-UXrHPLMGifOtAnB14YmJxVtJNSID05p3w8PJRFxJ8-oNDNNiPlCQKKwv~~uVux~zelJuY--7q0Bc6oTVXaT98jIf1NHxAy6xu558cBfcmD2xGD4QYNc8AdF7TSWvd3Z4qlFDGI2QeOqGdAxyJEc32oTLNFBESxs1BQhulfIgG~AOAI~O4YxF8idSGQhFy8~L~GYje0sZpIhtkPgpt1IcO3aoFKwnEMgrfKU1iJUfRRfUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The pipeline of PharaCon. (A) Conditional BERT is pre-trained using labeled data with label tokens (e.g. [BAC], [PHA]) attached during tokenization. This allows learning label-specific contextual representations via masked language modeling. (B) During fine-tuning, sequences are tokenized twice with different label tokens, creating “paradox” samples with incorrect labels. The model is trained on these augmented samples to predict the correct label and identify paradoxes. (C) For prediction, metagenomic sequences are split into 500-bp segments, each tokenized with [BAC] and [PHA] labels. PharaCon generates label class scores, using a label aggregation strategy to convert three-label scores (bacteria, phage, paradox) into binary scores. These scores are averaged across segments for final classification. (D) The label aggregation strategy combines the scores. Scores for bacteria and paradox are combined when tokenized with the [PHA] label, and scores for phage and paradox are combined when tokenized with the [BAC] label.
To estimate a masked token in a sequence, the model predicts the probability distribution of tokens at the masked position, conditioned on the label constraint and the unmasked context of the sequence. The masked language modeling task can be summarized by the following formula:
where is the estimate of the -th token at the masked position . is the set of unique tokens in the vocabulary, and is a token in . is the total number of tokens, and is the number of masked tokens. The model estimates the masked token, given the condition of unmasked tokens and label constraint .
2.1.3 Label class representations are learned during pre-training
Pre-training with attached label class tokens allows the model to learn contextual information conditioned on specific labels. The token representations of the labels differ after pretraining, demonstrating the ability of the model to distinguish between different types of sequences. To validate this, we pre-trained a traditional DNABERT model using the same bacterial and phage samples as conditional BERT. Detailed information on the dataset and settings is provided in Section 3.1.
To evaluate and visualize the differences, we randomly sampled 10,000 bacterial segments and 10,000 phage segments from the pre-training set. Using both conditional BERT and DNABERT, we generated the class token representations (last hidden states of the class tokens) and visualized them using the t-SNE plots, presented in Fig. 3.

The t-SNE plot of class token representations generated from conditional BERT and DNABERT. (A) The class token representations from Conditional BERT are well-clustered distribution and distinct for bacterial and phage sequences. This well-clustered distribution in conditional BERT highlights its ability to learn label-specific contextual information. (B) The class token representations of DNABERT are mixed with bacterial and phage data since it uses the same class token for all sequences.
Figure 3 highlights the distinction between DNABERT and conditional BERT. For DNABERT (Fig. 3A), the t-SNE plot shows overlapping bacterial and phage token representations, indicating a lack of separation. DNABERT uses the same class token ([CLS]) for all samples, failing to differentiate between bacterial and phage sequences during pre-training. In contrast, Fig. 3B shows that conditional BERT forms different clusters for bacterial and phage samples. By attaching label-specific class tokens ([BAC] for bacteria and [PHA] for phages) during pre-training, conditional BERT learns features specific to each label class, resulting in well-separated token representations as shown in the t-SNE plot.
This distinction is crucial for the downstream phage identification task. Conditional BERT leverages these separable label token representations to classify sequences effectively, demonstrating the benefit of incorporating label-specific tokens and enhancing its ability to encode meaningful label-conditioned representations.
2.2 PharaCon
Previously, conditional BERT models with similar structures have been used for data augmentation in natural language processing (Wu et al. 2019). However, few of them are directly used for downstream classification tasks. In this study, we propose a solution to use the conditional BERT for classification tasks, predicting the same label classes used in pre-training.
Here, we propose a method based on conditional BERT to identify phages from mixed bacterial and phage sequences, called PharaCon. This section introduces our novel design for fine-tuning PharaCon for the phage identification task.
2.2.1 Paradox samples
In our pre-training process, we employed two types of tokenization based on the sequence type. For bacterial sequences, we appended the label token [BAC], and for phage sequences, we appended the label token [PHA]. This approach allows us to pre-train the sequences with their respective ground truth labels. However, during applications, the ground truth is unknown, resulting in a 50% chance of appending an incorrect label token to the sequence. We refer to this scenario, where an incorrect label is appended, as a “paradox” because it deviates from the normal settings established during pre-training. This paradoxical scenario was not included in our pre-training process, as the model was trained to learn contextual information with the correct attachment (the segment tokens attached with their corresponding true labels). Therefore, it is necessary to design a model capable of understanding the relationship between the attached label token and the segment tokens, and distinguishing whether the attached label token is correct. The model should identify the paradox samples from those attached with their ground truth labels.
To address this, we implemented a special augmentation process called paradox augment during fine-tuning and testing. During tokenization and label attachment, each sample is tokenized twice, each time appending a different label token. Bacterial samples labeled with [BAC] are classified as bacteria, phage samples labeled with [PHA] are classified as phages, and metagenomic samples with incorrect label tokens are classified as paradox. This paradox augment process enables conditional BERT to classify bacterial and phage samples smoothly.
2.2.2 Fine-tuning
Our paradox augment process requires designing a model capable of understanding and differentiating the relationship between the attached label token and the segment tokens. To achieve this, we developed a conditional BERT + BiLSTM model to train on the paradox-augmented data within a multi-label classification scheme (see Fig. 2B).
During fine-tuning, each segment in the training set is tokenized using the paradox augment strategy. This means each sample is tokenized twice, each time with a different label token attached. These paradox-augmented samples are then fed into the conditional BERT, initialized with the pre-trained weights, to generate their representations.
The following BiLSTM layer processes these representations, learning the relationships between the label representations and the segment representations. This relationship helps the model to distinguish between correctly and incorrectly labeled samples. Finally, a multilayer perceptron is used to make the final prediction based on these learned features.
We named this model framework PharaCon. This model framework not only reinforces the representations of correctly labeled samples learned during pre-training but also helps distinguish them from incorrectly labeled samples, which were not seen during pre-training.
2.2.3 Prediction
Figure 2C illustrates the prediction process of PharaCon. Given a test metagenomic sequence, it is first divided into 500 bp-long segments. Each segment is then tokenized with both label tokens, following the paradox augment process, generating two sets of tokens for each segment. PharaCon processes these sets of tokens and generates label-class scores for each set. Since each segment generates two sets of tokens and each set of tokens is predicted with three scores, there are nine combinations of results for each segment. Developing a rule for each of these nine combinations would be too complex and not robust.
To address this, we propose a label aggregation strategy to simplify the process by aggregating the three-label scores into binarized scores with only bacteria and phage scores. The binarized scores can then be averaged together, regardless of which label token is attached.
Figure 2D details the label aggregation strategy. For a set of tokens, when the label token [PHA] is attached, predicting “paradox” carries the same meaning as predicting bacteria, since “paradox” indicates the attached label is not the ground-truth label. Therefore, we add the score of bacteria to the score of paradox. This same logic applies when the label token [BAC] is attached; we add the score of phage to the score of paradox. This approach allows us to convert the three-label scores into binarized scores, which contain only bacteria and phage scores. The binarized scores for each set of tokens are then averaged to obtain the final scores for the test sample. To compare with other methods, if the phage score is greater than 0.5, we predict the test sample as a phage; otherwise, it is predicted as a bacterium. This label aggregation strategy is simple yet effective in handling all possible results, allowing PharaCon to be compared effectively with other methods.
3 Numerical experiments
In this study, we conducted two experiments to evaluate the performance of PharaCon compared to several representative methods. We selected geNomad (Camargo et al. 2024), VirSorter2 (Guo et al. 2021), and VIBRANT (Kieft et al. 2020) for alignment-based approaches, and DeepVirFinder (CNN-based) (Ren et al. 2020), Seeker (LSTM-based) (Auslander et al. 2020), MetaPhaPred (dna2vec + attention mechanism) (Ma et al. 2023), PPR-Meta (BiPathCNN), PhaMer (Transformer encoded with protein-based tokens), and INHERIT (dual Transformer encoders) (Bai et al. 2022) for learning-based approaches. It is important to note that although geNomad integrates both alignment-free and gene-based approaches, it uses a protein marker database for its marker branch, so we classify it as an alignment-based method. Despite the alignment step during translation in PhaMer, we still regard it as a learning-based method due to the core role of Transformer in its architecture.
To compare these methods with PharaCon from different perspectives, we constructed two datasets: a simulated sequence dataset and a real metagenomic contig dataset. We evaluated the performance of these methods using several metrics: Area Under the Receiver Operating Characteristic Curve (AUROC), Area Under the Precision-Recall Curve (AUPRC), Accuracy, F1 score, and Matthews Correlation Coefficient (MCC). We did not evaluate AUROC and AUPRC for alignment-based methods to avoid unfair comparisons, as they do not generate scores for all test samples. Additionally, AUROC and AUPRC were also excluded for PPR-Meta, since it is a multi-label classifier that predicts metagenomic fragments as phages, plasmids, and chromosomes (bacteria).
3.1 Pre-training dataset and settings
There are two methods, INHERIT and PharaCon, that require pre-training. We used the same datasets as those used when pre-training INHERIT. For bacterial samples, we used the ncbi-genome-download tool (available at https://github.com/kblin/ncbi-genome-download) to download complete bacterial reference genomes published before 1 June 2021, from the National Center for Biotechnology Information (NCBI) FTP server. Due to physical memory limitations, we randomly sampled 4124 bacterial genomes, generating 15,975,346 segments.
For phage samples, we directly downloaded and cleaned the sequences longer than 500 bp from NCBI using the keyword “phage” for entries published before 22 June 2021. We also included samples used in Seeker (Auslander et al. 2020) and VIBRANT (Kieft et al. 2020). This dataset ultimately contains 26,920 phage sequences, generating 1,750,662 segments.
We pre-trained the conditional BERT model with mixed bacteria and phage datasets using masked language modeling with label constraints. We set the learning rate to 4e-4 and the total batch size to 2048. There were 10,000 warm-up steps, and the weight decay rate was 0.01.
For INHERIT, we directly used the pre-trained weights of the bacteria and phage models available at https://github.com/Celestial-Bai/INHERIT.
3.2 Evaluation on simulated sequence dataset
3.2.1 Dataset information
We built the simulated sequence dataset based on the information provided by MetaPhaPred (Ma et al. 2023). Using their guidelines, we downloaded bacterial and phage sequences released before 31 October 2022, from the NCBI database. Following their settings, we used 11,505 bacterial and 10,639 phage sequences released before 1 January 2020, as training sets. However, we split the sequences into fixed 1000 bp-long segments, since most learning-based methods (DeepVirFinder, Seeker, INHERIT, PharaCon) are trained with 500 bp or 1000 bp segments as default settings. We randomly sampled 100,000 bacterial and phage segments to build the training set. If the input length of a model should be set at 500 bp, we split those 1000 bp segments into 500 bp fragments.
To prevent overlap with the pre-training set, we selected 1302 bacterial and 327 phage sequences released between 1 July 2021, and 31 October 2022, for testing. We cleaned the redundant sequences in the test set by ensuring that if there were identical sequences with different accessions, only one was selected. Since the segments in the training sets are relatively short, we designed a data cleaning strategy specifically suited for short sequences. If a sequence in our selected set had a region overlapping with the segments in the pre-training set or the training set detected by BLASTN (McGinnis and Madden 2004) with 100% identity, we excluded it from the final test set. We separately calculated the Average Nucleotide Identity (ANI) for both bacterial and phage samples between different datasets. Specifically, we measured the ANI between the pre-training set and the test set, as well as between the training set and the test set. Detailed results and analysis can be found in Table S2, available as supplementary data at Bioinformatics online.
Ultimately, we cleaned 434 bacterial accessions from 61,632 accessions in the 1302 bacterial samples and 241 phage samples. We randomly split the remaining bacterial and phage samples into segments ranging from 500 to 10,000 bp in length. For phage samples, this generated 1032 segments. To balance the positive and negative samples, we randomly sampled 1032 segments from the generated bacterial segments.
3.2.2 Experimental results
Table 1 shows the overall performance of PharaCon and other representative methods in the simulated sequence dataset. PharaCon emerged as the best performer in all metrics, achieving the highest AUROC (0.9974), AUPRC (0.9964), accuracy (0.9830), F1 score (0.9831), and MCC (0.9661). This indicated that PharaCon was highly effective in distinguishing between phages and bacteria, maintaining a high balance between precision and recall.
Performance of PharaCon, INHERIT, PPR-Meta, MetaPhaPred, DeepVirFinder, PhaMer, Seeker, VIBRANT, VirSorter2, and geNomad in the simulated sequence dataset.a
| Method . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|
| Learning-based methods | |||||
| PharaCon | 0.9974 | 0.9964 | 0.9830 | 0.9831 | 0.9661 |
| INHERIT | 0.9965 | 0.9956 | 0.9690 | 0.9683 | 0.9388 |
| PPR-Meta | – | – | 0.9734 | 0.9736 | 0.9469 |
| MetaPhaPred | 0.9921 | 0.9934 | 0.9690 | 0.9693 | 0.9382 |
| DeepVirFinder | 0.9856 | 0.9868 | 0.9399 | 0.9401 | 0.8798 |
| PhaMer | 0.9100 | 0.9084 | 0.8939 | 0.8879 | 0.7924 |
| Seeker | 0.8741 | 0.8769 | 0.8081 | 0.8042 | 0.6168 |
| Alignment-based methods | |||||
| VIBRANT | – | – | 0.9598 | 0.9606 | 0.9210 |
| VirSorter2 | – | – | 0.9351 | 0.9326 | 0.8725 |
| geNomad | – | – | 0.9181 | 0.9123 | 0.8437 |
| Method . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|
| Learning-based methods | |||||
| PharaCon | 0.9974 | 0.9964 | 0.9830 | 0.9831 | 0.9661 |
| INHERIT | 0.9965 | 0.9956 | 0.9690 | 0.9683 | 0.9388 |
| PPR-Meta | – | – | 0.9734 | 0.9736 | 0.9469 |
| MetaPhaPred | 0.9921 | 0.9934 | 0.9690 | 0.9693 | 0.9382 |
| DeepVirFinder | 0.9856 | 0.9868 | 0.9399 | 0.9401 | 0.8798 |
| PhaMer | 0.9100 | 0.9084 | 0.8939 | 0.8879 | 0.7924 |
| Seeker | 0.8741 | 0.8769 | 0.8081 | 0.8042 | 0.6168 |
| Alignment-based methods | |||||
| VIBRANT | – | – | 0.9598 | 0.9606 | 0.9210 |
| VirSorter2 | – | – | 0.9351 | 0.9326 | 0.8725 |
| geNomad | – | – | 0.9181 | 0.9123 | 0.8437 |
PharaCon reached the highest performance with MCC at 0.9661. Bold values indicate the best performance in each metric across all evaluated methods.
Performance of PharaCon, INHERIT, PPR-Meta, MetaPhaPred, DeepVirFinder, PhaMer, Seeker, VIBRANT, VirSorter2, and geNomad in the simulated sequence dataset.a
| Method . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|
| Learning-based methods | |||||
| PharaCon | 0.9974 | 0.9964 | 0.9830 | 0.9831 | 0.9661 |
| INHERIT | 0.9965 | 0.9956 | 0.9690 | 0.9683 | 0.9388 |
| PPR-Meta | – | – | 0.9734 | 0.9736 | 0.9469 |
| MetaPhaPred | 0.9921 | 0.9934 | 0.9690 | 0.9693 | 0.9382 |
| DeepVirFinder | 0.9856 | 0.9868 | 0.9399 | 0.9401 | 0.8798 |
| PhaMer | 0.9100 | 0.9084 | 0.8939 | 0.8879 | 0.7924 |
| Seeker | 0.8741 | 0.8769 | 0.8081 | 0.8042 | 0.6168 |
| Alignment-based methods | |||||
| VIBRANT | – | – | 0.9598 | 0.9606 | 0.9210 |
| VirSorter2 | – | – | 0.9351 | 0.9326 | 0.8725 |
| geNomad | – | – | 0.9181 | 0.9123 | 0.8437 |
| Method . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|
| Learning-based methods | |||||
| PharaCon | 0.9974 | 0.9964 | 0.9830 | 0.9831 | 0.9661 |
| INHERIT | 0.9965 | 0.9956 | 0.9690 | 0.9683 | 0.9388 |
| PPR-Meta | – | – | 0.9734 | 0.9736 | 0.9469 |
| MetaPhaPred | 0.9921 | 0.9934 | 0.9690 | 0.9693 | 0.9382 |
| DeepVirFinder | 0.9856 | 0.9868 | 0.9399 | 0.9401 | 0.8798 |
| PhaMer | 0.9100 | 0.9084 | 0.8939 | 0.8879 | 0.7924 |
| Seeker | 0.8741 | 0.8769 | 0.8081 | 0.8042 | 0.6168 |
| Alignment-based methods | |||||
| VIBRANT | – | – | 0.9598 | 0.9606 | 0.9210 |
| VirSorter2 | – | – | 0.9351 | 0.9326 | 0.8725 |
| geNomad | – | – | 0.9181 | 0.9123 | 0.8437 |
PharaCon reached the highest performance with MCC at 0.9661. Bold values indicate the best performance in each metric across all evaluated methods.
PharaCon demonstrated a slight edge over INHERIT, highlighting the advantages of conditional pre-training. Specifically, when pre-training the same bacterial and phage samples, using a single BERT model with a conditional pre-training strategy can achieve better performance than using two separate BERT models pre-training bacteria and phages independently. This improvement is attributed to the ability of the conditional BERT to incorporate label-specific contextual information during pre-training, allowing the model to learn more distinct and relevant features for each label. As a result, PharaCon benefits from a more integrated and efficient representation of the data, leading to better performance in phage identification. To better illustrate the performance differences, we plotted the Precision-Recall Curves for the methods capable of calculating the AUPRC: PharaCon, INHERIT, MetaPhaPred, DeepVirFinder, Seeker, and PhaMer (see Figs S1 and S2, available as supplementary data at Bioinformatics online).
Figure 4 presents the confusion matrices of PharaCon, depicting results based on three-label scores and label-aggregated scores. In the three-label confusion matrix, PharaCon only misclassifies normal bacterial and phage samples (i.e. samples with correct labels) as paradox. Notably, all 17 paradox samples misclassified as bacteria were originally attached with the [BAC] label token, and all 17 misclassified as phages were attached with the [PHA] label token. In this dataset, PharaCon consistently predicts the sample as either the attached label or paradox, suggesting that the label token serves as a strong guiding constraint. This constraint enables PharaCon to discern whether the segment features align with the label token features and match the label-specific representations acquired from pre-training. Furthermore, PharaCon’s accuracy in predicting paradox samples is comparable to, or even slightly better than, its accuracy for normal samples. After applying label aggregation, the prediction of paradox samples enhanced PharaCon’s overall accuracy, resulting in predictions that are marginally more accurate than those for normal samples. This underscores the significance of paradox samples in the fine-tuning and prediction process and demonstrates the robustness of our label aggregation strategy.
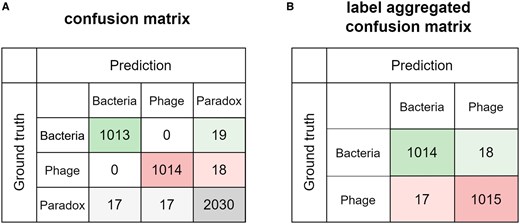
(A) Confusion matrix of three label scores generated from PharaCon shows label tokens as strong constraints. (B) Compared the label aggregated confusion matrix of PharaCon with the three-label score confusion matrix, paradox samples can help model better distinguish bacterial and phage samples to some extent.
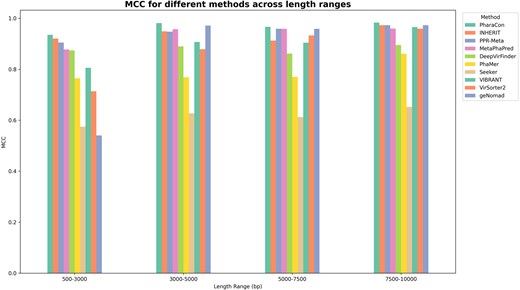
MCC score of PharaCon with other existing methods (Alignment-based: VIBRANT and VirSorter2, and Learning-based: Seeker, DeepVirFinder, and MetaPhaPred) in the simulated sequence dataset. We split the dataset into four subsets based on the length’s qualtiles. PharaCon achieved the highest MCC in all length ranges.
To evaluate the performance of the individual methods for samples of varying lengths within the test set, we divided the samples into four sub-test sets based on their lengths’ quartiles: 500–3000 bp, 3000–5000 bp, 5000–7500 bp, and 7500–10,000 bp. We then calculated the performance of each method within these four subsets. Figure 5 shows the MCC score for all the methods in different length ranges. From the results in Fig. 5, PharaCon consistently outperforms other methods in all length subsets, demonstrating its robustness and effectiveness in identifying phages from bacterial sequences.
For alignment-based methods, geNomad exhibited relatively poor performance on samples ranging from 500 to 3000 bp in length but showed improved results, performing second best in the other length ranges. VIBRANT and VirSorter2 demonstrated varying levels of performance across different sequence lengths, with steady improvements as the sequence lengths increased. These suggest that alignment-based methods are more effective with longer sequences. Especially, we recommend that when using geNomad for identifying phages and plasmids, it is advisable to focus on metagenomic fragments longer than 3000 bp for optimal results. Furthermore, the samples in the test set were submitted after 1 July 2021, which is later than the release of VIBRANT and VirSorter2. This temporal discrepancy may result in less overlap between these test samples and the sequences in the databases used by these alignment-based methods, thereby reducing the advantage of sequence alignment. This lack of overlap could be a significant factor contributing to the relatively poor performance of the alignment-based approaches in this experiment.
Overall, PharaCon consistently achieved the highest performance across all sequence lengths in the simulated sequence dataset, demonstrating robustness and effectiveness with MCC values ranging from 0.9350 to 0.9829. The performance of the methods in the four subsets evaluated with other metrics is shown in Table S3, available as supplementary data at Bioinformatics online.
3.2.3 Ablation study for label-conditioned pre-training and paradox-augmented fine-tuning
To better understand the contributions of different components within our proposed PharaCon framework, we conducted an ablation study by systematically removing or modifying key elements to evaluate their impact on performance. Specifically, we focused on evaluating the role of label-conditioned pre-training, the paradox-augmented fine-tuning scheme. We evaluated the ablated models on the simulated sequence dataset. To maintain structural comparability with PharaCon, we used BiLSTM as the downstream classifier in all ablated models.
First, we removed the paradox-augmented fine-tuning component from PharaCon, named Conditional BERT + BiLSTM. In this version, we fine-tuned the conditional BERT without generating paradox samples. For this ablation, we eliminated paradox augmentation by attaching a standard [CLS] token across all samples during fine-tuning instead of using label tokens, excluding the paradox samples. Then, we evaluated the effect of removing the label-conditioned pretraining scheme, named BERT + BiLSTM (w paradox ft). We pre-trained a DNABERT model in this experiment, with the same mixed data and hyperparameters as PharaCon, but using the traditional tokenization strategy and masked language modeling task. During fine-tuning, we introduced the [BAC] and [PHA] tokens for classification, following the paradox augmentation step. The model followed the paradox augmentation strategy, in which the sequences were tokenized with both label tokens ([BAC] and [PHA]) to create paradox samples. We also evaluated a version using standard BERT without conditional pre-training and paradox-augmented fine-tuning, named BERT + BiLSTM. We showed the performance of those ablated models on the simulated sequence dataset in Table 2.
Performance comparison of PharaCon and the ablated models with and without conditional pre-training and paradox-augmented fine-tuning.a
| Model name . | Conditional pre-training . | Paradox-augmented fine-tuning . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|---|---|
| PharaCon | 0.9974 | 0.9964 | 0.9830 | 0.9831 | 0.9661 | ||
| Conditional BERT+BiLSTM | 0.9965 | 0.9961 | 0.9724 | 0.9719 | 0.9453 | ||
| BERT+BiLSTM (w paradox ft) | 0.9928 | 0.9927 | 0.9462 | 0.9439 | 0.8956 | ||
| BERT+BiLSTM (standard) | 0.9947 | 0.9954 | 0.9356 | 0.9313 | 0.8778 |
| Model name . | Conditional pre-training . | Paradox-augmented fine-tuning . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|---|---|
| PharaCon | 0.9974 | 0.9964 | 0.9830 | 0.9831 | 0.9661 | ||
| Conditional BERT+BiLSTM | 0.9965 | 0.9961 | 0.9724 | 0.9719 | 0.9453 | ||
| BERT+BiLSTM (w paradox ft) | 0.9928 | 0.9927 | 0.9462 | 0.9439 | 0.8956 | ||
| BERT+BiLSTM (standard) | 0.9947 | 0.9954 | 0.9356 | 0.9313 | 0.8778 |
The results indicate each component of PharaCon: label-conditioned pre-training and paradox-augmented fine-tuning, contributes significantly to the overall performance and robustness of the model. Bold values indicate the highest score in each metric across all evaluated models.
Performance comparison of PharaCon and the ablated models with and without conditional pre-training and paradox-augmented fine-tuning.a
| Model name . | Conditional pre-training . | Paradox-augmented fine-tuning . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|---|---|
| PharaCon | 0.9974 | 0.9964 | 0.9830 | 0.9831 | 0.9661 | ||
| Conditional BERT+BiLSTM | 0.9965 | 0.9961 | 0.9724 | 0.9719 | 0.9453 | ||
| BERT+BiLSTM (w paradox ft) | 0.9928 | 0.9927 | 0.9462 | 0.9439 | 0.8956 | ||
| BERT+BiLSTM (standard) | 0.9947 | 0.9954 | 0.9356 | 0.9313 | 0.8778 |
| Model name . | Conditional pre-training . | Paradox-augmented fine-tuning . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|---|---|
| PharaCon | 0.9974 | 0.9964 | 0.9830 | 0.9831 | 0.9661 | ||
| Conditional BERT+BiLSTM | 0.9965 | 0.9961 | 0.9724 | 0.9719 | 0.9453 | ||
| BERT+BiLSTM (w paradox ft) | 0.9928 | 0.9927 | 0.9462 | 0.9439 | 0.8956 | ||
| BERT+BiLSTM (standard) | 0.9947 | 0.9954 | 0.9356 | 0.9313 | 0.8778 |
The results indicate each component of PharaCon: label-conditioned pre-training and paradox-augmented fine-tuning, contributes significantly to the overall performance and robustness of the model. Bold values indicate the highest score in each metric across all evaluated models.
Performance of PharaCon, INHERIT, PPR-Meta, MetaPhaPred, PhaMer, DeepVirFinder, Seeker, VirSorter2, geNomad, and VIBRANT in the real metagenomic contig dataset.a
| Method . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|
| Learning-based methods | |||||
| PharaCon | 0.9719 | 0.9638 | 0.9128 | 0.9097 | 0.8274 |
| INHERIT | 0.9592 | 0.9439 | 0.8985 | 0.8965 | 0.7975 |
| PPR-Meta | – | – | 0.8641 | 0.8526 | 0.7372 |
| MetaPhaPred | 0.9371 | 0.9217 | 0.8637 | 0.8693 | 0.7301 |
| PhaMer | 0.8414 | 0.8476 | 0.8312 | 0.8094 | 0.6805 |
| DeepVirFinder | 0.9155 | 0.9025 | 0.8082 | 0.7859 | 0.6301 |
| Seeker | 0.8430 | 0.8105 | 0.7648 | 0.7698 | 0.5301 |
| Alignment-based methods | |||||
| VirSorter2 | – | – | 0.9635 | 0.9639 | 0.9272 |
| geNomad | – | – | 0.9304 | 0.9264 | 0.8658 |
| VIBRANT | – | – | 0.9155 | 0.9199 | 0.8383 |
| Method . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|
| Learning-based methods | |||||
| PharaCon | 0.9719 | 0.9638 | 0.9128 | 0.9097 | 0.8274 |
| INHERIT | 0.9592 | 0.9439 | 0.8985 | 0.8965 | 0.7975 |
| PPR-Meta | – | – | 0.8641 | 0.8526 | 0.7372 |
| MetaPhaPred | 0.9371 | 0.9217 | 0.8637 | 0.8693 | 0.7301 |
| PhaMer | 0.8414 | 0.8476 | 0.8312 | 0.8094 | 0.6805 |
| DeepVirFinder | 0.9155 | 0.9025 | 0.8082 | 0.7859 | 0.6301 |
| Seeker | 0.8430 | 0.8105 | 0.7648 | 0.7698 | 0.5301 |
| Alignment-based methods | |||||
| VirSorter2 | – | – | 0.9635 | 0.9639 | 0.9272 |
| geNomad | – | – | 0.9304 | 0.9264 | 0.8658 |
| VIBRANT | – | – | 0.9155 | 0.9199 | 0.8383 |
PharaCon is the best performing learning-based method, while VirSorter2 reached the best performance. Bold values indicate the best performance in each metric across all evaluated methods.
Performance of PharaCon, INHERIT, PPR-Meta, MetaPhaPred, PhaMer, DeepVirFinder, Seeker, VirSorter2, geNomad, and VIBRANT in the real metagenomic contig dataset.a
| Method . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|
| Learning-based methods | |||||
| PharaCon | 0.9719 | 0.9638 | 0.9128 | 0.9097 | 0.8274 |
| INHERIT | 0.9592 | 0.9439 | 0.8985 | 0.8965 | 0.7975 |
| PPR-Meta | – | – | 0.8641 | 0.8526 | 0.7372 |
| MetaPhaPred | 0.9371 | 0.9217 | 0.8637 | 0.8693 | 0.7301 |
| PhaMer | 0.8414 | 0.8476 | 0.8312 | 0.8094 | 0.6805 |
| DeepVirFinder | 0.9155 | 0.9025 | 0.8082 | 0.7859 | 0.6301 |
| Seeker | 0.8430 | 0.8105 | 0.7648 | 0.7698 | 0.5301 |
| Alignment-based methods | |||||
| VirSorter2 | – | – | 0.9635 | 0.9639 | 0.9272 |
| geNomad | – | – | 0.9304 | 0.9264 | 0.8658 |
| VIBRANT | – | – | 0.9155 | 0.9199 | 0.8383 |
| Method . | AUROC . | AUPRC . | Accuracy . | F1 score . | MCC . |
|---|---|---|---|---|---|
| Learning-based methods | |||||
| PharaCon | 0.9719 | 0.9638 | 0.9128 | 0.9097 | 0.8274 |
| INHERIT | 0.9592 | 0.9439 | 0.8985 | 0.8965 | 0.7975 |
| PPR-Meta | – | – | 0.8641 | 0.8526 | 0.7372 |
| MetaPhaPred | 0.9371 | 0.9217 | 0.8637 | 0.8693 | 0.7301 |
| PhaMer | 0.8414 | 0.8476 | 0.8312 | 0.8094 | 0.6805 |
| DeepVirFinder | 0.9155 | 0.9025 | 0.8082 | 0.7859 | 0.6301 |
| Seeker | 0.8430 | 0.8105 | 0.7648 | 0.7698 | 0.5301 |
| Alignment-based methods | |||||
| VirSorter2 | – | – | 0.9635 | 0.9639 | 0.9272 |
| geNomad | – | – | 0.9304 | 0.9264 | 0.8658 |
| VIBRANT | – | – | 0.9155 | 0.9199 | 0.8383 |
PharaCon is the best performing learning-based method, while VirSorter2 reached the best performance. Bold values indicate the best performance in each metric across all evaluated methods.
From the results, it is evident that conditional BERT-based models outperform standard BERT-based models, demonstrating the contribution of conditional pre-training to the phage identification task. Conditional pre-training allows the model to capture learn label-specific contextual representations, enhancing its ability to differentiate between bacteria and phages. When paradox-augmented fine-tuning is removed, the performance slightly decreases across all metrics. According to the performance of Conditional BERT + BiLSTM, although conditional pre-training alone can effectively capture label-specific features, the absence of paradox samples limits the model’s ability to refine its decision-making when faced with ambiguous or mislabeled sequences. The smaller but notable decline highlights the added value of paradox-augmented fine-tuning.
Our results further proved that the combination of label-specific pre-training and paradox-augmented fine-tuning works as an integrated framework to enable effective classification. Although we applied paradox-augmented fine-tuning for BERT + BiLSTM, the absence of label tokens during pre-training limits the model’s ability to build meaningful representations aligned with specific classes. This result emphasizes that paradox fine-tuning alone cannot compensate for the loss of conditional pre-training. Moreover, the standard BERT + BiLSTM model achieves an MCC of 0.8778 and the lowest accuracy at 0.9356. This outcome demonstrates that both conditional pre-training and paradox-augmented fine-tuning are crucial to achieving the high performance observed in PharaCon.
3.2.4 Extended study on migrating data bias
Since we utilized a supervised pre-training task for PharaCon, the information PharaCon learns is derived from both a large pre-training set and a fine-tuning set. PharaCon uses these datasets as sources for acquiring information to learn features during both pre-training and fine-tuning. Compared to methods without pre-training (MetaPhaPred, DeepVirFinder, Seeker, PPR-Meta, PhaMer, etc.), PharaCon accesses a significantly larger source of information. This disparity may lead to an unfair comparison, as PharaCon’s advantage could stem from its access to more extensive datasets. It should be mentioned that MetaPhaPred uses pre-trained dna2vec (Ng 2017) as its embedding layer. However, since dna2vec was pre-trained in the human genome and not in the metagenome, we still consider MetaPhaPred as a method that does not use pre-training.
To eliminate the effect of data bias between methods that utilized pre-training and those that did not, we chose to re-train MetaPhaPred using both the pre-training set and the fine-tuning set while keeping the default hyperparameter settings. The pre-training set includes a large number of sequences, but it contains an imbalance between phage and bacterial samples. To address this, we implemented a down-sampling strategy for bacterial samples to ensure a balanced dataset suitable for training a classifier. First, we split the phage sequences in the pre-training set into 1000 bp fragments, generating 851,645 segments. Then, we split the bacterial sequences in the pre-training set into 1000 bp fragments and randomly sampled 851,645 segments from them. We combined these fragments with the samples in the fine-tuning set to re-train MetaPhaPred. By re-training MetaPhaPred with this extended dataset, we aimed to provide a fair comparison with methods that utilized pre-training, ensuring that the performance differences were not solely due to the amount of training data.
After testing with the same dataset, we observed that the MCC of MetaPhaPred improved from 0.9382 to 0.9535, indicating the advantage of increasing the number of training data. The performance of the re-trained MetaPhaPred with the extended dataset surpassed that of INHERIT but did not exceed PharaCon. This suggests that PharaCon remains the better-performing method even with similar amounts of information access.
It should be emphasized that training models on such extended datasets is not the usual practice. Typically, models are fine-tuned on datasets that are much smaller than the pre-training set to save computational costs. Additionally, the data used in the pre-training set is not filtered as rigorously as the fine-tuning set, and its quality is not as high. This may hinder models with smaller parameters from effectively extracting and learning features from a large dataset. For example, we similarly re-trained Seeker and found that its MCC decreased from 0.6168 to 0.4985. This suggests that in a real-world scenario, it is not always beneficial to train with an excessively large dataset to improve a method’s capability. Detailed results are provided in Tables S4 and S5, available as supplementary data at Bioinformatics online.
3.3 Evaluation on real metagenomic contig dataset
3.3.1 Dataset information
In this section, we focus on evaluating PharaCon and comparing it with other methods using metagenomic contigs from existing prokaryote and viral databases to simulate real-world application scenarios. We constructed datasets based on two phage metagenomic databases: the Gut Virome Database (GVD) (Gregory et al. 2020) and the Cenote Human Virome Database (CHVD) (Tisza and Buck 2021), as well as a bacterial metagenomic database called the Unified Human Gastrointestinal Genome (UHGG) (Almeida et al. 2021). We also constructed a training set for learning-based methods, as the feature distribution between reference genomes and real metagenomic contigs is different (see the Supplementary Materials, available as supplementary data at Bioinformatics online). Considering computational resources, we used the contigs published before 2019 in the GVD database for the training set. We randomly selected 20% of them as validation samples.
Since learning-based methods operate and predict at the segment level, we randomly selected bacterial sequences from UHGG to build the training and validation samples to match the total length of phage sequences in the training and validation sets, respectively. This ensured a balance between the bacterial and phage segments. In summary, there are 12,949 bacterial contigs and 12,585 phage contigs in the training set, and 3716 bacterial contigs and 3142 phage contigs in the validation set.
The phage samples in the test set were obtained from the CHVD database. We removed phages with cognate sequences from the training set from the CHVD database. The bacterial contigs in the test set were also randomly sampled from UHGG and did not overlap with those in the training set. The number of bacterial and phage contigs was the same in the test set. In summary, there are 36,012 bacterial contigs and 36,012 phage contigs in the test set. We also separately calculated the ANI for both bacterial and phage samples across different datasets for the real metagenomic contig dataset. Specifically, we measured the ANI between the pre-training set and the test set, as well as between the training set and the test set. Detailed results and analysis can be found in the Supplementary Materials, available as supplementary data at Bioinformatics online.
3.3.2 Experimental results
According to the results (see Table 3), the alignment-based methods, geNomad, VirSorter2, and VIBRANT, exhibited strong performance on the real metagenome dataset. Specifically, VirSorter2 outperformed all other methods, both learning-based and alignment-based. Among the learning-based methods, PharaCon led in all metrics, showcasing its ability to identify phages. The performance trend of learning-based methods in the real metagenomic contig dataset is consistent with the trend observed in the simulated sequence dataset, further highlighting the advantages of the label-specific pre-training strategy with conditional BERT. By leveraging label-specific information during pre-training and employing a paradox-augmented fine-tuning strategy, PharaCon effectively enhances identification ability and demonstrates advantages over other learning-based methods. To better show the performance differences, we plotted the Precision-Recall Curve for the methods capable of calculating the AUPRC: PharaCon, INHERIT, MetaPhaPred, DeepVirFinder, Seeker, and PhaMer (see Supplementary Materials, available as supplementary data at Bioinformatics online).
However, in this dataset, no learning-based method performed better than alignment-based methods. While PharaCon performed comparably to VIBRANT, it still had a 0.11% lower MCC than VIBRANT. The main reason why alignment-based methods performed better than all learning-based methods could be the identification process of viral contigs in the CHVD database. The viral contigs in the CHVD database were all identified by Cenote-Taker 2 (Tisza et al. 2021), a database-based method that includes profile Hidden Markov Models from the Pfam database (Mistry et al. 2021). These models are also referenced by geNomad, VIBRANT and VirSorter2. To validate our hypothesis, we first randomly selected 5000 sequences from the test set and used Prodigal (Hyatt et al. 2010) to predict the potential amino acid sequences that could be translated from the sequences in the test set. Next, we compared these predicted amino acid sequences with protein sequences in the Pfam database using BLASTp (McGinnis and Madden 2004), setting the e-value threshold at 0.001. Our results show that, on average, each sequence in our test set had 3817 hits, with the overall average e-value for these hits being 1.9e-5. This outcome suggests that there might be a strong correspondence between the protein sequences in the Pfam database and the amino acid sequences predicted from our test set, since the low average e-value indicates that many of the predicted sequences are highly similar to known proteins in Pfam.
4 Discussion
In this paper, we mainly focused on applying conditional BERT to phage identification, introducing PharaCon. The underlying principles could potentially be extended to other genomic classification challenges. The approach of leveraging label constraints during pre-training and the novel fine-tuning strategy may hold broader applicability. We hope that future research will explore the application of conditional BERT frameworks to other genomic classification tasks. Testing the conditional BERT and the novel pre-train-fine-tune paradigm across a variety of genomic classification tasks will help determine its robustness and versatility, which could provide valuable insights into its broader applicability.
Furthermore, the current work formulated phage identification as a binary classification problem, distinguishing bacterial from phage sequences. However, genomic data in real-world scenarios often exhibits multi-label characteristics, wherein sequences may simultaneously belong to multiple categories. Consequently, there is a pressing need for models capable of handling multi-label classification tasks. We hope that future research will investigate the extension of the conditional BERT framework and the proposed fine-tuning scheme to support multi-label classification, thereby enhancing their utility and effectiveness in more intricate genomic analyses.
For the current PharaCon framework, we employed a label aggregation strategy to integrate paradox predictions into a binary classification framework. This approach ensures robustness and interpretability while allowing the model to handle diverse real-world scenarios. However, we acknowledge that a more formal mathematical or statistical method might further improve this process. We have included this as a direction for future research and will continue exploring more advanced strategies for label aggregation.
5 Conclusion
In this study, we proposed a novel conditional BERT model for pre-training labeled data, incorporating label constraints during pre-training with modified language modeling tasks. Additionally, we applied conditional BERT to the phage identification task with a paradox-augmented fine-tuning strategy, introducing PharaCon. We tested PharaCon against other selected existing methods on both simulated sequence datasets and real metagenomic contig datasets. PharaCon outperformed other learning-based methods in multiple metrics in both datasets and achieved the best MCC score of 0.9661 in the simulated sequence dataset among all methods. In the real metagenomic contig dataset, it was the only learning-based method to achieve performance competitive with alignment-based methods like VIBRANT. The innovative use of label constraints during pre-training and the paradox-augmented fine-tuning process contributed to its enhanced accuracy and efficiency, making PharaCon an effective method for phage identification in metagenomic sequences.
Acknowledgements
The super-computing resource was provided by the Human Genome Center, Institute of Medical Science, The University of Tokyo (http://sc.hgc.jp/shirokane.html).
Supplementary data
Supplementary data are available at Bioinformatics online.
Conflict of interest: None declared.
Funding
This study was supported by the Japan Agency for Medical Research and Development (AMED) (JP21ae0121040) and the Japanese Society for the Promotion of Science (JSPS) (22H00477).
Data availability
The source code and associated data can be accessed at https://github.com/Celestial-Bai/PharaCon.



