-
PDF
- Split View
-
Views
-
Cite
Cite
Lexiang Wang, Jingli Zhou, Xuan Wang, Yadong Wang, Junyi Li, MCDHGN: heterogeneous network-based cancer driver gene prediction and interpretability analysis, Bioinformatics, Volume 40, Issue 6, June 2024, btae362, https://doi.org/10.1093/bioinformatics/btae362
Close - Share Icon Share
Abstract
Accurately predicting the driver genes of cancer is of great significance for carcinogenesis progress research and cancer treatment. In recent years, more and more deep-learning-based methods have been used for predicting cancer driver genes. However, deep-learning algorithms often have black box properties and cannot interpret the output results. Here, we propose a novel cancer driver gene mining method based on heterogeneous network meta-paths (MCDHGN), which uses meta-path aggregation to enhance the interpretability of predictions.
MCDHGN constructs a heterogeneous network by using several types of multi-omics data that are biologically linked to genes. And the differential probabilities of SNV, DNA methylation, and gene expression data between cancerous tissues and normal tissues are extracted as initial features of genes. Nine meta-paths are manually selected, and the representation vectors obtained by aggregating information within and across meta-path nodes are used as new features for subsequent classification and prediction tasks. By comparing with eight homogeneous and heterogeneous network models on two pan-cancer datasets, MCDHGN has better performance on AUC and AUPR values. Additionally, MCDHGN provides interpretability of predicted cancer driver genes through the varying weights of biologically meaningful meta-paths.
1 Introduction
Mutations occurring in somatic cells that confer a preferential growth advantage to tumor cells are termed driver mutations. The genes responsible for driving these mutations are referred to as driver genes. The overarching objective of the International Cancer Genome Consortium has consistently revolved around comprehending, elucidating, and pinpointing cancer driver genes. But finding cancer driver genes through traditional medical approaches requires significant time and research costs. With bioinformatics and high-throughput databases, we can accelerate this process through computational biology. Conventional machine learning approaches concentrate on genomic sequence data and identify cancer driver genes by evaluating gene sequence mutation probabilities, hotspots, and frequencies (Han et al. 2019), such as the MutsigCV method proposed by Lawrence et al. (2013). Dietlein et al. (2020) proposed a comprehensive oncogenomics workflow for constructing a compilation of cancer driver genes. This workflow integrates several advanced positive selection signal bias techniques and outlines three fundamental steps for handling cancer genomics data: preprocessing, implementing seven complementary driver gene identification methods, and combining candidate driver genes identified by each method through weighted voting. The TCGA (Tomczak et al. 2015) initiative has introduced a pan-cancer network database incorporating multi-omic data (Gibbons and Creighton 2018), standardizing expression within the field of cancer research. This initiative increases data volume and standardizes data formats, thereby facilitating the application of deep learning in predicting cancer driver genes. Elmarakeby et al. (2021) aggregated initial information from cases by building a feature aggregation architecture that is rich in biological information and conforms to a biological hierarchical system, and clinically validated it in the field of prostate cancer. Their work is more in line with clinical medicine’s need for explainability of cancer drug resistance. Compared with traditional network analysis, graph neural networks can obtain a variable number of neighbor node feature values and a local topology composed of neighbor nodes that are important to the classification result, and thus better perform the classification task of the nodes. Schulte-Sasse et al. (2021) introduced the EMOGI algorithm in their publication, which uses multi-omics data as node features, and uses graph neural network algorithms in a semi-supervised manner for training in protein interactions networks to learn the complex non-linear structures to identify the oncogenes and non-oncogenes. Building on the GCN method and homomorphic networks, Peng et al. (2022) made further extensions. Node classification is used as the primary task, with link prediction between pairs of nodes as a secondary task. These two tasks share two Chebyshev GCN layers and optimize two different objective functions. Zhao et al. (2022; MODIG) further enriched and expanded the gene network by constructing a multi-dimensional homogenous network of genes using five types of direct and indirect gene relationships. It obtains the representation vectors of gene nodes through the aggregated representations across these five networks. The MTGCL method (Peng et al. 2024) constructs three different gene network views from multi-omics data, essentially forming a multi-dimensional homogeneous network. This approach applies graph contrastive learning with shared parameters across these networks to learn consistent gene features across networks.
Existing network-based studies often involve only one type of node, blurring the labels of other molecular nodes or physiological roles that enable the two gene nodes to interact. This limitation hinders the interpretability of biological analyses. This study aims to make better use of biological high-throughput data and the interaction between genomics data and other histological data, and tightly integrate biological a priori knowledge in order to improve the predictive accuracy of cancer driver genes and the interpretability of the results. We constructed a heterogeneous network containing multi-omics data, nodes of multiple biological entity types and multiple interrelationships, and applied advanced heterogeneous network characterization algorithms to the characterization mining of cancer-related genes, with the main task of classifying and predicting cancer driver genes in the biological network. At the same time, we also pursue to combine the weights contributed by the meta-paths neighbor in the network to give some explanations for the cancer driver genes predicted by our model. Make the result to be more relevant to biological analysis, and to be more acceptable to the biological community.
2 Materials and methods
2.1 Datasets
Some cancer-driving genes not only affect one type of cancer but play a role in the development of various cancer types and subtypes (ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium 2020). Furthermore, in network-based studies, our main task is described as the classification of gene nodes. In this semi-supervised learning task, the quantity and quality of node labels significantly impact the classification outcomes. If we only use labels for driver genes related to a specific type of cancer, the number of positively labeled nodes is too small relative to the entire gene collection, which may cause the model to lean toward negative class discrimination. However, identifying potential positive samples is more valuable in our objectives. Therefore, we opt for a pan-cancer analysis to address this issue. Our study employs the same approach as the EMOGI method to extract multi-omics features of genes. We utilize clinical data from TCGA to calculate the mutation rate, methylation value, and gene expression data for each gene, resulting in 48D initial input features. For detailed procedures, please refer to the Supplementary Material. To represent the interactions between genes, we use protein–protein interaction (PPI) data obtained from the CPDB (Kamburov et al. 2011) database and encode the relationships between proteins as gene–gene interactions, forming a foundational homogenous network. We only retain edges in the PPI network where the likelihood of the relationship is greater than 0.5 as the basis for gene–gene relationships. To better leverage rich prior biological knowledge and increase the model’s interpretability, we introduce an heterogeneous network by incorporating various multi-omics data related to cancer from the MSigDB (Liberzon et al. 2015) database as nodes, in addition to gene nodes. The specific types and quantities of nodes we add are listed in Table 1. We consider the relationships between these newly added nodes and genes, treating them as edges within the heterogeneous network. The number and types of edges in the network are presented in Table 2. Please refer to the diagram of the heterogeneous network structure we have provided in Supplementary Fig. S7. Our research utilize the latest version of the NCG database (NCG7.0) (Dressler et al. 2022) to define positive samples as cancer driver genes, including candidate oncogenes. We obtain a collection of potential cancer driver genes from the set of genes supported by literature or research evidence of the CancerMine database (Lever et al. 2019). The remaining genes after removing CancerMineDB collection are considered as negative samples. This process results in a dataset with 2226 positive samples and 4240 negative samples. Our method does not employ the approach of generating negative sample genes by excluding the direct neighbors of known cancer driver genes in the network. Instead, we use data mining to avoid introducing additional label information that could impact model performance. We filter negative samples using existing research on cancer driver genes, selecting genes as negative samples that have no research supporting their classification as potential cancer driver genes. Given that the sample labels represent approximately half of the entire gene pool, the model training process falls under the category of semi-supervised learning.
| Nodes types . | No. of entities . |
|---|---|
| Gene | 12 944 |
| Positional gene sets | 293 |
| Pathways | 3089 |
| MicroRNA | 3708 |
| Computational gene sets | 858 |
| GO | 10 525 |
| HPO | 5404 |
| Oncogenic signature | 189 |
| Cell type signature | 829 |
| Nodes types . | No. of entities . |
|---|---|
| Gene | 12 944 |
| Positional gene sets | 293 |
| Pathways | 3089 |
| MicroRNA | 3708 |
| Computational gene sets | 858 |
| GO | 10 525 |
| HPO | 5404 |
| Oncogenic signature | 189 |
| Cell type signature | 829 |
| Nodes types . | No. of entities . |
|---|---|
| Gene | 12 944 |
| Positional gene sets | 293 |
| Pathways | 3089 |
| MicroRNA | 3708 |
| Computational gene sets | 858 |
| GO | 10 525 |
| HPO | 5404 |
| Oncogenic signature | 189 |
| Cell type signature | 829 |
| Nodes types . | No. of entities . |
|---|---|
| Gene | 12 944 |
| Positional gene sets | 293 |
| Pathways | 3089 |
| MicroRNA | 3708 |
| Computational gene sets | 858 |
| GO | 10 525 |
| HPO | 5404 |
| Oncogenic signature | 189 |
| Cell type signature | 829 |
| Type . | No. of entities . |
|---|---|
| Gene–gene | 314 032 |
| Gene–positional gene sets | 12 910 |
| Gene-pathways | 134 089 |
| Gene-microRNA | 596 322 |
| Gene-computational gene sets | 78 744 |
| Gene-GO | 701 315 |
| Gene-HPO | 385 926 |
| Gene-oncogenic signature | 24 034 |
| Gene-cell type signature | 112 393 |
| Type . | No. of entities . |
|---|---|
| Gene–gene | 314 032 |
| Gene–positional gene sets | 12 910 |
| Gene-pathways | 134 089 |
| Gene-microRNA | 596 322 |
| Gene-computational gene sets | 78 744 |
| Gene-GO | 701 315 |
| Gene-HPO | 385 926 |
| Gene-oncogenic signature | 24 034 |
| Gene-cell type signature | 112 393 |
| Type . | No. of entities . |
|---|---|
| Gene–gene | 314 032 |
| Gene–positional gene sets | 12 910 |
| Gene-pathways | 134 089 |
| Gene-microRNA | 596 322 |
| Gene-computational gene sets | 78 744 |
| Gene-GO | 701 315 |
| Gene-HPO | 385 926 |
| Gene-oncogenic signature | 24 034 |
| Gene-cell type signature | 112 393 |
| Type . | No. of entities . |
|---|---|
| Gene–gene | 314 032 |
| Gene–positional gene sets | 12 910 |
| Gene-pathways | 134 089 |
| Gene-microRNA | 596 322 |
| Gene-computational gene sets | 78 744 |
| Gene-GO | 701 315 |
| Gene-HPO | 385 926 |
| Gene-oncogenic signature | 24 034 |
| Gene-cell type signature | 112 393 |
2.2 Overview of MCDHGN
MCDHGN (Cancer Driver Gene Prediction using Multi-omics Heterogeneous Network and Meta-path Aggregation) consists of four main components: multi-omics heterogeneous network construction and manual selection of meta-paths; intra-meta-path information aggregation; inter-meta-path semantic weight aggregation; and the cancer prediction module based on a multi-layer linear classifier. The overall flowchart is illustrated in Fig. 1. Specifically, our model is an end-to-end architecture, starting with the input of nodes from the multi-omics heterogeneous network and the initial multi-omics features of each gene. The intra-meta-path information aggregation part resembles multiple parallel graph attention networks (GATs). It aims to learn the contribution weights of specific neighboring nodes within a certain meta-path for each gene node. On the other hand, the inter-meta-path semantic weight aggregation part employs a global attention mechanism to output the contributions of various meta-paths to the final prediction result. Finally, a multi-layer linear perceptron outputs a 2D vector representing the classification result. The model’s output is logarithmized and normalized, and the cross-entropy loss is then used to calculate the loss. Additionally, in the case analysis, the weights of the two parts in the meta-path aggregation module are output to explore the model’s interpretability.
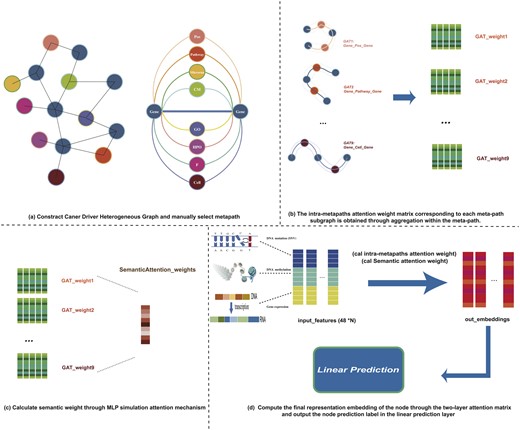
The framework of the MCDHGN model consists of four main components: First, we build a heterogeneous network of cancer driver genes and manually select meta-paths. Next, the model employs an attention mechanism to represent gene nodes through their neighboring nodes within specific meta-paths. Then, it aggregates the representations obtained from different meta-paths using attention weights. Finally, a linear classifier is used to complete the end-to-end output of the model’s predictions for cancer driver genes.
2.3 Meta-path selection and meta-path-based MFGs extraction
We provide a basic definition of a heterogeneous network, where a HG is defined as a graph . For each node and each edge , they are connected through mapping relationships and , where A and R, respectively, represent all types of nodes and edges. We consider that the network pattern of graph G can be defined as , which can be understood as the graph defined on node types A with connections derived from R. A meta-path is based on the network pattern S and is represented as , which can be abbreviated as , where the node types . The types of edges . We establish a heterogeneous network that includes genes and multi-omics biological entities. When two gene nodes are connected through a certain biological entity, such as being associated with a specific biological process described as Pathway1, we create the meta-path -Pathway1-. During the process of information transmission, the information passed to includes not only the characteristics of but also captures the semantics of Pathway1. Thus, during interpretability analysis, we can use the semantics and biological interpretation of Pathway1, along with the biological functions of , to conduct an interpretable analysis of the impact this meta-path has on . For specific details on manually selecting meta-paths, please refer to the Supplementary Material. In the given meta-path , we perform random walk sampling with limited steps based on neighbors along the meta-path to obtain a random walk sampling. During the process of random walks, the sampler selects the next node type strictly according to the meta-path specified. Starting from a particular node and following a meta-path of length l, we select l − 1 times to identify the sampling neighbors of the specific node based on this meta-path. The new graph of node relationships obtained by sampling a specific meta-path is referred to as the message flowing graph (MFG) of that meta-path. In this information transfer subgraph, the linkage between the initiating node and the destination node is determined by the intermediate nodes in the meta-path.
2.4 Internal message aggregation
To enhance the expressive and generalization capabilities of the model, we use a multi-head attention mechanism to enhance the information content and expressiveness of intermediate vectors. Specifically, we perform the previous process many times to get …. The results of the multi-head attention computations are directly concatenated. In the subsequent hyperparameter selection experiments, we will discuss the number of attention heads used for the intra-path aggregation process. The individual outputs of each meta-path for a single node will be concatenated to obtain the node’s representation vector. Finally, by concatenating the representation vectors of all nodes together, we obtain a final output matrix of dimensions called .
2.5 Global semantic attention computation
3. Results
3.1 Experiment
We have selected eight baseline methods based on heterogeneous and homogeneous networks, which include both advanced network approaches in this field and both classic and advanced heterogeneous network methods. For specific details, please refer to the Supplementary Material. To facilitate comparison with state-of-the-art models, besides evaluating on the dataset built in this article, we also conducted experiments on the labeled dataset generated by the EMOGI method. We get the comparison of MCDHGN’s ability to classify cancer driver genes with eight baseline methods on a training set of two datasets. Each method uses the corresponding model constructed in the respective homogeneous or heterogeneous graph for experimentation. The evaluation metrics used are AUC (area under the ROC curve) and AUPR (area under the precision–recall curve). For parameter sensitivity analyses and operating environments, see Supplementary Fig. S2, and the section “Evaluation index and parameter sensitivity analysis” in Supplementary Material.
From Table 3, it can be observed that our model performs optimally in all evaluation metrics. The smaller number of positive samples in the EMOGI dataset, without considering NCG’s candidate cancer genes, strengthens the correlation between positive samples, making the feature patterns more evident. Additionally, during the generation of negative samples in the EMOGI dataset, the potential negative sample genes directly connected to known cancer driver genes in the PPI network are removed, making the feature patterns of negative samples more apparent. In contrast, our dataset considers a broader range of genes, and the generation of positive and negative samples relies solely on literature data mining without incorporating additional prior knowledge. This leads to better performance in predicting potential cancer driver genes. Moreover, it can be observed that heterogeneous network models overall outperform homogeneous network models, and heterogeneous network models provide more significant room for interpretability. These observations collectively demonstrate the superior performance of our proposed MCDHGN model in predicting cancer driver genes, and the benefits of using heterogeneous networks for enhanced performance and interpretability. Figures 2 and 3 demonstrate the prediction performance of MCDHGN with the new labeled dataset, and Supplementary Fig. S3 shows its performance on the emogi dataset, it can be noticed that MCDHGN demonstrates better performance than the baseline method on the test set of both two datasets, showing strong generalization capabilities. The model’s ability to generalize well to unseen data is an important aspect of its effectiveness and reliability. Furthermore, the semantic weighting module’s output of the nine meta-paths’ weight proportions, which represent their contributions to the final prediction, is shown Supplementary Fig. 4. The figure illustrates significant differences in the contributions of different meta-paths. The MCDHGN model effectively identifies crucial semantic modules, allowing us to pinpoint relevant biological justifications more easily. The ability to identify and emphasize important semantic modules provides the MCDHGN model with an advantage in capturing biologically relevant information and contributing to its superior predictive performance.
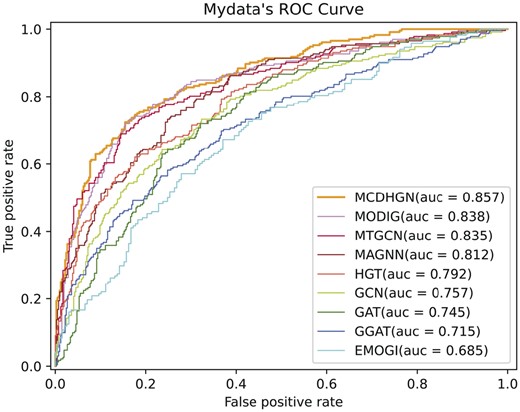
The performance of each method on our label dataset in terms of ROC curves.
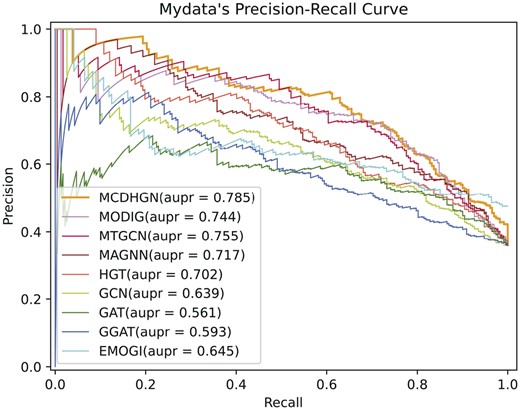
The performance of each method on our labeled dataset in terms of precision–recall (P–R) curves.
| Methods . | Mydata AUC . | Mydata AUPR . | EMOGI data AUC . | EMOGI data AUPR . |
|---|---|---|---|---|
| GCN (Kipf and Welling 2016) | 0.7744 ± 0.0129 | 0.6842 ± 0.0090 | 0.8455 ± 0.0128 | 0.7179 ± 0.0290 |
| GAT (Veličković et al. 2017) | 0.7578 ± 0.0244 | 0.6355 ± 0.0359 | 0.8441 ± 0.0170 | 0.7004 ± 0.0280 |
| GGAT (Qiu et al. 2021) | 0.7264 ± 0.0149 | 0.6213 ± 0.0190 | 0.7678 ± 0.0130 | 0.6213 ± 0.0343 |
| HGT (Hu et al. 2020) | 0.8262 ± 0.0064 | 0.7421 ± 0.0191 | 0.8725 ± 0.0089 | 0.7565 ± 0.0204 |
| EMOGI (Schulte-Sasse et al. 2021) | 0.6680 ± 0.0171 | 0.6883 ± 0.0151 | ||
| MTGCN (Peng et al. 2022) | 0.8384 ± 0.0136 | 0.7750 ± 0.0112 | 0.8935 ± 0.0200 | 0.8112 ± 0.0293 |
| MODIG (Zhao et al. 2022) | 0.8152 ± 0.0109 | 0.7421 ± 0.0117 | 0.8977 ± 0.0147 | 0.7922 ± 0.0124 |
| MAGNN (Fu et al. 2020) | 0.8272 ± 0.0084 | 0.7040 ± 0.0130 | 0.8902 ± 0.0109 | 0.7892 ± 0.0252 |
| MCDHGN (our method) | 0.8716 ± 0.0111 | 0.8025 ± 0.0111 | 0.9066 ± 0.0170 | 0.8265 ± 0.0170 |
| Methods . | Mydata AUC . | Mydata AUPR . | EMOGI data AUC . | EMOGI data AUPR . |
|---|---|---|---|---|
| GCN (Kipf and Welling 2016) | 0.7744 ± 0.0129 | 0.6842 ± 0.0090 | 0.8455 ± 0.0128 | 0.7179 ± 0.0290 |
| GAT (Veličković et al. 2017) | 0.7578 ± 0.0244 | 0.6355 ± 0.0359 | 0.8441 ± 0.0170 | 0.7004 ± 0.0280 |
| GGAT (Qiu et al. 2021) | 0.7264 ± 0.0149 | 0.6213 ± 0.0190 | 0.7678 ± 0.0130 | 0.6213 ± 0.0343 |
| HGT (Hu et al. 2020) | 0.8262 ± 0.0064 | 0.7421 ± 0.0191 | 0.8725 ± 0.0089 | 0.7565 ± 0.0204 |
| EMOGI (Schulte-Sasse et al. 2021) | 0.6680 ± 0.0171 | 0.6883 ± 0.0151 | ||
| MTGCN (Peng et al. 2022) | 0.8384 ± 0.0136 | 0.7750 ± 0.0112 | 0.8935 ± 0.0200 | 0.8112 ± 0.0293 |
| MODIG (Zhao et al. 2022) | 0.8152 ± 0.0109 | 0.7421 ± 0.0117 | 0.8977 ± 0.0147 | 0.7922 ± 0.0124 |
| MAGNN (Fu et al. 2020) | 0.8272 ± 0.0084 | 0.7040 ± 0.0130 | 0.8902 ± 0.0109 | 0.7892 ± 0.0252 |
| MCDHGN (our method) | 0.8716 ± 0.0111 | 0.8025 ± 0.0111 | 0.9066 ± 0.0170 | 0.8265 ± 0.0170 |
Bold value means the highest value in the column.
| Methods . | Mydata AUC . | Mydata AUPR . | EMOGI data AUC . | EMOGI data AUPR . |
|---|---|---|---|---|
| GCN (Kipf and Welling 2016) | 0.7744 ± 0.0129 | 0.6842 ± 0.0090 | 0.8455 ± 0.0128 | 0.7179 ± 0.0290 |
| GAT (Veličković et al. 2017) | 0.7578 ± 0.0244 | 0.6355 ± 0.0359 | 0.8441 ± 0.0170 | 0.7004 ± 0.0280 |
| GGAT (Qiu et al. 2021) | 0.7264 ± 0.0149 | 0.6213 ± 0.0190 | 0.7678 ± 0.0130 | 0.6213 ± 0.0343 |
| HGT (Hu et al. 2020) | 0.8262 ± 0.0064 | 0.7421 ± 0.0191 | 0.8725 ± 0.0089 | 0.7565 ± 0.0204 |
| EMOGI (Schulte-Sasse et al. 2021) | 0.6680 ± 0.0171 | 0.6883 ± 0.0151 | ||
| MTGCN (Peng et al. 2022) | 0.8384 ± 0.0136 | 0.7750 ± 0.0112 | 0.8935 ± 0.0200 | 0.8112 ± 0.0293 |
| MODIG (Zhao et al. 2022) | 0.8152 ± 0.0109 | 0.7421 ± 0.0117 | 0.8977 ± 0.0147 | 0.7922 ± 0.0124 |
| MAGNN (Fu et al. 2020) | 0.8272 ± 0.0084 | 0.7040 ± 0.0130 | 0.8902 ± 0.0109 | 0.7892 ± 0.0252 |
| MCDHGN (our method) | 0.8716 ± 0.0111 | 0.8025 ± 0.0111 | 0.9066 ± 0.0170 | 0.8265 ± 0.0170 |
| Methods . | Mydata AUC . | Mydata AUPR . | EMOGI data AUC . | EMOGI data AUPR . |
|---|---|---|---|---|
| GCN (Kipf and Welling 2016) | 0.7744 ± 0.0129 | 0.6842 ± 0.0090 | 0.8455 ± 0.0128 | 0.7179 ± 0.0290 |
| GAT (Veličković et al. 2017) | 0.7578 ± 0.0244 | 0.6355 ± 0.0359 | 0.8441 ± 0.0170 | 0.7004 ± 0.0280 |
| GGAT (Qiu et al. 2021) | 0.7264 ± 0.0149 | 0.6213 ± 0.0190 | 0.7678 ± 0.0130 | 0.6213 ± 0.0343 |
| HGT (Hu et al. 2020) | 0.8262 ± 0.0064 | 0.7421 ± 0.0191 | 0.8725 ± 0.0089 | 0.7565 ± 0.0204 |
| EMOGI (Schulte-Sasse et al. 2021) | 0.6680 ± 0.0171 | 0.6883 ± 0.0151 | ||
| MTGCN (Peng et al. 2022) | 0.8384 ± 0.0136 | 0.7750 ± 0.0112 | 0.8935 ± 0.0200 | 0.8112 ± 0.0293 |
| MODIG (Zhao et al. 2022) | 0.8152 ± 0.0109 | 0.7421 ± 0.0117 | 0.8977 ± 0.0147 | 0.7922 ± 0.0124 |
| MAGNN (Fu et al. 2020) | 0.8272 ± 0.0084 | 0.7040 ± 0.0130 | 0.8902 ± 0.0109 | 0.7892 ± 0.0252 |
| MCDHGN (our method) | 0.8716 ± 0.0111 | 0.8025 ± 0.0111 | 0.9066 ± 0.0170 | 0.8265 ± 0.0170 |
Bold value means the highest value in the column.
3.2 Ablation experiment
Study of ablation experiments was done using masking or changing part of the model architecture or input vectors. Firstly, the semantic aggregation module was replaced with a simple averaging module, removing the influence of the semantic module. The results of the nine meta-paths were summed using a simple average and denoted as MCDHGNinterf. Next, the attention mechanism within each meta-path was removed, and a simple Graphconv module was used instead of GAT for intra-path aggregation, denoted as MCDHGNintraf. Based on the previous analysis, it was found that four meta-paths contributed more than 10% to the results. Therefore, these four significantly important meta-paths were separately used to construct a heterogeneous network to explore their impact on the model’s performance, referred to as . Finally, the influence of the initial multi-omics input on the results was considered. Random vectors of the same dimension (48 dimensions) were used as new inputs to the model after normalization. This experiment was denoted as MCDHGNrandomfeat. The final experimental results are presented in Table 4. The experimental outcomes reveal that diminishing or substituting various modules within the model results in a reduction of AUC and AUPR values. Among the ablation experiments, the most significant impact on the results comes from removing the attention mechanism within each meta-path. This indicates that identifying important neighboring nodes in the meta-path message-passing subgraph is crucial for predicting cancer driver genes. Additionally, replacing the initial features with random vectors also results in a noticeable performance drop. This information directly reflects the abnormal expression and mutation phenomena in cancerous tissues. Given the complexity of the heterogeneous semantics used in our model, enhancing the relevance of the initial information to the prediction task can significantly improve the model’s generalization ability. This enhancement enables the node features to possess stronger representational power, aiding the model in better capturing the characteristics and patterns associated with cancer driver genes. Regarding the changes in semantic weights between meta-paths and using only the top four meta-paths with the highest weights, the impact on performance is not as evident as observed with the two previous factors (removing intra-path attention and replacing initial features). However, preserving more meta-paths and the semantic-level attention contributes to enhancing the model’s interpretability.
| Methods . | Five folds AUC . | Five folds AUPR . |
|---|---|---|
| MCDHGN | 0.8716 ± 0.0111 | 0.8025 ± 0.0111 |
| 0.8591 ± 0.0071 | 0.7768 ± 0.0155 | |
| 0.8109 ± 0.0046 | 0.6906 ± 0.0124 | |
| 0.8521 ± 0.0296 | 0.7642 ± 0.0540 | |
| 0.8121 ± 0.0072 | 0.7208 ± 0.0194 |
| Methods . | Five folds AUC . | Five folds AUPR . |
|---|---|---|
| MCDHGN | 0.8716 ± 0.0111 | 0.8025 ± 0.0111 |
| 0.8591 ± 0.0071 | 0.7768 ± 0.0155 | |
| 0.8109 ± 0.0046 | 0.6906 ± 0.0124 | |
| 0.8521 ± 0.0296 | 0.7642 ± 0.0540 | |
| 0.8121 ± 0.0072 | 0.7208 ± 0.0194 |
| Methods . | Five folds AUC . | Five folds AUPR . |
|---|---|---|
| MCDHGN | 0.8716 ± 0.0111 | 0.8025 ± 0.0111 |
| 0.8591 ± 0.0071 | 0.7768 ± 0.0155 | |
| 0.8109 ± 0.0046 | 0.6906 ± 0.0124 | |
| 0.8521 ± 0.0296 | 0.7642 ± 0.0540 | |
| 0.8121 ± 0.0072 | 0.7208 ± 0.0194 |
| Methods . | Five folds AUC . | Five folds AUPR . |
|---|---|---|
| MCDHGN | 0.8716 ± 0.0111 | 0.8025 ± 0.0111 |
| 0.8591 ± 0.0071 | 0.7768 ± 0.0155 | |
| 0.8109 ± 0.0046 | 0.6906 ± 0.0124 | |
| 0.8521 ± 0.0296 | 0.7642 ± 0.0540 | |
| 0.8121 ± 0.0072 | 0.7208 ± 0.0194 |
3.3 Results
In the cancer driver gene prediction phase, we used the entire training dataset for training and then outputted the prediction scores for all gene nodes. We considered samples with scores greater than 0.8 as potential cancer driver gene predictions, resulting in 602 suspected cancer driver genes (as shown in Fig. 4). Analysing the 602 suspected cancer driver genes, we found that 323 genes were already present in the positive sample set, specifically in the NCG7.0 database. Among them, 156 genes were known cancer driver genes (KCGs), and 160 genes were candidate cancer driver genes (CGs) according to NCG set. Then, we continued our analysis on the remaining 254 genes. Among these, 48 genes were mentioned in the Oncokb database (Chakravarty et al. 2017), Ongene database, and a comprehensive cancer analysis paper from TCGA (Ding et al. 2018), indicating some evidence of their involvement in cancer. Using the Cancer Miner data mining tool, they further explored potential cancer genes. Cancer Miner identified 153 genes as high-confidence cancer driver genes and 36 unfiltered genes were mined for which some studies have shown that these genes are associated with cancer but no direct evidence. In summary, 91.86% of cancer driver genes classified by MCDHGN have at least one paper or database evidence mentioning them as cancer driver genes or cancer-related genes. The remaining 49 genes (8.14%) are predicted as novel cancer driver genes that require further validation since there is currently no direct research evidence supporting their role in cancer.
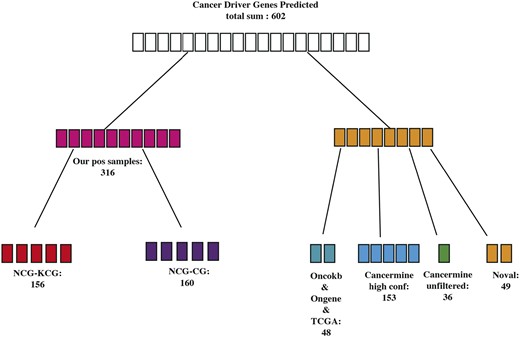
3.4 Case study
We will illustrate how MCDHGN demonstrates biological interpretability in predicting cancer driver genes by presenting case studies on the SETD2 and ZBTB17 genes.
From Table 5, it is evident that the non-positive sample gene ZBTB17 is most strongly associated with the cancer driver gene PTEN. Both ZBTB17 and PTEN are part of the WP_SMALL_CELL_LUNG_CANCER pathway, indicating their relevance to small cell lung cancer. The PTEN protein primarily functions by regulating the PI3K/Akt signaling pathway. When PTEN function is impaired, excessive activation of the PI3K/Akt signaling pathway may lead to abnormal cell proliferation and survival, promoting tumor development. It can be inferred that ZBTB17 is also involved in the regulation of cell signaling pathways, as research (Ikegaki et al. 2007) suggests its association with tumor occurrence and development. ZBTB17 interacts with the transcription factor Myc, influencing the expression of Myc target genes. Myc is an important cancer-related gene associated with tumor cell proliferation, survival, and metastasis. Both Myc and the PI3K/AKT signaling pathway can jointly promote tumor cell proliferation, survival, and invasion, leading to tumor progression. Hence, it is reasonable to believe that PTEN is a cancer driver gene that interacts or mutually influences ZBTB17 gene. Our model can determine a gene as a cancer driver gene based on the neighbors of specific genes in the heterogeneous network. It can also discuss the functional similarity of potential oncogenes to known oncogenes based on the biological annotations of the intermediate nodes of the meta-paths connecting different genes. By identifying the more influential meta-pathways and their intermediate nodes, the interpretability of the model can be improved. Please refer to the Supplementary Material for the case analysis of the SETD2 gene.
Heterogeneous graph nodes and paths judging the top 10 contributions of ZBTB17 as a cancer driver gene.
| Order . | Genename . | Meta-path type . | Relevant biological explanations . |
|---|---|---|---|
| No. 1 | PTEN | Gene–CM–Gene | The primary function of the PTEN (Álvarez-Garcia et al. 2019) protein involves the regulation of the PI3K/Akt signaling pathway, PTEN and the target gene belong to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 2 | TP53 | Gene–Pathways–Gene | The mutation of TP53 gene is closely related to the occurrence and development of various cancers (Petitjean et al. 2007) |
| No. 3 | PTEN | Gene–Pathways–Gene | Both belongs to Pathway: WP_SMALL_CELL_LUNG_CANCER |
| No. 4 | GRIK5 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 5 | RUNX1 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 6 | PGR | Gene–Gene | In breast cancer, PGR also plays an important role |
| No. 7 | ESR1 | Gene–Gene | The research (Gu and Fuqua 2016) shows ESR1 gene encodes estrogen receptorα which related to the occurrence of breast cancer |
| No. 8 | SIM1 | Gene–F–Gene | Observing the functional compensation phenomenon of RB1 and P130 genes In the experiment, after knocking out the P130 gene, both expression levels increased in the process of cell mitosis. |
| No. 9 | TRIM63 | Gene–Gene | (Peris-Moreno et al. 2020) shows TRIM63 plays an important regulatory role in the process of muscle atrophy |
| No. 10 | PAX9 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| Order . | Genename . | Meta-path type . | Relevant biological explanations . |
|---|---|---|---|
| No. 1 | PTEN | Gene–CM–Gene | The primary function of the PTEN (Álvarez-Garcia et al. 2019) protein involves the regulation of the PI3K/Akt signaling pathway, PTEN and the target gene belong to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 2 | TP53 | Gene–Pathways–Gene | The mutation of TP53 gene is closely related to the occurrence and development of various cancers (Petitjean et al. 2007) |
| No. 3 | PTEN | Gene–Pathways–Gene | Both belongs to Pathway: WP_SMALL_CELL_LUNG_CANCER |
| No. 4 | GRIK5 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 5 | RUNX1 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 6 | PGR | Gene–Gene | In breast cancer, PGR also plays an important role |
| No. 7 | ESR1 | Gene–Gene | The research (Gu and Fuqua 2016) shows ESR1 gene encodes estrogen receptorα which related to the occurrence of breast cancer |
| No. 8 | SIM1 | Gene–F–Gene | Observing the functional compensation phenomenon of RB1 and P130 genes In the experiment, after knocking out the P130 gene, both expression levels increased in the process of cell mitosis. |
| No. 9 | TRIM63 | Gene–Gene | (Peris-Moreno et al. 2020) shows TRIM63 plays an important regulatory role in the process of muscle atrophy |
| No. 10 | PAX9 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
Heterogeneous graph nodes and paths judging the top 10 contributions of ZBTB17 as a cancer driver gene.
| Order . | Genename . | Meta-path type . | Relevant biological explanations . |
|---|---|---|---|
| No. 1 | PTEN | Gene–CM–Gene | The primary function of the PTEN (Álvarez-Garcia et al. 2019) protein involves the regulation of the PI3K/Akt signaling pathway, PTEN and the target gene belong to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 2 | TP53 | Gene–Pathways–Gene | The mutation of TP53 gene is closely related to the occurrence and development of various cancers (Petitjean et al. 2007) |
| No. 3 | PTEN | Gene–Pathways–Gene | Both belongs to Pathway: WP_SMALL_CELL_LUNG_CANCER |
| No. 4 | GRIK5 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 5 | RUNX1 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 6 | PGR | Gene–Gene | In breast cancer, PGR also plays an important role |
| No. 7 | ESR1 | Gene–Gene | The research (Gu and Fuqua 2016) shows ESR1 gene encodes estrogen receptorα which related to the occurrence of breast cancer |
| No. 8 | SIM1 | Gene–F–Gene | Observing the functional compensation phenomenon of RB1 and P130 genes In the experiment, after knocking out the P130 gene, both expression levels increased in the process of cell mitosis. |
| No. 9 | TRIM63 | Gene–Gene | (Peris-Moreno et al. 2020) shows TRIM63 plays an important regulatory role in the process of muscle atrophy |
| No. 10 | PAX9 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| Order . | Genename . | Meta-path type . | Relevant biological explanations . |
|---|---|---|---|
| No. 1 | PTEN | Gene–CM–Gene | The primary function of the PTEN (Álvarez-Garcia et al. 2019) protein involves the regulation of the PI3K/Akt signaling pathway, PTEN and the target gene belong to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 2 | TP53 | Gene–Pathways–Gene | The mutation of TP53 gene is closely related to the occurrence and development of various cancers (Petitjean et al. 2007) |
| No. 3 | PTEN | Gene–Pathways–Gene | Both belongs to Pathway: WP_SMALL_CELL_LUNG_CANCER |
| No. 4 | GRIK5 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 5 | RUNX1 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
| No. 6 | PGR | Gene–Gene | In breast cancer, PGR also plays an important role |
| No. 7 | ESR1 | Gene–Gene | The research (Gu and Fuqua 2016) shows ESR1 gene encodes estrogen receptorα which related to the occurrence of breast cancer |
| No. 8 | SIM1 | Gene–F–Gene | Observing the functional compensation phenomenon of RB1 and P130 genes In the experiment, after knocking out the P130 gene, both expression levels increased in the process of cell mitosis. |
| No. 9 | TRIM63 | Gene–Gene | (Peris-Moreno et al. 2020) shows TRIM63 plays an important regulatory role in the process of muscle atrophy |
| No. 10 | PAX9 | Gene–CM–Gene | Both belongs to CM1: MORF_MT4 CM2: MORF_PSMF1 |
4. Conclusion
In this study, we introduce a cancer driver gene prediction model called MCDHGN, which is based on heterogeneous network meta-path analysis. We employ random walks to generate informative subgraphs for each meta-path and aggregate information within and between meta-paths to produce the final gene representation vectors. We compare MCDHGN against state-of-the-art methods on two cancer driver gene label datasets and achieve superior performance. Subsequently, we use the output from MCDHGN for predicting cancer driver genes and attempt to provide some interpretable analysis of our prediction results. In our subsequent work, we plan to enhance the network’s expressive capabilities by using methods that augment the semi-supervised label dataset. We also consider incorporating the model’s topological features within the network to strengthen the initial multi-omics features. Once the model’s representational and generalization capabilities are well-developed, we will initiate efforts toward predicting specific types of cancer driver genes with small positive sample labels.
Supplementary data
Supplementary data are available at Bioinformatics online.
Conflict of interest
None declared.
Funding
This work was supported by the grants from the National Key R&D Program of China [2021YFA0910700], Shenzhen Science and Technology University Stable Support Program [GXWD20201230155427003-20200821222112001], Shenzhen Science and Technology Program [JCYJ20200109113201726], Guangdong Basic and Applied Basic Research Foundation [2021A1515012461 and 2021A1515220115], Guangdong Provincial Key Laboratory of Novel Security Intelligence Technologies [2022B1212010005].



