-
PDF
- Split View
-
Views
-
Cite
Cite
Simon G. Coetzee, Gerhard A. Coetzee, Dennis J. Hazelett, motifbreakR: an R/Bioconductor package for predicting variant effects at transcription factor binding sites, Bioinformatics, Volume 31, Issue 23, December 2015, Pages 3847–3849, https://doi.org/10.1093/bioinformatics/btv470
Close - Share Icon Share
Abstract
Summary: Functional annotation represents a key step toward the understanding and interpretation of germline and somatic variation as revealed by genome-wide association studies (GWAS) and The Cancer Genome Atlas (TCGA), respectively. GWAS have revealed numerous genetic risk variants residing in non-coding DNA associated with complex diseases. For sequences that lie within enhancers or promoters of transcription, it is not straightforward to assess the effects of variants on likely transcription factor binding sites. Consequently we introduce motifbreakR, which allows the biologist to judge whether the sequence surrounding a polymorphism or mutation is a good match, and how much information is gained or lost in one allele of the polymorphism or mutation relative to the other. MotifbreakR is flexible, giving a choice of algorithms for interrogation of genomes with motifs from many public sources that users can choose from. MotifbreakR can predict effects for novel or previously described variants in public databases, making it suitable for tasks beyond the scope of its original design. Lastly, it can be used to interrogate any genome curated within bioconductor.
Availability and implementation: https://github.com/Simon-Coetzee/MotifBreakR, www.bioconductor.org.
Contact: [email protected]
1 Introduction
Transcription factor binding sites (TFBS) are typically short DNA sequence motifs that facilitate binding of a specific transcription factors via protein–DNA interactions (Stormo, 2000). There are some software tools that facilitate the scoring of non-coding variants with respect to either predefined or user-specified motifs. RegulomeDB, HaploReg and FunSeq each enable assessment of the effects of single nucleotide variants on predicted binding sites (Boyle etal., 2012; Khurana et al., 2013; Ward and Kellis, 2012). Each of these packages has strengths, but does not provide the analysis independent of its other functions. Many users generate their own motifs, but users are limited to built-in motif collections and functions. The functions are largely unavailable to non-human data sets. We hereby introduce an R/bioconductor software package called motifbreakR that addresses these major concerns. Implementation in R has the advantage of universality: R and bioconductor are widely used for bioinformatics and well supported across different platforms including Galaxy (Tenenbaum, 2015).
2 Features
2.1 Germline or somatic variants
Single nucleotide polymorphisms (SNPs) can be generated from another R package such as FunciSNP (Coetzee etal., 2012), or read in directly from .bed or .vcf files. The SNPs then need to be converted to GRanges objects. This is accomplished within motifbreakR in one of two ways, via the snps.from.rsid and snps.from.file functions. MotifbreakR will produce a GRanges table with match statistics describing the percent of maximum score for a matrix for both alleles of the SNP, the matrix values for each allele (useful for determining the severity of the disruption), the strand, whether the disruption is strong or weak. The package also includes a plotting function based on the gviz and motifstack packages as shown for SNP rs10486567 in Figure 1.
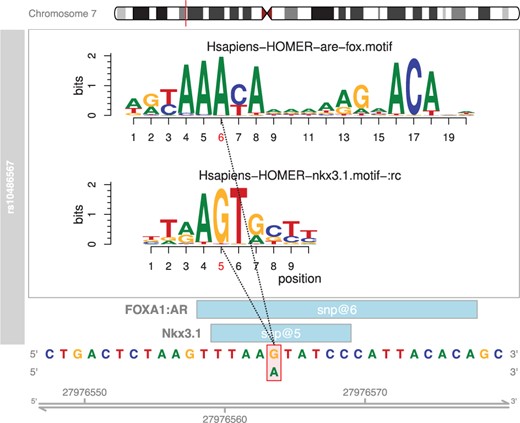
Example of motifbreakR output from plotMB function for a previously published SNP (Hazelett et al., 2014). Genomic sequence and coordinates are at the bottom of the display; the positions of the matches represented (light blue boxes). The position of the SNP within the motif is indicated with red bounding box and alternate allele below, and as red text on the motif logo position bar above. The motif logos generated from motifstack are shown above using the color conventions of the genomic sequence below
2.2 Comprehensive positional weight matrices
Once a SNP list is converted to GRanges, selection of PWMs proceeds by subsetting from the MotifDb package or from one of the included motifList-compatible libraries. Any motifList object can be passed to motifbreakR.MotifDb provides access to ScerTF (Spivak and Stormo, 2012), FlyFactorSurvey (Zhu etal., 2011), hPDI (Xie etal., 2010), all the selex-generated motifs from (Jolma etal., 2013), UniPROBE (Newburger and Bulyk, 2009) and JASPAR (Stormo, 2000). In addition to these, we have included motifs from Encode (Kheradpour and Kellis, 2014), Homer (Heinz etal., 2010), Factorbook (Wang etal., 2012) and HOCOMOCO (Kulakovskiy etal., 2013). Importantly, MotifbreakR generates pseudocounts based on the number of observations listed for each motif in these databases, but defaults to 20 observations when this information is lacking.
2.3 Choice of algorithms
There are three algorithms accessible via the ‘method’ argument. The first is the standard sum of log probabilities (method = ‘log’). The second and third are the weighted sum and information content methods (method = ‘default’ and method = ‘ic’). The default was previously described (Hazelett etal., 2014); the ic method is based on relative entropy but renders very similar conclusions. The methods are extensively documented in the vignette. For all three methods, motifbreakR scores and reports the reference and alternate alleles of the sequence, and the effect (strong, weak or neutral). The match scores are scaled as a fraction of scoring range [0,1] of the motif matrix.
2.4 Extensible to model organisms
It is straightforward within the motifbreakR package to specify any of the research model organism genomes in BSgenome (currently 22 in number), including mouse, zebrafish, and fruit fly. This may be done both at the level of reading in SNP lists and during invocations of motifbreakR. To our knowledge, no other software currently offers this functionality.
3 Conclusion
In principle, a SNP label contains all the information necessary to characterize a variant or mutation, since it points to information in external databases somewhere. Our package makes it possible to rapidly explore TFBS disruptions for a large number of SNPs within the R framework, with no need to install third party software or massage arcane output files for downstream analysis. Although the intention is to study the relationship of human variation to disease, use of motifbreakR is not limited to this application. Indeed, one may access any genome in BSgenome, and query it with custom SNPs and PWMs, or specify organism-specific sets of PWMs from MotifDb.motifbreakR uses a highly efficient information content-based algorithm for discriminating between truly disruptive variants versus neutral. Because motifbreakR is designed to work with the existing bioconductor framework, we believe it to be the most flexible and extensible package available for this type of analysis.
Acknowledgements
The authors acknowledge Benjamin Berman and Huy Q. Dinh for editing and discussions.
Funding
This work was supported by the National Institutes of Health [grant numbers R01CA136924, R01CA190182 to G.A.C.] and National Cancer Institute [grant number UO1CA184826] supporting [D.J.H. and S.G.C.]. Funding for open access charge: [National Cancer Institute UO1CA184826].
Conflict of interest: none declared.
References
Author notes
Associate Editor: John Hancock



