-
PDF
- Split View
-
Views
-
Cite
Cite
Miguel Juliá, Amalio Telenti, Antonio Rausell, Sincell: an R/Bioconductor package for statistical assessment of cell-state hierarchies from single-cell RNA-seq, Bioinformatics, Volume 31, Issue 20, October 2015, Pages 3380–3382, https://doi.org/10.1093/bioinformatics/btv368
Close - Share Icon Share
Abstract
Summary: Cell differentiation processes are achieved through a continuum of hierarchical intermediate cell states that might be captured by single-cell RNA seq. Existing computational approaches for the assessment of cell-state hierarchies from single-cell data can be formalized under a general framework composed of (i) a metric to assess cell-to-cell similarities (with or without a dimensionality reduction step) and (ii) a graph-building algorithm (optionally making use of a cell clustering step). The Sincell R package implements a methodological toolbox allowing flexible workflows under such a framework. Furthermore, Sincell contributes new algorithms to provide cell-state hierarchies with statistical support while accounting for stochastic factors in single-cell RNA seq. Graphical representations and functional association tests are provided to interpret hierarchies. The functionalities of Sincell are illustrated in a real case study, which demonstrates its ability to discriminate noisy from stable cell-state hierarchies.
Availability and implementation: Sincell is an open-source R/Bioconductor package available at http://bioconductor.org/packages/sincell. A detailed manual and a vignette are provided with the package.
Contact: [email protected]
Supplementary information: Supplementary data are available at Bioinformatics online.
1 Introduction
Unbiased profiling of individual cells through single-cell RNA-seq allows assessing heterogeneity of transcriptional states within a cell population (Wu et al., 2014). In the context of a cell population’s differentiation or activation process, such transcriptional heterogeneity might reflect a continuum of intermediate cell states and lineages resulting from dynamic regulatory programs. Such a continuum might be captured through the assessment of cell-state hierarchies, where each cell is placed in a relative ordering in the transcriptional landscape. Additionally, statistical support should be provided to discriminate reliable hierarchies from stochastic heterogeneity, arising from both technical and biological factors.
A number of algorithms have been used to assess cell-state hierarchies from single-cell data (Amir et al., 2013; Bendall et al., 2014; Buettner et al., 2015; Jaitin et al., 2014; Moignard et al., 2015; Qiu et al., 2011; Trapnell et al., 2014). These approaches can be formalized under a general framework (Supplementary Table S1). Here we present Sincell, an R/Bioconductor package where the various building blocks of that general workflow are extended and combined (Fig. 1). Notably, Sincell implements algorithms to provide statistical support to the cell-state hierarchies derived from single-cell RNA-seq. The package is complemented with graphical representations and functional association tests to help interpret the results.
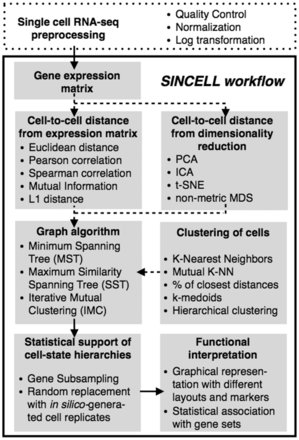
Overall workflow for the statistical assessment of cell-state hierarchies implemented by the Sincell R package. Dashed arrows correspond to optional steps in the analysis
2 Description
As input, Sincell requires an expression matrix with user-defined normalized gene expression levels per single cell (Fig. 1). Variance stabilization is recommended (e.g. through log transformation). First, a cell-to-cell distance matrix is calculated using a metric of choice. Sincell provides both linear and non-linear distances: Euclidean, Mutual Information, L1 distance, Pearson and Spearman correlation. Optionally, the distance matrix may be obtained from the leading dimensions of a data reduction technique, performed to keep the most informative parts of the data while excluding noise. Both linear and non-linear algorithms are provided: Principal Component Analysis, Independent Component Analysis, t-Distributed Stochastic Neighbor Embedding and non-metric Multidimensional Scaling.
Second, a cell-state hierarchy is obtained by applying a graph-building algorithm on the cell-to-cell distance matrix. Graph-building algorithms may consider cells both individually and in clusters of highly similar cells. Sincell provides different clustering methods (K-Mutual Nearest Neighbors, k-medoids, agglomerative clustering, etc.) and graph-building algorithms (MST, SST and IMC; Supplementary Text).
Stochastic technical and biological factors may drive cell-state heterogeneity observed in single-cell RNA seq data. Additionally, hierarchies derived from experiments with a low number of individual cells (e.g. 96 cells when using a Fluidigm C1™ Single-Cell Auto Prep System) are more susceptible to noise artifacts than experiments profiling thousands of individual cells (e.g. flow cytometry data). Sincell implements two algorithms to discriminate reliable hierarchies from noise-driven ones. The first strategy relies on a gene resampling procedure. The second one is based on random cell substitution with in silico-generated cell replicates. These replicates are built by perturbing observed gene expression levels with random noise, following patterns of stochasticity observed in single-cell RNA-seq (Supplementary Text). Either approach generates a population of hierarchies whose similarities to the reference show the distribution of the hierarchy stability against changes in the data.
To help interpret hierarchies in functional terms, Sincell provides graphical representations of cell-to-cell similarities in low-dimensional space as well as different graph displays of hierarchies, coloring cells, e.g. by expression levels of a marker of choice. Furthermore, Sincell implements an algorithm to determine the statistical significance of the association of the hierarchy with the expression levels of a given gene set (Supplementary Text). Gene set collections (e.g. Gene Ontology terms) can be systematically evaluated.
3 Application
The Sincell R package includes a detailed vignette illustrating all functionalities using real single-cell RNA-seq data. We use data from (Trapnell et al., 2014) quantifying gene expression levels in differentiating myoblast at 0, 24, 48 and 72 h. Here, we analyze each time-point separately and evaluate the statistical evidence of cell-state heterogeneity within them (Supplementary Fig. 1). Our results show that early differentiation timepoints produce unstable hierarchies suggesting a low level of cell-state heterogeneity. However, late differentiation timepoints produce statistically significant hierarchies that reflect cell-state diversity along the differentiation process (Supplementary Text).
4 Discussion
The landscape of computational approaches to assess cell-state hierarchies from single-cell data is far from being fully explored. The diversity of biological studies and rapid evolution of single-cell technologies require a comprehensive toolbox where users may easily tailor workflows and compare alternative methods and assumptions. Furthermore, cell-state hierarchies should be statistically supported before being used as input in subsequent analyses. The Sincell R package addresses these needs by providing a general analysis framework, new algorithms for statistical support as well as tools for functional interpretation of cell-state hierarchies.
Funding
This research was supported by the European Union's Seventh Framework Programme FP7/2007-2013/ under grant agreement no. 305762 and the Swiss National Science Foundation grant no. 149724. Computations were performed at the Vital-IT (http://www.vital-it.ch) Center for high-performance computing of the SIB Swiss Institute of Bioinformatics.
Conflict of interest: none declared.
References
Author notes
Associate Editor: Ivo Hofacker



