-
PDF
- Split View
-
Views
-
Cite
Cite
Fan Yang, Shuaijie Zhang, Wei Pan, Ruiyuan Yao, Weiguo Zhang, Yanchun Zhang, Guoyin Wang, Qianghua Zhang, Yunlong Cheng, Jihua Dong, Chunyang Ruan, Lizhen Cui, Hao Wu, Fuzhong Xue, Signaling repurposable drug combinations against COVID-19 by developing the heterogeneous deep herb-graph method, Briefings in Bioinformatics, Volume 23, Issue 5, September 2022, bbac124, https://doi.org/10.1093/bib/bbac124
Close - Share Icon Share
Abstract
Coronavirus disease 2019 (COVID-19) has spurred a boom in uncovering repurposable existing drugs. Drug repurposing is a strategy for identifying new uses for approved or investigational drugs that are outside the scope of the original medical indication.
Current works of drug repurposing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are mostly limited to only focusing on chemical medicines, analysis of single drug targeting single SARS-CoV-2 protein, one-size-fits-all strategy using the same treatment (same drug) for different infected stages of SARS-CoV-2. To dilute these issues, we initially set the research focusing on herbal medicines. We then proposed a heterogeneous graph embedding method to signaled candidate repurposing herbs for each SARS-CoV-2 protein, and employed the variational graph convolutional network approach to recommend the precision herb combinations as the potential candidate treatments against the specific infected stage.
We initially employed the virtual screening method to construct the ‘Herb-Compound’ and ‘Compound-Protein’ docking graph based on 480 herbal medicines, 12,735 associated chemical compounds and 24 SARS-CoV-2 proteins. Sequentially, the ‘Herb-Compound-Protein’ heterogeneous network was constructed by means of the metapath-based embedding approach. We then proposed the heterogeneous-information-network-based graph embedding method to generate the candidate ranking lists of herbs that target structural, nonstructural and accessory SARS-CoV-2 proteins, individually. To obtain precision synthetic effective treatments forvarious COVID-19 infected stages, we employed the variational graph convolutional network method to generate candidate herb combinations as the recommended therapeutic therapies.
There were 24 ranking lists, each containing top-10 herbs, targeting 24 SARS-CoV-2 proteins correspondingly, and 20 herb combinations were generated as the candidate-specific treatment to target the four infected stages. The code and supplementary materials are freely available at https://github.com/fanyang-AI/TCM-COVID19.
1 Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2019 has caused an ongoing global pandemic of the severe pneumonia-like disease known as coronavirus disease 2019 (COVID-19) [1–3]. The COVID-19 outbreak has swept across the world, causing severe human mortality as well as loss of property in many countries. SARS-CoV-2 is an enveloped, positive-sense, single-stranded ribonucleic acid (RNA) betacoronavirus related to the viruses that caused the SARS outbreaks in 2002 to 2004 and the outbreaks of Middle East respiratory syndrome (MERS) that have occurred since 2012. The World Health Organization (WHO) declared COVID-19 a pandemic on 11 March 2020. The infection process of SARS-CoV-2 is shown in Figure 1. As of 10 January 2022, more than 310 million people worldwide had been diagnosed with COVID-19, and more than 5.4 million people had died (https://coronavirus.jhu.edu/map.html).
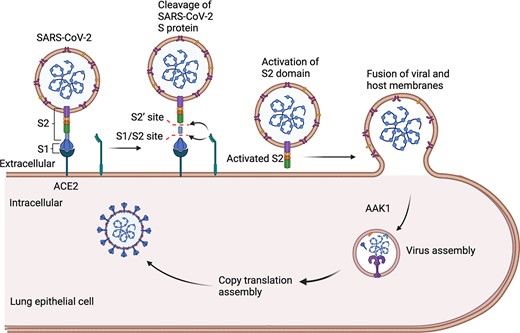
Entry of SARS-CoV-2 into host cells. SARS-CoV-2 spike (S) glycoprotein binds to the ACE2 receptor on the host cell surface.
Drugs of natural origin, including preparations obtained from medicinal herbs, are commonly used in many countries [4]. They are considered safer compared with chemical medicines, and have less side effects on human organism [5]. Traditional Chinese medicine (TCM, also referred to as herbal medicine or herb) has been successfully applied to treat various diseases over the past several thousand years [6]. In addition, TCM has produced many effective prescriptions and led to the accumulation of rich clinical experiences [7]. Chemical medicines, which are typically developed based on a single active compound, target specific biological activities, and it is therefore relatively simple to determine their mechanisms of actions [8–10]. In contrast, TCM relies on diverse herbs in formulations that have synthetic therapeutic effects on the human body. Currently, the use of TCM in COVID-19 treatment has earned wide recognition and positive evaluations from the medical community [11–13].
On 22 January 2020, the National Health Commission (NHC) of the People’s Republic of China first formally incorporated TCM-related treatments into the ‘Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 3)’ (http://www.nhc.gov.cn/xcs/yqfkdt/202001/f492c9153ea9437bb587ce2ffcbee1fa.shtml). Based on numerous clinical therapeutic evidence [14–16], Huashi-Baidu Formula (HSBD) and Xuanfei-Baidu Formula (XFBD) were admitted into the Trial Version 7 guiding to treat the syndrome of lung with dampness toxin retention [17]. Additionally, NHC published the composition of Qingfei-Paidu Decoction (QFPD) in Trial Version 7, which was used in the treatment of mild to sever stages of COVID-19 patients [17]. Simultaneously, NHC also recommended Jinhua-Qinggan Granule (JHQG) and Lianhua-Qingwen Capsule (LHQW) for reducing fever of SARS-CoV-2-infected patients during the period of clinical observation. And NHC also issued Xuebijing Injection (XBJ) for treating patients in sever and critical pathological periods. Eventually, the six officially issued therapies (drug combinations) were collectively referred to as ‘Three TCM formulations and three medicines’ (abbreviated as 3F3M) [14, 18]. The brief description referring 3F3M is shown in Figure 2 and the details are presented in Section 3.2.
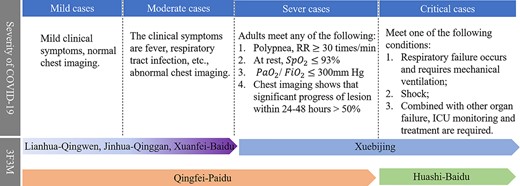
The descriptions of issued ‘Three TCM formulations and three medicines’ (3F3M) targeting the four infected stages of COVID-19.
At present, no specific effective therapeutic drugs (or drug combinations) are available to treat against SARS-CoV-2. The traditional strategies for drug development are considered unfavorable for developing new drugs for treating COVID-19 because of the time-consuming nature of drug discovery along with the high costs and unforeseen high failure rates. The development of a new drug usually takes over 5 years [19], and the time required for approval of a new antiviral therapeutic agent can exceed 5 years [20, 21]. Conversely, drug repurposing can be approved for clinical or generic drugs with well-defined structures and known biological activities. Therefore, to fight for the rapid spread of COVID-19 in the absence of specific antiviral therapeutic drugs, repurposing known drugs could substantially accelerate the implementation of new therapeutic treatments.
TCM repurposing is the study of herb compounds that are approved for clinical use to expand their clinical indications and discover new targets [22]. The SARS-CoV-2 genome encodes 29 proteins, including four structural, 16 nonstructural and nine accessory proteins. Clarifying the interactions between these 29 proteins and various compounds is a critical task in drug development. Because SARS-CoV-2 acts on the human body through various proteins, treatment focusing on a single target hardly produces positive therapeutic effects. Thus, researchers have primarily used multitarget therapies to treat COVID-19 patients [23, 24]. Additionally, TCM inherently has multicomponent and multitarget characteristics [25, 26]. This multicomponent–multitarget approach can enhance therapeutic effectiveness, thereby increasing the success rate of drug repurposing [27, 28].
In addition, combination therapies (termed as ‘drug/herb combinations’ in this study) have been shown to be more effective than single drug for diseases that lack effective drug treatment [29]. Drug combinations with synergistic compounds can improve therapeutic potency, as well as reduce side effects. Accordingly, repurposing herb combinations could be an effective treatment for COVID-19.
High-throughput screening technically could help to find candidate drug(s) for COVID-19. However, exploring the space of combinations is deterring due to the exceedingly huge number of unique chemical combinations. Therefore, computational-based approaches are the engaging alternative. And the recent computational-based approaches against COVID-19 include the following categories: (1) Virtual screening is the computational approach used in the early stages of a drug repurposing strategy to explore a compound library for bioactive molecules against a certain drug target [30]. Elfiky et al. [31] employed conventional molecular docking approaches involving SARS-CoV-2 RNA polymerase to screen anti-polymerase drugs and found ribavirin, remdesivir, sofosbuvir, galidesivir and tenofovir as candidate inhibitors; (2) Data-driven approaches. Richardson et al. [32] identified Baricitinib as a candidate drug to target SARS-CoV-2 Spike protein by employing text-mining approaches from substantial literature. Esmail et al. [33] employed a transfer learning-based approach to identify 30 drugs with strong inhibitory potencies to the Angiotensin Converting Enzyme 2 (ACE2) receptor (ACE2 is the Major Cell Entry Receptor for SARS-CoV-2.) and the transmembrane protease serine 2 based on data from DrugBank and ZINC. (3) Graph-based approaches. Hsieh et al. [34] proposed a simplified drug repurposing workflow that incorporated interactions between SARS-CoV-2 and drugs by employing the deep graph neural network approach, in which a set of 22 chemical drugs and related drug-combinations have been signaled as the candidate treatments against COVID-19. Researchers from AWS-AI developed a drug–disease knowledge graph to identify 41 repurposable chemical drugs that may accelerate therapeutic response against COVID-19 [35].
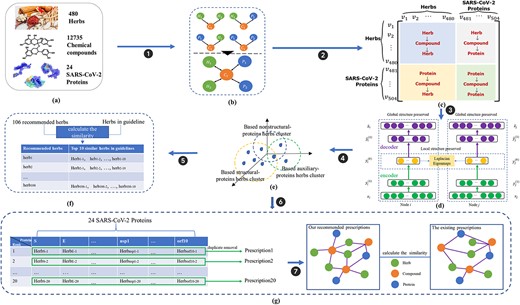
The work-flow of recommending herb combinations as the candidate therapy against COVID-19. (A) Data collection. (B) Heterogeneous ‘Herb-Compound-Protein’ network. (C) Matepath-based proximity matrix. (D) HCP-DGE model (inspired from SDNE approach). (E) Signaled herb clusters for SARS-CoV-2 structural, nonstructural and accessory proteins, individually. (F) Recommended ranking list of top-20 candidate herbs w.r.t. each single SARS-CoV-2 protein. (G) The process of recommending specific herb combinations referring certain infect-stage by employing VGAE approach.
Aforementioned studies contributed in drug repurposing to fight against COVID-19, though there exist a few limitations yet. Partial studies are based on the previous knowledge that the potential candidates had a strong inhibitory effect on MERS and SARS-CoV; it was not guaranteed that these candidates could strongly fight against SARS-CoV-2. Besides, most of the current issued methods (1) only focused on chemical medicines without innate synthetic effects, comparing multicomponent-based herbal medicines; (2) relied on the strategy of targeting single drug on single SARS-CoV-2 protein (e.g. S protein), which is neither binding all protein sites of the virus nor owning the therapeutic power; (3) simply adopted the ‘one-size-fits-all’ strategy that is utilizing the same drug to treat patients in various infected stages. In such a case, infected patients cannot obtain the precision treatment; and (4) no experimental validation was provided to the results.
In this work, we initially employed the virtual drug screening with an effective filtering strategy to implement ‘Herb-Compound’ and ‘Compound-Protein’ docking graphs based on 480 herbs, 13,448 compounds and 24 SARS-CoV-2 proteins. The effective filtering strategy consists of ADME (described in Section 3.2) and postdocking filtering process (In postdocking filtering process the chemicals identified through docking simulations were evaluated, considering the docking energy and the similarity of the protein–ligand interactions with the known active compounds.) [36], which can improve the hit rate. There were 12,735 compounds remaining after the filtering process, and the ‘Herb-Compound-Protein’ docking graph was then constructed based on the two docking graphs. We then proposed a heterogeneous deep graph approach termed HCPGraph, which was modeled based on the ‘Herb-Compound-Protein’ graph for the purposes of (1) signaling candidate specifig repurposing herbs targeting allSARS-CoV-2 proteins and (2) recommending candidate precision herb combinations referring the specific infected stages.
The HCPGraph approach consists of two components. The first component is the ‘Herb-Compound-Protein’ graph-based deep graph embedding method (abbr. HCP-DGE). HCP-DGE firstly generates the following metapaths: ‘Herb|$\rightarrow $|Compound|$\rightarrow $|Herb’, ‘Herb|$\rightarrow $|Compound|$\rightarrow $|Protein’, ‘Protein|$\rightarrow $|Compound|$\rightarrow $|Herb’ and ‘Protein|$\rightarrow $|Compound|$\rightarrow $|Protein’. Sequentially, the ‘Herb-Compound-Protein’ heterogeneous embedding graph can be generated based on the four types of metapath. A heterogeneous embedding graph contains rich semantics, in which a metapath connects different types of nodes (e.g. herbs, compounds or proteins) and edges (docking relations). HCP-DGE then generated the unique list of top-10 candidate herbs for each of the 24 SARS-CoV-2 proteins.
Patients should be better to take the tailored herb combination as the precision treatment when they suffered from the certain infected stage. To achieve this purpose, we employed the variational graph autoencoders (VGAE) method, as well as the second component of HCPGraph. We initially composed 20 herb combinations from the signaled |$24\times $|Top-10 candidate repurposing herb sets. Then we computed the ‘Herb-Compound-Protein’ graph-based similarity between the recommended 20 herb combinations and the six official issued herb combinations (3F3M) to obtain specific candidate herb combinations for certain infected stages. Since each ‘Herb-Compound-Protein’ graph has a variable size of unordered nodes and each node in a graph has a different number of neighbors, so we cannot just use convolution-like approach directly. To conquer this issue, VGAE applies the idea of variational autoencoder on graph-structured data, which significantly improves predictive performance on graph-based similarity tasks. (Results are described in the Supplementary files S6 and S7). The schema of our proposed method HCPGraph is shown in Figure 3.
The remainder of this paper is organized as follows. Section 2 discusses related works, including the graph-embedding and data-driven approaches, as well as their limitations in treating against COVID-19 comparing our work. In Section 3, the notations used in this study and the basic background are introduced. In Section 4, we present the details of ‘Herb-Compound’ and ‘Compound-Protein’ docking graph constructed process. In Section 5, the details of signaling candidate repurposable herbs for each protein by employing the HCP-DGE method are described. We present the content of employing the VGAE approach to recommend candidate precision herb combinations in Section 6. The materials and experimental details are provided in Sections 7 and 8, respectively. Section 9 presents the conclusion.
2 Related work
Drug repurposing has become the main strategy for COVID-19 drug development. Many effective methods have been applied for drug repurposing, such as graph embedding and data-driven approaches. The graph embedding methods usually can be categorized into three broad categories: (1) Matrix factorization based, (2) Random Walk based and (3) Deep Learning based. Data mining is the commonly used data-driven approach. In this section, we presented the characteristics of each of these categories and provided a summary of a few representative approaches for each category.
2.1 Matrix-factorization-based embedding approaches
Matrix factorization (MF) is defined as the factorization of a matrix into a product of matrices. A graph is denoted by |$\mathcal{G}=\left (\mathcal{V}, \mathcal{E}, w\right )$|, where |$\mathcal{V}=\left \{v_1,v_2,\ldots ,v_n\right \}$| represents |$n$| nodes, |$\mathcal{E}=\left \{e_{ij}\right \}_{i,j=1}^n$| represents the set of edges and |$w$| denotes the set of edge weights. If |$v_{i}$| and |$v_{j}$| are not linked by an edge, then |${w}_{ij}=0$|; otherwise, |$0<{w}_{ij}<1$|. Based on the |$n$| nodes in |$\mathcal{G}$|, we can use an |$n\times n$| adjacency matrix to represent the topology of the graph. Each element of the adjacency matrix represents the connection relationship between a pair of nodes. The row or column vectors of the adjacency matrix form |$n$|-dimensional representations of the nodes. However, because the dimensionality of these representations is usually high, the node embedding approach is usually employed to obtain low-dimensional vector representations of the nodes. To this end, the high-dimensional original matrix can be decomposed through matrix factorization into lower dimensional representations.
MF-based approaches for COVID-19. Charilaos [42] leveraged matrix decomposition to learn concise representations of entities and relations in knowledge bases and employed these representations to perform drug repurposing against COVID-19. Sadeghi [43] presented a method based on non-negative matrix factorization for drug repurposing to predict novel drug indications by integrating drugs and diseases related data sources. Tang [44] developed the indicator regularized non-negative matrix factorization algorithm to predict the potential drug against COVID-19.
2.2 Random walk-based embedding approaches
Random walk-based graph embedding approaches enable the application of classical algorithms for high-dimensional data to graph-based downstream tasks (e.g. link prediction). These embedding methods learn vector representations for nodes based on some notion of topological similarity (or proximity). A node embedding is a function |$\phi : \mathcal{V} \rightarrow \mathbb{R}^{d}$| that maps each node |$v$| to a |$d$|-dimensional condition on |$(d < n)$| vector |$\boldsymbol{u}_{v}$|. Random walk-based embedding methods use a random walk process to embed nodes |$u$| and |$v$| such that a similarity metric is preserved by dot products |$\boldsymbol{u}_{u}^{T}\cdot \boldsymbol{u}_{v}^{T}$|.
node2vec. Similar to DeepWalk approach, node2vec [47] can learn low-dimensional representations for nodes in a graph by optimizing a neighborhood preserving objective. node2vec creates a series of random walks of the nodes in a network and uses those sequences as the input data for the embedding algorithm, in this case the skipgram model of word2vec. The key difference between DeepWalk and node2vec that is node2vec employs biased-random walks that provides a trade-off between breadth-first and depth-first graph searches. In this way, node2vec is able to generate higher quality and more informative embeddings than DeepWalk.
struc2vec. Compared with DeepWalk and node2vec, struc2vec [48] focuses more on the network’s structure and does not need to rely on the properties of nodes or edges. The struc2vec method identifies nodes that play a similar role based solely on the structure of the graph. In particular, struc2vec employs a degree-based method to measure a pairwise structural role similarity, which is then adopted to build a multilayer graph.
metapath2vec [49] is designed to sample random walks from heterogeneous graphs, in which the random walks are restricted to only transition between particular types of nodes.
Radom walk-based approaches for COVID-19. Sibilio [50] leveraged the Random Walk with Restart algorithm to measure the closeness between the COVID-19 module and other disease modules in the human interactome network and to identify potentially repurposable drugs for COVID-19. Fiscon [51] exploited a Bi-Random walk-based approach to infer potential reuse for existing drugs.
2.3 Deep Learning-based embedding approaches
Deep learning (DL) has shown outstanding performance in a wide variety of research fields, such as computer vision, disease diagnosis, etc. [52]. DL-based graph embedding applies DL models on graphs. These models are either a direct adoption from other fields or a new neural network designed for embedding graph data. The neural network architecture in DL that is a robust and effective solution to encode the graph into a low-dimensional space.
Autoencoder aims to minimize the reconstruction error of the output and input by its encoder and decoder. Both encoder and decoder contain multiple nonlinear functions [53]. The encoder maps input data to a representation space and the decoder maps the representation space to a reconstruction space. The idea of adopting autoencoder for graph embedding is similar to the above-mentioned matrix factorization in terms of neighborhood preservation.
Graph embedding. Graph embedding methods aim at learning low-dimensional latent representation of nodes in a network. Structural deep network embedding (SDNE) [54] learns node representations that preserve the proximity between 2-hop neighbors with a deep autoencoder. It further preserves the proximity between adjacent nodes by minimizing the Euclidean distance between their representations.
Deep Learning-based approaches for COVID-19. Doshi [55] proposed a dedicated graph neural network based drug repurposing model and provided 150 potential drugs against COVID-19. Su [56] proposed a sequence combined attentive network embedding model SANE for identifying COVID-19 drugs based on sequence features and network features.
2.4 TCM data-driven approaches for COVID-19
Besides the above-mentioned embedding-based methods, we will present a few data mining-based approaches by utilizing the public TCM dataset to signaling candidate herbs. To explore potential alternative therapies for COVID-19, Ren and co-workers [57] developed an association network to mine high-frequency herbs from ancient prescriptions. Luo et al. [58] employed complex system entropy and unsupervised hierarchical clustering to identify eight core herb combinations and 10 new formulae as potentially useful candidates for treating COVID-19.
3 Notations and preliminaries
3.1 Notations
All vectors are represented by bold lowercase letters (e.g. |$\boldsymbol{f}_{i}$|). All matrices are represented by bold uppercase letters (e.g. |$\mathcal{A}$|). Row vectors are denoted by the presence of a superscript T, indicating transposition (e.g. |$\boldsymbol{f}_{i}^{T}$|); otherwise, by default, vectors are assumed to take the form of column vectors. The symbol |$\rho $| denotes a metapath. Table 1 provides a summary of the notations used in this paper.
| Notation . | Description . |
|---|---|
| |$\mathcal{G}=\left (\mathcal{V}, \mathcal{E}, w\right )$| | A graph |$\mathcal{G}$| constructed of nodes |$\mathcal{V}$| and edges |$\mathcal{E}$|, with |$w$| denoting the set of edge weights |
| |$v_{i}$| | The |$i$|-th node |
| |$n$| | The number of nodes |
| |$\rho $| | A metapath |
| |$R$| | Edge types |
| |$\mathcal{A}$| | Node types |
| |$s\left (v_{i},v_{j}|\rho \right )$| | The proximity between node |$v_{i}$| and node |$v_{j}$| in path instance |$\rho $| |
| |$\mathcal{S}=\left \lbrace s_{i}\right \rbrace _{n}^{i=1}$| | The herb-protein transfer probability matrix and the input data for the deep autoencoder |
| |$K$| | The number of layers of the deep autoencoder |
| |$\mathcal{W}^{k}, \hat{\mathcal{W}}^{k}$| | The |$k$|-th layer weight matrix of the deep autoencoder |
| |$b^{k}, \hat{b}^{k}$| | The |$k$|-th layer biases |
| |$\boldsymbol{y}_{i}^{k}$| | The |$k$|-th layer hidden representation for |$v_{i}$| |
| Notation . | Description . |
|---|---|
| |$\mathcal{G}=\left (\mathcal{V}, \mathcal{E}, w\right )$| | A graph |$\mathcal{G}$| constructed of nodes |$\mathcal{V}$| and edges |$\mathcal{E}$|, with |$w$| denoting the set of edge weights |
| |$v_{i}$| | The |$i$|-th node |
| |$n$| | The number of nodes |
| |$\rho $| | A metapath |
| |$R$| | Edge types |
| |$\mathcal{A}$| | Node types |
| |$s\left (v_{i},v_{j}|\rho \right )$| | The proximity between node |$v_{i}$| and node |$v_{j}$| in path instance |$\rho $| |
| |$\mathcal{S}=\left \lbrace s_{i}\right \rbrace _{n}^{i=1}$| | The herb-protein transfer probability matrix and the input data for the deep autoencoder |
| |$K$| | The number of layers of the deep autoencoder |
| |$\mathcal{W}^{k}, \hat{\mathcal{W}}^{k}$| | The |$k$|-th layer weight matrix of the deep autoencoder |
| |$b^{k}, \hat{b}^{k}$| | The |$k$|-th layer biases |
| |$\boldsymbol{y}_{i}^{k}$| | The |$k$|-th layer hidden representation for |$v_{i}$| |
| Notation . | Description . |
|---|---|
| |$\mathcal{G}=\left (\mathcal{V}, \mathcal{E}, w\right )$| | A graph |$\mathcal{G}$| constructed of nodes |$\mathcal{V}$| and edges |$\mathcal{E}$|, with |$w$| denoting the set of edge weights |
| |$v_{i}$| | The |$i$|-th node |
| |$n$| | The number of nodes |
| |$\rho $| | A metapath |
| |$R$| | Edge types |
| |$\mathcal{A}$| | Node types |
| |$s\left (v_{i},v_{j}|\rho \right )$| | The proximity between node |$v_{i}$| and node |$v_{j}$| in path instance |$\rho $| |
| |$\mathcal{S}=\left \lbrace s_{i}\right \rbrace _{n}^{i=1}$| | The herb-protein transfer probability matrix and the input data for the deep autoencoder |
| |$K$| | The number of layers of the deep autoencoder |
| |$\mathcal{W}^{k}, \hat{\mathcal{W}}^{k}$| | The |$k$|-th layer weight matrix of the deep autoencoder |
| |$b^{k}, \hat{b}^{k}$| | The |$k$|-th layer biases |
| |$\boldsymbol{y}_{i}^{k}$| | The |$k$|-th layer hidden representation for |$v_{i}$| |
| Notation . | Description . |
|---|---|
| |$\mathcal{G}=\left (\mathcal{V}, \mathcal{E}, w\right )$| | A graph |$\mathcal{G}$| constructed of nodes |$\mathcal{V}$| and edges |$\mathcal{E}$|, with |$w$| denoting the set of edge weights |
| |$v_{i}$| | The |$i$|-th node |
| |$n$| | The number of nodes |
| |$\rho $| | A metapath |
| |$R$| | Edge types |
| |$\mathcal{A}$| | Node types |
| |$s\left (v_{i},v_{j}|\rho \right )$| | The proximity between node |$v_{i}$| and node |$v_{j}$| in path instance |$\rho $| |
| |$\mathcal{S}=\left \lbrace s_{i}\right \rbrace _{n}^{i=1}$| | The herb-protein transfer probability matrix and the input data for the deep autoencoder |
| |$K$| | The number of layers of the deep autoencoder |
| |$\mathcal{W}^{k}, \hat{\mathcal{W}}^{k}$| | The |$k$|-th layer weight matrix of the deep autoencoder |
| |$b^{k}, \hat{b}^{k}$| | The |$k$|-th layer biases |
| |$\boldsymbol{y}_{i}^{k}$| | The |$k$|-th layer hidden representation for |$v_{i}$| |
3.2 Preliminaries
SARS-CoV-2 proteins. Drug targets refer to biological macromolecules in the body that have pharmacodynamic functions and can be acted upon by drugs, such as particular proteins and nucleic acids [59, 60]. Additionally, these proteins are the starting points for drug design. The interactions between drugs and targeting proteins are the basis on which many drugs perform their biological functions [61, 62].
The S protein, which is an important determinant of viral entry into host cells, has a high binding strength with ACE2 [63]. Among the nonstructural proteins,
The coronavirus protease nsp5 (3CLpro) is an approximately 30kDa, 3domain cysteine protease conserved in structure and function in all known coronaviruses and serves as the main protease for proteolytic processing of the replicase polyproteins [64]. The papain-like protease (PLpro) is an essential coronavirus enzyme that is required for processing viral polyproteins to generate a functional replicase complex and enable viral spread [65].
Three formulations and three medicines, 3F3M. COVID-19 belongs to the ‘plague’ category in TCM [66] and is classified into four stages (mild, moderate, severe and critical) based on the severity of illness and the symptoms that are present (see Figure 2). According to NHC guidelines, the following six therapeutic treatments are used for different stages of COVID-19: Jinhua-Qinggan Granule (JHQG), Lianhua-Qingwen Capsule (LHQW) and Xuanfei-Baidu Decoction (XFBD) are recommended for mild and moderate cases, Huashi-Baidu Decoction (HSBD) for clinical cases, Xuebijng Injection (XBJ) for severe and critical cases and Qingfei-Paidu Decoction (QFPD) for all stages except critical stage.
The three medicines (3M) are JHQG, LHQW and XBJ. These drugs were previously approved by NHC for treating respiratory diseases. The three TCM formulations (3F) are QFPD, HSBD and XFBD. As demonstrated by clinical data, the 3F3M performed well in treating COVID-19 and are recommended in the Diagnosis and Treatment Protocol for COVID-19 [67, 68]. All of the recommended formulas are comprised of traditional formulas that have been used to treat pulmonary and respiratory diseases for thousands of years.
Precision herbal medicine. Precision medicine is ‘an approach for disease treatment and prevention that takes into account individual variability in genes, environment and lifestyle for each person’ [69]. One important special feature of biology is its diversity, its variation. Personalized medicine refers to the right treatment for the right individual at the right time in the health-care realm [70]. Precision herbal medicine (or herb combinations) that is based on the individual’s physiology and the holistic characteristics has been used in Asian countries for thousands of years. And the recent advanced system biology approach that can boost herbal medicine to signal causal biomarkers [71], which own the power to offer ‘the right therapy for the right patient’ [72].
ADME. ADME is an abbreviation in pharmacokinetics and pharmacology for ‘absorption, distribution, metabolism and excretion’, which describes the disposition of a pharmaceutical compound within an organism. The four criteria all influence the drug levels and kinetics of drug exposure to the tissues and hence influence the performance and pharmacological activity of the compound as a drug. Similar to the chemical medicines, herbal medicines are associated with the chemical constituents of herbs including prototype components and their metabolites in the circulation, which are directly associated with the whole process of ADME[73].
Heterogeneous Graph. A heterogeneous graph is defined as a graph |$\mathcal{G} = (\mathcal{V}, \mathcal{E})$|, where |$\mathcal{V}$| and |$\mathcal{E}$| represent the node set and the link set, respectively, and each node |$v_i\in \mathcal{V}$| belongs to a certain node type. |$\varphi \left (v_i\right )\in \mathcal{A}$|, where |$\varphi \left (v_i\right )$| stands for the type of |$v_i$| and |$\mathcal{A}$| means the set of node types. Each edge |$e_{ij}\in E$| belongs to a specific relationship type in the relationship type set |$\mathcal{R}: \phi \left (e\right )\in \mathcal{R}$|. A graph is heterogeneous when the total number of object types satisfies |$| \mathcal{A} |> 1$| or the total number of edge types satisfies |$| \mathcal{R} |> 1$|.
Heterogeneous graph not only memorizes the graph structure of the original data, but also preserves a higher level semantics of the data. An example of heterogeneous graph in this study is illustrated in Figure 3(B), which consists of three node types (Herb, Compound and Protein) and two link types (Herb-Compound and Compound-Protein); Figure 3(B) illustrates the network schema. Based on the constructed heterogeneous graph ‘Herb-Compound-Protein’, to formulate the semantics of higher order relationships among entities, metapath is further proposed whose definition is given below.
First-Order Proximity. The first-order proximity represents the local proximity between two nodes. For a connection between two nodes, the edge weight represents the first-order proximity between them.
Second-Order Proximity. The second-order proximity is used to measure the similarity between neighborhoods of the two interested nodes |$u$| and |$v$|. We use |$p_{u}=\left (\boldsymbol{w}_{1},\ \cdots ,\ \boldsymbol{w}_{\left |u\right |}\right )$| to represent the first-order similarities between |$u$| and all other nodes. The second-order proximity between |$u$| and |$v$| is then determined by |$p_{u}$| and |$p_{v}$|. The more neighboring node |$u$| and |$v$| share, the more similar the two nodes are. Since the first-order proximity cannot preserve the link information between any pair of nodes, the second-order proximity is used to preserve global information by memorizing nodes’ adjacent network structure.
This notation represents a composite relationship between object types of the form |$R=R_{1}\circ R_{2}\circ \cdots \circ R_{l}$|, where |$\circ $| represents the composition operator between relationships. Metapaths describe the entity relationships between objects, and different metapaths describe different entity relationships between objects. The mining of these entity relationships is the cornerstone of various subsequent tasks [75].
In this study, we generated the following four metapaths: (1) Herb-Compound-Herb indicates that two herbs contain the same compound, (2) Herb-Compound-Protein indicates that an herb targets a certain protein through a certain compound, (3) Protein-Compound-Herb indicates that a protein binds to a certain compound in a certain herb and (4) Protein-Compound-Protein indicates that one compound targets two different proteins.
4 Herb-Compound and Compound-Protein docking graphs
In this section, we presented the process of constructing the two docking graphs, ‘Herb-Compound’ and ‘Compound-Protein’, by employing the virtual screening-based approach.
4.1 Herb-Compound docking graph
We employed ADME as the filtering approach to identify chemical compounds from herbs. It has widely been accepted that ADME is the critical metric for determining which chemical components are likely to be active and what mode of action they may adopt to achieve their therapeutic effects. In addition, the ADME properties are defined as the dynamic changes in drugs within an animal or the human body, such as oral bioavailability (OB), drug-likeness and half-life, which are critical in drug discovery and development [76].
Therefore, we employed the in silico ADME profiling to identify effective chemical compounds from herbal medicines. There existed 12,735 compounds remaining from the original set of 13,448 chemical compounds by conducting the filtering threshold as oral bioavailability (|$> 30\%$|), caco-2 (|$> -0.4$|), drug-like value (|$>0.18$|) and drug half-life (|$> 3h$|). The ‘Herb-Compound’ graph sequentially to be generated based on the ADME filtered docking value. The details of ADME identification are presented in Supplementary file S1.
4.2 Compound-Protein docking graph
We downloaded SARS-CoV-2 protein structures (S, nsp5, nsp7, nsp8, nsp9, nsp10, nsp12, nsp15 and nsp16) from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) (http://www.rcsb.org/). Referring to SARS-CoV-2 proteins with no clear structures (E, N, M, orf3a, orf6, orf7a, orf8, orf10, nsp1, nsp2, nsp3, nsp4, nsp6, nsp13 and nsp14), we downloaded the predicted protein structures from Zhang-Lab (https://zhanglab.ccmb.med.umich.edu/COVID-19/) at the University of Michigan. Thus, we retrieved a total of 24 protein structures. We employed OpenBabel (http://openbabel.org/wiki/Main_Page) to perform dewatering and hydrogenation operations on the downloaded PDB files. The PDB files were subsequently converted into the PDBQT format for molecular docking.
After processing the aforementioned properties of the molecule, the binding of the herbal medicine compounds and the SARS-CoV-2 proteins is calculated using the scoring function Eq. 10. The compound would be judged as being effectively binded to the protein if the binding free energy (binding-affinity) |$< -7$| kcal/mol [31]. Since the ligand–receptor interaction is an integrated equilibrium process, the resulting herbal medicine molecule conformation has the lowest free energy.
We then docked 12,735 compounds with 24 SARS-CoV-2 proteins (containing four structural proteins, 15 nonstructural proteins and five auxiliary proteins) 10 times for reducing the docking bias. The ‘Compound-Protein’ docking graph consequently can be constructed based on the average docking value. The docking results are recorded in the Supplementary file S2.
5 Signaling candidate repurposing herbs targeting each SARS-CoV-2 protein
5.1 Overview
We first constructed a nonlinear heterogeneous ‘Herb-Compound-Protein’ graph |$\mathcal{G}=(\mathcal{V}, \mathcal{E})$| based on ‘Herb-Compound’ and ‘Compound-Protein’ docking graphs, in which the nodes are connected through the four above-mentioned TCM metapaths. To preserve the information of the neighborhood structure of each node, we generated a sparse herb-protein adjacency matrix |$\mathcal{S}$| by employing the random walk method to calculate the similarity between each two nodes in |$\mathcal{G}$|.
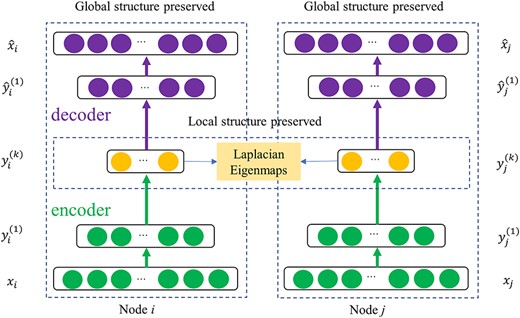
Since the ‘Herb-Compound-Protein’ is a second-order topological structure heterogeneous graph, which has two inherent characteristics: highly nonlinear network structure and low parallelizability, graph embedding approach has the following benefits: (1) graph embedding is the data structure that is used for fast comparison of similar data structures; (2) graph embedding approaches help in converting high-dimensional sparse graphs into low-dimensional, dense and continuous vector spaces effectively; (3) graph embeddings are calculated by employing machine/deep learning algorithms. Given the ‘Herb-Compound-Protein’ heterogeneous graph, we borrowed the model architecture from SDNE to develop ‘Herb-Compound-Protein’ deep graph embedding (HCP-DGE) approach. By employing HCP-DGE, we are able to signal top-10 candidate repurposing herbs for all SARS-CoV-2 proteins. The schema of the architecture is shown in Figure 4.
5.2 Metapath-based proximity
The proximity between any two nodes |$v_{i}$| and |$v_j$| was obtained by means of the random walk-based process. A random-walk is a Markov chain over the set of nodes |$\mathcal{V}$|[79]. The transition probability of the walker jumping to node |$v_{i}$| is based solely on its previous location.
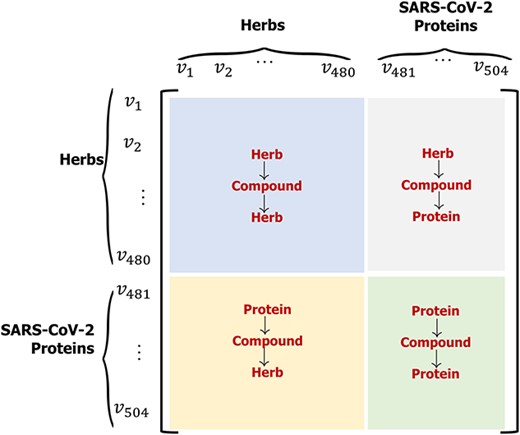
Toy example. Given an assumptive metapath |$p: \text{Herb}\rightarrow \text{Compound}\rightarrow \text{Herb}$|, where |$h_{1}$| represents a node of herb that is connected to three compounds, one of the compounds is connected to herb |$h_{2}$|. Then, the |$h_{1}$|-to-|$h_{2}$| transition probability can be obtained by employing the Eq. 13, which is |$s(h_{1}, h_{2}|\rho _{h_{1}\rightarrow h_{2}})=\dfrac{1}{6}$|. Thereby we can generate the ‘Herb-Protein’ adjacency matrix |$\mathcal{S}$| (see Figure 5). Each cell |$s_{ij}|_{i\in \left \lbrace 1\dots 480\right \rbrace , j\in \left \lbrace 481\dots 504\right \rbrace }$| in matrix |$\mathcal{S}$| represents the transition probability between the |$i$|-th herb and the |$j$|-th SARS-CoV-2 protein.
5.3 Herb-Compound-Protein graph embedding
Given the network |$\mathcal{G}=(\mathcal{V}, \mathcal{E})$|, we identified the mapping |$f:v_{i}\rightarrow y_{i}$|, where the dimensionality of |$y_i$| is |$d$|, i.e. |$y_i\in \mathcal{R}^d$|. The mapping |$f$| reduces the dimensionality of each node vector, furthermore mapping nodes from various types to the same dimensional space. This process acts as an embedding process for the ‘Herb-Compound-Protein’ graph. Importantly, the embedding vectors retain the structure and entity relationships of the original graph.
5.4 HCP-DGE approach
5.4.1 Framework
HCP-DGE borrows the idea from SDNE model that is a semisupervised model for performing graph embedding. This method is capable of capturing nonlinear structures. Insighting from this architecture, HCP-DGE exploits both first-order proximity and second-order proximity to represent the local and global ‘Herb-Compound-Protein’ graph structures, respectively. In detail, HCP-DGE comprises a supervised component and an unsupervised component. The supervised component preserves the first-order proximity using Laplacian eigenmaps (LEs). Additionally, the unsupervised component is a deep autoencoder that captures the second-order proximity by reconstructing the input adjacency matrix |$\mathcal{S}$|. The second-order proximity preserves the global network structure. After obtaining the low-dimensional embedding representations, we clustered the herb nodes and protein nodes.
5.4.2 The input
The inputs for HCP-DGE are divided into two parts, the adjacency matrix of ‘Herb-Protein’ and the weight matrix for nodes of herbs and proteins.
The weight matrix is composed of Herb–Herb weight matrix, Protein–Protein weight matrix and Herb–Protein weight matrix, where Herb–Herb weight matrix, Protein–Protein weight matrix are generated based on random walk-based meatapaths ‘Herb-Compound-Herb’ and ‘Protein-Compound-Protein’, individually. Furthermore, Herb-Protein weight matrix consists of three components: (1) predefined weights for structural, nonstructural and accessory SARS-CoV-2 proteins, (2) normalized Herb-Compound ADME property value (Caco-2 cell permeability (Caco-2 permeability stands for absorption of the herb in the intestine, and is closely related to its permeability in the intestinal epithelial cells. The human intestinal cell line Caco-2 is often used as an effective in vitro model to study the passive diffusion of drugs through the intestinal epithelium.)) and (3) normalized Compound-Protein docking value.
5.4.3 Objective function
In this section, we presented the details of the objective function, together with the process of model learning and optimization. Since HCP-DGE follows SDNE model architecture, the objective function is composed of three components:
the loss function for second-order proximity that enables capturing the global ‘Herb-Compound-Protein’ graph structure;
the loss function for first-order proximity that owns the power preserving the local ‘Herb-Compound-Protein’ graph structure;
the Frobenius norm-based regularizer that reduces the model bias and avoiding over-fitting.
Loss function for the second-order proximity. Firstly, we introduce the loss function for the second-order proximity. The second-order proximity refers to the similarity w.r.t. the neighborhood structure of a pair of nodes. To preserve the second-order proximity, the autoencoder was exploited. The autoencoder model is an unsupervised learning technique that imposes a bottleneck in the neural network forcing a compressed knowledge representation of the original input. In such a case, the neighborhood structure that existed in the ‘Herb-Compound-Protein’ graph can be learned and consequently leveraged when forcing the adjacency matrix and weight matrix through the neural network bottleneck layer.
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| S | Mori Folium, Hippophae Fructus, Carthami Flos, Peucedani Radix, Herbal Ephedra, Perilla, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Flower of Lobed Kudzuvine, Amomi Fructus | Mori Folium, Hippophae Fructus, Carthami Flos, Peucedani Radix, Herbal Ephedra, Perilla, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Flower of Lobed Kudzuvine, Amomi Fructus, Fructus Tribuli, Zanthoxyli Radix, Coralhead Plant Seed, Caulis Trachelospermi, Chicory Herb, Anemarrhenae Rhizoma, Common Cephalanoplos Herb, Radix Gentianae, Ussuriensis Fritillary Bulb, Inulae Flos, Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Smilacis Chinae Rhizoma, Farfarae Flos, Smilacis Glabrae Rhizoma, Herba Gnathali Affinis |
| E | Fructus Tribuli, Zanthoxyli Radix, Coralhead Plant Seed, Caulis Trachelospermi, Chicory Herb, Anemarrhenae Rhizoma, Common Cephalanoplos Herb, Radix Gentianae, Ussuriensis Fritillary Bulb, Inulae Flos | |
| M | Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Smilacis Chinae Rhizoma, Farfarae Flos, Smilacis Glabrae Rhizoma | |
| N | Carthami Flos, Peucedani Radix, Mori Folium, Hippophae Fructus, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Herbal Ephedra, Flower of Lobed Kudzuvine, Herba Gnathali Affinis, Perilla |
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| S | Mori Folium, Hippophae Fructus, Carthami Flos, Peucedani Radix, Herbal Ephedra, Perilla, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Flower of Lobed Kudzuvine, Amomi Fructus | Mori Folium, Hippophae Fructus, Carthami Flos, Peucedani Radix, Herbal Ephedra, Perilla, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Flower of Lobed Kudzuvine, Amomi Fructus, Fructus Tribuli, Zanthoxyli Radix, Coralhead Plant Seed, Caulis Trachelospermi, Chicory Herb, Anemarrhenae Rhizoma, Common Cephalanoplos Herb, Radix Gentianae, Ussuriensis Fritillary Bulb, Inulae Flos, Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Smilacis Chinae Rhizoma, Farfarae Flos, Smilacis Glabrae Rhizoma, Herba Gnathali Affinis |
| E | Fructus Tribuli, Zanthoxyli Radix, Coralhead Plant Seed, Caulis Trachelospermi, Chicory Herb, Anemarrhenae Rhizoma, Common Cephalanoplos Herb, Radix Gentianae, Ussuriensis Fritillary Bulb, Inulae Flos | |
| M | Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Smilacis Chinae Rhizoma, Farfarae Flos, Smilacis Glabrae Rhizoma | |
| N | Carthami Flos, Peucedani Radix, Mori Folium, Hippophae Fructus, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Herbal Ephedra, Flower of Lobed Kudzuvine, Herba Gnathali Affinis, Perilla |
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| S | Mori Folium, Hippophae Fructus, Carthami Flos, Peucedani Radix, Herbal Ephedra, Perilla, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Flower of Lobed Kudzuvine, Amomi Fructus | Mori Folium, Hippophae Fructus, Carthami Flos, Peucedani Radix, Herbal Ephedra, Perilla, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Flower of Lobed Kudzuvine, Amomi Fructus, Fructus Tribuli, Zanthoxyli Radix, Coralhead Plant Seed, Caulis Trachelospermi, Chicory Herb, Anemarrhenae Rhizoma, Common Cephalanoplos Herb, Radix Gentianae, Ussuriensis Fritillary Bulb, Inulae Flos, Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Smilacis Chinae Rhizoma, Farfarae Flos, Smilacis Glabrae Rhizoma, Herba Gnathali Affinis |
| E | Fructus Tribuli, Zanthoxyli Radix, Coralhead Plant Seed, Caulis Trachelospermi, Chicory Herb, Anemarrhenae Rhizoma, Common Cephalanoplos Herb, Radix Gentianae, Ussuriensis Fritillary Bulb, Inulae Flos | |
| M | Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Smilacis Chinae Rhizoma, Farfarae Flos, Smilacis Glabrae Rhizoma | |
| N | Carthami Flos, Peucedani Radix, Mori Folium, Hippophae Fructus, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Herbal Ephedra, Flower of Lobed Kudzuvine, Herba Gnathali Affinis, Perilla |
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| S | Mori Folium, Hippophae Fructus, Carthami Flos, Peucedani Radix, Herbal Ephedra, Perilla, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Flower of Lobed Kudzuvine, Amomi Fructus | Mori Folium, Hippophae Fructus, Carthami Flos, Peucedani Radix, Herbal Ephedra, Perilla, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Flower of Lobed Kudzuvine, Amomi Fructus, Fructus Tribuli, Zanthoxyli Radix, Coralhead Plant Seed, Caulis Trachelospermi, Chicory Herb, Anemarrhenae Rhizoma, Common Cephalanoplos Herb, Radix Gentianae, Ussuriensis Fritillary Bulb, Inulae Flos, Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Smilacis Chinae Rhizoma, Farfarae Flos, Smilacis Glabrae Rhizoma, Herba Gnathali Affinis |
| E | Fructus Tribuli, Zanthoxyli Radix, Coralhead Plant Seed, Caulis Trachelospermi, Chicory Herb, Anemarrhenae Rhizoma, Common Cephalanoplos Herb, Radix Gentianae, Ussuriensis Fritillary Bulb, Inulae Flos | |
| M | Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Smilacis Chinae Rhizoma, Farfarae Flos, Smilacis Glabrae Rhizoma | |
| N | Carthami Flos, Peucedani Radix, Mori Folium, Hippophae Fructus, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Herbal Ephedra, Flower of Lobed Kudzuvine, Herba Gnathali Affinis, Perilla |
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| nsp1 | Calendula officinalis, Common Cephalanoplos Herb, Meliae Cortex, Lasiosphaera, Inulae Herba, Black Nightshade Herb, Akebia Stem, Eupatorii Herba, Bistortae Rhizoma, Fruit of Tree-of-heaven Ailanthus | Calendula officinalis, Common Cephalanoplos Herb, Meliae Cortex, Lasiosphaera, Inulae Herba, Black Nightshade Herb, Akebia Stem, Eupatorii Herba, Bistortae Rhizoma, Fruit of Tree-of-heaven Ailanthus, Carthami Flos, Common Macrocarpium Fruit, Mori Folium, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Chinese Fevervine Herb, Fructus Rosae Laevigatae, Modern Rose, Hippophae Fructus, Perilla, Notopterygii Rhizoma Et Radix, Rhododendri Daurici Folium, Forsythia Suspensa, Ginkgo Folium, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Honeysuckle Flower, Amur Corktree Bark, Chrysanthemum, Fructus Jujubae, Achyranthes Bidentata, Fagopyri Dibotryis Rhizoma, Indigowoad Root, Salviae Miltiorrhizae, Hyperici Perforati Herba, Allii Macrostemonis Bulbus, Curcumae Radix, Aucklandiae Radix, Asparagi Radix, Ginkgo Seed, Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae, Indigo Naturalis, Sophorae Flavescentis Radix, Swertiae Mileensis Herba, Omphalia, Coptis Root, Flos Daturae, Bulb of Thunberg Fritillary, Mung Bean, Daylily Root, Rhizoma Atractylodis, Mistletoe, Rhizoma Dioscoreae Bulbiferae, Smilacis Glabrae Rhizoma, Stigma Maydis, Farfarae Flos |
| nsp2 | Carthami Flos, Common Macrocarpium Fruit, Mori Folium, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Chinese Fevervine Herb, Fructus Rosae Laevigatae, Modern Rose | |
| nsp3 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Hippophae Fructus, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Perilla, Notopterygii Rhizoma Et Radix, Chinese Fevervine Herb | |
| nsp4 | Common Macrocarpium Fruit, Carthami Flos, Mori Folium, Peucedani Radix, Herba Glechomae, Modern Rose, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Fructus Rosae Laevigatae, Rhododendri Daurici Folium | |
| nsp5 | Forsythia Suspensa, Ginkgo Folium, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Honeysuckle Flower, Amur Corktree Bark, Chrysanthemum, Fructus Jujubae, Achyranthes Bidentata, Fagopyri Dibotryis Rhizoma | |
| nsp6 | Indigowoad Root, Amur Corktree Bark, Salviae Miltiorrhizae, Hyperici Perforati Herba, Smilacis Chinae Rhizoma, Allii Macrostemonis Bulbus, Curcumae Radix, Aucklandiae Radix, Asparagi Radix, Ginkgo Seed | |
| nsp7 | Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae | |
| nsp8 | Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae | |
| nsp9 | Indigo Naturalis, Sophorae Flavescentis Radix, Swertiae Mileensis Herba, Omphalia, Coptis Root, Flos Daturae, Bulb of Thunberg Fritillary, Mung Bean, Daylily Root, Rhizoma Atractylodis | |
| nsp10 | Amur Corktree Bark, Indigowoad Root, Salviae Miltiorrhizae, Smilacis Chinae Rhizoma, Hyperici Perforati Herba, Forsythia Suspensa, Herba Hyperici Japonici, Curcumae Radix, Achyranthes Bidentata, Mistletoe | |
| nsp12 | Common Macrocarpium Fruit, Carthami Flos, Mori Folium, Modern Rose, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Chinese Fevervine Herb | |
| nsp13 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Flower of Lobed Kudzuvine, Herba Glechomae, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Notopterygii Rhizoma Et Radix, Chinese Fevervine Herb | |
| nsp14 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Flower of Lobed Kudzuvine, Herba Glechomae, Herba Gnathali Affinis, Notopterygii Rhizoma Et Radix, Fructus Rosae Laevigatae, Perilla | |
| nsp15 | Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Rhizoma Dioscoreae Bulbiferae, Smilacis Glabrae Rhizoma, Chrysanthemum, Carthami Flos, Forsythia Suspensa, Stigma Maydis | |
| nsp16 | Common Macrocarpium Fruit, Ginkgo Folium, Modern Rose, Honeysuckle Flower, Chrysanthemum, Forsythia Suspensa, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Farfarae Flos, Smilacis Chinae Rhizoma |
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| nsp1 | Calendula officinalis, Common Cephalanoplos Herb, Meliae Cortex, Lasiosphaera, Inulae Herba, Black Nightshade Herb, Akebia Stem, Eupatorii Herba, Bistortae Rhizoma, Fruit of Tree-of-heaven Ailanthus | Calendula officinalis, Common Cephalanoplos Herb, Meliae Cortex, Lasiosphaera, Inulae Herba, Black Nightshade Herb, Akebia Stem, Eupatorii Herba, Bistortae Rhizoma, Fruit of Tree-of-heaven Ailanthus, Carthami Flos, Common Macrocarpium Fruit, Mori Folium, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Chinese Fevervine Herb, Fructus Rosae Laevigatae, Modern Rose, Hippophae Fructus, Perilla, Notopterygii Rhizoma Et Radix, Rhododendri Daurici Folium, Forsythia Suspensa, Ginkgo Folium, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Honeysuckle Flower, Amur Corktree Bark, Chrysanthemum, Fructus Jujubae, Achyranthes Bidentata, Fagopyri Dibotryis Rhizoma, Indigowoad Root, Salviae Miltiorrhizae, Hyperici Perforati Herba, Allii Macrostemonis Bulbus, Curcumae Radix, Aucklandiae Radix, Asparagi Radix, Ginkgo Seed, Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae, Indigo Naturalis, Sophorae Flavescentis Radix, Swertiae Mileensis Herba, Omphalia, Coptis Root, Flos Daturae, Bulb of Thunberg Fritillary, Mung Bean, Daylily Root, Rhizoma Atractylodis, Mistletoe, Rhizoma Dioscoreae Bulbiferae, Smilacis Glabrae Rhizoma, Stigma Maydis, Farfarae Flos |
| nsp2 | Carthami Flos, Common Macrocarpium Fruit, Mori Folium, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Chinese Fevervine Herb, Fructus Rosae Laevigatae, Modern Rose | |
| nsp3 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Hippophae Fructus, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Perilla, Notopterygii Rhizoma Et Radix, Chinese Fevervine Herb | |
| nsp4 | Common Macrocarpium Fruit, Carthami Flos, Mori Folium, Peucedani Radix, Herba Glechomae, Modern Rose, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Fructus Rosae Laevigatae, Rhododendri Daurici Folium | |
| nsp5 | Forsythia Suspensa, Ginkgo Folium, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Honeysuckle Flower, Amur Corktree Bark, Chrysanthemum, Fructus Jujubae, Achyranthes Bidentata, Fagopyri Dibotryis Rhizoma | |
| nsp6 | Indigowoad Root, Amur Corktree Bark, Salviae Miltiorrhizae, Hyperici Perforati Herba, Smilacis Chinae Rhizoma, Allii Macrostemonis Bulbus, Curcumae Radix, Aucklandiae Radix, Asparagi Radix, Ginkgo Seed | |
| nsp7 | Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae | |
| nsp8 | Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae | |
| nsp9 | Indigo Naturalis, Sophorae Flavescentis Radix, Swertiae Mileensis Herba, Omphalia, Coptis Root, Flos Daturae, Bulb of Thunberg Fritillary, Mung Bean, Daylily Root, Rhizoma Atractylodis | |
| nsp10 | Amur Corktree Bark, Indigowoad Root, Salviae Miltiorrhizae, Smilacis Chinae Rhizoma, Hyperici Perforati Herba, Forsythia Suspensa, Herba Hyperici Japonici, Curcumae Radix, Achyranthes Bidentata, Mistletoe | |
| nsp12 | Common Macrocarpium Fruit, Carthami Flos, Mori Folium, Modern Rose, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Chinese Fevervine Herb | |
| nsp13 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Flower of Lobed Kudzuvine, Herba Glechomae, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Notopterygii Rhizoma Et Radix, Chinese Fevervine Herb | |
| nsp14 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Flower of Lobed Kudzuvine, Herba Glechomae, Herba Gnathali Affinis, Notopterygii Rhizoma Et Radix, Fructus Rosae Laevigatae, Perilla | |
| nsp15 | Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Rhizoma Dioscoreae Bulbiferae, Smilacis Glabrae Rhizoma, Chrysanthemum, Carthami Flos, Forsythia Suspensa, Stigma Maydis | |
| nsp16 | Common Macrocarpium Fruit, Ginkgo Folium, Modern Rose, Honeysuckle Flower, Chrysanthemum, Forsythia Suspensa, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Farfarae Flos, Smilacis Chinae Rhizoma |
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| nsp1 | Calendula officinalis, Common Cephalanoplos Herb, Meliae Cortex, Lasiosphaera, Inulae Herba, Black Nightshade Herb, Akebia Stem, Eupatorii Herba, Bistortae Rhizoma, Fruit of Tree-of-heaven Ailanthus | Calendula officinalis, Common Cephalanoplos Herb, Meliae Cortex, Lasiosphaera, Inulae Herba, Black Nightshade Herb, Akebia Stem, Eupatorii Herba, Bistortae Rhizoma, Fruit of Tree-of-heaven Ailanthus, Carthami Flos, Common Macrocarpium Fruit, Mori Folium, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Chinese Fevervine Herb, Fructus Rosae Laevigatae, Modern Rose, Hippophae Fructus, Perilla, Notopterygii Rhizoma Et Radix, Rhododendri Daurici Folium, Forsythia Suspensa, Ginkgo Folium, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Honeysuckle Flower, Amur Corktree Bark, Chrysanthemum, Fructus Jujubae, Achyranthes Bidentata, Fagopyri Dibotryis Rhizoma, Indigowoad Root, Salviae Miltiorrhizae, Hyperici Perforati Herba, Allii Macrostemonis Bulbus, Curcumae Radix, Aucklandiae Radix, Asparagi Radix, Ginkgo Seed, Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae, Indigo Naturalis, Sophorae Flavescentis Radix, Swertiae Mileensis Herba, Omphalia, Coptis Root, Flos Daturae, Bulb of Thunberg Fritillary, Mung Bean, Daylily Root, Rhizoma Atractylodis, Mistletoe, Rhizoma Dioscoreae Bulbiferae, Smilacis Glabrae Rhizoma, Stigma Maydis, Farfarae Flos |
| nsp2 | Carthami Flos, Common Macrocarpium Fruit, Mori Folium, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Chinese Fevervine Herb, Fructus Rosae Laevigatae, Modern Rose | |
| nsp3 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Hippophae Fructus, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Perilla, Notopterygii Rhizoma Et Radix, Chinese Fevervine Herb | |
| nsp4 | Common Macrocarpium Fruit, Carthami Flos, Mori Folium, Peucedani Radix, Herba Glechomae, Modern Rose, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Fructus Rosae Laevigatae, Rhododendri Daurici Folium | |
| nsp5 | Forsythia Suspensa, Ginkgo Folium, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Honeysuckle Flower, Amur Corktree Bark, Chrysanthemum, Fructus Jujubae, Achyranthes Bidentata, Fagopyri Dibotryis Rhizoma | |
| nsp6 | Indigowoad Root, Amur Corktree Bark, Salviae Miltiorrhizae, Hyperici Perforati Herba, Smilacis Chinae Rhizoma, Allii Macrostemonis Bulbus, Curcumae Radix, Aucklandiae Radix, Asparagi Radix, Ginkgo Seed | |
| nsp7 | Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae | |
| nsp8 | Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae | |
| nsp9 | Indigo Naturalis, Sophorae Flavescentis Radix, Swertiae Mileensis Herba, Omphalia, Coptis Root, Flos Daturae, Bulb of Thunberg Fritillary, Mung Bean, Daylily Root, Rhizoma Atractylodis | |
| nsp10 | Amur Corktree Bark, Indigowoad Root, Salviae Miltiorrhizae, Smilacis Chinae Rhizoma, Hyperici Perforati Herba, Forsythia Suspensa, Herba Hyperici Japonici, Curcumae Radix, Achyranthes Bidentata, Mistletoe | |
| nsp12 | Common Macrocarpium Fruit, Carthami Flos, Mori Folium, Modern Rose, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Chinese Fevervine Herb | |
| nsp13 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Flower of Lobed Kudzuvine, Herba Glechomae, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Notopterygii Rhizoma Et Radix, Chinese Fevervine Herb | |
| nsp14 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Flower of Lobed Kudzuvine, Herba Glechomae, Herba Gnathali Affinis, Notopterygii Rhizoma Et Radix, Fructus Rosae Laevigatae, Perilla | |
| nsp15 | Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Rhizoma Dioscoreae Bulbiferae, Smilacis Glabrae Rhizoma, Chrysanthemum, Carthami Flos, Forsythia Suspensa, Stigma Maydis | |
| nsp16 | Common Macrocarpium Fruit, Ginkgo Folium, Modern Rose, Honeysuckle Flower, Chrysanthemum, Forsythia Suspensa, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Farfarae Flos, Smilacis Chinae Rhizoma |
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| nsp1 | Calendula officinalis, Common Cephalanoplos Herb, Meliae Cortex, Lasiosphaera, Inulae Herba, Black Nightshade Herb, Akebia Stem, Eupatorii Herba, Bistortae Rhizoma, Fruit of Tree-of-heaven Ailanthus | Calendula officinalis, Common Cephalanoplos Herb, Meliae Cortex, Lasiosphaera, Inulae Herba, Black Nightshade Herb, Akebia Stem, Eupatorii Herba, Bistortae Rhizoma, Fruit of Tree-of-heaven Ailanthus, Carthami Flos, Common Macrocarpium Fruit, Mori Folium, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Chinese Fevervine Herb, Fructus Rosae Laevigatae, Modern Rose, Hippophae Fructus, Perilla, Notopterygii Rhizoma Et Radix, Rhododendri Daurici Folium, Forsythia Suspensa, Ginkgo Folium, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Honeysuckle Flower, Amur Corktree Bark, Chrysanthemum, Fructus Jujubae, Achyranthes Bidentata, Fagopyri Dibotryis Rhizoma, Indigowoad Root, Salviae Miltiorrhizae, Hyperici Perforati Herba, Allii Macrostemonis Bulbus, Curcumae Radix, Aucklandiae Radix, Asparagi Radix, Ginkgo Seed, Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae, Indigo Naturalis, Sophorae Flavescentis Radix, Swertiae Mileensis Herba, Omphalia, Coptis Root, Flos Daturae, Bulb of Thunberg Fritillary, Mung Bean, Daylily Root, Rhizoma Atractylodis, Mistletoe, Rhizoma Dioscoreae Bulbiferae, Smilacis Glabrae Rhizoma, Stigma Maydis, Farfarae Flos |
| nsp2 | Carthami Flos, Common Macrocarpium Fruit, Mori Folium, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Chinese Fevervine Herb, Fructus Rosae Laevigatae, Modern Rose | |
| nsp3 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Hippophae Fructus, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Perilla, Notopterygii Rhizoma Et Radix, Chinese Fevervine Herb | |
| nsp4 | Common Macrocarpium Fruit, Carthami Flos, Mori Folium, Peucedani Radix, Herba Glechomae, Modern Rose, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Fructus Rosae Laevigatae, Rhododendri Daurici Folium | |
| nsp5 | Forsythia Suspensa, Ginkgo Folium, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Honeysuckle Flower, Amur Corktree Bark, Chrysanthemum, Fructus Jujubae, Achyranthes Bidentata, Fagopyri Dibotryis Rhizoma | |
| nsp6 | Indigowoad Root, Amur Corktree Bark, Salviae Miltiorrhizae, Hyperici Perforati Herba, Smilacis Chinae Rhizoma, Allii Macrostemonis Bulbus, Curcumae Radix, Aucklandiae Radix, Asparagi Radix, Ginkgo Seed | |
| nsp7 | Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae | |
| nsp8 | Caulis Trachelospermi, Coralhead Plant Seed, Strychni Semen, Ecliptae Herba, Rhubarb, Zanthoxyli Radix, Anemarrhenae Rhizoma, Commelinae Herba, Platycodonis Radix, Radix Gentianae | |
| nsp9 | Indigo Naturalis, Sophorae Flavescentis Radix, Swertiae Mileensis Herba, Omphalia, Coptis Root, Flos Daturae, Bulb of Thunberg Fritillary, Mung Bean, Daylily Root, Rhizoma Atractylodis | |
| nsp10 | Amur Corktree Bark, Indigowoad Root, Salviae Miltiorrhizae, Smilacis Chinae Rhizoma, Hyperici Perforati Herba, Forsythia Suspensa, Herba Hyperici Japonici, Curcumae Radix, Achyranthes Bidentata, Mistletoe | |
| nsp12 | Common Macrocarpium Fruit, Carthami Flos, Mori Folium, Modern Rose, Herba Glechomae, Flower of Lobed Kudzuvine, Peucedani Radix, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Chinese Fevervine Herb | |
| nsp13 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Flower of Lobed Kudzuvine, Herba Glechomae, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Notopterygii Rhizoma Et Radix, Chinese Fevervine Herb | |
| nsp14 | Mori Folium, Carthami Flos, Common Macrocarpium Fruit, Peucedani Radix, Flower of Lobed Kudzuvine, Herba Glechomae, Herba Gnathali Affinis, Notopterygii Rhizoma Et Radix, Fructus Rosae Laevigatae, Perilla | |
| nsp15 | Common Macrocarpium Fruit, Modern Rose, Ginkgo Folium, Honeysuckle Flower, Rhizoma Dioscoreae Bulbiferae, Smilacis Glabrae Rhizoma, Chrysanthemum, Carthami Flos, Forsythia Suspensa, Stigma Maydis | |
| nsp16 | Common Macrocarpium Fruit, Ginkgo Folium, Modern Rose, Honeysuckle Flower, Chrysanthemum, Forsythia Suspensa, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Farfarae Flos, Smilacis Chinae Rhizoma |
Since |$\boldsymbol{y}^{(K)}$| is with the characteristic that the data lie in a low-dimensional manifold in a high-dimensional space, the loss function is learned by borrowing the idea from the Laplacian eigenmaps. Specifically, the loss function will be penalized when similar nodes are with dissimilar encoded embedding vectors. Accordingly, the Laplacian eigenmaps approach preserves the representations of two nodes connected by an edge that are relatively close in the embedding space.
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| orf3a | Ginkgo Folium, Common Macrocarpium Fruit, Modern Rose, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Herba Hyperici Japonici, Smilacis Chinae Rhizoma | Ginkgo Folium, Common Macrocarpium Fruit, Modern Rose, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Bletilla Striata, Arecae Pericarpium, Greater Calandine Herb, Garlic, Herba Solani Lyrati, Radix Physochlainae, Root of Garden Eggplant, Euphorbiae Pekinensis Radix, Cortex Erythrinae Seu Kalopanacis, Cyrtomium Rhizome, Stemonae Radix, Imperatae Rhizoma, Herbal Ephedra Root, Akebia Stem, Cynanchi Atrati Radix Et Rhizoma, Fruit of Tree-of-heaven Ailanthus, Beartiful Sweetgum Fruit, Cortex Periplocae, Kaki Calyx, Clematidis Radix Et Rhizoma, Hyperici Perforati Herba, Indigowoad Root, Ilex latifolia Thunb, Amur Corktree Bark, Platycladi Cacumen, Ginkgo Seed, Glycyrrhiza, Artemisiae Annuae Herba, Fritillariae Cirrhosae Bulbus, Curcumae Radix, Vigna umbellate, Radix Tinosporae, Aconiti Kusnezoffii Folium, Rhizome of Decumbent Corydalis, Polyporus, Radix Trichosanthis, Menispermi Rhizoma, Spina Date Seed, Spiderflower Seed |
| orf6 | Bletilla Striata, Arecae Pericarpium, Greater Calandine Herb, Garlic, Herba Solani Lyrati, Radix Physochlainae, Root of Garden Eggplant, Euphorbiae Pekinensis Radix, Cortex Erythrinae Seu Kalopanacis, Cyrtomium Rhizome | |
| orf7a | Stemonae Radix, Imperatae Rhizoma, Herbal Ephedra Root, Akebia Stem, Cynanchi Atrati Radix Et Rhizoma, Fruit of Tree-of-heaven Ailanthus, Beartiful Sweetgum Fruit, Cortex Periplocae, Kaki Calyx, Clematidis Radix Et Rhizoma | |
| orf8 | Hyperici Perforati Herba, Indigowoad Root, Ilex latifolia Thunb, Amur Corktree Bark, Platycladi Cacumen, Ginkgo Seed, Glycyrrhiza, Artemisiae Annuae Herba, Fritillariae Cirrhosae Bulbus, Curcumae Radix | |
| orf10 | Vigna umbellate, Radix Tinosporae, Aconiti Kusnezoffii Folium, Rhizome of Decumbent Corydalis, Polyporus, Radix Trichosanthis, Menispermi Rhizoma, Spina Date Seed, Spiderflower Seed, Root of Garden Eggplant |
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| orf3a | Ginkgo Folium, Common Macrocarpium Fruit, Modern Rose, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Herba Hyperici Japonici, Smilacis Chinae Rhizoma | Ginkgo Folium, Common Macrocarpium Fruit, Modern Rose, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Bletilla Striata, Arecae Pericarpium, Greater Calandine Herb, Garlic, Herba Solani Lyrati, Radix Physochlainae, Root of Garden Eggplant, Euphorbiae Pekinensis Radix, Cortex Erythrinae Seu Kalopanacis, Cyrtomium Rhizome, Stemonae Radix, Imperatae Rhizoma, Herbal Ephedra Root, Akebia Stem, Cynanchi Atrati Radix Et Rhizoma, Fruit of Tree-of-heaven Ailanthus, Beartiful Sweetgum Fruit, Cortex Periplocae, Kaki Calyx, Clematidis Radix Et Rhizoma, Hyperici Perforati Herba, Indigowoad Root, Ilex latifolia Thunb, Amur Corktree Bark, Platycladi Cacumen, Ginkgo Seed, Glycyrrhiza, Artemisiae Annuae Herba, Fritillariae Cirrhosae Bulbus, Curcumae Radix, Vigna umbellate, Radix Tinosporae, Aconiti Kusnezoffii Folium, Rhizome of Decumbent Corydalis, Polyporus, Radix Trichosanthis, Menispermi Rhizoma, Spina Date Seed, Spiderflower Seed |
| orf6 | Bletilla Striata, Arecae Pericarpium, Greater Calandine Herb, Garlic, Herba Solani Lyrati, Radix Physochlainae, Root of Garden Eggplant, Euphorbiae Pekinensis Radix, Cortex Erythrinae Seu Kalopanacis, Cyrtomium Rhizome | |
| orf7a | Stemonae Radix, Imperatae Rhizoma, Herbal Ephedra Root, Akebia Stem, Cynanchi Atrati Radix Et Rhizoma, Fruit of Tree-of-heaven Ailanthus, Beartiful Sweetgum Fruit, Cortex Periplocae, Kaki Calyx, Clematidis Radix Et Rhizoma | |
| orf8 | Hyperici Perforati Herba, Indigowoad Root, Ilex latifolia Thunb, Amur Corktree Bark, Platycladi Cacumen, Ginkgo Seed, Glycyrrhiza, Artemisiae Annuae Herba, Fritillariae Cirrhosae Bulbus, Curcumae Radix | |
| orf10 | Vigna umbellate, Radix Tinosporae, Aconiti Kusnezoffii Folium, Rhizome of Decumbent Corydalis, Polyporus, Radix Trichosanthis, Menispermi Rhizoma, Spina Date Seed, Spiderflower Seed, Root of Garden Eggplant |
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| orf3a | Ginkgo Folium, Common Macrocarpium Fruit, Modern Rose, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Herba Hyperici Japonici, Smilacis Chinae Rhizoma | Ginkgo Folium, Common Macrocarpium Fruit, Modern Rose, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Bletilla Striata, Arecae Pericarpium, Greater Calandine Herb, Garlic, Herba Solani Lyrati, Radix Physochlainae, Root of Garden Eggplant, Euphorbiae Pekinensis Radix, Cortex Erythrinae Seu Kalopanacis, Cyrtomium Rhizome, Stemonae Radix, Imperatae Rhizoma, Herbal Ephedra Root, Akebia Stem, Cynanchi Atrati Radix Et Rhizoma, Fruit of Tree-of-heaven Ailanthus, Beartiful Sweetgum Fruit, Cortex Periplocae, Kaki Calyx, Clematidis Radix Et Rhizoma, Hyperici Perforati Herba, Indigowoad Root, Ilex latifolia Thunb, Amur Corktree Bark, Platycladi Cacumen, Ginkgo Seed, Glycyrrhiza, Artemisiae Annuae Herba, Fritillariae Cirrhosae Bulbus, Curcumae Radix, Vigna umbellate, Radix Tinosporae, Aconiti Kusnezoffii Folium, Rhizome of Decumbent Corydalis, Polyporus, Radix Trichosanthis, Menispermi Rhizoma, Spina Date Seed, Spiderflower Seed |
| orf6 | Bletilla Striata, Arecae Pericarpium, Greater Calandine Herb, Garlic, Herba Solani Lyrati, Radix Physochlainae, Root of Garden Eggplant, Euphorbiae Pekinensis Radix, Cortex Erythrinae Seu Kalopanacis, Cyrtomium Rhizome | |
| orf7a | Stemonae Radix, Imperatae Rhizoma, Herbal Ephedra Root, Akebia Stem, Cynanchi Atrati Radix Et Rhizoma, Fruit of Tree-of-heaven Ailanthus, Beartiful Sweetgum Fruit, Cortex Periplocae, Kaki Calyx, Clematidis Radix Et Rhizoma | |
| orf8 | Hyperici Perforati Herba, Indigowoad Root, Ilex latifolia Thunb, Amur Corktree Bark, Platycladi Cacumen, Ginkgo Seed, Glycyrrhiza, Artemisiae Annuae Herba, Fritillariae Cirrhosae Bulbus, Curcumae Radix | |
| orf10 | Vigna umbellate, Radix Tinosporae, Aconiti Kusnezoffii Folium, Rhizome of Decumbent Corydalis, Polyporus, Radix Trichosanthis, Menispermi Rhizoma, Spina Date Seed, Spiderflower Seed, Root of Garden Eggplant |
| Protein . | Top 10 herbs . | Unique drug set . |
|---|---|---|
| orf3a | Ginkgo Folium, Common Macrocarpium Fruit, Modern Rose, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Herba Hyperici Japonici, Smilacis Chinae Rhizoma | Ginkgo Folium, Common Macrocarpium Fruit, Modern Rose, Honeysuckle Flower, Forsythia Suspensa, Chrysanthemum, Rhizoma Dioscoreae Bulbiferae, Stigma Maydis, Herba Hyperici Japonici, Smilacis Chinae Rhizoma, Bletilla Striata, Arecae Pericarpium, Greater Calandine Herb, Garlic, Herba Solani Lyrati, Radix Physochlainae, Root of Garden Eggplant, Euphorbiae Pekinensis Radix, Cortex Erythrinae Seu Kalopanacis, Cyrtomium Rhizome, Stemonae Radix, Imperatae Rhizoma, Herbal Ephedra Root, Akebia Stem, Cynanchi Atrati Radix Et Rhizoma, Fruit of Tree-of-heaven Ailanthus, Beartiful Sweetgum Fruit, Cortex Periplocae, Kaki Calyx, Clematidis Radix Et Rhizoma, Hyperici Perforati Herba, Indigowoad Root, Ilex latifolia Thunb, Amur Corktree Bark, Platycladi Cacumen, Ginkgo Seed, Glycyrrhiza, Artemisiae Annuae Herba, Fritillariae Cirrhosae Bulbus, Curcumae Radix, Vigna umbellate, Radix Tinosporae, Aconiti Kusnezoffii Folium, Rhizome of Decumbent Corydalis, Polyporus, Radix Trichosanthis, Menispermi Rhizoma, Spina Date Seed, Spiderflower Seed |
| orf6 | Bletilla Striata, Arecae Pericarpium, Greater Calandine Herb, Garlic, Herba Solani Lyrati, Radix Physochlainae, Root of Garden Eggplant, Euphorbiae Pekinensis Radix, Cortex Erythrinae Seu Kalopanacis, Cyrtomium Rhizome | |
| orf7a | Stemonae Radix, Imperatae Rhizoma, Herbal Ephedra Root, Akebia Stem, Cynanchi Atrati Radix Et Rhizoma, Fruit of Tree-of-heaven Ailanthus, Beartiful Sweetgum Fruit, Cortex Periplocae, Kaki Calyx, Clematidis Radix Et Rhizoma | |
| orf8 | Hyperici Perforati Herba, Indigowoad Root, Ilex latifolia Thunb, Amur Corktree Bark, Platycladi Cacumen, Ginkgo Seed, Glycyrrhiza, Artemisiae Annuae Herba, Fritillariae Cirrhosae Bulbus, Curcumae Radix | |
| orf10 | Vigna umbellate, Radix Tinosporae, Aconiti Kusnezoffii Folium, Rhizome of Decumbent Corydalis, Polyporus, Radix Trichosanthis, Menispermi Rhizoma, Spina Date Seed, Spiderflower Seed, Root of Garden Eggplant |
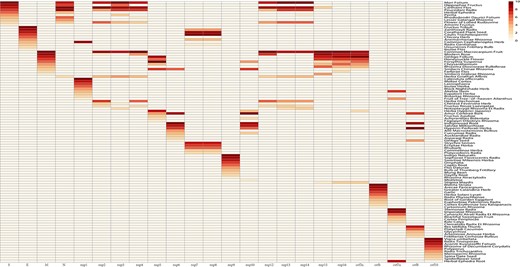
Herb cluster heatmap based on SARS-CoV-2 proteins. The darker the red color is, the closer the distance between the herb and protein.
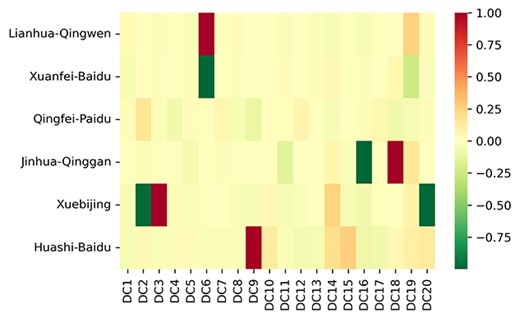
Heatmap of describing the similarity between recommended 20 herb combinations and the six official issued (3F3M) drug combinations. The recommended herb combinations are recorded in Table 5, and the details are presented in the Supplementary file S6.
5.4.4 Optimization
6 Recommending personalized candidate herb combinations
6.1 Overview
Since the clinical symptoms of SARS-CoV-2 divided into mild, moderate, sever and critical cases [17], the six officially issued herb combinations (3F3M) have certain effects for the specific infected stage that is shown in Table 6. And the descriptions of 3F3M are presented in Supplement S3.
| Index . | Herb combinations . |
|---|---|
| DC1 | Mori Folium, Fructus Tribuli, Common Macrocarpium Fruit, Carthami Flos, Calendula officinalis, Forsythia Suspensa, Indigowoad Root, Caulis Trachelospermi, Indigo Naturalis, Amur Corktree Bark, Ginkgo Folium, Bletilla Striata, Stemonae Radix, Hyperici Perforati Herba, Vigna umbellata |
| DC2 | Hippophae Fructus, Zanthoxyli Radix, Modern Rose, Peucedani Radix, Common Cephalanoplos Herb, Common Macrocarpium Fruit, Carthami Flos, Ginkgo Folium, Amur Corktree Bark, Coralhead Plant Seed, Sophorae Flavescentis Radix, Indigowoad Root, Arecae Pericarpium, Imperatae Rhizoma, Radix Tinosporae |
| DC3 | Carthami Flos, Coralhead Plant Seed, Ginkgo Folium, Mori Folium, Meliae Cortex, Herba Hyperici Japonici, Strychni Semen, Swertiae Mileensis Herba, Salviae Miltiorrhizae, Common Macrocarpium Fruit, Modern Rose, Greater Calandine Herb, Herbal Ephedra Root, Ilex latifolia Thunb, Aconiti Kusnezoffii Folium |
| DC4 | Peucedani Radix, Caulis Trachelospermi, Honeysuckle Flower, Hippophae Fructus, Lasiosphaera, Herba Glechomae, Smilacis Chinae Rhizoma, Ecliptae Herba, Hyperici Perforati Herba, Omphalia, Modern Rose, Garlic, Akebia Stem, Amur Corktree Bark, Rhizome of Decumbent Corydalis |
| DC5 | Herbal Ephedra, Chicory Herb, Forsythia Suspensa, Rhododendri Daurici Folium, Inulae Herba, Hippophae Fructus, Honeysuckle Flower, Smilacis Chinae Rhizoma, Rhubarb, Coptis Root, Hyperici Perforati Herba, Herba Glechomae, Flower of Lobed Kudzuvine, Rhizoma Dioscoreae Bulbiferae, Chrysanthemum, Herba Solani Lyrati, Cynanchi Atrati Radix Et Rhizoma, Platycladi Cacumen, Polyporus |
| DC6 | Perilla, Anemarrhenae Rhizoma, Chrysanthemum, Lesser Galangal Rhizome, Black Nightshade Herb, Peucedani Radix, Herba Gnathali Affinis, Modern Rose, Amur Corktree Bark, Allii Macrostemonis Bulbus, Zanthoxyli Radix, Forsythia Suspensa, Flos Daturae, Flower of Lobed Kudzuvine, Herba Glechomae, Smilacis Glabrae Rhizoma, Radix Physochlainae, Fruit of Tree-of-heaven Ailanthus, Ginkgo Seed, Radix Trichosanthis |
| DC7 | Rhododendri Daurici Folium, Common Cephalanoplos Herb, Rhizoma Dioscoreae Bulbiferae, Herbal Ephedra, Akebia Stem, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Chrysanthemum, Curcumae Radix, Anemarrhenae Rhizoma, Bulb of Thunberg Fritillary, Herba Hyperici Japonici, Peucedani Radix, Root of Garden Eggplant, Beartiful Sweetgum Fruit, Glycyrrhiza, Menispermi Rhizoma |
| DC8 | Lesser Galangal Rhizome, Radix Gentianae, Smilacis Chinae Rhizoma, Flower of Lobed Kudzuvine, Eupatorii Herba, Chinese Fevervine Herb, Perilla, Fructus Jujubae, Aucklandiae Radix, Commelinae Herba, Mung Bean, Curcumae Radix, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Notopterygii Rhizoma Et Radix, Carthami Flos, Stigma Maydis, Euphorbiae Pekinensis Radix, Cortex Periplocae, Artemisiae Annuae Herba |
| DC9 | Flower of Lobed Kudzuvine, Ussuriensis Fritillary Bulb, Farfarae Flos, Herba Gnathali Affinis, Bistortae Rhizoma, Fructus Rosae Laevigatae, Achyranthes Bidentata, Asparagi Radix, Platycodonis Radix, Daylily Root, Forsythia Suspensa, Herba Hyperici Japonici, Notopterygii Rhizoma Et Radix, Cortex Erythrinae Seu Kalopanacis, Kaki Calyx, Fritillariae Cirrhosae Bulbus, Spiderflower Seed |
| DC10 | Amomi Fructus, Inulae Flos, Perilla, Smilacis Glabrae Rhizoma, Modern Rose, Chinese Fevervine Herb, Fruit of Tree-of-heaven Ailanthus, Rhododendri Daurici Folium, Fagopyri Dibotryis Rhizoma, Radix Gentianae, Ginkgo Seed, Rhizoma Atractylodis, Mistletoe, Stigma Maydis, Smilacis Chinae Rhizoma, Cyrtomium Rhizome, Clematidis Radix Et Rhizoma, Curcumae Radix, Root of Garden Eggplant |
| DC11 | Notopterygii Rhizoma Et Radix, Eupatorii Herba, Fructus Rosae Laevigatae, Achyranthes Bidentata, Imperatae Rhizoma, Mistletoe, Platycladi Cacumen, Lemongrass Herb, Anemones Raddeanae Rhizoma, Fimbriate Orostachys, Modern Rose, Chinese Fevervine Herb, Herba Glechomae, Herba Hyperici Japonici, Smilacis Glabrae Rhizoma, Cow-Bezoar, India Madder Root, Aucklandiae Radix, Greater Calandine Herb |
| DC12 | Inulae Herba, Grassleaf Sweetflag Rhizome, Stigma Maydis, Palmleaf Raspberry Fruit, Rhododendri Daurici Folium, Fructus Rosae Laevigatae, Cortex Phellodendri Chinensis, Modern Rose, Grosvenor Momordica Fruit, Yanhusuo, Vietnamese Sophora Root, Hydnocarpus anthelmintica Pier, Fagopyri Dibotryis Rhizoma, Perilla, Hippophae Fructus, Smilacis Glabrae Rhizoma, Germinated Barley, Achyranthes Bidentata, Spiderflower Seed, Common Fenugreek Seed, Epimedium Herb, Arecae Pericarpium |
| DC13 | Herba Gnathali Affinis, Fourleaf Ladybell Root, Tatarian Aster Root, Herba Glechomae, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Kaki Calyx, Germinated Barley, Salviae Miltiorrhizae, Dyers Woad Leaf, Forsythia Suspensa, Tabasheer, Fructus Jujubae, Grosvenor Momordica Fruit, Farfarae Flos, Mistletoe, Ricepaperplant Pith, Sanqi, Giant Knotweed Rhizome, Garlic |
| DC14 | Lasiosphaera, Peppermint, Mistletoe, Stemonae Radix, Smilacis Glabrae Rhizoma, Lesser Galangal Rhizome, Notopterygii Rhizoma Et Radix, Stigma Maydis, Fimbriate Orostachys, Fructus Tribuli, Golden Larch Bark, Allii Macrostemonis Bulbus, Perilla, Hippophae Fructus, Grosvenor Momordica Fruit, Raisin Tree Seed, Tatarian Aster Root, Fructus Jujubae, Mulberry Twig, Largeleaf Gentian Root, Salviae Miltiorrhizae, Bletilla Striata |
| DC15 | Chinese Fevervine Herb, Fourstamen Stephania Root, Raisin Tree Seed, Glabrous Sarcandra Herb, Rhizoma Polygonati, Perilla, Grosvenor Momordica Fruit, Lesser Galangal Rhizome, Humuli Scandentis Herba, Giant Knotweed Rhizome, Lotus Plumule, Green Tangerine Peel, Dogbane Leaf, Rhododendri Daurici Folium, Smilacis Glabrae Rhizoma, Modern Rose, Mori Folium, Chinese Honeylocust Spine, Tatarian Aster Root, Cicada Slough, Fortune’s Drynaria Rhizome, Herba Solani Lyrati |
| DC16 | Fineleaf Schizonepeta Herb, Meliae Cortex, Herba Hyperici Japonici, Notopterygii Rhizoma Et Radix, Beartiful Sweetgum Fruit, Pharbitis Seed, Peppermint, Farfarae Flos, Perilla, Benzoin, Artemisiae Annuae Herba, Nelumbinis Receptaculum, Ginkgo Folium, Grosvenor Momordica Fruit, Lesser Galangal Rhizome, Tatarian Aster Root, Raisin Tree Seed, Kadsura Pepper Stem, Akebia Stem, Dried Ginger, Radix Physochlainae |
| DC17 | Herba Glechomae, Bistortae Rhizoma, Chinese Honeylocust Spine, Amomi Fructus, Large Thistle, Grosvenor Momordica Fruit, Herbal Ephedra, Smilacis Glabrae Rhizoma, Rhizoma Dioscoreae Bulbiferae, Ilex latifolia Thunb, Indian Stringbush Root, Ricini Semen, Gambir Plant Nod, Pharbitis Seed, Herba Euphorbiae Helioscopiae, Achyranthes Bidentata, Spina Date Seed, Chinese Cinquefoil, Wild Chrysanthemum, Euphorbiae Pekinensis Radix |
| DC18 | Palmleaf Raspberry Fruit, Platycodonis Radix, Ilicis Chinensis Folium, Fructus Jujubae, Himalayan Teasel Root, Hippophae Fructus, Pharbitis Seed, Dogbane Leaf, Tree-of-Heaven Bark, Chicory Herb, Herb of Crescent-shaped Euphorbia, Asparagi Radix, Notopterygii Rhizoma Et Radix, Lesser Galangal Rhizome, Smilacis Glabrae Rhizoma, Smilacis Chinae Rhizoma, Humuli Scandentis Herba, Stem Pith of Himalayan Stachyurus, Common Fenugreek Seed, Cortex Erythrinae Seu Kalopanacis |
| DC19 | Fructus Rosae Laevigatae, Blackberrylily Rhizome, Herbal Ephedra Root, Descolor Cinquefoil Herb, Common Macrocarpium Fruit, Medicinal Evodia Fruit, Perilla Fruit, Hippophae Fructus, Red Peony Root, Figwort Root, Herba Selaginellae Doederleinii, Bomeol, Lesser Galangal Rhizome, Germinated Barley, Peppermint, Flower of Lobed Kudzuvine, Humuli Scandentis Herba, Fagopyri Dibotryis Rhizoma, Japanese Ginseng, Chinese Pulsatilla Root, Ramulus Euonymi Alati, Cyrtomium Rhizome |
| DC20 | Japanese Ampelopsis Root, Benzoin, Glossy Privet Fruit, Coix Seed, Amomi Fructus, Chinese Fevervine Herb, Purslane Herb, Wild Chrysanthemum, Fourleaf Ladybell Root, Purslane Speedwell Herb, Yanhusuo, Rhizoma Dioscoreae Bulbiferae, Morinda Root, Germinated Barley, Humuli Scandentis Herba, Mistletoe, Chinese Gall, Calendula officinalis, Ginseng, Cow-Bezoar |
| Index . | Herb combinations . |
|---|---|
| DC1 | Mori Folium, Fructus Tribuli, Common Macrocarpium Fruit, Carthami Flos, Calendula officinalis, Forsythia Suspensa, Indigowoad Root, Caulis Trachelospermi, Indigo Naturalis, Amur Corktree Bark, Ginkgo Folium, Bletilla Striata, Stemonae Radix, Hyperici Perforati Herba, Vigna umbellata |
| DC2 | Hippophae Fructus, Zanthoxyli Radix, Modern Rose, Peucedani Radix, Common Cephalanoplos Herb, Common Macrocarpium Fruit, Carthami Flos, Ginkgo Folium, Amur Corktree Bark, Coralhead Plant Seed, Sophorae Flavescentis Radix, Indigowoad Root, Arecae Pericarpium, Imperatae Rhizoma, Radix Tinosporae |
| DC3 | Carthami Flos, Coralhead Plant Seed, Ginkgo Folium, Mori Folium, Meliae Cortex, Herba Hyperici Japonici, Strychni Semen, Swertiae Mileensis Herba, Salviae Miltiorrhizae, Common Macrocarpium Fruit, Modern Rose, Greater Calandine Herb, Herbal Ephedra Root, Ilex latifolia Thunb, Aconiti Kusnezoffii Folium |
| DC4 | Peucedani Radix, Caulis Trachelospermi, Honeysuckle Flower, Hippophae Fructus, Lasiosphaera, Herba Glechomae, Smilacis Chinae Rhizoma, Ecliptae Herba, Hyperici Perforati Herba, Omphalia, Modern Rose, Garlic, Akebia Stem, Amur Corktree Bark, Rhizome of Decumbent Corydalis |
| DC5 | Herbal Ephedra, Chicory Herb, Forsythia Suspensa, Rhododendri Daurici Folium, Inulae Herba, Hippophae Fructus, Honeysuckle Flower, Smilacis Chinae Rhizoma, Rhubarb, Coptis Root, Hyperici Perforati Herba, Herba Glechomae, Flower of Lobed Kudzuvine, Rhizoma Dioscoreae Bulbiferae, Chrysanthemum, Herba Solani Lyrati, Cynanchi Atrati Radix Et Rhizoma, Platycladi Cacumen, Polyporus |
| DC6 | Perilla, Anemarrhenae Rhizoma, Chrysanthemum, Lesser Galangal Rhizome, Black Nightshade Herb, Peucedani Radix, Herba Gnathali Affinis, Modern Rose, Amur Corktree Bark, Allii Macrostemonis Bulbus, Zanthoxyli Radix, Forsythia Suspensa, Flos Daturae, Flower of Lobed Kudzuvine, Herba Glechomae, Smilacis Glabrae Rhizoma, Radix Physochlainae, Fruit of Tree-of-heaven Ailanthus, Ginkgo Seed, Radix Trichosanthis |
| DC7 | Rhododendri Daurici Folium, Common Cephalanoplos Herb, Rhizoma Dioscoreae Bulbiferae, Herbal Ephedra, Akebia Stem, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Chrysanthemum, Curcumae Radix, Anemarrhenae Rhizoma, Bulb of Thunberg Fritillary, Herba Hyperici Japonici, Peucedani Radix, Root of Garden Eggplant, Beartiful Sweetgum Fruit, Glycyrrhiza, Menispermi Rhizoma |
| DC8 | Lesser Galangal Rhizome, Radix Gentianae, Smilacis Chinae Rhizoma, Flower of Lobed Kudzuvine, Eupatorii Herba, Chinese Fevervine Herb, Perilla, Fructus Jujubae, Aucklandiae Radix, Commelinae Herba, Mung Bean, Curcumae Radix, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Notopterygii Rhizoma Et Radix, Carthami Flos, Stigma Maydis, Euphorbiae Pekinensis Radix, Cortex Periplocae, Artemisiae Annuae Herba |
| DC9 | Flower of Lobed Kudzuvine, Ussuriensis Fritillary Bulb, Farfarae Flos, Herba Gnathali Affinis, Bistortae Rhizoma, Fructus Rosae Laevigatae, Achyranthes Bidentata, Asparagi Radix, Platycodonis Radix, Daylily Root, Forsythia Suspensa, Herba Hyperici Japonici, Notopterygii Rhizoma Et Radix, Cortex Erythrinae Seu Kalopanacis, Kaki Calyx, Fritillariae Cirrhosae Bulbus, Spiderflower Seed |
| DC10 | Amomi Fructus, Inulae Flos, Perilla, Smilacis Glabrae Rhizoma, Modern Rose, Chinese Fevervine Herb, Fruit of Tree-of-heaven Ailanthus, Rhododendri Daurici Folium, Fagopyri Dibotryis Rhizoma, Radix Gentianae, Ginkgo Seed, Rhizoma Atractylodis, Mistletoe, Stigma Maydis, Smilacis Chinae Rhizoma, Cyrtomium Rhizome, Clematidis Radix Et Rhizoma, Curcumae Radix, Root of Garden Eggplant |
| DC11 | Notopterygii Rhizoma Et Radix, Eupatorii Herba, Fructus Rosae Laevigatae, Achyranthes Bidentata, Imperatae Rhizoma, Mistletoe, Platycladi Cacumen, Lemongrass Herb, Anemones Raddeanae Rhizoma, Fimbriate Orostachys, Modern Rose, Chinese Fevervine Herb, Herba Glechomae, Herba Hyperici Japonici, Smilacis Glabrae Rhizoma, Cow-Bezoar, India Madder Root, Aucklandiae Radix, Greater Calandine Herb |
| DC12 | Inulae Herba, Grassleaf Sweetflag Rhizome, Stigma Maydis, Palmleaf Raspberry Fruit, Rhododendri Daurici Folium, Fructus Rosae Laevigatae, Cortex Phellodendri Chinensis, Modern Rose, Grosvenor Momordica Fruit, Yanhusuo, Vietnamese Sophora Root, Hydnocarpus anthelmintica Pier, Fagopyri Dibotryis Rhizoma, Perilla, Hippophae Fructus, Smilacis Glabrae Rhizoma, Germinated Barley, Achyranthes Bidentata, Spiderflower Seed, Common Fenugreek Seed, Epimedium Herb, Arecae Pericarpium |
| DC13 | Herba Gnathali Affinis, Fourleaf Ladybell Root, Tatarian Aster Root, Herba Glechomae, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Kaki Calyx, Germinated Barley, Salviae Miltiorrhizae, Dyers Woad Leaf, Forsythia Suspensa, Tabasheer, Fructus Jujubae, Grosvenor Momordica Fruit, Farfarae Flos, Mistletoe, Ricepaperplant Pith, Sanqi, Giant Knotweed Rhizome, Garlic |
| DC14 | Lasiosphaera, Peppermint, Mistletoe, Stemonae Radix, Smilacis Glabrae Rhizoma, Lesser Galangal Rhizome, Notopterygii Rhizoma Et Radix, Stigma Maydis, Fimbriate Orostachys, Fructus Tribuli, Golden Larch Bark, Allii Macrostemonis Bulbus, Perilla, Hippophae Fructus, Grosvenor Momordica Fruit, Raisin Tree Seed, Tatarian Aster Root, Fructus Jujubae, Mulberry Twig, Largeleaf Gentian Root, Salviae Miltiorrhizae, Bletilla Striata |
| DC15 | Chinese Fevervine Herb, Fourstamen Stephania Root, Raisin Tree Seed, Glabrous Sarcandra Herb, Rhizoma Polygonati, Perilla, Grosvenor Momordica Fruit, Lesser Galangal Rhizome, Humuli Scandentis Herba, Giant Knotweed Rhizome, Lotus Plumule, Green Tangerine Peel, Dogbane Leaf, Rhododendri Daurici Folium, Smilacis Glabrae Rhizoma, Modern Rose, Mori Folium, Chinese Honeylocust Spine, Tatarian Aster Root, Cicada Slough, Fortune’s Drynaria Rhizome, Herba Solani Lyrati |
| DC16 | Fineleaf Schizonepeta Herb, Meliae Cortex, Herba Hyperici Japonici, Notopterygii Rhizoma Et Radix, Beartiful Sweetgum Fruit, Pharbitis Seed, Peppermint, Farfarae Flos, Perilla, Benzoin, Artemisiae Annuae Herba, Nelumbinis Receptaculum, Ginkgo Folium, Grosvenor Momordica Fruit, Lesser Galangal Rhizome, Tatarian Aster Root, Raisin Tree Seed, Kadsura Pepper Stem, Akebia Stem, Dried Ginger, Radix Physochlainae |
| DC17 | Herba Glechomae, Bistortae Rhizoma, Chinese Honeylocust Spine, Amomi Fructus, Large Thistle, Grosvenor Momordica Fruit, Herbal Ephedra, Smilacis Glabrae Rhizoma, Rhizoma Dioscoreae Bulbiferae, Ilex latifolia Thunb, Indian Stringbush Root, Ricini Semen, Gambir Plant Nod, Pharbitis Seed, Herba Euphorbiae Helioscopiae, Achyranthes Bidentata, Spina Date Seed, Chinese Cinquefoil, Wild Chrysanthemum, Euphorbiae Pekinensis Radix |
| DC18 | Palmleaf Raspberry Fruit, Platycodonis Radix, Ilicis Chinensis Folium, Fructus Jujubae, Himalayan Teasel Root, Hippophae Fructus, Pharbitis Seed, Dogbane Leaf, Tree-of-Heaven Bark, Chicory Herb, Herb of Crescent-shaped Euphorbia, Asparagi Radix, Notopterygii Rhizoma Et Radix, Lesser Galangal Rhizome, Smilacis Glabrae Rhizoma, Smilacis Chinae Rhizoma, Humuli Scandentis Herba, Stem Pith of Himalayan Stachyurus, Common Fenugreek Seed, Cortex Erythrinae Seu Kalopanacis |
| DC19 | Fructus Rosae Laevigatae, Blackberrylily Rhizome, Herbal Ephedra Root, Descolor Cinquefoil Herb, Common Macrocarpium Fruit, Medicinal Evodia Fruit, Perilla Fruit, Hippophae Fructus, Red Peony Root, Figwort Root, Herba Selaginellae Doederleinii, Bomeol, Lesser Galangal Rhizome, Germinated Barley, Peppermint, Flower of Lobed Kudzuvine, Humuli Scandentis Herba, Fagopyri Dibotryis Rhizoma, Japanese Ginseng, Chinese Pulsatilla Root, Ramulus Euonymi Alati, Cyrtomium Rhizome |
| DC20 | Japanese Ampelopsis Root, Benzoin, Glossy Privet Fruit, Coix Seed, Amomi Fructus, Chinese Fevervine Herb, Purslane Herb, Wild Chrysanthemum, Fourleaf Ladybell Root, Purslane Speedwell Herb, Yanhusuo, Rhizoma Dioscoreae Bulbiferae, Morinda Root, Germinated Barley, Humuli Scandentis Herba, Mistletoe, Chinese Gall, Calendula officinalis, Ginseng, Cow-Bezoar |
| Index . | Herb combinations . |
|---|---|
| DC1 | Mori Folium, Fructus Tribuli, Common Macrocarpium Fruit, Carthami Flos, Calendula officinalis, Forsythia Suspensa, Indigowoad Root, Caulis Trachelospermi, Indigo Naturalis, Amur Corktree Bark, Ginkgo Folium, Bletilla Striata, Stemonae Radix, Hyperici Perforati Herba, Vigna umbellata |
| DC2 | Hippophae Fructus, Zanthoxyli Radix, Modern Rose, Peucedani Radix, Common Cephalanoplos Herb, Common Macrocarpium Fruit, Carthami Flos, Ginkgo Folium, Amur Corktree Bark, Coralhead Plant Seed, Sophorae Flavescentis Radix, Indigowoad Root, Arecae Pericarpium, Imperatae Rhizoma, Radix Tinosporae |
| DC3 | Carthami Flos, Coralhead Plant Seed, Ginkgo Folium, Mori Folium, Meliae Cortex, Herba Hyperici Japonici, Strychni Semen, Swertiae Mileensis Herba, Salviae Miltiorrhizae, Common Macrocarpium Fruit, Modern Rose, Greater Calandine Herb, Herbal Ephedra Root, Ilex latifolia Thunb, Aconiti Kusnezoffii Folium |
| DC4 | Peucedani Radix, Caulis Trachelospermi, Honeysuckle Flower, Hippophae Fructus, Lasiosphaera, Herba Glechomae, Smilacis Chinae Rhizoma, Ecliptae Herba, Hyperici Perforati Herba, Omphalia, Modern Rose, Garlic, Akebia Stem, Amur Corktree Bark, Rhizome of Decumbent Corydalis |
| DC5 | Herbal Ephedra, Chicory Herb, Forsythia Suspensa, Rhododendri Daurici Folium, Inulae Herba, Hippophae Fructus, Honeysuckle Flower, Smilacis Chinae Rhizoma, Rhubarb, Coptis Root, Hyperici Perforati Herba, Herba Glechomae, Flower of Lobed Kudzuvine, Rhizoma Dioscoreae Bulbiferae, Chrysanthemum, Herba Solani Lyrati, Cynanchi Atrati Radix Et Rhizoma, Platycladi Cacumen, Polyporus |
| DC6 | Perilla, Anemarrhenae Rhizoma, Chrysanthemum, Lesser Galangal Rhizome, Black Nightshade Herb, Peucedani Radix, Herba Gnathali Affinis, Modern Rose, Amur Corktree Bark, Allii Macrostemonis Bulbus, Zanthoxyli Radix, Forsythia Suspensa, Flos Daturae, Flower of Lobed Kudzuvine, Herba Glechomae, Smilacis Glabrae Rhizoma, Radix Physochlainae, Fruit of Tree-of-heaven Ailanthus, Ginkgo Seed, Radix Trichosanthis |
| DC7 | Rhododendri Daurici Folium, Common Cephalanoplos Herb, Rhizoma Dioscoreae Bulbiferae, Herbal Ephedra, Akebia Stem, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Chrysanthemum, Curcumae Radix, Anemarrhenae Rhizoma, Bulb of Thunberg Fritillary, Herba Hyperici Japonici, Peucedani Radix, Root of Garden Eggplant, Beartiful Sweetgum Fruit, Glycyrrhiza, Menispermi Rhizoma |
| DC8 | Lesser Galangal Rhizome, Radix Gentianae, Smilacis Chinae Rhizoma, Flower of Lobed Kudzuvine, Eupatorii Herba, Chinese Fevervine Herb, Perilla, Fructus Jujubae, Aucklandiae Radix, Commelinae Herba, Mung Bean, Curcumae Radix, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Notopterygii Rhizoma Et Radix, Carthami Flos, Stigma Maydis, Euphorbiae Pekinensis Radix, Cortex Periplocae, Artemisiae Annuae Herba |
| DC9 | Flower of Lobed Kudzuvine, Ussuriensis Fritillary Bulb, Farfarae Flos, Herba Gnathali Affinis, Bistortae Rhizoma, Fructus Rosae Laevigatae, Achyranthes Bidentata, Asparagi Radix, Platycodonis Radix, Daylily Root, Forsythia Suspensa, Herba Hyperici Japonici, Notopterygii Rhizoma Et Radix, Cortex Erythrinae Seu Kalopanacis, Kaki Calyx, Fritillariae Cirrhosae Bulbus, Spiderflower Seed |
| DC10 | Amomi Fructus, Inulae Flos, Perilla, Smilacis Glabrae Rhizoma, Modern Rose, Chinese Fevervine Herb, Fruit of Tree-of-heaven Ailanthus, Rhododendri Daurici Folium, Fagopyri Dibotryis Rhizoma, Radix Gentianae, Ginkgo Seed, Rhizoma Atractylodis, Mistletoe, Stigma Maydis, Smilacis Chinae Rhizoma, Cyrtomium Rhizome, Clematidis Radix Et Rhizoma, Curcumae Radix, Root of Garden Eggplant |
| DC11 | Notopterygii Rhizoma Et Radix, Eupatorii Herba, Fructus Rosae Laevigatae, Achyranthes Bidentata, Imperatae Rhizoma, Mistletoe, Platycladi Cacumen, Lemongrass Herb, Anemones Raddeanae Rhizoma, Fimbriate Orostachys, Modern Rose, Chinese Fevervine Herb, Herba Glechomae, Herba Hyperici Japonici, Smilacis Glabrae Rhizoma, Cow-Bezoar, India Madder Root, Aucklandiae Radix, Greater Calandine Herb |
| DC12 | Inulae Herba, Grassleaf Sweetflag Rhizome, Stigma Maydis, Palmleaf Raspberry Fruit, Rhododendri Daurici Folium, Fructus Rosae Laevigatae, Cortex Phellodendri Chinensis, Modern Rose, Grosvenor Momordica Fruit, Yanhusuo, Vietnamese Sophora Root, Hydnocarpus anthelmintica Pier, Fagopyri Dibotryis Rhizoma, Perilla, Hippophae Fructus, Smilacis Glabrae Rhizoma, Germinated Barley, Achyranthes Bidentata, Spiderflower Seed, Common Fenugreek Seed, Epimedium Herb, Arecae Pericarpium |
| DC13 | Herba Gnathali Affinis, Fourleaf Ladybell Root, Tatarian Aster Root, Herba Glechomae, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Kaki Calyx, Germinated Barley, Salviae Miltiorrhizae, Dyers Woad Leaf, Forsythia Suspensa, Tabasheer, Fructus Jujubae, Grosvenor Momordica Fruit, Farfarae Flos, Mistletoe, Ricepaperplant Pith, Sanqi, Giant Knotweed Rhizome, Garlic |
| DC14 | Lasiosphaera, Peppermint, Mistletoe, Stemonae Radix, Smilacis Glabrae Rhizoma, Lesser Galangal Rhizome, Notopterygii Rhizoma Et Radix, Stigma Maydis, Fimbriate Orostachys, Fructus Tribuli, Golden Larch Bark, Allii Macrostemonis Bulbus, Perilla, Hippophae Fructus, Grosvenor Momordica Fruit, Raisin Tree Seed, Tatarian Aster Root, Fructus Jujubae, Mulberry Twig, Largeleaf Gentian Root, Salviae Miltiorrhizae, Bletilla Striata |
| DC15 | Chinese Fevervine Herb, Fourstamen Stephania Root, Raisin Tree Seed, Glabrous Sarcandra Herb, Rhizoma Polygonati, Perilla, Grosvenor Momordica Fruit, Lesser Galangal Rhizome, Humuli Scandentis Herba, Giant Knotweed Rhizome, Lotus Plumule, Green Tangerine Peel, Dogbane Leaf, Rhododendri Daurici Folium, Smilacis Glabrae Rhizoma, Modern Rose, Mori Folium, Chinese Honeylocust Spine, Tatarian Aster Root, Cicada Slough, Fortune’s Drynaria Rhizome, Herba Solani Lyrati |
| DC16 | Fineleaf Schizonepeta Herb, Meliae Cortex, Herba Hyperici Japonici, Notopterygii Rhizoma Et Radix, Beartiful Sweetgum Fruit, Pharbitis Seed, Peppermint, Farfarae Flos, Perilla, Benzoin, Artemisiae Annuae Herba, Nelumbinis Receptaculum, Ginkgo Folium, Grosvenor Momordica Fruit, Lesser Galangal Rhizome, Tatarian Aster Root, Raisin Tree Seed, Kadsura Pepper Stem, Akebia Stem, Dried Ginger, Radix Physochlainae |
| DC17 | Herba Glechomae, Bistortae Rhizoma, Chinese Honeylocust Spine, Amomi Fructus, Large Thistle, Grosvenor Momordica Fruit, Herbal Ephedra, Smilacis Glabrae Rhizoma, Rhizoma Dioscoreae Bulbiferae, Ilex latifolia Thunb, Indian Stringbush Root, Ricini Semen, Gambir Plant Nod, Pharbitis Seed, Herba Euphorbiae Helioscopiae, Achyranthes Bidentata, Spina Date Seed, Chinese Cinquefoil, Wild Chrysanthemum, Euphorbiae Pekinensis Radix |
| DC18 | Palmleaf Raspberry Fruit, Platycodonis Radix, Ilicis Chinensis Folium, Fructus Jujubae, Himalayan Teasel Root, Hippophae Fructus, Pharbitis Seed, Dogbane Leaf, Tree-of-Heaven Bark, Chicory Herb, Herb of Crescent-shaped Euphorbia, Asparagi Radix, Notopterygii Rhizoma Et Radix, Lesser Galangal Rhizome, Smilacis Glabrae Rhizoma, Smilacis Chinae Rhizoma, Humuli Scandentis Herba, Stem Pith of Himalayan Stachyurus, Common Fenugreek Seed, Cortex Erythrinae Seu Kalopanacis |
| DC19 | Fructus Rosae Laevigatae, Blackberrylily Rhizome, Herbal Ephedra Root, Descolor Cinquefoil Herb, Common Macrocarpium Fruit, Medicinal Evodia Fruit, Perilla Fruit, Hippophae Fructus, Red Peony Root, Figwort Root, Herba Selaginellae Doederleinii, Bomeol, Lesser Galangal Rhizome, Germinated Barley, Peppermint, Flower of Lobed Kudzuvine, Humuli Scandentis Herba, Fagopyri Dibotryis Rhizoma, Japanese Ginseng, Chinese Pulsatilla Root, Ramulus Euonymi Alati, Cyrtomium Rhizome |
| DC20 | Japanese Ampelopsis Root, Benzoin, Glossy Privet Fruit, Coix Seed, Amomi Fructus, Chinese Fevervine Herb, Purslane Herb, Wild Chrysanthemum, Fourleaf Ladybell Root, Purslane Speedwell Herb, Yanhusuo, Rhizoma Dioscoreae Bulbiferae, Morinda Root, Germinated Barley, Humuli Scandentis Herba, Mistletoe, Chinese Gall, Calendula officinalis, Ginseng, Cow-Bezoar |
| Index . | Herb combinations . |
|---|---|
| DC1 | Mori Folium, Fructus Tribuli, Common Macrocarpium Fruit, Carthami Flos, Calendula officinalis, Forsythia Suspensa, Indigowoad Root, Caulis Trachelospermi, Indigo Naturalis, Amur Corktree Bark, Ginkgo Folium, Bletilla Striata, Stemonae Radix, Hyperici Perforati Herba, Vigna umbellata |
| DC2 | Hippophae Fructus, Zanthoxyli Radix, Modern Rose, Peucedani Radix, Common Cephalanoplos Herb, Common Macrocarpium Fruit, Carthami Flos, Ginkgo Folium, Amur Corktree Bark, Coralhead Plant Seed, Sophorae Flavescentis Radix, Indigowoad Root, Arecae Pericarpium, Imperatae Rhizoma, Radix Tinosporae |
| DC3 | Carthami Flos, Coralhead Plant Seed, Ginkgo Folium, Mori Folium, Meliae Cortex, Herba Hyperici Japonici, Strychni Semen, Swertiae Mileensis Herba, Salviae Miltiorrhizae, Common Macrocarpium Fruit, Modern Rose, Greater Calandine Herb, Herbal Ephedra Root, Ilex latifolia Thunb, Aconiti Kusnezoffii Folium |
| DC4 | Peucedani Radix, Caulis Trachelospermi, Honeysuckle Flower, Hippophae Fructus, Lasiosphaera, Herba Glechomae, Smilacis Chinae Rhizoma, Ecliptae Herba, Hyperici Perforati Herba, Omphalia, Modern Rose, Garlic, Akebia Stem, Amur Corktree Bark, Rhizome of Decumbent Corydalis |
| DC5 | Herbal Ephedra, Chicory Herb, Forsythia Suspensa, Rhododendri Daurici Folium, Inulae Herba, Hippophae Fructus, Honeysuckle Flower, Smilacis Chinae Rhizoma, Rhubarb, Coptis Root, Hyperici Perforati Herba, Herba Glechomae, Flower of Lobed Kudzuvine, Rhizoma Dioscoreae Bulbiferae, Chrysanthemum, Herba Solani Lyrati, Cynanchi Atrati Radix Et Rhizoma, Platycladi Cacumen, Polyporus |
| DC6 | Perilla, Anemarrhenae Rhizoma, Chrysanthemum, Lesser Galangal Rhizome, Black Nightshade Herb, Peucedani Radix, Herba Gnathali Affinis, Modern Rose, Amur Corktree Bark, Allii Macrostemonis Bulbus, Zanthoxyli Radix, Forsythia Suspensa, Flos Daturae, Flower of Lobed Kudzuvine, Herba Glechomae, Smilacis Glabrae Rhizoma, Radix Physochlainae, Fruit of Tree-of-heaven Ailanthus, Ginkgo Seed, Radix Trichosanthis |
| DC7 | Rhododendri Daurici Folium, Common Cephalanoplos Herb, Rhizoma Dioscoreae Bulbiferae, Herbal Ephedra, Akebia Stem, Herba Gnathali Affinis, Flower of Lobed Kudzuvine, Chrysanthemum, Curcumae Radix, Anemarrhenae Rhizoma, Bulb of Thunberg Fritillary, Herba Hyperici Japonici, Peucedani Radix, Root of Garden Eggplant, Beartiful Sweetgum Fruit, Glycyrrhiza, Menispermi Rhizoma |
| DC8 | Lesser Galangal Rhizome, Radix Gentianae, Smilacis Chinae Rhizoma, Flower of Lobed Kudzuvine, Eupatorii Herba, Chinese Fevervine Herb, Perilla, Fructus Jujubae, Aucklandiae Radix, Commelinae Herba, Mung Bean, Curcumae Radix, Herba Gnathali Affinis, Fructus Rosae Laevigatae, Notopterygii Rhizoma Et Radix, Carthami Flos, Stigma Maydis, Euphorbiae Pekinensis Radix, Cortex Periplocae, Artemisiae Annuae Herba |
| DC9 | Flower of Lobed Kudzuvine, Ussuriensis Fritillary Bulb, Farfarae Flos, Herba Gnathali Affinis, Bistortae Rhizoma, Fructus Rosae Laevigatae, Achyranthes Bidentata, Asparagi Radix, Platycodonis Radix, Daylily Root, Forsythia Suspensa, Herba Hyperici Japonici, Notopterygii Rhizoma Et Radix, Cortex Erythrinae Seu Kalopanacis, Kaki Calyx, Fritillariae Cirrhosae Bulbus, Spiderflower Seed |
| DC10 | Amomi Fructus, Inulae Flos, Perilla, Smilacis Glabrae Rhizoma, Modern Rose, Chinese Fevervine Herb, Fruit of Tree-of-heaven Ailanthus, Rhododendri Daurici Folium, Fagopyri Dibotryis Rhizoma, Radix Gentianae, Ginkgo Seed, Rhizoma Atractylodis, Mistletoe, Stigma Maydis, Smilacis Chinae Rhizoma, Cyrtomium Rhizome, Clematidis Radix Et Rhizoma, Curcumae Radix, Root of Garden Eggplant |
| DC11 | Notopterygii Rhizoma Et Radix, Eupatorii Herba, Fructus Rosae Laevigatae, Achyranthes Bidentata, Imperatae Rhizoma, Mistletoe, Platycladi Cacumen, Lemongrass Herb, Anemones Raddeanae Rhizoma, Fimbriate Orostachys, Modern Rose, Chinese Fevervine Herb, Herba Glechomae, Herba Hyperici Japonici, Smilacis Glabrae Rhizoma, Cow-Bezoar, India Madder Root, Aucklandiae Radix, Greater Calandine Herb |
| DC12 | Inulae Herba, Grassleaf Sweetflag Rhizome, Stigma Maydis, Palmleaf Raspberry Fruit, Rhododendri Daurici Folium, Fructus Rosae Laevigatae, Cortex Phellodendri Chinensis, Modern Rose, Grosvenor Momordica Fruit, Yanhusuo, Vietnamese Sophora Root, Hydnocarpus anthelmintica Pier, Fagopyri Dibotryis Rhizoma, Perilla, Hippophae Fructus, Smilacis Glabrae Rhizoma, Germinated Barley, Achyranthes Bidentata, Spiderflower Seed, Common Fenugreek Seed, Epimedium Herb, Arecae Pericarpium |
| DC13 | Herba Gnathali Affinis, Fourleaf Ladybell Root, Tatarian Aster Root, Herba Glechomae, Rhododendri Daurici Folium, Lesser Galangal Rhizome, Kaki Calyx, Germinated Barley, Salviae Miltiorrhizae, Dyers Woad Leaf, Forsythia Suspensa, Tabasheer, Fructus Jujubae, Grosvenor Momordica Fruit, Farfarae Flos, Mistletoe, Ricepaperplant Pith, Sanqi, Giant Knotweed Rhizome, Garlic |
| DC14 | Lasiosphaera, Peppermint, Mistletoe, Stemonae Radix, Smilacis Glabrae Rhizoma, Lesser Galangal Rhizome, Notopterygii Rhizoma Et Radix, Stigma Maydis, Fimbriate Orostachys, Fructus Tribuli, Golden Larch Bark, Allii Macrostemonis Bulbus, Perilla, Hippophae Fructus, Grosvenor Momordica Fruit, Raisin Tree Seed, Tatarian Aster Root, Fructus Jujubae, Mulberry Twig, Largeleaf Gentian Root, Salviae Miltiorrhizae, Bletilla Striata |
| DC15 | Chinese Fevervine Herb, Fourstamen Stephania Root, Raisin Tree Seed, Glabrous Sarcandra Herb, Rhizoma Polygonati, Perilla, Grosvenor Momordica Fruit, Lesser Galangal Rhizome, Humuli Scandentis Herba, Giant Knotweed Rhizome, Lotus Plumule, Green Tangerine Peel, Dogbane Leaf, Rhododendri Daurici Folium, Smilacis Glabrae Rhizoma, Modern Rose, Mori Folium, Chinese Honeylocust Spine, Tatarian Aster Root, Cicada Slough, Fortune’s Drynaria Rhizome, Herba Solani Lyrati |
| DC16 | Fineleaf Schizonepeta Herb, Meliae Cortex, Herba Hyperici Japonici, Notopterygii Rhizoma Et Radix, Beartiful Sweetgum Fruit, Pharbitis Seed, Peppermint, Farfarae Flos, Perilla, Benzoin, Artemisiae Annuae Herba, Nelumbinis Receptaculum, Ginkgo Folium, Grosvenor Momordica Fruit, Lesser Galangal Rhizome, Tatarian Aster Root, Raisin Tree Seed, Kadsura Pepper Stem, Akebia Stem, Dried Ginger, Radix Physochlainae |
| DC17 | Herba Glechomae, Bistortae Rhizoma, Chinese Honeylocust Spine, Amomi Fructus, Large Thistle, Grosvenor Momordica Fruit, Herbal Ephedra, Smilacis Glabrae Rhizoma, Rhizoma Dioscoreae Bulbiferae, Ilex latifolia Thunb, Indian Stringbush Root, Ricini Semen, Gambir Plant Nod, Pharbitis Seed, Herba Euphorbiae Helioscopiae, Achyranthes Bidentata, Spina Date Seed, Chinese Cinquefoil, Wild Chrysanthemum, Euphorbiae Pekinensis Radix |
| DC18 | Palmleaf Raspberry Fruit, Platycodonis Radix, Ilicis Chinensis Folium, Fructus Jujubae, Himalayan Teasel Root, Hippophae Fructus, Pharbitis Seed, Dogbane Leaf, Tree-of-Heaven Bark, Chicory Herb, Herb of Crescent-shaped Euphorbia, Asparagi Radix, Notopterygii Rhizoma Et Radix, Lesser Galangal Rhizome, Smilacis Glabrae Rhizoma, Smilacis Chinae Rhizoma, Humuli Scandentis Herba, Stem Pith of Himalayan Stachyurus, Common Fenugreek Seed, Cortex Erythrinae Seu Kalopanacis |
| DC19 | Fructus Rosae Laevigatae, Blackberrylily Rhizome, Herbal Ephedra Root, Descolor Cinquefoil Herb, Common Macrocarpium Fruit, Medicinal Evodia Fruit, Perilla Fruit, Hippophae Fructus, Red Peony Root, Figwort Root, Herba Selaginellae Doederleinii, Bomeol, Lesser Galangal Rhizome, Germinated Barley, Peppermint, Flower of Lobed Kudzuvine, Humuli Scandentis Herba, Fagopyri Dibotryis Rhizoma, Japanese Ginseng, Chinese Pulsatilla Root, Ramulus Euonymi Alati, Cyrtomium Rhizome |
| DC20 | Japanese Ampelopsis Root, Benzoin, Glossy Privet Fruit, Coix Seed, Amomi Fructus, Chinese Fevervine Herb, Purslane Herb, Wild Chrysanthemum, Fourleaf Ladybell Root, Purslane Speedwell Herb, Yanhusuo, Rhizoma Dioscoreae Bulbiferae, Morinda Root, Germinated Barley, Humuli Scandentis Herba, Mistletoe, Chinese Gall, Calendula officinalis, Ginseng, Cow-Bezoar |
| Stages . | Prescriptions . |
|---|---|
| Mild cases [13, 17, 25, 80, 81] | Lianhua-Qingwen, Jinhua-Qinggan, Qingfei-Paidu, and Xuanfei-Baidu |
| Moderate cases [13, 25, 80, 81] | Lianhua-Qingwen, Jinhua-Qinggan, Qingfei-Paidu, and Xuanfei-Baidu |
| Sever cases [81–83] | Xuebijing and Qingfei-Paidu |
| Critical cases [82–84] | Xuebijing, Huashi-Baidu |
| Stages . | Prescriptions . |
|---|---|
| Mild cases [13, 17, 25, 80, 81] | Lianhua-Qingwen, Jinhua-Qinggan, Qingfei-Paidu, and Xuanfei-Baidu |
| Moderate cases [13, 25, 80, 81] | Lianhua-Qingwen, Jinhua-Qinggan, Qingfei-Paidu, and Xuanfei-Baidu |
| Sever cases [81–83] | Xuebijing and Qingfei-Paidu |
| Critical cases [82–84] | Xuebijing, Huashi-Baidu |
| Stages . | Prescriptions . |
|---|---|
| Mild cases [13, 17, 25, 80, 81] | Lianhua-Qingwen, Jinhua-Qinggan, Qingfei-Paidu, and Xuanfei-Baidu |
| Moderate cases [13, 25, 80, 81] | Lianhua-Qingwen, Jinhua-Qinggan, Qingfei-Paidu, and Xuanfei-Baidu |
| Sever cases [81–83] | Xuebijing and Qingfei-Paidu |
| Critical cases [82–84] | Xuebijing, Huashi-Baidu |
| Stages . | Prescriptions . |
|---|---|
| Mild cases [13, 17, 25, 80, 81] | Lianhua-Qingwen, Jinhua-Qinggan, Qingfei-Paidu, and Xuanfei-Baidu |
| Moderate cases [13, 25, 80, 81] | Lianhua-Qingwen, Jinhua-Qinggan, Qingfei-Paidu, and Xuanfei-Baidu |
| Sever cases [81–83] | Xuebijing and Qingfei-Paidu |
| Critical cases [82–84] | Xuebijing, Huashi-Baidu |
Consequently, in order to recommend precision herb combinations targeting the specific infected stage, we employed the VGAE model to compute the similarity between the recommended Top-20 herb combinations and 3F3M. In such case, we signaled the specific candidate herbs combinations to treat SARS-CoV-2 at the certain cases. The ‘Herb-Compound-Protein’ docking results of the six prescriptions are shown in Figures S1–S6 in Supplementary file S4. The results of the similarity between our recommended 20 candidate herb combinations and 3F3M are depicted in Figure 7, and the details are recorded in the Supplementary file S6.
Repurposable candidate herbs for treating COVID-19. The signaled herbs are located in the inner circle, while the outer circle shows the top-5 issued herbs with most potential repurposing possibility. And the repurposing herbs assigned by the same color.
6.2 Variational graph autoencoder
We constructed the ‘Herb-Compound-Protein’ graphs of top-20 recommended herb combinations and 3F3M, individually. To be more specific, a graph is represented as |$\mathcal{G} = (\mathcal{V}, \mathcal{E}, \mathcal{X})$|, where |$\mathcal{V}$| represents all nodes consisting of herbs, compounds and proteins; |$\mathcal{E}$| represents the interaction between nodes; |$\mathcal{X}$| indicates the interaction characteristic associated with each node. Given the ‘Herbs-Compounds-Proteins’ graph, we employed the VGAE-based graph convolution network (GCN) to learn the potential representation of a node. The VAE utilizes these representations to minimize the reconstructed error.
We employed ReLU as the active function in the first layer and used the linear function as the active function in the second layer. The mean |$\mu $| and variance |$\sigma ^2$| can be learned by means of the GCN, which is further to embedding nodes to be mapped to the low-dimensional space. |$\mu _i$| and |$\sigma _i^2$| only share parameter weights of the first layer.
7 Data
In this section, we presented the process of crawling and processing the data used in this work. We utilized the python-based crawler tool (The code of this python-based crawler tool can be downloaded from https://github.com/fanyang-AI/TCM-COVID19) to obtain 480 herbs and 13 448 associated chemical compounds from Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (https://tcmspw.com/tcmsp.php). And we converted them into the PDBQT format using OpenBabel. The PDBQT file was then input into the AutoDock software for molecular docking.
8 Results
For repurposing candidate herbs of each specific SARS-CoV-2 protein. There were |$24 \times $|Top-20 herbs signaled by means of HCP-DGE for structural, nonstructural and accessory proteins, individually. The results are depicted in Figure 6. Further, we designed the following procedures to measure the repurposing: (1) downloading the data of herb attributes from HerbNet (http://www.openkg.cn/dataset/herb-net) (details presented in Supplement S8) to construct the signaled herb feature matrix |$\mathcal{F}_{{\tiny S}}\in \mathbb{R}^{106\times 48}$| and the issued herb feature matrix |$\mathcal{F}_{{\tiny I}}\in \mathbb{R}^{73\times 48}$|, where 106 stands for the number of deduplicated signaled herbs, 73 means the number of issued herbs (Issued by “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8)”) , and 48 represents the dimension of herb feature space; (2) performing the Cosine similarity with matrix decomposition |$\mathcal{F}_{{\tiny S}}^{T}{\mathcal{F}_{{\tiny I}}}$| to generate the repurposing herb matrix |$\mathcal{M}_{{\tiny herb}}\in \mathbb{R}^{106\times 73}$| (Brief description is shown in Figure 8, and the details are recorded in the Supplement S5).
For precision candidate herb combinations. We initially selected herbs from the 24|$\times $|Top-10 signaled herb sets to construct 20 candidate herb combinations. By employing the VGAE to signal the precision candidate herb combinations for each specific infected stages. The ‘Herb-Compound-Protein’ docking graph of the recommended 20 herb combinations are depicted in the Supplementary file S7. The detailed results of the similarity between the recommended 20 herb combinations and 3F3M are described in the Supplementary file S6.
9 Conclusions
In this study, we proposed a ‘Herb-Compound-Protein’ heterogeneous graph-based approach to investigate the relationships among herbs, compounds and SARS-CoV-2 proteins. A total of 106 candidate repurposing herbs were signaled by the proposed HCP-DGE approach. The VGAE model was employed to recommend 20 herb combinations as the candidate treatments for specific infected stages for the precision treating. In the future, additional experiments are needed to evaluate the results to provide a clinical evidence for finding antiviral herbs or herb combinations.
We proposed a heterogeneous graph-based deep network embedding method to signal candidate herbs for each SARS-CoV-2 protein.
We improved the recommended repurposing of herbs by employing the virtual screening-based docking method.
We recommended precision herb combinations for specific SARS-CoV-2 infected stage.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No. 2021YFF0704100, 2021YFF0704101, 2020YFC2003500), the China Postdoctoral Science Foundation (No. 2019M662373), and the National Natural Science Foundation of China (Grant No. 61972322).
Author contributions statement
F.Y., S.Z. and W.P. conceived the experiments. S.Z., W.P., R.Y. and W.Z. conducted the experiments. W.P., Y.Z., F.X. and H.W. analysed the results. F.Y. and S.Z. wrote the manuscript. H.W., G.W., Q.Z., J.D., Y.C., L.C. and F.X. reviewed the manuscript.
Fan Yang, Ph.D., Professor, Department of Epidemiology and Biostatistics, School of Public Health, Cheeloo College of Medicine, Shandong University, China. He is also the Professor of National Health Medical Data North Center China and National Institute of Health Data Science of China. His main research interests focus on Causal Machine Learning, Causaliyt, and Pan-cancer. He has published over 15 papers in professional journals and conferences, including TKDE, BIB, BIBM and so on. His research has been funded by NSF, NIH, NSFC, National Key Research and Development Program of China and others. He has served for various journals as reviewer (BIB, JBHI, INSC, HISC and so on) or guest editor.
Shuaijie Zhang Master candidate, School of Public Health, Cheeloo College of Medicine, Shandong University, China.
Wei Pan Master candidate, School of Public Health, Cheeloo College of Medicine, Shandong University, China.
Ruiyuan Yao M.M. Shandong University of Chinese Medicine. Her main research interests are compatibility law and effectiveness mechanism of prescription.
Weiguo Zhang M.M. Shandong University of Chinese Medicine. His main research interests are compatibility law and effectiveness mechanism of prescription.
Yanchun Zhang Ph.D., Professor, Department of New Networks, Peng Cheng Laboratory, Shenzhen, China and Cyberspace Institute of Advanced Technology (CIAT), Guangzhou University, Guangzhou, China. He is also an emeritus professor at Victoria University, Melbourne, VIC, Australia. He has published over 400 research articles in international journals and conference proceedings. Dr. Zhang is a Founding Editor and the Editor-in-Chief of World Wide Web Journal and Health Information Science and Systems Journal. He is also the Chairman of the International Web Information Systems Engineering Society.
Guoyin Wang Ph.D., Professor, Chongqing University of Posts and Telecommunications. He is currently the director of the Chongqing Key Laboratory of Computational Intelligence, the Vice-President of the University and the dean of the School of Graduate. He is the author of over 10 books, the editor of dozens of proceedings of international and national conferences, and has more than 300 reviewed research publications. His research interests include rough sets, granular computing, knowledge technology, data mining, neural network, and cognitive computing, etc.
Qianghua Zhang is currently a professor and serves as the President of the Institute of advanced technology and Director of the science and technology department. His research interests include rough sets, fuzzy sets, granular computing and uncertain information processing.
Yunlong Cheng is currently an Associate Professor with College of Mobile Telecommunications, Chongqing University of Posts and Telecommunications. His research interests include analysis and processing of uncertain data, data mining, three-way decisions, granular computing and rough sets.
Jihua Dong is Professor, Qilu Young Scholar, and Taishan Young Scholar in the School of Foreign Languages and Literature at Shandong University, China. Her research interests include corpus linguistics, corpus-based teaching, academic writing, and discourse analysis.
Chunyang Ruan Ph.D. lecturer, Shanghai International Studies University, Shanghai, China.
Lizhen Cui is a professor in the School of Software and Joint SDU-NTU Centre for Artificial Intelligence Research (C-FAIR) at the Shandong University, and also a visiting professor at Nanyang Technological University Singapore. He published over 100 papers in journals and refereed conference proceedings. His research interests include big data management and analysis, AI theory and application.
Hao Wu is currently an associate professor in the School of Software, Shandong University. He has published over 30 works in professional journals and conferences, including BIB, TCBB, EAAI and so on. His main research interests include data mining and bioinformatics. He has also served for various journals as reviewer or guest editor.
Fuzhong Xue is a professor with the School of Public Health at Shandong University. He is currently the Dean of the National Institute of Health Data Science of China. His research interests focus on causal inference, Statistical analysis of omics big data. He has published over 160 papers and 3 books.
References
Hazem M, Gentile F, Perez C, and Cherkasov A.
Zhang M, Wang W, and He Y. GraSP: Optimizing Graph-based Nearest Neighbor Search with Subgraph Sampling and Pruning. In:
Manaal F, Dyer C.
Liu, H., Lin, H., Shen, C., Yang, L., Lin, Y., Xu, B., Yang, Z., Wang, J. and Sun, Y.
Chen Z, Wang G, Yu B, Xie Y, Pan K.
Author notes
Fan Yang, Shuaijie Zhang, and Wei Pan contributed equally to this work.


