-
PDF
- Split View
-
Views
-
Cite
Cite
Qun Dong, Feng Li, Yanjun Xu, Jing Xiao, Yingqi Xu, Desi Shang, Chunlong Zhang, Haixiu Yang, Zihan Tian, Kai Mi, Xia Li, Yunpeng Zhang, RNAactDrug: a comprehensive database of RNAs associated with drug sensitivity from multi-omics data, Briefings in Bioinformatics, Volume 21, Issue 6, November 2020, Pages 2167–2174, https://doi.org/10.1093/bib/bbz142
Close - Share Icon Share
Abstract
Drug sensitivity has always been at the core of individualized cancer chemotherapy. However, we have been overwhelmed by large-scale pharmacogenomic data in the era of next-generation sequencing technology, which makes it increasingly challenging for researchers, especially those without bioinformatic experience, to perform data integration, exploration and analysis. To bridge this gap, we developed RNAactDrug, a comprehensive database of RNAs associated with drug sensitivity from multi-omics data, which allows users to explore drug sensitivity and RNA molecule associations directly. It provides association data between drug sensitivity and RNA molecules including mRNAs, long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) at four molecular levels (expression, copy number variation, mutation and methylation) from integrated analysis of three large-scale pharmacogenomic databases (GDSC, CellMiner and CCLE). RNAactDrug currently stores more than 4 924 200 associations of RNA molecules and drug sensitivity at four molecular levels covering more than 19 770 mRNAs, 11 119 lncRNAs, 438 miRNAs and 4155 drugs. A user-friendly interface enriched with various browsing sections augmented with advance search facility for querying the database is offered for users retrieving. RNAactDrug provides a comprehensive resource for RNA molecules acting in drug sensitivity, and it could be used to prioritize drug sensitivity–related RNA molecules, further promoting the identification of clinically actionable biomarkers in drug sensitivity and drug development more cost-efficiently by making this knowledge accessible to both basic researchers and clinical practitioners.
Database URL: http://bio-bigdata.hrbmu.edu.cn/RNAactDrug.
Introduction
Drug sensitivity study in cancer is vital for achieving personalized treatment of cancer and moving precision medicine forward. Great variation in drug sensitivity due to interindividual heterogeneity results in inefficient utilization of limited healthcare resources [1, 2], and hence, there is a great need to study drug response–related molecules for optimizing drug therapies.
It has been well recognized that molecular alterations at multiple levels including expression, copy number variation, mutation and methylation in protein-coding messenger RNAs (mRNAs) strongly influence clinical drug sensitivity to treatment and in many instances are potent biomarkers for response to drugs [3, 4]. Misale et al. have revealed that point mutations of KRAS are causally associated with the onset of acquired resistance to cetuximab in colorectal cancers [5]. Besides, increasing evidences suggest that non-coding RNAs (ncRNAs) including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) are responsible for drug sensitivity as they could regulate drug sensitivity–related genes, induce alternative signaling pathways and further affect drug efficacy [6–8]. For instance, miR-634 overexpression sensitizes resistant primary ovarian cancer cells to cisplatin, carboplatin and doxorubicin [9]. HOTAIR, a long intergenic non-coding RNA, affects chemoresistance by regulating HOXA1 methylation in small cell lung cancer cells [10]. Long non-coding RNA TUG1 is involved in chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2 [11]. Therefore, integrating associations of ncRNAs and drug sensitivity will provide a promising direction to identify clinically actionable biomarkers and understand underlying mechanisms in individualized drug responses.
The detection and profiling of more and more RNA molecules at unprecedented sensitivity and depth with advances in high-throughput next-generation sequencing technology offer new opportunities for deeply researching their biological functions [12], especially their roles in drug sensitivity [13–15]. However, the high-throughput sequencing technology with rapid advances and reduced costs has generated an overwhelming amount of cancer pharmacogenomic data, making it increasingly complex to perform data mining. Information on the associations between biomolecules and drug sensitivity is fragmented and hidden in scattered pharmacogenomic resources not yet to be systematically collected and analyzed, which limits opportunities for basic researchers without experience in bioinformatics [16]. It needs to be identified, collected and properly integrated to promote the development of precision medicine. Furthermore, there are very few publicly available databases or tools to help make use of the full breadth and depth of existing pharmacogenomic data.
To help remove the barriers, we have developed the RNAactDrug, a comprehensive database of RNAs including lncRNAs, miRNAs and mRNAs, associated with drug sensitivity from multi-omics data, which allows the user to explore drug sensitivity and RNA molecule associations directly. RNAactDrug integrated three wide-used resources from pharmacogenomic studies that profiled large panels of cell lines against a broad range of approved drugs and experimental chemical compounds, Genomics of Drug Sensitivity in Cancer (GDSC) [17], Cancer Cell Line Encyclopedia (CCLE) [18] and CellMiner [19]. To date, RNAactDrug contains 4 924 260 RNA molecule–drug sensitivity associations at multidimensional molecular levels (expression, copy number variation, mutation and methylation) covering 19 773 mRNAs, 11 119 lncRNAs, 438 miRNAs and 4155 drugs. We created a user-friendly interface that can be used to rapidly query potentially relevant RNA molecules associated with drug effectiveness in expression, copy number variation (CNV), mutation and methylation, respectively. The database will help experimental researchers in having an overview about the relationship between RNAs and drug sensitivity, and will be a valuable resource in identifying actionable biomarkers of drug response in cancer. The RNAactDrug database can be freely accessed through http://bio-bigdata.hrbmu.edu.cn/RNAactDrug.
Materials and methods
Data collection and preprocessing
In order to extract more associations of RNA molecules and drug sensitivity, RNAactDrug integrated three well-known pharmacogenomic databases, CCLE, GDSC and CellMiner (Figure 1). In addition to drug sensitivity data, we collected multi-omics data including expression, CNV, methylation and mutation data of mRNAs and lncRNAs, and expression data of miRNAs, considering the availability of data (Supplementary Table S1). We separated multi-omics data of mRNAs and lncRNAs according to the annotation files of human mRNA and lncRNA from GENCODE [20]. Based on the fact that more than one probe might be annotated to the same gene, we calculated the mean value as molecular value (expression value, methylation value or CNV) of the gene at corresponding omics. We excluded genes whose molecular values were ‘NA’ in greater than 20% cell lines and then filled the remaining with the mean of the data in the row.
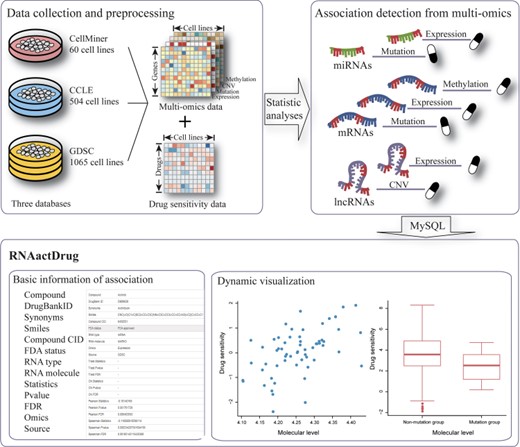
Flowchart of the RNAactDrug pipeline. The pipeline starts with data collection and preprocessing from CellMiner, CCLE and GDSC, which consists of expression, copy number variation, mutation, methylation and drug sensitivity data. Next, the preprocessed data are sent to statistical analyses to identify significant associations between RNAs and drug sensitivity at different omics levels. In the final step, RNAactDrug is constructed for storing the comprehensive information about the associations.
Identifying associations of RNA molecules and drug sensitivity
To identify the association between RNA molecule and drug sensitivity hidden in large-scale data, we performed two different statistic analyses to measure the correlation between multi-omics and drug sensitivity data according to data types.
where subscripts 1 and 2 are for mutation group and not-mutation group, respectively, n is the number of cell lines, |$\overline{X}$| (|$\overline{X}=\frac{\sum_{\mathrm{i}=1}^n{x}_{\mathrm{i}}}{n}$|) and |${S}^2$| (|${S}^2=\frac{\sum_{\mathrm{i}=1}^n{({x}_{\mathrm{i}}-\overline{X})}^2}{n-1}$|) are the mean value and variance of drug sensitivity, respectively, and xi is the given drug sensitivity data in corresponding cell line i.
where n is the number of cell lines, Xi and Yi are drug sensitivity data of given drug and molecular value (expression, CNV or methylation value) of considered gene in sample i, respectively, and |$\overline{X}$|, |$\overline{Y}$| are averages of the corresponding data.
Finally, only the RNA molecule–drug sensitivity associations whose P-values and false positive rates (FDRs) from t-test or Pearson correlation analysis that passed the cut-off of P < 0.05 and FDR < 0.05 were identified and included in RNAactDrug (Figure 1).
Database architecture
The RNAactDrug website was developed in JSP using a Struts2 framework and deployed on a Tomcat 6.0.44 web server that ran under a Red Hat 6.4 system. All data in RNAactDrug were stored and managed using MySQL (version 5.7.18). The web server of RNAactDrug was developed based on Java. jQuery was used to manage the result views. RNAactDrug was fully tested in Google Chrome.
Results
Database content
By integrating data from three well-known pharmacogenomic databases, RNAactDrug totally identified more than 4 924 200 RNA molecule–drug sensitivity associations at four molecular levels (expression, CNV, mutation and methylation) covering more than 19 770 mRNAs, 11 119 lncRNAs, 438 miRNAs and 4155 drugs (Table 1). mRNA is the major type of RNA molecules related to drug sensitivity in all three data sources, 91% in CCLE, 96% in CellMiner and 87% in GDSC (Figure 2A–C), as described previously [25, 26]. Obviously, quite a lot of associations between RNA molecule and drug sensitivity were identified at every one of four molecular levels (Figure 2A–C), 73% of lncRNAs identified in expression level from CCLE, 47% of mRNAs identified in mutation level from CCLE, 37% of mRNAs identified in methylation level from CellMiner and 97% of lncRNAs identified in CNV level from GDSC. These findings indicate that both genetic and epigenetic events play considerable roles in drug sensitivity and RNAactDrug incorporating multi-omics data could provide more comprehensive information to dissect drug response of individuals [27, 28].
| Source . | Omics . | Drug–mRNA . | Drug–lncRNA . | Drug–miRNA . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Associations . | mRNA . | drug . | Associations . | lncRNA . | drug . | Associations . | miRNA . | drug . | ||
| CCLE | Expression | 39 835 | 10371 | 24 | 7502 | 2229 | 23 | 419 | 210 | 19 |
| CNV | 5368 | 3796 | 16 | 361 | 271 | 12 | – | – | – | |
| Methylation | 8204 | 4609 | 17 | 453 | 230 | 17 | – | – | – | |
| Mutation | 47 858 | 15 517 | 24 | 1979 | 712 | 22 | – | – | – | |
| GDSC | Expression | 1 315 879 | 16 456 | 198 | 7922 | 113 | 197 | – | – | – |
| CNV | 1 063 322 | 17 759 | 155 | 496 299 | 8110 | 154 | – | – | – | |
| Methylation | 1 167 600 | 15 926 | 197 | 7855 | 133 | 196 | – | – | – | |
| Mutation | 392 | 307 | 77 | 13 | 11 | 10 | – | – | – | |
| CellMiner | Expression | 1 182 814 | 15 853 | 3321 | 35 793 | 801 | 2373 | 28 536 | 232 | 1980 |
| Methylation | 730 575 | 18 002 | 2138 | 19 108 | 465 | 1749 | – | – | – | |
| Mutation | 84 712 | 4245 | 1821 | – | – | – | – | – | – | |
| Source . | Omics . | Drug–mRNA . | Drug–lncRNA . | Drug–miRNA . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Associations . | mRNA . | drug . | Associations . | lncRNA . | drug . | Associations . | miRNA . | drug . | ||
| CCLE | Expression | 39 835 | 10371 | 24 | 7502 | 2229 | 23 | 419 | 210 | 19 |
| CNV | 5368 | 3796 | 16 | 361 | 271 | 12 | – | – | – | |
| Methylation | 8204 | 4609 | 17 | 453 | 230 | 17 | – | – | – | |
| Mutation | 47 858 | 15 517 | 24 | 1979 | 712 | 22 | – | – | – | |
| GDSC | Expression | 1 315 879 | 16 456 | 198 | 7922 | 113 | 197 | – | – | – |
| CNV | 1 063 322 | 17 759 | 155 | 496 299 | 8110 | 154 | – | – | – | |
| Methylation | 1 167 600 | 15 926 | 197 | 7855 | 133 | 196 | – | – | – | |
| Mutation | 392 | 307 | 77 | 13 | 11 | 10 | – | – | – | |
| CellMiner | Expression | 1 182 814 | 15 853 | 3321 | 35 793 | 801 | 2373 | 28 536 | 232 | 1980 |
| Methylation | 730 575 | 18 002 | 2138 | 19 108 | 465 | 1749 | – | – | – | |
| Mutation | 84 712 | 4245 | 1821 | – | – | – | – | – | – | |
| Source . | Omics . | Drug–mRNA . | Drug–lncRNA . | Drug–miRNA . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Associations . | mRNA . | drug . | Associations . | lncRNA . | drug . | Associations . | miRNA . | drug . | ||
| CCLE | Expression | 39 835 | 10371 | 24 | 7502 | 2229 | 23 | 419 | 210 | 19 |
| CNV | 5368 | 3796 | 16 | 361 | 271 | 12 | – | – | – | |
| Methylation | 8204 | 4609 | 17 | 453 | 230 | 17 | – | – | – | |
| Mutation | 47 858 | 15 517 | 24 | 1979 | 712 | 22 | – | – | – | |
| GDSC | Expression | 1 315 879 | 16 456 | 198 | 7922 | 113 | 197 | – | – | – |
| CNV | 1 063 322 | 17 759 | 155 | 496 299 | 8110 | 154 | – | – | – | |
| Methylation | 1 167 600 | 15 926 | 197 | 7855 | 133 | 196 | – | – | – | |
| Mutation | 392 | 307 | 77 | 13 | 11 | 10 | – | – | – | |
| CellMiner | Expression | 1 182 814 | 15 853 | 3321 | 35 793 | 801 | 2373 | 28 536 | 232 | 1980 |
| Methylation | 730 575 | 18 002 | 2138 | 19 108 | 465 | 1749 | – | – | – | |
| Mutation | 84 712 | 4245 | 1821 | – | – | – | – | – | – | |
| Source . | Omics . | Drug–mRNA . | Drug–lncRNA . | Drug–miRNA . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Associations . | mRNA . | drug . | Associations . | lncRNA . | drug . | Associations . | miRNA . | drug . | ||
| CCLE | Expression | 39 835 | 10371 | 24 | 7502 | 2229 | 23 | 419 | 210 | 19 |
| CNV | 5368 | 3796 | 16 | 361 | 271 | 12 | – | – | – | |
| Methylation | 8204 | 4609 | 17 | 453 | 230 | 17 | – | – | – | |
| Mutation | 47 858 | 15 517 | 24 | 1979 | 712 | 22 | – | – | – | |
| GDSC | Expression | 1 315 879 | 16 456 | 198 | 7922 | 113 | 197 | – | – | – |
| CNV | 1 063 322 | 17 759 | 155 | 496 299 | 8110 | 154 | – | – | – | |
| Methylation | 1 167 600 | 15 926 | 197 | 7855 | 133 | 196 | – | – | – | |
| Mutation | 392 | 307 | 77 | 13 | 11 | 10 | – | – | – | |
| CellMiner | Expression | 1 182 814 | 15 853 | 3321 | 35 793 | 801 | 2373 | 28 536 | 232 | 1980 |
| Methylation | 730 575 | 18 002 | 2138 | 19 108 | 465 | 1749 | – | – | – | |
| Mutation | 84 712 | 4245 | 1821 | – | – | – | – | – | – | |
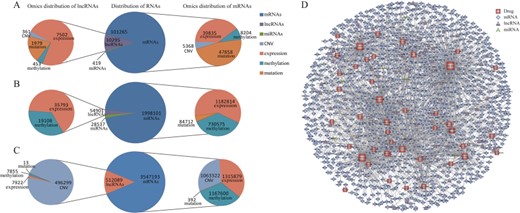
Proportions of mRNAs, lncRNAs and miRNAs in CCLE (A), CellMiner (B) and GDSC (C). (D) The bipartite network of associations with correlation strength greater than 0.4 and FDR less than 0.00001 between RNA molecules and FDA-approved drugs. Node represents RNA molecule (miRNA, mRNAs or lncRNA) or drug, and the thickness of edge corresponds to correlation strength. The size of the node corresponds to the node degree.
Notably, when comparing drugs from these different pharmacogenomic databases, we observed limited overlap of drugs among three databases, only 32 common drugs in GDSC and CellMiner and 7 common drugs shared by CellMiner and CCLE (Supplementary Figure S1A). Therefore, RNAactDrug incorporated the three resources to cover more comprehensive drugs. We then compared the RNA molecules associated with the common drug sensitivity from diverse data sources, and different data sources all have some unique results (Supplementary Figure S1B and S1C). Except differences of the cell lines, the results could be partly explained by technology preferences. The high degree of data heterogeneity poses difficulties in data mining, integration and further analysis. It thus highlights the advantage of RNAactDrug integrating databases with complementary information.
To explore the data in RNAactDrug from a global perspective, we constructed a bipartite network composed of RNAs (miRNAs, mRNAs, lncRNAs) and drugs based on association data we identified (Figure 2D). The bipartite network consists of associations with correlation strength greater than 0.4 [29] and FDR less than 0.00001 between RNA molecules and Food and Drug Administration (FDA)-approved drugs, given the size of data in RNAactDrug. Tight connection of this network indicates the clinical drug sensitivity as a complex phenotype is influenced by numerous genetic elements. For example, the number of associations with higher correlation (|r| > 0.4) of nelarabine and RNA molecules reaches up to 263, followed by imatinib (199) and methotrexate (193) (Supplementary Figure S2A). It is noticeable, however, that 11 drugs are connected with only one RNA molecule with higher correlation (|r| > 0.4) (Supplementary Figure S2A). The critical roles of some RNA molecules connected to these drugs have been confirmed, such as ABCB1 in carfilzomib [30], NTRK1 in crizotinib [31, 32] and SLFN11 in irinotecan [33]. Besides, there are WDR34 in 5-fluorouracil, CCDC102B in olaparib, PRSS57 in ponatinib, etc., that could be the driving regulators in drug sensitivity and reflect essential research value (Supplementary Table S2). To give further insights into associations of drugs and RNAs, we provided a correlation landscape of these associations (Supplementary Figure S2B). Combining drug mechanism data from DrugBank, we found that sensitivities of drugs with the same mode of action were affected by similar RNA molecules—for example, topotecan and irinotecan. In many instances, drug sensitivity showed relations to multiple RNA molecules with different correlations. Note that there are some drugs related to RNAs with consistent correlation including hydroxyurea and LDK−378 positively correlated with RNAs consistently, with imiquimod in the opposite. To inspect the functional mechanisms for these RNAs in drug sensitivity, we performed functional enrichment analysis and got some enriched gene ontology (GO) terms (Supplementary Figure S3). We found that the RNA molecules were enriched to immune-related functions, such as leukocyte migration, leukocyte cell–cell adhesion and T cell activation in Carmustine.

Comparison with other databases
In the past decades, enormous effort has been devoted, and some pharmacogenomic databases cataloguing and curating relationships between genomic elements and drug sensitivity are developed [34–37]. GEAR (genomic elements associated with drug resistance) similarly provides associations of genetic elements and drug efficacy but extracted from the pharmacogenomic literatures [37]. GEAR includes 1631 associations between 201 human drugs and 758 genes, 106 associations between 29 human drugs and 66 miRNAs and 44 associations between 17 human drugs and 22 SNPs. The number of association in this database is limited (1781 in GEAR, 4924260 in RNAactDrug). Compared with GEAR, RNAactDrug contains more drugs including not only 65 drugs whose sensitivities have been reported in existing literatures but also more than 3000 drugs to be studied (Supplementary Figure S4A). These additional data extracted by statistical analysis could provide guidance for future research to economize manpower and material resources. As for mRNAs responsible for sensitivity of the same drugs in both databases, RNAactDrug incorporated almost all of mRNAs (98%) included in GEAR (Supplementary Figure S4B). Furthermore, abundant mRNAs and miRNAs involved in drug sensitivity of these same drugs recorded specifically in RNAactDrug have not yet been identified experimentally (Supplementary Figure S4B and S4C), which could serve as important complementary for current knowledge and facilitate to prioritize drug sensitivity–related RNA molecules. Besides, the cancer drug resistance database (CancerDR) provides information about mutations on 116 drug targets that lead to resistance of 148 anticancer drugs [34]. The Mutation To Cancer Therapy Scan (mTCTScan) web server provides a platform to systematically analyze mutations affecting cancer drug sensitivity based on individual genomic profiles [38]. These resources often only focus on one type of molecular alterations, although they greatly benefit basic researchers and clinicians for interrogating mechanism of drug sensitivity. However, clinical drug sensitivity represents a complex phenotype associated with alterations of multiple features of molecules [39–42]. Therefore, RNAactDrug, a comprehensive database of RNAs associated with drug sensitivity from multi-omics data, allows basic researchers to make full use of existing large-scale data directly to discover clinically actionable biomarkers related to their compound or RNA molecule of interest and enables rapid hypothesis generation or in vitro validation which will have a significant impact on cancer precision medicine.
Web interface
The RNAactDrug provides a user-friendly web interface that enables users to browse, search, submit and download the records of drug resistance associated RNA molecules (Figure 3). On the ‘Browse’ page, users can browse all results in the database by selecting a specific RNA molecule or drug. The ‘Quick Search’ enables users to search information easily by one keyword of interest, RNA molecule or drug. Also, an advanced search is provided in the ‘Search’ page for more specific requirements where users could obtain desired associations restricted to interested RNA molecules, drug, significance level (0.01, 0.001 and 0.0001), omics and data source. The browse and search results include basic information of associations, such as drug name, FDA status, RNA type, RNA name, statistics/correlation, P-value, FDR, molecular level and data source. Notably, the detailed P-value and FDR of association are provided in return results, and users could obtain desired associations restricted to a specific significance level. Based on the fact that a drug may have many synonyms, RNAactDrug provides the DrugBank ID [43] and PubChem CID [44] of drug for the convenience of research. Besides, a hyperlink named ‘details’ in this return result provides detailed information about each association, besides the above still including drug synonyms and SMILES strings from DrugBank, and data visualization for correlation between drug sensitivity data (GI50 in CellMiner, IC50 in GDSC and CCLE) and molecule values of the RNA molecule at a corresponding omics level (expression value, CNV, methylation level or mutation status). For a more comprehensive collection of associations between RNAs and drugs, RNAactDrug offers a submission page for researchers to submit experimentally supported RNA molecule–drug sensitivity association data. The data submitted require experimental method, a brief description of the association and literature. Once approved by the submission review committee, the submitted record will be included in the RNAactDrug database and made available to the public in updated releases. Finally, all data in RNAactDrug can be downloaded in the ‘Download’ page, and a detailed guide showing users how to use RNAactDrug is available on the ‘Help’ page.
Discussion and future directions
Increasing studies have shown that RNA molecules, including miRNAs, lncRNAs and mRNAs, have important functions and play vital roles in drug sensitivity. In this study, we developed the RNAactDrug, a comprehensive database which contains more than 4 924 200 RNA molecules–drug sensitivity associations at multidimensional molecular levels (expression, CNV, mutation and methylation), covering more than 19 770 mRNAs, 11 119 lncRNAs, 438 miRNAs and 4155 drugs. By combining three wide-used databases (CCLE, CellMiner and GDSC), RNAactDrug with comprehensive search and visualizations provides a user-friendly interface which allows basic researchers to quickly access the direct associations between RNA molecules and drugs. The major advantages of RNAactDrug over other similar databases include (i) inclusions of not only mRNAs and but also numerous lncRNAs and miRNAs, (ii) consideration of four omics of RNA molecules, (iii) comprehensive curation of large-scale associations between RNA molecules and drug and (iv) a convenient interface for basic researchers, especially those without bioinformatic experience, to explore the associations between drug sensitivity–RNA molecules directly. In addition, RNAactDrug also applied the chi-square test to mutation data and Spearman correlation analysis to other data (expression, CNV and methylation). Significant associations (P < 0.05 and FDR < 0.05) between RNA molecules and drugs have been identified and included in RNAactDrug. Users can obtain desired associations restricted to specific significance level and test type. RNAactDrug facilitates to make the most of existing large-scale pharmacogenomic resources. More importantly, RNAactDrug could be used to prioritize drug sensitivity–related RNAs to make drug development more cost-efficient and further promote the identification of clinically actionable biomarkers in personalized precision medicine by making this knowledge accessible to both basic researchers and clinical practitioners.
The extensions of RNAactDrug will continue, and newly reliable RNA molecules–drug sensitivity will be involved in later updated release. Other classes of RNA molecules such as small interfering RNA [45–49] and circular RNA [50, 51] will be incorporated in the future. Besides, we anticipate further development and improvement of the high-throughput sequencing–based technology on drug screening. It will generate more pharmacogenomic data and enable novel investigations into drug sensitivity–related factors. We will maintain and keep update RNAactDrug as more data are generated in the future. We believe that RNAactDrug will be a valuable resource for the future research of drug development.
- (i)
RNAs including mRNAs, miRNAs and lncRNAs play essential roles in clinical drug sensitivity at multi-omics levels and in many instances are potent biomarkers for response to drugs.
- (ii)
RNAactDrug currently stores more than 4 924 200 associations of RNA molecules and drug sensitivity at four molecular levels (expression, CNV, mutation and methylation) from integrated analysis of three large-scale pharmacogenomic databases (GDSC, CellMiner and CCLE), covering more than 19 770 mRNAs, 11 119 lncRNAs, 438 miRNAs and 4155 drugs.
- (iii)
Implementing networks to overview RNAactDrug database helps make impression on drug mechanism and provides novel insights in drug development, including identification of potential biomarkers for drug sensitivity.
- (iv)
RNAactDrug can be an important resource when prioritizing drug sensitivity–related RNA molecules, further promoting the identification of clinically actionable biomarkers in drug sensitivity and drug development more cost-efficiently.
Funding
National Key R&D Program of China (2018YFC2000100); National Natural Science Foundation of China (61873075, 31801107, 61603116); Fundamental Research Funds for the Provincial Universities; Heilongjiang Postdoctoral Science Foundation (LBHZ16123).
Qun Dong is an MS student in the College of Bioinformatics Science and Technology at Harbin Medical University.
Feng Li is an assistant researcher in the College of Bioinformatics Science and Technology at Harbin Medical University.
Yanjun Xu is an instructor in the College of Bioinformatics Science and Technology at Harbin Medical University.
Jing Xiao is an undergraduate in the College of Bioinformatics Science and Technology at Harbin Medical University.
Yingqi Xu is an instructor in the College of Bioinformatics Science and Technology at Harbin Medical University.
Desi Shang is an associate professor in the College of Bioinformatics Science and Technology at Harbin Medical University.
Chunlong Zhang is an associate professor in the College of Bioinformatics Science and Technology at Harbin Medical University.
Haixiu Yang is an instructor in the College of Bioinformatics Science and Technology at Harbin Medical University.
Zihan Tian is an undergraduate in the College of Bioinformatics Science and Technology at Harbin Medical University.
Kai Mi is an MS student in the College of Bioinformatics Science and Technology at Harbin Medical University.
Xia Li is a professor and head of the Chair in the College of Bioinformatics Science and Technology at Harbin Medical University.
Yunpeng Zhang is an associate professor in the College of Bioinformatics Science and Technology at Harbin Medical University.
References
Dummy.
Author notes
These authors contributed equally to this work.


