-
PDF
- Split View
-
Views
-
Cite
Cite
Fang Xu, Chunfa Tong, Yafei Lyu, Wenhao Bo, Xiaoming Pang, Rongling Wu, Allotetraploid and autotetraploid models of linkage analysis, Briefings in Bioinformatics, Volume 16, Issue 1, January 2015, Pages 32–38, https://doi.org/10.1093/bib/bbt075
Close - Share Icon Share
Abstract
As a group of important plant species in agriculture and biology, polyploids have been increasingly studied in terms of their genome structure and organization. There are two types of polyploids, allopolyploids and autopolyploids, each resulting from a different genetic origin, which undergo meiotic divisions of a distinct complexity. A set of statistical models has been developed for linkage analysis, respectively for each type, by taking into account their unique meiotic behavior, i.e. preferential pairing for allopolyploids and double reduction for autopolyploids. We synthesized these models and modified them to accommodate the linkage analysis of less informative dominant markers. By reanalysing a published data set of varying ploidy in Arabidopsis, we corrected the estimates of the meiotic recombination frequency aimed to study the significance of polyploidization.
INTRODUCTION
Because of their biological and economic importance, genetic analysis of polyploids has received a surge of interest in the recent years [1–5]. Tremendous efforts have been made to develop statistical models for linkage analysis used to study the genome structure and organization of polyploids and map quantitative trait loci for polyploid traits [6–13]. These models can be biologically relevant only when they have taken into account cytological properties of meiosis characterized by polyploids, which are qualitatively different from those in diploids.
According to their different origins, polyploids can be classified into two groups, allopolyploids and autopolyploids [1]. Wu et al. [12,13] have for the first time developed a series of models for linkage analysis in allopolyploids arising from hybridization of two different species. Allopolyploids frequently display bivalent pairings of chromosomes at meiosis, in which more similar chromosomes pair with each other at a higher likelihood than less similar chromosomes. Wu et al.’s models incorporated a parameter that describes this cytological property, called the preferential pairing factor [14], into the linkage analysis framework, increasing the biological interpretation of linkage mapping models [15].
For autopolyploids derived from genome combinations of different species, chromosomes pair among more than two homologous copies at meiosis [16,17], leading to a phenomenon called double reduction, i.e. two sister chromatids of a chromosome sort into the same gamete [18]. Thus, models for autopolyploid linkage analysis are qualitatively different from those for allopolyploids, because the double reduction affects the relative proportions of recombinant and non-recombinant gametes in a way differently from the preferential pairing factor. Fisher [19] was the first who pioneered a conceptual model that characterizes the individual probabilities of 11 different modes of gamete formation for an autotetraploid in terms of the recombination fraction between two different loci and their double reductions. By assuming that the chromosomal distribution of double reduction follows a particular pattern, Luo et al. [9] was able to develop linkage analysis models for autotetraploids. Wu et al. [10] derived the EM algorithm for simultaneous estimation of the linkage and double reductions based on Fisher’s model, with no assumption about the genomic distribution of double reduction. This algorithm was extended to analyse simplex or multiplex markers [11] and three-point linkage relationships [20].
Owing to the implementation of unique meiotic properties of polyploids, these algorithms have been shown to perform better than traditional approaches derived from the recombination event alone [10–13]. Despite this, the existing algorithms aimed at allotetraploid and autotetraploid characteristics have not well been used in most linkage analysis for this group of species partly because of their statistical complexities. For example, in a recent well-designed study for Arabidopsis, Pecinka et al. [5] attempted to detect the evolutionary merit of polyploidization by estimating and comparing the recombination frequencies of two dominant markers among segregating populations of diploids, allotetraploids and autotetraploids. They used a traditional diploid model to perform linkage analysis for all these three types of populations, which may inevitably lead to an imprecise inference about the linkage for the two tetraploid populations. In this Note, we modify the existing tetraploid linkage analysis algorithms to accommodate dominant markers and use them to reanalyse marker data for Arabidopsis by Pecinka et al. [5]. We have not only corrected the estimates of the recombination frequency in the tetraploid populations, but also provided a recommendation for the choice of informative molecular markers used to study the evolutionary significance of polyploids.
MODEL
Allotetraploid model
Consider an allotetraploid plant derived from the chromosomal combination of distinct genomes and subsequent chromosomal doubling [14]. Four sets of chromosomes for the allotetraploid are labelled as 1, 2, 3 and 4, respectively. There are three different homologous patterns:
Chromosomes 1 and 2 are homologous while chromosomes 3 and 4 are homologous. Chromosomes from different homologous pairs, i.e. 1 and 3, 1 and 4, 2 and 3 and 2 and 4, are homoeologous.
Chromosomes 1 and 3 and, therefore, chromosomes 2 and 4 are homologous. Chromosome pairs, 1 and 2, 1 and 4, 2 and 3 and 3 and 4, are homoeologous.
Chromosomes 1 and 4 and, therefore, chromosomes 2 and 3 are homologous. Chromosome pairs, 1 and 2, 1 and 3, 2 and 4 and 3 and 4, are homoeologous.
The affinity of chromosomal pairing may be higher between homologous pairs than between the homoeologues. Two-third of this difference is defined as the preferential pairing factor (p) [14]. By considering all possible pairs, i.e. 1 pairs with 2, then 3 must pair 4; if 1 pairs with 3, then 2 must pair 4; if 1 pairs 4, then 2 must pair 3. Of the three possibilities, the first is homologous and the rest are homoeologous for the first configuration. The homologous combination has a probability of  and the two homoeologous combinations each have a probability of
and the two homoeologous combinations each have a probability of  . If p =
. If p =  , then homoeologous chromosomes do not pair, i.e. chromosomal pairings happen strictly between the homologues. When p = 0, then all the four chromosomes act as if they are homologous, and they will pair randomly. Thus, the value of p ranges from 0 to
, then homoeologous chromosomes do not pair, i.e. chromosomal pairings happen strictly between the homologues. When p = 0, then all the four chromosomes act as if they are homologous, and they will pair randomly. Thus, the value of p ranges from 0 to  .
.
Consider a heterozygous allotetraploid that undergoes bivalent pairings during meiosis. A fully informative codominant marker has four alleles, labelled as 1, 2, 3 and 4, that locate on chromosomes 1, 2, 3 and 4, respectively. If meiotic pairing strictly occurs between two homologous chromosomes, we will have four diploid gametes 13, 14, 23 and 24 under homologous pattern I where chromosomes 1 and 2 are homologous and chromosomes 3 and 4 are homologous. Because homoeologous chromosomes may also pair, but with a lesser extent compared with homologous pairing, we essentially have six diploid gametes 12, 13, 14, 23, 24 and 34 with frequencies  ,
,  ,
,  ,
,  ,
,  and
and  , respectively.
, respectively.
For a particular allelic configuration of two given markers and homologous pattern, Wu et al. [12] derived the frequencies of 36 two-marker gametes for the allotetraploid in terms of θ1, θ2 and the recombination frequency between the two markers. When two markers are dominant markers, 36 gametes are collapsed into four genotypically distinguishable groups. In this case, the preferential pairing factor (p) either disappears or is impossible to be estimated so that only the recombination frequency (r) can be estimated. An optimal combination of allelic configuration and homologous pattern corresponds to one in which the smallest estimate of r is obtained.
Autotetraploid model
For an autotetraploid with genotype 1234 at a fully informative codominant marker, it generates 10 gametes through multivalent pairing, i.e. 11, 22, 33, 44, 12, 13, 14, 23, 24 and 34. The first four are the gametes resulting from double reduction and the second six are those from non-double reduction. Fisher [19] classified gamete formation for two codominant markers into 11 types distributed within four blocks of a 10 × 10 gamete matrix expressed in Matrix (2) of Wu et al. [10]. Block one is composed of two gamete formations resulting from double reduction at both markers, block two composed of two gamete formations resulting from double reduction at the first marker and non-double reduction at the second marker, block three composed of two gamete formations resulting from double reduction at the second marker and non-double reduction at the first marker, and block four composed of five gamete formations resulting from non-double reduction at both markers. However, in block four only three gametes can be observed, two of which each include two gamete formations. In total, 11 gamete formations can be observed in terms of nine distinguishable gametes whose frequencies are defined as f1,…, f9 (see [10]).
 produces can be derived by collapsing the frequencies of indistinguishable gametes (see [11]). Let 1 denote dominant allele and 0 denote recessive allele. Thus, the collapsed gamete frequencies are expressed as
produces can be derived by collapsing the frequencies of indistinguishable gametes (see [11]). Let 1 denote dominant allele and 0 denote recessive allele. Thus, the collapsed gamete frequencies are expressed as 
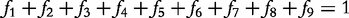
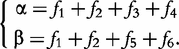
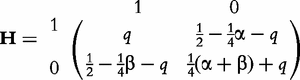
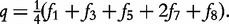
 ×
× , we can obtain the frequencies of genotypes at the two dominant markers through merging the elements of matrix H
, we can obtain the frequencies of genotypes at the two dominant markers through merging the elements of matrix H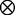 H with the same genotypes, expressed as
H with the same genotypes, expressed as 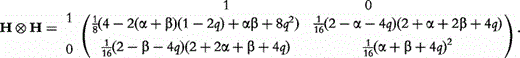
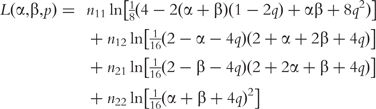
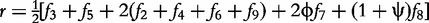
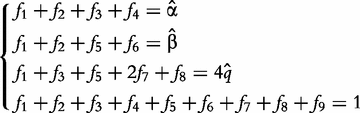
For a cross of two heterozygous tetraploids, there are four possible combinations of tetraploid configurations, i.e.  ×
× ,
,  ×
× ,
,  ×
× and
and  ×
× .
.
An optimal allelic configuration combination corresponds to one in which the lowest estimate of r is obtained.
RESULTS
To investigate whether polyploidization increases the frequency of meiotic recombination and, therefore, the genetic variation of a species as a fuel for evolution, Pecinka et al. [5] genotyped two genetically linked transgenes that provide seed-specific green and red fluorescence, respectively, for the progeny derived from diploid and tetraploid Arabidopsis plants. Staring with a diploid plant that is homozygous for green and red transgenes, they made three different crosses (see Figure 1 in [5]). First, this homozygote (parent 1) was crossed with a homozygous wild-type (parent 2) that was not inserted by the transgenic genes, producing the F1 heterozygotes. The F1 was then selfed to generate an F2 population and backcrossed with the second parent to generate a backcross population, in both of which four types of seed colors were observed, green, red, merged color and normal (because the transgenic genes are dominant to wild types). Second, the transgenic plant was polyploidized to form a homozygous tetraploid, which was crossed with a homozygous non-transgenic tetraploid of the same species, producing a heterozygous F1 autotetraploid. The selfing and backcrossing of this F1 produce a segregating population with four different types of colors. Third, the polyploidized tetraploid was crossed with a homozygous wild-type tetraploid of a different species, producing a heterozygous F1 allotetraploid. By selfing and backcrossing, the F2 and backcross populations were generated with four different types of colors. To examine the effect of sex on meiotic recombination frequencies, reciprocal matings for each backcross cross were made.
The linkage between the two transgenes was analysed for each cross derived from diploids, autotetraploids or allotetraploids using the data supplied in Table 1 of Pecinka et al. [5]. According to their cross experiment, the allelic configuration for the individual that generates segregating progeny populations is known, which is  for the diploids,
for the diploids,  for the allotetraploids and
for the allotetraploids and  for the autotetraploids. The allotetraploid model that incorporates the preferential pairing factor for an allopolyploid’s meiosis was used to estimate the recombination frequency for the two backcrosses and F2. Because of insufficient information of segregation from the two dominant markers, we could not estimate this factor. In this case, our allotetraploid model is reduced to the traditional diploid model. However, as the principle that leads to the occurrence of preferential pairings has been considered, our estimate of the recombination frequency, r, for codominant markers is biologically more relevant. It turns out that our estimate of r for each backcross population is the same as those obtained by Pecinka et al. [5] but for the selfed F2 population is different from their result (Table 1). Our estimate in the F2 is 0.2770 ± 0.0110, in a contrast of 0.241 ± 0.018. This difference is owing to their incorrect use of the backcross model to analyse the F2 data.
for the autotetraploids. The allotetraploid model that incorporates the preferential pairing factor for an allopolyploid’s meiosis was used to estimate the recombination frequency for the two backcrosses and F2. Because of insufficient information of segregation from the two dominant markers, we could not estimate this factor. In this case, our allotetraploid model is reduced to the traditional diploid model. However, as the principle that leads to the occurrence of preferential pairings has been considered, our estimate of the recombination frequency, r, for codominant markers is biologically more relevant. It turns out that our estimate of r for each backcross population is the same as those obtained by Pecinka et al. [5] but for the selfed F2 population is different from their result (Table 1). Our estimate in the F2 is 0.2770 ± 0.0110, in a contrast of 0.241 ± 0.018. This difference is owing to their incorrect use of the backcross model to analyse the F2 data.
Estimates of the recombination frequency in diploid, autotetraploid and allotetraploid populations of Arabidopsis, in comparison with traditional diploid backcross models
| . | . | . | Proposed Model . | ||||
|---|---|---|---|---|---|---|---|
| Ploidy . | Meiosis . | Traditional . | α . | β . | r . | rmin . | rmax . |
| Diploid | Female | 0.074 ± 0.019 | 0.0740 ± 0.0190 | ||||
| Selfed | 0.154 ± 0.009 | 0.1643 ± 0.0062 | |||||
| Male | 0.202 ± 0.003 | 0.2020 ± 0.0030 | |||||
| Autotetraploid | Female | 0.150 ± 0.032 | 0.0873 ± 0.0156 | 0.0326 ± 0.0169 | 0.1672 | 0.1932 | |
| Selfed | 0.205 ± 0.011 | 0.0682 ± 0.0085 | 0.0000 ± 0.0089 | 0.2534 | 0.2761 | ||
| Male | 0.280 ± 0.003 | 0.0583 ± 0.0215 | 0.0739 ± 0.0213 | 0.2960 | 0.3685 | ||
| Allotetraploid | Female | 0.130 ± 0.025 | 0.1300 ± 0.0250 | ||||
| Selfed | 0.241 ± 0.018 | 0.2770 ± 0.0110 | |||||
| Male | 0.298 ± 0.031 | 0.2980 ± 0.0310 | |||||
| . | . | . | Proposed Model . | ||||
|---|---|---|---|---|---|---|---|
| Ploidy . | Meiosis . | Traditional . | α . | β . | r . | rmin . | rmax . |
| Diploid | Female | 0.074 ± 0.019 | 0.0740 ± 0.0190 | ||||
| Selfed | 0.154 ± 0.009 | 0.1643 ± 0.0062 | |||||
| Male | 0.202 ± 0.003 | 0.2020 ± 0.0030 | |||||
| Autotetraploid | Female | 0.150 ± 0.032 | 0.0873 ± 0.0156 | 0.0326 ± 0.0169 | 0.1672 | 0.1932 | |
| Selfed | 0.205 ± 0.011 | 0.0682 ± 0.0085 | 0.0000 ± 0.0089 | 0.2534 | 0.2761 | ||
| Male | 0.280 ± 0.003 | 0.0583 ± 0.0215 | 0.0739 ± 0.0213 | 0.2960 | 0.3685 | ||
| Allotetraploid | Female | 0.130 ± 0.025 | 0.1300 ± 0.0250 | ||||
| Selfed | 0.241 ± 0.018 | 0.2770 ± 0.0110 | |||||
| Male | 0.298 ± 0.031 | 0.2980 ± 0.0310 | |||||
The autotetraploid model provides the estimates of double reductions at the green (α) and red transgenes (β) and the minimum and maximum values of the recombination frequency.
Estimates of the recombination frequency in diploid, autotetraploid and allotetraploid populations of Arabidopsis, in comparison with traditional diploid backcross models
| . | . | . | Proposed Model . | ||||
|---|---|---|---|---|---|---|---|
| Ploidy . | Meiosis . | Traditional . | α . | β . | r . | rmin . | rmax . |
| Diploid | Female | 0.074 ± 0.019 | 0.0740 ± 0.0190 | ||||
| Selfed | 0.154 ± 0.009 | 0.1643 ± 0.0062 | |||||
| Male | 0.202 ± 0.003 | 0.2020 ± 0.0030 | |||||
| Autotetraploid | Female | 0.150 ± 0.032 | 0.0873 ± 0.0156 | 0.0326 ± 0.0169 | 0.1672 | 0.1932 | |
| Selfed | 0.205 ± 0.011 | 0.0682 ± 0.0085 | 0.0000 ± 0.0089 | 0.2534 | 0.2761 | ||
| Male | 0.280 ± 0.003 | 0.0583 ± 0.0215 | 0.0739 ± 0.0213 | 0.2960 | 0.3685 | ||
| Allotetraploid | Female | 0.130 ± 0.025 | 0.1300 ± 0.0250 | ||||
| Selfed | 0.241 ± 0.018 | 0.2770 ± 0.0110 | |||||
| Male | 0.298 ± 0.031 | 0.2980 ± 0.0310 | |||||
| . | . | . | Proposed Model . | ||||
|---|---|---|---|---|---|---|---|
| Ploidy . | Meiosis . | Traditional . | α . | β . | r . | rmin . | rmax . |
| Diploid | Female | 0.074 ± 0.019 | 0.0740 ± 0.0190 | ||||
| Selfed | 0.154 ± 0.009 | 0.1643 ± 0.0062 | |||||
| Male | 0.202 ± 0.003 | 0.2020 ± 0.0030 | |||||
| Autotetraploid | Female | 0.150 ± 0.032 | 0.0873 ± 0.0156 | 0.0326 ± 0.0169 | 0.1672 | 0.1932 | |
| Selfed | 0.205 ± 0.011 | 0.0682 ± 0.0085 | 0.0000 ± 0.0089 | 0.2534 | 0.2761 | ||
| Male | 0.280 ± 0.003 | 0.0583 ± 0.0215 | 0.0739 ± 0.0213 | 0.2960 | 0.3685 | ||
| Allotetraploid | Female | 0.130 ± 0.025 | 0.1300 ± 0.0250 | ||||
| Selfed | 0.241 ± 0.018 | 0.2770 ± 0.0110 | |||||
| Male | 0.298 ± 0.031 | 0.2980 ± 0.0310 | |||||
The autotetraploid model provides the estimates of double reductions at the green (α) and red transgenes (β) and the minimum and maximum values of the recombination frequency.
We used our autotetraploid model to estimate the linkage between the same pair of markers for the progeny derived from the autotetraploid parent. It is found that our estimates are different from those obtained by the original authors who simply used the traditional backcross linkage model (Table 1). Our method allows the estimation and test of double reduction at individual transgenes. There is a significant double reduction (6–9%, P < 0.05) for the green transgene in all backcrosses and F2 populations, but non-significant double reduction is observed (P > 0.05) at the red transgene for the female parent-derived backcross and F2. The autotetraploid model provides the estimates of the interval of the recombination frequency between the two transgenes, with the lower bound strikingly larger than the estimates obtained by Pecinka et al. [5].
Pecinka et al. [5] used a backcross diploid model to estimate the F2 diploid population for dominant markers, which provided an incorrect result. Using the EM algorithm given in Wu et al. [21], we estimated the recombination frequency as 0.1643 ± 0.0062, different from their estimate 0.0154 ± 0.009 (Table 1).
DISCUSSION
The estimation of meiotic recombination frequencies between different markers in polyploids is challenging because their meiotic properties complicate the pattern of genotypic segregation. A set of statistical models has been available in the literature [6–13], which integrate parametric aspects of polyploidy meiotic behavior into a linkage analysis framework. For example, the preferential pairing factor that specifies bivalent pairing in allotetraploids is embedded in the derivation of gamete frequencies [12,13]. In autotetraploids, double reduction resulting from multivalent pairing is integrated [10,11]. However, many studies in polyploidy linkage mapping have not well used these cytologically more meaningful models partly owing to the complexity of the models.
In this Note, we describe two basic frameworks for linkage analysis in allotetraploids and autotetraploids and demonstrate their usefulness by reanalysing a published data set for diploid and tetraploid Arabidopsis plants by Pecinka et al. [5]. In particular, we pinpoint the limitation of the results obtained by inappropriate linkage analysis models, making the conclusions more precise and appealing for biological interpretations.
The dominant feature of the two transgenes used by Pecinka et al. [5] loses much information for linkage analysis. However, the autotetraploid model can still provide informative estimates of double reduction for dominant genes. By producing more gametes, double reduction may play a significant role in the maintenance of genetic diversity that favours evolution under natural populations. Thus, the estimation of double reduction becomes essential for shedding light on the genetic variation and organization of natural populations in autopolyploids [22]. Based on our previous computer simulation, the estimation of the linkage among markers would be biased for a progeny of autotetraploids that undergo double reduction if double reduction was neglected during the estimation procedure [10,11].
Although it is impossible to provide a point estimate of the recombination fraction between dominant markers in an autotetraploid population, the autotetraploid model can obtain an estimate of the interval for the recombination fraction, facilitating the widespread use of dominant markers to study the genetic variation of autotetraploid populations. The use of this model to reanalyse the data of dominant markers in an autotetraploid of Arabidopsis [5] has well validated its practical application. Our estimates of the recombination frequency are different from those reported in the original article, but the conclusion that polyploidization leads to an increase of meiotic recombination frequencies does not change. Indeed, to precisely address this fundamental question related to polyploidization, we recommend the use of codominant markers that will contain more informativeness for the estimation of the recombination frequencies and double reduction in autotetraploids [10,11].
Our models focus on the derivations of tetraploids, but the idea behind can be used for model development in polyploids at higher ploidy levels, such as hexaploid (i.e. wheat, kiwifruit), octaploid (i.e. dahlias), decaploid (i.e. strawberries) and dodecaploid (i.e. plumed cockscomb). Although polyploidy is more common in plants than in animals, linkage analysis of animal polyploids, such as sturgeon and the uganda clawed frog, can gain additional insight into evolution and speciation. All the models described can also find their immediate applications in animals as long as their segregating populations are available. With a set of models available for linkage analysis of polyploids at any ploidy level, we are in a better position to study the genetics and evolution of polyploids [23]. In addition, despite their importance, statistical models for mapping quantitative trait loci (QTLs) in polyploids are still in their infancy (but see [24–26]). Li et al. [24] has presented a first model that incorporates Fisher’s [18] gamete classification patterns into a QTL mapping framework, allowing the QTLs for autotetraploids that experience double reduction to be characterized. This incorporation provides a powerful tool for QTL mapping and understanding the genetic control of a quantitative trait in a multivalent autotetraploid.
The statistical model for allotetraploid and autotetraploid linkage analysis described in this article has been packed into an R platform, available at http://statgen.psu.edu/software.html. By inputting marker data for segregating population from allotetraploids or autotetraploids, this platform provides users with the estimates of pair-wise recombination fractions and the standard errors of these estimates. The platform allows the recombination fraction, preferential pairing factor and double reduction to be tested.
Polyploids can be broadly classified into allopolyploids and autopolyploids based on their origins.
Several models developed for linkage analysis in each of these two types of polyploids are sporadically distributed in the literature, lacking the synthetic description and interpretation of their utility.
In this Opinion Note, we synthesize the models of linkage analysis for allotetraploids and autotetraploids and interpret differences of these models using a case study.
Acknowledgments
This work is partially supported by NSF/IOS-0923975, Changjiang Scholars Award and ‘Thousand-person Plan’ Award.
References
Author notes
*These authors contributed to this work equally.


