-
PDF
- Split View
-
Views
-
Cite
Cite
Michael L. Schummer, Scott A. Petrie, Robert C. Bailey, Dietary Overlap of Sympatric Diving Ducks During Winter on Northeastern Lake Ontario, The Auk, Volume 125, Issue 2, 1 April 2008, Pages 425–433, https://doi.org/10.1525/auk.2008.06117
Close - Share Icon Share
Abstract
Food resources available to diving ducks wintering on the Great Lakes have changed dramatically since the introduction of dreissenid mussels (Dreissena bugensis and D. polymorpha). We investigated the diets of Buffleheads (Bucephala albeola), Common Goldeneyes (B. clangula), and Long-tailed Ducks (Clangula hyemalis) during winter, 2002–2004, on northeastern Lake Ontario and determined the levels of dietary overlap. Dietary niche-breadth values were low, and dietary overlap values (prey size and type) were high for all species. Ducks primarily consumed high-quality, energy-dense prey (Amphipoda, Chironomidae), which were abundant. Our results highlighted three patterns: (1) dreissenid mussels constituted 85% of the macroinvertebrate community in Lake Ontario but were consumed in relatively low amounts during winter, (2) foods of high energy-density such as Amphipoda and Chironomidae were likely abundant enough for ducks to selectively feed on them, and (3) some constraint caused ducks to select energy-dense prey instead of the most available items (dreissenid mussels). Although the abundance of prey may have allowed numbers of diving ducks to increase in the past few decades on the Great Lakes, the long-term implications of high levels of dietary overlap among diving ducks is relatively unknown and warrants continued monitoring.
Chevauchement du régime alimentaire de canards plongeurs sympatriques pendant l'hiver dans le nord-est du lac Ontario
Résumé.—Les ressources alimentaires disponibles pour les canards plongeurs hivernant sur les Grands Lacs ont dramatiquement changé depuis l'introduction des bivalves dreissénidés (Dreissena bugensis et D. polymorpha). Nous avons étudié les régimes alimentaires de Bucephala albeola, B. clangula et Clangula hyemalis au cours des hivers 2002–2004 au nord-est du lac Ontario et déterminé leurs niveaux de chevauchement. Les valeurs de l'étendue des niches alimentaires étaient faibles et les valeurs de chevauchement des régimes alimentaires (taille et type de proie) étaient élevées pour toutes les espèces. Les canards ont principalement consommé des proies de grande qualité et denses en énergie (Amphipoda, Chironomidae), lesquelles étaient abondantes. Nos résultats ont mis en lumière trois patrons: (1) les bivalves dreissénidés constituent 85% de la communauté de macroinvertébrés du lac Ontario mais ils étaient consommés en relativement petites quantités au cours de l'hiver, (2) la nourriture dense en énergie (e.g. Amphipoda et Chironomidae) était apparemment assez abondante pour que les canards s'en nourrissent sélectivement, (3) quelques contraintes ont poussé les canards à sélectionner des proies très denses en énergie plutôt que les items les plus disponibles (bivalves dreissénidés). Malgré que l'abondance de proies ait pu permettre l'augmentation du nombre de canards plongeurs sur les Grands Lacs dans les dernières décennies, les implications à long terme associées à des niveaux élevés de chevauchement de régime alimentaire chez les canards plongeurs sont relativement peu connues et nécessitent un suivi continu.
Food available to diving ducks using the Great Lakes has increased since Quagga Mussels (Dreissena bugensis) and Zebra Mussels (D. polymorpha) (hereafter "dreissenid mussels") were introduced (Wormington and Leach 1992, Hamilton and Ankney 1994, Petrie and Knapton 1999, Mitchell et al. 2000). In addition, shells of live and dead dreissenid mussels increase the surface area of the benthos, which can increase densities of Amphipoda and Chironomidae (Dermott et al. 1993, Stewart and Haynes 1994, Wisenden and Bailey 1995, Kuhns and Berg 1999). Diving ducks congregate in areas of abundant foods on the Great Lakes (Wormington and Leach 1992, Hamilton and Ankney 1994, Petrie and Knapton 1999, Mitchell et al. 2000, Schummer et al. 2008), though few studies have documented how the diet of diving ducks has changed in response to increases in dreissenid mussels, amphipods, and chironomids. Dietary convergence among diving ducks caused by the introduction of exotic prey species on the Great Lakes could have energetic, contaminant, behavioral, and habitat-use implications (Ross et al. 2005). Ultimately, dietary convergence can influence competition, species ranges, population dynamics, and community structure (Arthur 1987, Newton 1998). Conversely, when birds use similar habitats, ecological segregation can still occur if birds forage on different items or on items of different size (Nudds and Bowlby 1984, Goudie and Ankney 1986, McKnight and Hepp 1998).
We examined patterns of seasonal dietary overlap and change among Buffleheads (Bucephala albeola), Common Goldeneyes (B. clangula), and Long-tailed Ducks (Clangula hyemalis) that used northeastern Lake Ontario throughout winter. Dreissenid mussels, Amphipoda, and Chironomidae were abundant and common in benthic samples from northeastern Lake Ontario (Schummer 2005). Buffleheads, Common Goldeneyes, and Long-tailed Ducks regularly forage on these items within the Great Lakes (Hamilton and Ankney 1994, Custer and Custer 1996, Ross et al. 2005). Therefore, because it was likely that food was highly abundant (Schummer 2005, Schummer et al. 2008) and some were high-energy foods (Sugden 1973, Driver et al. 1974), we predicted that ducks would specialize on high-energy foods, resulting in narrow niche-breadths and high dietary overlap throughout winter. We also compared size distributions of commonly consumed prey, because previous work indicated that waterfowl may segregate by eating different size classes of the same prey (Nudds and Bowlby 1984, Goudie and Ankney 1986, Hamilton and Ankney 1994, de Leeuw et al. 1999, Benoy et al. 2002). Dietary shifts by ducks can occur as food resources decline seasonally (Newton 1998, Sekiya et al. 2000, Badzinski and Petrie 2006).
Methods
Study area.—The present study was conducted along the southeast shoreline of Prince Edward County, Ontario (44°00′N, 77°55′W), during December to March, 2002–2003 and 2003–2004. Substrate and vegetation varied across the study area. Prince Edward Bay was mostly mud substrate and abundant aquatic macrophytes in the west and mostly limestone rock substrate with little or no vegetation in the east (Barton 1986). In 1990, dreissenid mussels colonized the study area (Griffiths et al. 1991). Lake Ontario remains relatively ice-free throughout winter compared with the other Lower Great Lakes (Assel and Rodionov 1998, Assel 2003) and, thus, provides wintering habitat for large congregations of diving ducks (Petrie and Schummer 2002).
Specimen collection and dissection.—We collected 269 Buffleheads, 224 Common Goldeneyes, and 256 Long-tailed Ducks during December to March, 2002–2003 and 2003–2004. Ducks were collected opportunistically by shooting over decoys (n = 558), jump shooting from shore (n = 95), pass shooting (n = 5), and floating into flocks with a canoe and shooting (n = 91). We attempted to collect 50 ducks per species each month (December–March). We also attempted to collect ducks from throughout the study area by moving among 26 shooting locations, depending on ice conditions and presence of birds. Seventy-percent ethanol was injected into the esophagus of each duck within 5 min of collection. Ducks were then double bagged, frozen, and transferred to the University of Western Ontario, London, Ontario, where they were thawed and dissected.
Specimens were aged and sexed by external and internal characteristics (Bellrose 1980). Esophagus and proventriculus contents were sorted, and prey were identified and counted under a dissection microscope. Food items were dried at 60°C for 24 h and weighed (±0.0001 g) on a digital balance. Data were summarized for each category of food as aggregate percent dry mass and percent frequency of occurrence (Swanson et al. 1974). We randomly selected and measured (±0.01 mm) 50 whole individuals (when that many were available) of Chironomidae, Amphipoda, and dreissenid mussels from each bird. Dreissenid mussels were often broken in the surf because of heavy wave action and ice-scouring along the north shore of Lake Ontario during winter. Shell-free tissue from dreissenid mussels was available, so we analyzed separately the shell-free tissue and whole dreissenid mussels consumed by ducks. Damage to Amphipoda and Chironomidae, their attachment to other ingested materials (i.e., shell fragments), and their small size often precluded accurate measurement of dry weight of individuals. Therefore, we measured dry weight of 10 whole individuals and then estimated individual dry weight. We conducted a power analysis using an allowable error of 5% to determine the sample size required for accurate estimation of mean dry weights of individuals (Shiver and Borders 1996). Sample-size requirements were calculated for individual birds when enough invertebrates were found in the esophagus and proventriculus. However, when we found too few Amphipoda or Chironomidae in the esophagus and proventriculus of an individual, we estimated dry weight (± SE) of these prey as 1.0179 ± 0.0482 mg and 0.2428 ± 0.0251 mg, respectively (n = 280 samples per prey from all three duck species collected). Sample size required to accurately estimate these weights for Amphipoda and Chironomidae was 73 and 174, respectively. We estimated total dry weight of Amphipoda and Chironomidae ingested by individual birds as mean dry weight (1.0179 or 0.2428 mg, respectively) times the number ingested.
We quantitatively estimated the amount of available soft tissue (g dry) in selected prey of diving ducks using predictive models from the literature. We estimated that 26% of intact Gastropoda was available soft tissue (equations in Mackie and Flippance [1983] and Ross et al. [2005]). Soft tissue of Zebra Mussels was determined using shell measurements and equations in (Draulans 1982). We estimated soft-tissue mass of Quagga Mussels using the same equations as for Zebra Mussels, but multiplied by 1.11 because Quagga Mussels have consistently more soft tissue than Zebra Mussels across a wide range of size classes (Stoeckmann 2003).

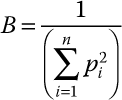
Statistical analyses.—Data were tested for normality using a Kolmogorov-Smirnov test for goodness-of-fit and, subsequently, data were transformed using an arcsine square-root transformation to increase normality (Sokal and Rohlf 1981). Multivariate analysis of variance (MANOVA) was used to determine whether overall diets differed among species, year, sex, and age (PROC GLM; SAS Institute 1990). Species, year, sex, age, and biologically plausible interactions were included as class variables to examine overall variation in aggregate proportion of dry mass for shell-free dreissenid mussel tissue, whole dreissenid mussels (Quagga and Zebra mussels combined), Amphipoda, Chironomidae, Trichoptera, Gastropoda, plant matter (tubers, miscellaneous vegetation, and seeds combined), fish, fish eggs, Decapoda (crayfish), and Isopoda. For multivariate analyses, Wilks' lambda was used as the test statistic. Because an observational approach was used, statistical models and differences between or among means are considered worthy of biological interpretation and discussion at P ≤ 0.10. Following a significant MANOVA (P < 0.10) for species, a discriminant function analysis was used to depict overlap of diets between species (PROC DISCRIM, DFA; SAS Institute 1990). Discriminant scores were generated from the MANOVA for the entire winter and on a monthly basis (December–February). The first two axes were used for plots of species position in discriminant space. Standardized coefficients of the eigenvectors indicated the relative importance of different taxa in determining species position on each axis.
To compare overlap in prey sizes between commonly ingested items, we assigned prey to categories by rounding measured size of invertebrates to the nearest millimeter. We used Kolmogorov-Smirnov two-sample tests and Kendall's tau distance (Dmax) based on cumulative frequency distributions of dreissenid mussels, Amphipoda, and Chironomidae to quantify differences among species in prey size eaten. Kendall's tau distance values range from 0 to 1, and values closer to 0 indicate a greater similarity between distributions (Sokal and Rohlf 1981). Differences are considered worthy of biological interpretation and discussion at P ≤ 0.10. All species combinations were compared for Amphipoda and Chironomidae, but only Common Goldeneyes and Long-tailed Ducks were used for comparisons of distributions of dreissenid size classes because only two Buffleheads consumed mussels. We also compared distributions of dreissenid sizes consumed by males and females of each duck species, because previous research has indicated that males and females may eat different size classes of mussels (Draulans 1982, Hamilton and Ankney 1994, de Leuww et al. 1999). Dreissenid mussel, Amphipoda, and Chironomidae sizes (±0.01 mm) were regressed against study day (study day 0 = 14 December, study day 100 = 22 March; PROC REG; SAS Institute 1990) to determine whether size of prey consumed changed during winter.
Results
Prey type.—We collected 90 Buffleheads, 78 Common Goldeneyes, and 153 Long-tailed Ducks that contained food in their esophagus and proventriculus (Tables 1 and 2). Chironomidae and Amphipoda constituted the largest portion of diets in all species. Common Goldeneyes and Long-tailed Ducks also commonly ingested dreissenid mussels, but only Long-tailed Ducks ate shell-free dreissenid mussel tissue. Buffleheads primarily ate Chironomidae and Amphipoda, rarely ingesting dreissenid mussels. Diets (December–March inclusive) of the three species were different (MANOVA, species effect: Wilks' λ = 0.86, P = 0.0055; Fig. 1) but were not affected by collection year (including all interaction terms, P > 0.10). However, niche breadths were narrow for all species, and niche overlap between species was high, especially in midwinter (January and February; Table 3). Differences in diet (December–March inclusive) among species were based on prey that comprised a lower percentage of total diet, not on primary prey of Amphipoda and Chironomidae (Table 1 and Fig. 1). All age–sex classes of Buffleheads and Long-tailed Ducks ate similar items, but adult and immature Common Goldeneyes differed (F = 1.19, df = 3 and 74, P < 0.05) in consumption of Chironomidae and plant tubers (Table 1). Diet of Common Goldeneyes and Long-tailed Ducks differed primarily in the amount of shell-free dreissenid-mussel tissue consumed (Tables 1 and 2; Fig. 1) and the amount of plant matter consumed during early winter (Fig. 1). Buffleheads did not segregate as much from other species and had larger niche breadths than Common Goldeneyes and Long-tailed Ducks each month (Fig. 1 and Table 3). When Buffleheads segregated from other species, they did so on the axis heavily weighted toward fish eggs and Gastropoda (Fig. 1). Common Goldeneyes foraged on more plant matter (mostly Vallisneria americana tubers) and Decapoda (crayfish) in December but shifted toward a diet entirely of animal matter (e.g., dreissenid mussels, Amphipoda, and Chironomidae) later in winter, which resulted in increased dietary overlap with Buffleheads and Long-tailed Ducks (Fig. 1 and Table 3).
Aggregate percent dry mass of food items (mean ± SD; TR = trace, <0.01%) consumed by immature (Imm.) and adult Buffleheads, Common Goldeneyes, and Long-tailed Ducks on northeastern Lake Ontario during winter, 2002–2003 and 2003–2004.
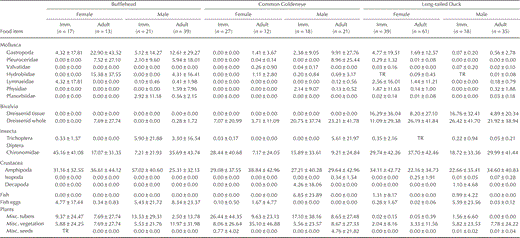
Aggregate percent dry mass of food items (mean ± SD; TR = trace, <0.01%) consumed by immature (Imm.) and adult Buffleheads, Common Goldeneyes, and Long-tailed Ducks on northeastern Lake Ontario during winter, 2002–2003 and 2003–2004.

Frequency of occurrence (%) of food items consumed by Buffleheads, Common Goldeneyes, and Long-tailed Ducks on northeastern Lake Ontario during winter, 2002–2003 and 2003–2004.
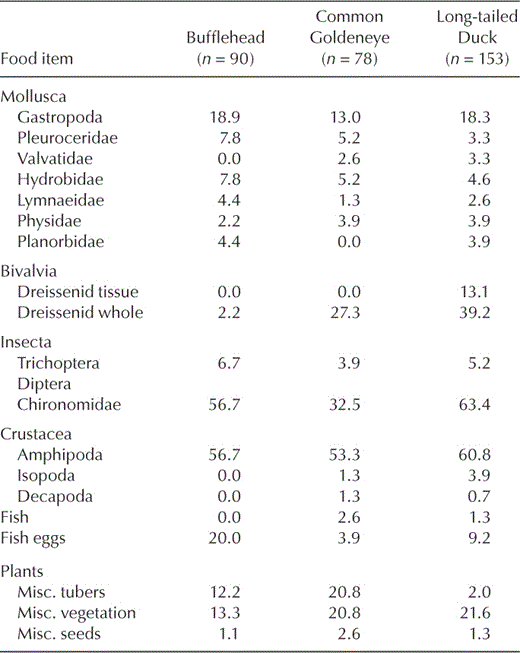
Frequency of occurrence (%) of food items consumed by Buffleheads, Common Goldeneyes, and Long-tailed Ducks on northeastern Lake Ontario during winter, 2002–2003 and 2003–2004.

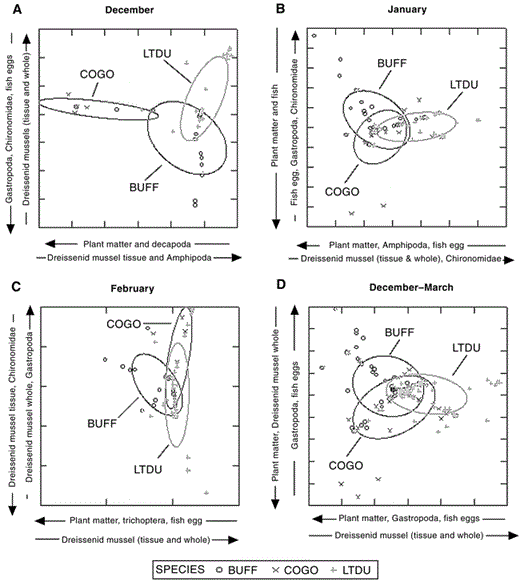
Discriminant score plots of foods eaten by diving ducks collected during (A) December, (B) January, (C) February, and (D) December–March combined on northeastern Lake Ontario, 2002–2003 and 2003–2004. Fifty-percent confidence ellipses are based on individual observations. BUFF = Bufflehead, COGO = Common Goldeneye, and LTDU = Long-tailed Duck.
Monthly and pooled dietary overlap and breadth values for Buffleheads (BUFF), Common Goldeneyes (COGO), and Long-tailed Ducks (LTDU) on northeastern Lake Ontario, December–March, 2002–2003 and 2003–2004.
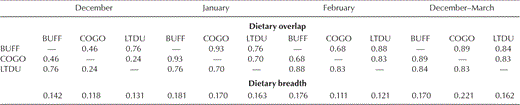
Monthly and pooled dietary overlap and breadth values for Buffleheads (BUFF), Common Goldeneyes (COGO), and Long-tailed Ducks (LTDU) on northeastern Lake Ontario, December–March, 2002–2003 and 2003–2004.

Prey size.—The sizes of Chironomidae and Amphipoda eaten by the three species were very similar. Prey size distributions of Chironomidae eaten by Buffleheads did not differ (P > 0.10) from those for Common Goldeneyes (Dmax = 0.054) or Long-tailed Ducks (Dmax = 0.063). Common Goldeneyes and Long-tailed Ducks differed in size distribution of Chironomidae eaten (P < 0.05), but size overlap remained relatively high (Dmax = 0.098). Buffleheads consumed smaller amphipods than Common Goldeneyes and Long-tailed Ducks (P < 0.05), but the distribution of sizes consumed overlapped greatly (Dmax = 0.197 and 0.165, respectively). Long-tailed Ducks consumed smaller mussels than Common Goldeneyes (Dmax = 0.386; Fig. 2), and female Common Goldeneyes ate smaller mussels than males (Fig. 2 and Table 4), but this sex effect was not evident in Long-tailed Ducks (Table 4).
![Length frequency distributions of dreissenid mussels consumed by Common Goldeneyes ([A] female, n = 41; [B] male, n = 129) and Long-tailed Ducks ([C] female, n = 569; [D] male, n = 343) during winter on northeastern Lake Ontario, December–March, 2002–2004.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/auk/125/2/10.1525_auk.2008.06117/4/m_i0004-8038-125-2-425-f02.gif?Expires=1750196522&Signature=MKhMYmE-OfV08NNojtUMa7u1wjanYxb6TodZmN4d-7Wp6ouI9Rj~zCFGOIC0y7ioImew4USSrlJjcfHvJHzO6fXcrQuudX-aYxaNjHAmmBjHwEpTyajOQA6PPEfVCNGv~q6-C9yBPYIuoM4jxkK46pI4jFmw4HKq8dczp5q5lxn7MWE7eE-dkOmFhnzo0FO6H4sTaHCfoTDKscPn-ADQ4iUGf1I7el7U2q~~6ba39YrJQuDxIFT9XtvOUv9M7eBFwkyVS5qD338u7cvxC4aq3Xuz9qYYmwPj-PqRL4I0ZP2fPdyTQaYH~JqAHePbm7H2aDpgJVeXCrjnttq-He~JFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Length frequency distributions of dreissenid mussels consumed by Common Goldeneyes ([A] female, n = 41; [B] male, n = 129) and Long-tailed Ducks ([C] female, n = 569; [D] male, n = 343) during winter on northeastern Lake Ontario, December–March, 2002–2004.
Indices (Dmax) of dreissenid-mussel size overlap between Common Goldeneyes and Long-tailed Ducks during winter on northeastern Lake Ontario, December–March, 2002–2003 and 2003–2004. Low Dmax values correspond to high prey-size overlap. Dmax values followed by an asterisk are significant at P < 0.10, indicating a difference in size distributions consumed.
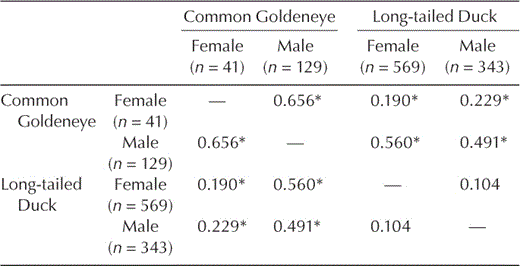
Indices (Dmax) of dreissenid-mussel size overlap between Common Goldeneyes and Long-tailed Ducks during winter on northeastern Lake Ontario, December–March, 2002–2003 and 2003–2004. Low Dmax values correspond to high prey-size overlap. Dmax values followed by an asterisk are significant at P < 0.10, indicating a difference in size distributions consumed.

Mean size of amphipods eaten did not change throughout winter for any of the three duck species (P > 0.10). Size of chironomids eaten by Common Goldeneyes (t = −3.09, df = 255, P = 0.022) and Long-tailed Ducks (t = −13.55, df = 1,821, P < 0.0001) decreased throughout winter, but this relationship was not observed in Buffleheads (t = 0.13, df = 356, P = 0.8959). Size of dreissenid mussels eaten by male Common Goldeneyes (t = 2.49, df = 127, P = 0.0140) and female Long-tailed Ducks (t = 4.50, df = 576, P < 0.0001) increased throughout winter, but size selection did not change for other species–sex classes (P > 0.10).
Discussion
Food resources available to diving ducks on the Great Lakes have changed substantially since the introduction of dreissenid mussels (Wormington and Leach 1992, Hamilton and Ankney 1994, Petrie and Knapton 1999, Mitchell et al. 2000). However, little research has been conducted on resource use and partitioning of wintering diving ducks at northern latitudes (but see Goudie and Ankney 1988). Therefore, ecologists had little idea of the implications of large-scale changes in food availability on diets of diving ducks wintering on the Great Lakes (Ross et al. 2005). We found high overlap in type and size of prey consumed by Buffleheads, Common Goldeneyes, and Long-tailed Ducks, with little indication of dietary shift throughout winter. We also found that size distributions of certain consumed prey changed throughout winter, but these changes did not occur in all species–sex–age classes of ducks. Overall, our results suggest that depletion of prey or of specific size classes of prey had not occurred. Dreissenid mussels (85%) dominated the macroinvertebrate community in the study area, whereas Amphipoda (5%) and Chironomidae (3%) constituted a relatively small portion of the macroinvertebrates sampled during the period in which ducks were collected for dietary analyses (Schummer 2005). We found that ducks primarily ate Amphipoda and Chironomidae, and not dreissenid mussels, which is inconsistent with other studies on food habits of ducks that were conducted in the Great Lakes since dreissenid mussels were introduced (Hamilton and Ankney 1994, Custer and Custer 1996, Petrie and Knapton 1999, Badzinski and Petrie 2006).
Selection of prey by predators involves tradeoffs among various factors, including energy density, abundance, and prey-handling time (Krebs and Davies 1991). Low-energy returns, as a result of a low ratio of flesh content to shell size, could make dreissenid mussels a less profitable prey compared with more digestible macroinvertebrates (de Leeuw et al. 1999). Although total energy values are similar between dreissenid mussels (about 4.8–5.4 kcal g–1 dry weight; Ricciardi et al. 1996) and other commonly consumed prey (Amphipoda: 4.6–4.7 kcal g–1 dry weight; Chironomidae: 4.6–6.1 kcal g–1 dry weight; Driver et al. 1974), indigestible shell and water within shells could make dreissenid mussels a less profitable prey (Hamilton and Ankney 1994, de Leeuw et al. 1999) than smaller prey without shells (i.e., Amphipoda and Chironomidae). Indigestible shell decreases the space available in the digestive tract and requires time for grinding in the gizzard (Draulans 1987, de Leeuw et al. 1990). Also, water within dreissenid mussels must be warmed after ingestion, again increasing energy expenditure (de Leeuw et al. 1999). However, handling and capture time should be greater for more mobile prey such as Amphipoda, which would also increase energy expenditure. Therefore, we argue that (1) dreissenid mussels were avoided regularly, possibly because they were a less profitable prey than Amphipoda and Chironomidae; (2) high-energy-density prey, such as Amphipoda and Chironomidae, were likely abundant enough for ducks to selectively forage on them; and (3) some constraint increased the likelihood that ducks would select high-energy-density prey instead of the most abundant items.
Common Goldeneyes and Long-tailed Ducks regularly ate dreissenid mussels, though they ate different sizes of mussels and foraged on mussels differently. Mussels were regularly dislodged from substrate and broken in the surf, which resulted in shell-free dreissenid mussel tissue within the water column. Long-tailed Ducks often foraged on shell-free dreissenid mussel tissue, whereas Common Goldeneyes did not. Ingestion of mussel shells requires mechanical reduction by the gizzard and provides little energy (Draulans 1987, Hamilton and Ankney 1994, de Leeuw et al. 1999). Long-tailed Ducks are known to feed on nektonic prey (i.e., crustaceans, fish; Sanger and Jones 1984, Robertson and Savard 2002), but we found no other published studies that reported Long-tailed Ducks feeding on shell-free dreissenid mussel tissue. Long-tailed Ducks use their wings (Schorger 1951) to propel them during foraging dives, and this behavior, which increases mobility within the water column, may allow them to forage on nektonic or free-floating prey (i.e., shell-free dreissenid mussel tissue). Buffleheads and Common Goldeneyes propel themselves with their feet while foraging (Gauthier 1993, Eadie et al. 1995), and we found that they did not eat shell-free dreissenid mussel tissue. Long-tailed Ducks may have a foraging advantage over other diving ducks because they are able to feed throughout the water column at various depths (Schummer 2005, Schummer et al. 2008), as well as on benthic prey. Differences in foraging behaviors appear to allow Long-tailed Ducks to segregate and exploit a wider range of resources.
Increased abundance of diving ducks during winter on Lake Ontario (Petrie and Schummer 2002) may not be attributable exclusively to increased abundance of dreissenid mussels. Although dreissenid mussels directly compete with and can cause decreases in traditional duck foods such as Gastropoda (Wisenden and Bailey 1995, Kuhns and Berg 1999, Ross et al. 2005), increased surface area provided by shells of live and dead dreissenid mussels can also increase surface area available for colonization by other macroinvertebrates, including Amphipoda and Chironomidae (Dermott et al. 1993, Stewart and Haynes 1994, Wisenden and Bailey 1995). Therefore, we argue that increases in Buffleheads, Common Goldeneyes, and Long-tailed Ducks have been at least partially caused by facilitation of colonization by high-energy prey such as Amphipoda and Chironomidae and that birds supplement with dreissenid mussels (or other alternative prey) during periods when high-quality foods are relatively unavailable. This is likely true for Buffleheads, which increased in number during winter on Lake Ontario since the 1980s (Petrie and Schummer 2002), showed stable to slight decreases on breeding areas during the same period (U.S. Fish and Wildlife Service 2004), and rarely fed on dreissenid mussels during winter in the present study. The extent to which mussels have facilitated increases in the numbers of Common Goldeneyes and Long-tailed Ducks is difficult to ascertain, because we found that they forage regularly on dreissenid mussels. However, if dreissenid mussels have facilitated increases in high-energy macroinvertebrates within mussel beds, patch quality has likely also increased (Newton 1998). (Wisenden and Bailey 1995) described this process as a Zebra Mussel–amphipod community that developed on rocks, increasing microhabitat stability and food supply for Amphipoda. Overall, this scenario would likely contribute to more birds remaining on wintering areas at northern latitudes, where relatively harsh environmental conditions regularly occur.
We recommend continued monitoring of the interaction between dreissenid mussels, macroinvertebrates (e.g., Amphipoda, Chironomidae), and diving ducks that inhabit the Great Lakes. Although few studies documented diets of ducks prior to dreissenid mussel introduction, our findings suggest that dietary convergence among at least three species of diving ducks has occurred coincidentally with large-scale shifts in benthic community structure. The implications of high dietary overlap among diving ducks, combined with narrow foraging breadths, over several decades, are relatively unknown.
Acknowledgments
Financial support was provided by Long Point Waterfowl and Wetlands Research Fund, through funding provided by Bluff's Hunting Club; Ducks Unlimited Canada; Ontario Federation of Anglers and Hunters; and a Natural Sciences and Engineering Research Council of Canada research grant awarded to R.C.B. Gagnon Sports, Lefevbre Outdoor Sports, and B.H. Consultants donated field equipment. Bird Studies Canada and the Canadian Wildlife Service provided logistical support. Specimens were collected under Canadian Wildlife Service Scientific-Capture Permit no. CA 0166. We thank E. Craigie, G. Dunn, S. Fleming, R. Lancaster, S. A. Highmeadow, S. Meyer, B. Shroyer, F. G. Tanker, R. Ward, Sr., R. Ward, Jr., and D. Wight for field assistance. J. Donald, K. S. Fleming, S. Gil, A. Kosloski, C. LaFrance, J. Musada, S. Perez, and B. Shojaei provided laboratory assistance. K. Abraham, D. Ankney, S. Badzinski, G. Baldassarre, D. Dennis, G. MacCullough, S. McWilliams, J. Millar, B. Scott, and two anonymous reviewers provided helpful comments on the manuscript.
Literature Cited
Author notes
Present address: Department of Wildlife and Fisheries, Mississippi State University, Box 9690, Mississippi State, Mississippi 39762, USA. E-mail: [email protected]
>Associate Editor: S. R. McWilliams



