-
PDF
- Split View
-
Views
-
Cite
Cite
Shervin A Etemad, Melissa M Poh, Enhanced Recovery After Gender-Affirming Surgery, Aesthetic Surgery Journal, Volume 44, Issue Supplement_1, September 2024, Pages S3–S14, https://doi.org/10.1093/asj/sjae082
Close - Share Icon Share
Abstract
The adoption of enhanced recovery after surgery (ERAS) protocols in multiple surgical disciplines has revolutionized perioperative care, demonstrating reduced complications and shorter hospital stays across surgical specialties. ERAS protocols have increasingly been incorporated in plastic surgery, yet a notable gap in the literature on ERAS for gender-affirming surgery (GAS) still exists. A scoping review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify studies on ERAS protocols in GAS. The aim of this review was to assess the current status of ERAS adoption in GAS, evaluate its impact on perioperative care, and provide recommendations for future research and clinical practice. While there is an overall scarcity of evidence-based ERAS protocols across GAS, published studies on the application of ERAS in GAS have demonstrated promising early outcomes and illustrate an area for further investigation and innovation in plastic surgery.

The integration of enhanced recovery after surgery (ERAS) protocols signifies a paradigm shift in perioperative care across surgical specialties and has resulted in reduced complications, lower hospital length of stay (LOS), and cost savings for health systems.1,2 ERAS is a multidisciplinary commitment to the implementation of patient-centered, evidence-based pathways that address and alleviate the surgical stress response, optimize physical function, and streamline postoperative recovery. While initially developed for colorectal surgery, the adoption of ERAS protocols has gained traction in plastic surgery in recent years—most notably in breast reconstruction.3,4
Gender-affirming surgery (GAS) encompasses a broad set of operations that aim to align a patient's anatomy with their gender identity. These procedures can be categorized as facial surgery, chest surgery, and genitourinary surgery. Patients undergoing GAS are particularly vulnerable, because they face systemic barriers to healthcare and higher rates of mental health comorbidities, including substance use disorder, anxiety, and depression.5 This baseline risk is often exacerbated by multiple operative interventions and related complications encountered in the process of transitioning.
Despite the evident benefits of ERAS protocols across surgical domains, there is little literature on ERAS specifically for GAS. Through a scoping review of the ERAS-GAS literature, we aimed to assess the present use of ERAS principles in GAS, evaluate their effectiveness in enhancing perioperative care and patient outcomes in this population, identify potential challenges and disparities in their implementation, and provide recommendations for future research and clinical practice.
The goal of this study was to provide an overview of the current published literature regarding ERAS protocols in the gender-affirming surgery field and highlight some of the unique features in this population that warrant additional attention, such as previous substance use and high prevalence of mental health concerns that hold true across the different procedures. Given the paucity of studies to date, this paper is a call to action to start implementing more systemic methods for perioperative care in this field. Presently, even for just 1 procedure (eg, vaginoplasty), there is a wide range of perioperative care protocols. We aim to make gender surgeons aware of the importance and effectiveness of ERAS protocols and provide a framework for the development of these protocols for the various gender surgery options to aid in streamlining and standardizing care.
METHODS
A scoping review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1). A literature search was conducted on March 8, 2024, to identify all English-language articles pertaining to ERAS studies for patients undergoing gender-affirming surgery with 5 databases: PubMed (National Institutes of Health, Bethesda, MD); Medline (National Institutes of Health); Scopus (Elsevier, Amsterdam, the Netherlands); Embase (Elsevier); and Web of Science (Clarivate, London, UK). Selected search terms included terminology related to gender-affirming surgery, which included “gender affirming,” “facial feminization,” “facial masculinization,” “chest wall masculinization,” “top surgery,” “bottom surgery,” “vaginoplasty,” “metoidioplasty,” “phalloplasty,” “rhinoplasty,” “transgender,” “gender surgery,” “gender affirmation,” “gender confirmation,” and “gender confirming.” Terminology associated with ERAS management was also included, which included “Enhanced recovery after surgery” and “ERAS.” All observational studies, case reports, reviews, case series, and editorials describing ERAS protocols pertaining to gender-affirming surgery were included. Exclusion criteria consisted of duplicate studies, abstracts, conference proceedings, and studies that did not reference ERAS protocols because they pertained to the operative procedure. Author S.E. conducted the search, and all search results were reviewed by authors S.E. and M.P. The full texts for all remaining articles were then read in full and assessed for subject or content relevance. Additional studies from other sources were identified through a review of included study references. The included articles were categorized according to operative procedure and were reviewed by study authors for content related to the development and implementation of ERAS protocols for patients undergoing gender-affirming surgery.
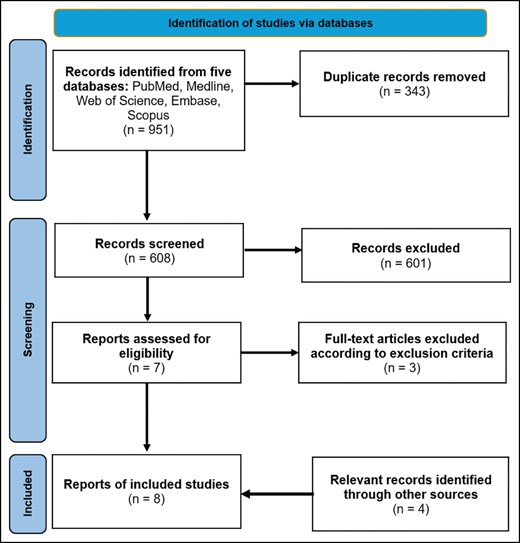
RESULTS
The initial search yielded a total of 951 articles. After excluding duplicates, 608 titles and abstracts were screened by both authors. Seven full-text studies were assessed for eligibility, of which 4 met the inclusion criteria. Four additional relevant articles were identified through other sources, yielding a total of 8 included articles specifically pertaining to ERAS in GAS.6-13 Specific details and characteristics for the included studies are provided in Table 1.
| Study (year) . | Study design . | Study details . | Outcome . |
|---|---|---|---|
| Aquino (2023)6 | Retrospective observational cohort study | ERAS protocol with a focus on anesthesiology-led interventions in top surgery | ERAS group with shorter length of stay, decreased hematoma requiring operative intervention, lower drain output |
| Bedar (2023)7 | Retrospective review | ERAS protocol consisting of opioid-reducing multimodal pain regimen for facial feminization surgery | Reduced opioid use, lower pain score, shorter length of stay in ERAS group |
| Edalatpour (2024)8 | Retrospective review | Tranexamic acid and liposomal bupivacaine in patients undergoing top surgery | Lower opioid use following liposomal bupivacaine. No difference in complications with TXA |
| Tirrell (2022)9 | Retrospective review | Vaginoplasty with and without PCA | Non-PCA group with lower postoperative opioid use and shorter LOS |
| Aquino (2022)10 | Retrospective case series | Development of a Gender Affirming Surgical Perioperative Program | Description of postoperative pain scores, spectrum of cases, and the overview of program development |
| Rifkin (2022)11 | Retrospective review | Phalloplasty with standardized protocol | Low overall complication profile |
| Coon (2023)12 | Semistructured interviews | NA | Variable LOS for vaginoplasty (1-9 days) |
| Salgado (2023)13 | Retrospective review | Vaginoplasty with and without epidural | Epidural group with reduction in pain scores and no difference in LOS |
| Study (year) . | Study design . | Study details . | Outcome . |
|---|---|---|---|
| Aquino (2023)6 | Retrospective observational cohort study | ERAS protocol with a focus on anesthesiology-led interventions in top surgery | ERAS group with shorter length of stay, decreased hematoma requiring operative intervention, lower drain output |
| Bedar (2023)7 | Retrospective review | ERAS protocol consisting of opioid-reducing multimodal pain regimen for facial feminization surgery | Reduced opioid use, lower pain score, shorter length of stay in ERAS group |
| Edalatpour (2024)8 | Retrospective review | Tranexamic acid and liposomal bupivacaine in patients undergoing top surgery | Lower opioid use following liposomal bupivacaine. No difference in complications with TXA |
| Tirrell (2022)9 | Retrospective review | Vaginoplasty with and without PCA | Non-PCA group with lower postoperative opioid use and shorter LOS |
| Aquino (2022)10 | Retrospective case series | Development of a Gender Affirming Surgical Perioperative Program | Description of postoperative pain scores, spectrum of cases, and the overview of program development |
| Rifkin (2022)11 | Retrospective review | Phalloplasty with standardized protocol | Low overall complication profile |
| Coon (2023)12 | Semistructured interviews | NA | Variable LOS for vaginoplasty (1-9 days) |
| Salgado (2023)13 | Retrospective review | Vaginoplasty with and without epidural | Epidural group with reduction in pain scores and no difference in LOS |
ERAS, enhanced recovery after surgery; LOS, length of stay; NA, not applicable; PCA, patient-controlled analgesia; TXA, tranexamic acid.
| Study (year) . | Study design . | Study details . | Outcome . |
|---|---|---|---|
| Aquino (2023)6 | Retrospective observational cohort study | ERAS protocol with a focus on anesthesiology-led interventions in top surgery | ERAS group with shorter length of stay, decreased hematoma requiring operative intervention, lower drain output |
| Bedar (2023)7 | Retrospective review | ERAS protocol consisting of opioid-reducing multimodal pain regimen for facial feminization surgery | Reduced opioid use, lower pain score, shorter length of stay in ERAS group |
| Edalatpour (2024)8 | Retrospective review | Tranexamic acid and liposomal bupivacaine in patients undergoing top surgery | Lower opioid use following liposomal bupivacaine. No difference in complications with TXA |
| Tirrell (2022)9 | Retrospective review | Vaginoplasty with and without PCA | Non-PCA group with lower postoperative opioid use and shorter LOS |
| Aquino (2022)10 | Retrospective case series | Development of a Gender Affirming Surgical Perioperative Program | Description of postoperative pain scores, spectrum of cases, and the overview of program development |
| Rifkin (2022)11 | Retrospective review | Phalloplasty with standardized protocol | Low overall complication profile |
| Coon (2023)12 | Semistructured interviews | NA | Variable LOS for vaginoplasty (1-9 days) |
| Salgado (2023)13 | Retrospective review | Vaginoplasty with and without epidural | Epidural group with reduction in pain scores and no difference in LOS |
| Study (year) . | Study design . | Study details . | Outcome . |
|---|---|---|---|
| Aquino (2023)6 | Retrospective observational cohort study | ERAS protocol with a focus on anesthesiology-led interventions in top surgery | ERAS group with shorter length of stay, decreased hematoma requiring operative intervention, lower drain output |
| Bedar (2023)7 | Retrospective review | ERAS protocol consisting of opioid-reducing multimodal pain regimen for facial feminization surgery | Reduced opioid use, lower pain score, shorter length of stay in ERAS group |
| Edalatpour (2024)8 | Retrospective review | Tranexamic acid and liposomal bupivacaine in patients undergoing top surgery | Lower opioid use following liposomal bupivacaine. No difference in complications with TXA |
| Tirrell (2022)9 | Retrospective review | Vaginoplasty with and without PCA | Non-PCA group with lower postoperative opioid use and shorter LOS |
| Aquino (2022)10 | Retrospective case series | Development of a Gender Affirming Surgical Perioperative Program | Description of postoperative pain scores, spectrum of cases, and the overview of program development |
| Rifkin (2022)11 | Retrospective review | Phalloplasty with standardized protocol | Low overall complication profile |
| Coon (2023)12 | Semistructured interviews | NA | Variable LOS for vaginoplasty (1-9 days) |
| Salgado (2023)13 | Retrospective review | Vaginoplasty with and without epidural | Epidural group with reduction in pain scores and no difference in LOS |
ERAS, enhanced recovery after surgery; LOS, length of stay; NA, not applicable; PCA, patient-controlled analgesia; TXA, tranexamic acid.
Enhanced Recovery After Surgery (ERAS) Society Guidelines
The ERAS Society is a nonprofit, multidisciplinary medical academic society with the aim of advancing perioperative care and improving recovery through research, education, audit, and implementation of evidence-based practice.14 With the proliferation of ERAS literature, the ERAS Society has put forth important considerations for ERAS Society guidelines (Table 2) and an outline of core ERAS elements that have scientific support (Table 3).1,15 While initially developed for patients undergoing colorectal surgery, these core elements are easily translatable to other surgical fields with specialty-specific modifications and serve as a logical starting point for the development and implementation of ERAS protocols in GAS.
| ERAS Society Guidelines Requirements . |
|---|
| Target specific surgical procedures or a group of similar surgical procedures |
| Multidisciplinary and multiprofessional |
| Developed by individuals from different health settings and different professions, with consideration for patient involvement |
| Holistic and should address elements of preoperative, intraoperative, and postoperative care |
| Address multiple patient outcomes |
| Require endorsement from ERAS Society leadership |
| Creation of ERAS guidelines should follow ERAS Society methods |
| Be presented, when possible, with ERAS formatting, including an ERAS diagram |
| ERAS guidelines should be created with a plan for implementation, audit, and evaluation |
| ERAS Society Guidelines Requirements . |
|---|
| Target specific surgical procedures or a group of similar surgical procedures |
| Multidisciplinary and multiprofessional |
| Developed by individuals from different health settings and different professions, with consideration for patient involvement |
| Holistic and should address elements of preoperative, intraoperative, and postoperative care |
| Address multiple patient outcomes |
| Require endorsement from ERAS Society leadership |
| Creation of ERAS guidelines should follow ERAS Society methods |
| Be presented, when possible, with ERAS formatting, including an ERAS diagram |
| ERAS guidelines should be created with a plan for implementation, audit, and evaluation |
From Brindle et al, which is freely available under a Creative Commons CC BY license, and appeared in BJS Open published by John Wiley & Sons Ltd on behalf of the British Journal of Surgery Society.15 ERAS, enhanced recovery following surgery.
| ERAS Society Guidelines Requirements . |
|---|
| Target specific surgical procedures or a group of similar surgical procedures |
| Multidisciplinary and multiprofessional |
| Developed by individuals from different health settings and different professions, with consideration for patient involvement |
| Holistic and should address elements of preoperative, intraoperative, and postoperative care |
| Address multiple patient outcomes |
| Require endorsement from ERAS Society leadership |
| Creation of ERAS guidelines should follow ERAS Society methods |
| Be presented, when possible, with ERAS formatting, including an ERAS diagram |
| ERAS guidelines should be created with a plan for implementation, audit, and evaluation |
| ERAS Society Guidelines Requirements . |
|---|
| Target specific surgical procedures or a group of similar surgical procedures |
| Multidisciplinary and multiprofessional |
| Developed by individuals from different health settings and different professions, with consideration for patient involvement |
| Holistic and should address elements of preoperative, intraoperative, and postoperative care |
| Address multiple patient outcomes |
| Require endorsement from ERAS Society leadership |
| Creation of ERAS guidelines should follow ERAS Society methods |
| Be presented, when possible, with ERAS formatting, including an ERAS diagram |
| ERAS guidelines should be created with a plan for implementation, audit, and evaluation |
From Brindle et al, which is freely available under a Creative Commons CC BY license, and appeared in BJS Open published by John Wiley & Sons Ltd on behalf of the British Journal of Surgery Society.15 ERAS, enhanced recovery following surgery.
| ERAS Society Guideline Elements . |
|---|
| Preadmission |
| Smoking cessation |
| Optimization of nutritional status and comorbid conditions |
| Preoperative |
| Structured patient counseling |
| Preoperative carbohydrate treatment |
| Prophylaxis against infection, thrombosis, and nausea and vomiting |
| Intraoperative |
| Minimize drains and invasive techniques |
| Standardized anesthesia; limit long-acting opioids; nasogastric tube removal before anesthesia reversal |
| Body temperature warming |
| Locoregional adjunct use |
| Postoperative |
| Early mobilization and protein-rich oral intake |
| Early removal of catheters and drains |
| Multimodal opioid-sparing pain control |
| Multimodal nausea and vomiting control |
| Preparation for early discharge |
| Multidisciplinary audit of outcomes |
| ERAS Society Guideline Elements . |
|---|
| Preadmission |
| Smoking cessation |
| Optimization of nutritional status and comorbid conditions |
| Preoperative |
| Structured patient counseling |
| Preoperative carbohydrate treatment |
| Prophylaxis against infection, thrombosis, and nausea and vomiting |
| Intraoperative |
| Minimize drains and invasive techniques |
| Standardized anesthesia; limit long-acting opioids; nasogastric tube removal before anesthesia reversal |
| Body temperature warming |
| Locoregional adjunct use |
| Postoperative |
| Early mobilization and protein-rich oral intake |
| Early removal of catheters and drains |
| Multimodal opioid-sparing pain control |
| Multimodal nausea and vomiting control |
| Preparation for early discharge |
| Multidisciplinary audit of outcomes |
| ERAS Society Guideline Elements . |
|---|
| Preadmission |
| Smoking cessation |
| Optimization of nutritional status and comorbid conditions |
| Preoperative |
| Structured patient counseling |
| Preoperative carbohydrate treatment |
| Prophylaxis against infection, thrombosis, and nausea and vomiting |
| Intraoperative |
| Minimize drains and invasive techniques |
| Standardized anesthesia; limit long-acting opioids; nasogastric tube removal before anesthesia reversal |
| Body temperature warming |
| Locoregional adjunct use |
| Postoperative |
| Early mobilization and protein-rich oral intake |
| Early removal of catheters and drains |
| Multimodal opioid-sparing pain control |
| Multimodal nausea and vomiting control |
| Preparation for early discharge |
| Multidisciplinary audit of outcomes |
| ERAS Society Guideline Elements . |
|---|
| Preadmission |
| Smoking cessation |
| Optimization of nutritional status and comorbid conditions |
| Preoperative |
| Structured patient counseling |
| Preoperative carbohydrate treatment |
| Prophylaxis against infection, thrombosis, and nausea and vomiting |
| Intraoperative |
| Minimize drains and invasive techniques |
| Standardized anesthesia; limit long-acting opioids; nasogastric tube removal before anesthesia reversal |
| Body temperature warming |
| Locoregional adjunct use |
| Postoperative |
| Early mobilization and protein-rich oral intake |
| Early removal of catheters and drains |
| Multimodal opioid-sparing pain control |
| Multimodal nausea and vomiting control |
| Preparation for early discharge |
| Multidisciplinary audit of outcomes |
ERAS Implementation in Gender-Affirming Surgery
To date, no formalized ERAS Society guidelines exist for GAS. However, efforts to study and implement ERAS interventions in GAS are underway, with several studies explicitly referencing ERAS.6,7,9-13 This section will provide an overview of the ERAS-GAS team and highlight general considerations for the preoperative, intraoperative, and postoperative phases.
The gender-affirming process includes social, medical, and surgical components. GAS is naturally situated for incorporation of ERAS protocols given its foundation in multidisciplinary care. Patients often enter the care continuum through primary care physicians, who may work in collaboration with endocrinology, obstetrics and gynecology, surgery, and mental health specialists to provide comprehensive patient-centered care. When patients are either referred or self-present for surgical intervention, the surgeon must understand the need for the multidisciplinary care team in managing any comorbid condition and ensuring that the whole patient is being cared for. The recent publication of the World Professional Association for Transgender Health (WPATH) Standards of Care Version 8 emphasizes the importance of informed consent in the execution of GAS, with emphasis on stability of confounding conditions and the inclusion of additional providers to assist with the patient's readiness for surgery.16
The perioperative ERAS team should be a partnership between the surgeon and anesthesiologist, who plays a critical role in preoperative risk assessment, appropriate analgesia and anxiolytic adjuncts, and perioperative pain management. Approximately 16% of transgender individuals have a history of prescription drug misuse that can influence the anesthetic and pain management plan.17 Perioperative nursing and support staff should receive trans-competent training, including an awareness of the patient's name and pronouns, because these may not be accurately reflected in the patient chart. For genital surgery, preoperative shaving should be reserved for the operating room given the dysphoria related to the patient's genitalia and the possible history of sexual trauma which is greater in this population cohort.18
The goals of the intraoperative phase should be faster recovery, lower levels of pain, and a reduction in complications. Similar to most surgical procedures, to achieve these goals, minimally invasive surgical techniques should be performed (when possible), the patient's body temperature should be kept normothermic, and euvolemia should be maintained (as opposed to hypervolemia as recommended in the past). The anesthesiologist should be familiar with any history of facial feminization surgery (FFS) and chondrolaryngoplasty that may complicate approaches to airway management due to scar tissue and altered laryngeal and facial anatomy.19,20 The anesthesia team should also be aware of chest binding by patients, which has been associated with cough, dyspnea, recurrent pulmonary infections, chest pain, and the potential for chronic lung function changes.21,22 Urinary catheterization may be a challenge due to urethral stenosis and postsurgical anatomic changes, which are more commonly complications after transmasculine genital surgery.23
The postoperative phase begins in the postanesthesia care unit, where a multimodal opioid-sparing pain regimen and varied approach to prevent nausea and vomiting are paramount. Postoperative nursing staff should be aware of implemented protocols specific for GAS, because they may differ from recovery guidelines for other operations. Patients who remain admitted for recovery (most commonly those undergoing genital surgery) often have a multidisciplinary team that includes the floor nurse, licensed clinical social workers, advanced practice providers, and physical therapists. Early intake of oral fluids and solids and prompt mobilization (if feasible) should be introduced in the early postoperative period. The length of stay for the various gender-affirming procedures available is not standardized and is highly surgeon or center dependent.12 A data driven consensus may be elucidated as more centers audit and publish their experiences.
All team members should have an awareness of ERAS interventions being employed, especially when it pertains to changes in the postoperative pain regimen, because mixed messaging around pain control has the potential to undermine the therapeutic alliance with the patient. The ERAS coordinator, often a nurse or advanced practice provider, serves a key role in the audit and evaluation of the protocols, which is a crucial component of the evidence-based ERAS protocols. This audit process includes the institutionalization of protocols, gaining hospital-wide stakeholder engagement, providing feedback to team members, and management of the ERAS process.
In the postoperative setting, the challenges of a physical recovery can be compounded by an exacerbation of existing mental health issues or a newly presenting adjustment disorder for the patient. To address this, our institution has implemented a postoperative inpatient “mental health check-in” by a licensed mental health specialist for every genital gender-affirming patient. The goal of this check is to provide continuity from the preoperative mental health team throughout the hospitalization, to offer patients a space to process physical and psychological changes, and to raise any concerns that should be communicated to the larger team. For outpatient GAS, we have implemented an early virtual or in-person check-in visit with advance practice providers to answer questions and triage any concerns.
Hormone Therapy
Hormone therapy is an important component of gender-affirming medical care, with an increasing number of patients presenting for surgery while receiving estrogen, progesterone, or testosterone. Patients on hormone therapy may be hesitant about temporary cessation because it can lead to increased dysphoria and mood changes that are disruptive to their well-being. Increased concern for venous thromboembolism (VTE) while on estrogen has led to inconsistent recommendations—typically guided by individual risk factors, ranging from estrogen cessation for 1 to 4 weeks before surgery to continuation of estrogen through surgery with no change in dosing.24-27 While WPATH Standards of Care (SOC) 8 recommends safe continuation of estrogen therapy in most patients, there is a role for future studies to provide risk-stratified recommendations for perioperative estrogen therapy in GAS.16 WPATH SOC 8 also recommends administration of transdermal estrogen in patients > 45 years of age or previous VTE. Studies suggest that testosterone therapy is safe to continue through surgery, with no increased risk of VTE or wound complications.21-31 In the setting of phalloplasty, testosterone levels and blood counts may be measured preoperatively to determine if the patient has testosterone-induced erythrocytosis, which may contribute to the risk of thrombosis in free tissue transfer.32
Facial Surgery
Facial GAS involves a host of procedures dependent on the patient's anatomy and appearance. Bone and soft tissue are remodeled, sometimes with the aid of synthetic or biologic implants to yield a more feminine or masculine face. Facial feminization surgery (FFS)—the more common type of facial GAS—can include single or multistage frontal bone contouring, hairline lowering, rhinoplasty, malar augmentation, genioplasty, mandibular angle reduction, and tracheal shave. Complications include hematoma, epistaxis, ophthalmic injury, cerebrospinal fluid leak, pulmonary edema, nerve injury, seroma, sinus dysfunction, and VTE.33
Because FFS is typically performed as either same-day surgery or with an overnight stay, there are limited studies focused on ERAS guidelines. Preoperative counseling should set expectations regarding the recovery timeline, particularly related to early pain, bruising, and swelling, which are often points of early postoperative distress. To date, 1 study has reported on investigated outcomes of an ERAS protocol specifically for FFS, demonstrating reduced perioperative opioid usage, decreased patient-reported pain, shortened length of stay.7 Elements of this protocol are outlined in Table 4. Interestingly, study authors elected to omit gabapentin from their ERAS protocol due to increasing concerns about gabapentin misuse as an underrecognized entity.34,35
| Facial Feminization Surgery ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding postoperative protocol |
| Intraoperative |
| 0.25% bupivacaine with 1:200,000 epinephrine 10 minutes before each incision |
| IV opioids as needed |
| IV ketorolac (15-30 mg) |
| IV acetaminophen (1000 mg) |
| Postoperative |
| Scheduled IV ketorolac (15 mg) and oral acetaminophen (650 mg) every 6 hours until discharge |
| Oral tramadol (50 mg) for breakthrough pain |
| Oral oxycodone (5 mg) or IV hydromorphone based on clinical judgment |
| Post discharge (5-day supply) |
| Acetaminophen (650 mg) and ibuprofen (400 mg) every 6 hours alternating until no longer needed |
| 5-day supply of tramadol (50 mg) for breakthrough pain |
| Facial Feminization Surgery ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding postoperative protocol |
| Intraoperative |
| 0.25% bupivacaine with 1:200,000 epinephrine 10 minutes before each incision |
| IV opioids as needed |
| IV ketorolac (15-30 mg) |
| IV acetaminophen (1000 mg) |
| Postoperative |
| Scheduled IV ketorolac (15 mg) and oral acetaminophen (650 mg) every 6 hours until discharge |
| Oral tramadol (50 mg) for breakthrough pain |
| Oral oxycodone (5 mg) or IV hydromorphone based on clinical judgment |
| Post discharge (5-day supply) |
| Acetaminophen (650 mg) and ibuprofen (400 mg) every 6 hours alternating until no longer needed |
| 5-day supply of tramadol (50 mg) for breakthrough pain |
| Facial Feminization Surgery ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding postoperative protocol |
| Intraoperative |
| 0.25% bupivacaine with 1:200,000 epinephrine 10 minutes before each incision |
| IV opioids as needed |
| IV ketorolac (15-30 mg) |
| IV acetaminophen (1000 mg) |
| Postoperative |
| Scheduled IV ketorolac (15 mg) and oral acetaminophen (650 mg) every 6 hours until discharge |
| Oral tramadol (50 mg) for breakthrough pain |
| Oral oxycodone (5 mg) or IV hydromorphone based on clinical judgment |
| Post discharge (5-day supply) |
| Acetaminophen (650 mg) and ibuprofen (400 mg) every 6 hours alternating until no longer needed |
| 5-day supply of tramadol (50 mg) for breakthrough pain |
| Facial Feminization Surgery ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding postoperative protocol |
| Intraoperative |
| 0.25% bupivacaine with 1:200,000 epinephrine 10 minutes before each incision |
| IV opioids as needed |
| IV ketorolac (15-30 mg) |
| IV acetaminophen (1000 mg) |
| Postoperative |
| Scheduled IV ketorolac (15 mg) and oral acetaminophen (650 mg) every 6 hours until discharge |
| Oral tramadol (50 mg) for breakthrough pain |
| Oral oxycodone (5 mg) or IV hydromorphone based on clinical judgment |
| Post discharge (5-day supply) |
| Acetaminophen (650 mg) and ibuprofen (400 mg) every 6 hours alternating until no longer needed |
| 5-day supply of tramadol (50 mg) for breakthrough pain |
Gender-Affirming Mastectomy
Gender-affirming mastectomy (GAM) is the most commonly performed masculinizing operation for transmen or gender-diverse individuals, often as a same-day surgery or with overnight observation. The operation can range from a periareolar approach to a more extensive double incision mastectomy with or without free nipple grafts. Complications include hematoma, seroma, nipple loss, infection, contour deformities, and wound dehiscence.
Existing studies in cisgender mastectomy and breast reconstruction report that ERAS pathways result in improved patient outcomes and reduced LOS, but few studies reported on investigation of ERAS elements in GAM specifically.3,36-38 Compared to cisgender counterparts undergoing oncologic mastectomy or breast reduction, patients undergoing GAM are prescribed a greater number of opioids postoperatively—resulting in a compounded risk of opioids misuse for an already high-risk patient population.39 One study describes a GAM-specific ERAS protocol, demonstrating shortened length of stay and fewer returns to the operating room for hematoma with the administration of tranexamic acid (TXA).6 Both topical and systemic TXA in breast reconstruction have been associated with reduced risk of postoperative hematoma and seroma, representing a promising area for further research into risk-reducing intraoperative measures.40,41 Additional intraoperative measures that may be considered in the development of GAM ERAS protocols include an intraoperative blood pressure challenge to reduce hematoma rates and a drain-free approach to GAM that was associated with lower rates of hematoma and shorter hospital LOS.42,43
Vaginoplasty
The goal of feminizing vaginoplasty is the creation of a natural-appearing vulva, an adequate vaginal canal for dilation or receptive intercourse, a sensate clitoris, and a perineal urethra capable of a downward urinary stream. While the penile inversion vaginoplasty is the most utilized technique worldwide, the robotic-assisted vaginoplasty which utilizes peritoneal flaps for the creation of the proximal vaginal canal is gaining traction. A comparative study of vaginoplasty practices across 17 high-volume centers identified a wide range of heterogeneity in preoperative, intraoperative, and postoperative approaches, notably with regard to length of stay—varying from overnight observation to a 9-day inpatient stay.12
In the preoperative phase, standard ERAS principles should be applied, including smoking and alcohol cessation, nutritional screening, and medical optimization of any preexisting comorbidities. In a survey study of patients undergoing vaginoplasty, 93% identified possible complications as an “extremely important” topic to be discussed in the preoperative setting.44 Another study reported that patients want to see more preoperative and postoperative images of previous patients with anticipated wound-healing courses.45 Preoperative pelvic floor physical therapy may play an important role in physically and psychologically preparing patients for postoperative dilation.46 Surgeons vary regarding a preoperative bowel preparation, ranging from complete clearance to self-administered enemas with or without a clear liquid diet for 1 to 2 days before the procedure. Given the low rate of rectal injury and rectovaginal fistula and the data provided by urology literature for open or robotic prostatectomy, a full bowel clearance may be unnecessary and merely lead to dehydration before surgery.12,47
One retrospective study comparing vaginoplasty with combined epidural and general anesthesia vs general anesthesia alone reported less opioid use and lower mean pain scores in the combined group, with no difference in length of stay and time to ambulation, suggesting potential benefit with intraoperative analgesia adjuncts.13 Pudendal nerve blocks have also been found to result in lower postoperative pain scores for patients undergoing penile inversion vaginoplasty.48 With regard to postoperative pain control, a non-PCA ERAS protocol after vaginoplasty resulted in decreased opioid use and shorter hospital length of stay (6.2 vs 7.3 days).9
At our institution, we have implemented ERAS principles in our perioperative vaginoplasty protocol, which has facilitated a transition from a previous 7-day hospitalization stay to a standardized 2-night stay with planned discharge on postoperative day 2. An antibiotic-impregnated roll gauze is tightly packed in the neovaginal canal to act as a skin graft bolster and the labia majora are sewn together to keep the packing in place and allow for early mobilization. We outline this protocol in Table 5.
| Vaginoplasty Perioperative ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding hospital stay and postoperative protocol |
| Cefazolin 2 gm (weight-based dosing) |
| Metronidazole 500 mg IV |
| Heparin 5000U SC |
| Postoperative medication regimen |
| Enoxaparin 40 mg SC daily if no concern of bleeding |
| Cefazolin 2 gm IV Q8H for 24 hours |
| Acetaminophen 650 mg PO Q8H |
| Gabapentin 100 mg PO Q8H |
| Ibuprofen 600 mg PO Q6-8H |
| Hydrocodone-acetaminophen 5-325 PO Q6H PRN |
| Senna 17.2 mg PO nightly and docusate sodium PO 100 mg BID |
| Flomax 0.4 mg nightly |
| Postoperative labs |
| Complete blood count, basic metabolic panel, BUN/creatinine, magnesium |
| Recheck only if following abnormal values |
| Hospitalization |
| Diet |
| Clear liquid diet on POD0; Advance to regular diet on POD1 |
| Activity/Mobilization |
| Head of bed elevated at 30 degrees, no sitting upright for 2 weeks |
| Out of bed standing on POD0, ambulate in room |
| Increasing ambulation on POD1 |
| Surgical Site Care |
| Ice packs to labia and perineum every 2 hours for 15 minutes |
| Discharge POD2 |
| Sponge bath only; keep surgical site clean and dry |
| No sitting upright in chair for 2 weeks |
| Ambulate at least 3 times per day |
| Record drain outputs |
| Continue ice packs |
| Follow-up in clinic in 1 week |
| Discharge medication regimen |
| Acetaminophen 650 mg PO Q8H PRN |
| Ibuprofen 600 mg PO Q6H PRN |
| Gabapentin 100 mg PO TID |
| Hydrocodone-acetaminophen PO 5-325 mg Q6H PRN |
| Nitrofurantoin 100 mg daily while catheter in place |
| Colace 100 mg twice a day; hold for loose stools |
| Resume estrogen at 1/2 preoperative dose; discontinue home spironolactone |
| Vaginoplasty Perioperative ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding hospital stay and postoperative protocol |
| Cefazolin 2 gm (weight-based dosing) |
| Metronidazole 500 mg IV |
| Heparin 5000U SC |
| Postoperative medication regimen |
| Enoxaparin 40 mg SC daily if no concern of bleeding |
| Cefazolin 2 gm IV Q8H for 24 hours |
| Acetaminophen 650 mg PO Q8H |
| Gabapentin 100 mg PO Q8H |
| Ibuprofen 600 mg PO Q6-8H |
| Hydrocodone-acetaminophen 5-325 PO Q6H PRN |
| Senna 17.2 mg PO nightly and docusate sodium PO 100 mg BID |
| Flomax 0.4 mg nightly |
| Postoperative labs |
| Complete blood count, basic metabolic panel, BUN/creatinine, magnesium |
| Recheck only if following abnormal values |
| Hospitalization |
| Diet |
| Clear liquid diet on POD0; Advance to regular diet on POD1 |
| Activity/Mobilization |
| Head of bed elevated at 30 degrees, no sitting upright for 2 weeks |
| Out of bed standing on POD0, ambulate in room |
| Increasing ambulation on POD1 |
| Surgical Site Care |
| Ice packs to labia and perineum every 2 hours for 15 minutes |
| Discharge POD2 |
| Sponge bath only; keep surgical site clean and dry |
| No sitting upright in chair for 2 weeks |
| Ambulate at least 3 times per day |
| Record drain outputs |
| Continue ice packs |
| Follow-up in clinic in 1 week |
| Discharge medication regimen |
| Acetaminophen 650 mg PO Q8H PRN |
| Ibuprofen 600 mg PO Q6H PRN |
| Gabapentin 100 mg PO TID |
| Hydrocodone-acetaminophen PO 5-325 mg Q6H PRN |
| Nitrofurantoin 100 mg daily while catheter in place |
| Colace 100 mg twice a day; hold for loose stools |
| Resume estrogen at 1/2 preoperative dose; discontinue home spironolactone |
BID, 2 times a day; BUN, blood urea nitrogen; IV, intravenous; PO, by mouth; POD, postoperative day; PRN, as needed; Q8H, every 8 hours; SC, subcutaneous; TID, 3 times a day.
| Vaginoplasty Perioperative ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding hospital stay and postoperative protocol |
| Cefazolin 2 gm (weight-based dosing) |
| Metronidazole 500 mg IV |
| Heparin 5000U SC |
| Postoperative medication regimen |
| Enoxaparin 40 mg SC daily if no concern of bleeding |
| Cefazolin 2 gm IV Q8H for 24 hours |
| Acetaminophen 650 mg PO Q8H |
| Gabapentin 100 mg PO Q8H |
| Ibuprofen 600 mg PO Q6-8H |
| Hydrocodone-acetaminophen 5-325 PO Q6H PRN |
| Senna 17.2 mg PO nightly and docusate sodium PO 100 mg BID |
| Flomax 0.4 mg nightly |
| Postoperative labs |
| Complete blood count, basic metabolic panel, BUN/creatinine, magnesium |
| Recheck only if following abnormal values |
| Hospitalization |
| Diet |
| Clear liquid diet on POD0; Advance to regular diet on POD1 |
| Activity/Mobilization |
| Head of bed elevated at 30 degrees, no sitting upright for 2 weeks |
| Out of bed standing on POD0, ambulate in room |
| Increasing ambulation on POD1 |
| Surgical Site Care |
| Ice packs to labia and perineum every 2 hours for 15 minutes |
| Discharge POD2 |
| Sponge bath only; keep surgical site clean and dry |
| No sitting upright in chair for 2 weeks |
| Ambulate at least 3 times per day |
| Record drain outputs |
| Continue ice packs |
| Follow-up in clinic in 1 week |
| Discharge medication regimen |
| Acetaminophen 650 mg PO Q8H PRN |
| Ibuprofen 600 mg PO Q6H PRN |
| Gabapentin 100 mg PO TID |
| Hydrocodone-acetaminophen PO 5-325 mg Q6H PRN |
| Nitrofurantoin 100 mg daily while catheter in place |
| Colace 100 mg twice a day; hold for loose stools |
| Resume estrogen at 1/2 preoperative dose; discontinue home spironolactone |
| Vaginoplasty Perioperative ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding hospital stay and postoperative protocol |
| Cefazolin 2 gm (weight-based dosing) |
| Metronidazole 500 mg IV |
| Heparin 5000U SC |
| Postoperative medication regimen |
| Enoxaparin 40 mg SC daily if no concern of bleeding |
| Cefazolin 2 gm IV Q8H for 24 hours |
| Acetaminophen 650 mg PO Q8H |
| Gabapentin 100 mg PO Q8H |
| Ibuprofen 600 mg PO Q6-8H |
| Hydrocodone-acetaminophen 5-325 PO Q6H PRN |
| Senna 17.2 mg PO nightly and docusate sodium PO 100 mg BID |
| Flomax 0.4 mg nightly |
| Postoperative labs |
| Complete blood count, basic metabolic panel, BUN/creatinine, magnesium |
| Recheck only if following abnormal values |
| Hospitalization |
| Diet |
| Clear liquid diet on POD0; Advance to regular diet on POD1 |
| Activity/Mobilization |
| Head of bed elevated at 30 degrees, no sitting upright for 2 weeks |
| Out of bed standing on POD0, ambulate in room |
| Increasing ambulation on POD1 |
| Surgical Site Care |
| Ice packs to labia and perineum every 2 hours for 15 minutes |
| Discharge POD2 |
| Sponge bath only; keep surgical site clean and dry |
| No sitting upright in chair for 2 weeks |
| Ambulate at least 3 times per day |
| Record drain outputs |
| Continue ice packs |
| Follow-up in clinic in 1 week |
| Discharge medication regimen |
| Acetaminophen 650 mg PO Q8H PRN |
| Ibuprofen 600 mg PO Q6H PRN |
| Gabapentin 100 mg PO TID |
| Hydrocodone-acetaminophen PO 5-325 mg Q6H PRN |
| Nitrofurantoin 100 mg daily while catheter in place |
| Colace 100 mg twice a day; hold for loose stools |
| Resume estrogen at 1/2 preoperative dose; discontinue home spironolactone |
BID, 2 times a day; BUN, blood urea nitrogen; IV, intravenous; PO, by mouth; POD, postoperative day; PRN, as needed; Q8H, every 8 hours; SC, subcutaneous; TID, 3 times a day.
Metoidioplasty/Phalloplasty
Masculinizing genital surgery encompasses a spectrum of procedures ranging from vaginectomy to urethral lengthening, suspensory ligament release, scrotoplasty, and phalloplasty. For those patients that do not desire a phalloplasty, a metoidioplasty is another option and can range from simple to complex or full. Patients who demonstrate sufficient clitoral enlargement from testosterone can achieve standing micturition and penetrative intercourse. A metoidioplasty can be easily and safely combined with a hysterectomy or oophorectomy and benefits the patient by reducing anesthetic events and the time needed off work. Similarly, at our institution, the patients are discharged on the day of surgery and provided a multimodal postoperative pain regimen. To date, there is no literature reporting on ERAS protocols specifically for metoidioplasty.
A phalloplasty involves the same steps of a full metoidioplasty (vaginectomy, urethral lengthening, scrotoplasty) but adds additional tissue from a distant donor site to make a phallus. The 2 most common donor sites utilized are the radial forearm or the anterolateral thigh (ALT). Literature on ERAS in phalloplasty is limited, with 1 study that outlines a postoperative phalloplasty protocol with incorporation of ERAS elements.11
For this procedure, setting expectations is paramount in the preoperative setting. Phalloplasty is a complex operation that requires patient commitment to not only a long operation, but also prolonged immobilization, lengthy postoperative flap monitoring, intricate wound care, and the potential for complications and future operations. An important component in preparing patients for a phalloplasty is outlining expectations for the hospital course and recovery period, more so than other surgeries, given the limited mobility, the restrictions on position, and the multiple donor sites (flap and skin graft). Given the small inset of the phallus at the mons and the location of the recipient vessels (lower abdomen or groin), the patient must avoid overflexion at the hip and keep the phallus elevated at all times for the first month to prevent unwanted venous congestion. If a radial forearm flap is performed, the patient's donor arm is splinted and restricted for activity immediately after surgery. Given these limitations, the patient experience is improved if they are aware of these circumstances and their anxiety is lower.
Collaboration with the anesthesia team is imperative for a phalloplasty given the length of the procedure, the intraoperative patient position change, the possible need to temporarily clamp the femoral vessels if required for recipient vessels, and the desire to maintain euvolemia (almost hypovolemia) to minimize postoperative edema and risk of congestion of the flap. In our practice, a 2-team approach is utilized to minimize anesthesia time. The patient is changed in a sterile fashion from dorsal lithotomy to supine once the perineal components of the procedure are completed to avoid prolonged positioning, which can increase the risk of nerve injury or compartment syndrome.49 Dual blood pressure cuffs are placed either on both arms (if ALT is planned) or an arm and the calf (if a radial forearm flap is planned) to avoid repeated compression in 1 area for the length of the procedure.
Postoperatively, the typical multimodal regimen for pain control is ordered. Other standard ERAS measures are implemented, with the exception of early mobilization at some centers due to the location of the recipient vessels for the free flap phallus. If a double tube phalloplasty is performed, the patient's fluid status is monitored closely to minimize postoperative swelling in the phallus, which can quickly lead to congestion and compromise of the inner tube (neourethra). Our postoperative protocol for phalloplasty is outlined in Table 6.
| Phalloplasty Perioperative ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding hospital stay and postoperative protocol |
| Cefazolin 2 gm (weight-based dosing) |
| Heparin 5000U SC |
| Intraoperative |
| Maintain euvolemia |
| Transition from lithotomy to supine once perineal procedures are completed |
| Two-surgeon approach |
| Postoperative medication regimen |
| Pantoprazole 40 mg IV daily |
| Enoxaparin 40 mg SC daily POD1 if no concern for bleeding |
| Cefazolin 2 gm IV Q8H; switch to nitrofurantoin 100 mg PO daily on POD2 |
| Acetaminophen 650 mg PO Q8H |
| Gabapentin 100 mg PO Q8H |
| Ibuprofen 600 mg PO Q6-8H PRN |
| PCA for 2 days; transition to hydrocodone-acetaminophen 5-325 mg PO Q6H as needed POD2 |
| Senna 17.2 mg PO QHS and docusate sodium PO 100 mg BID |
| Postoperative Labs |
| Complete blood count, basic metabolic panel, BUN/creatinine, magnesium |
| Recheck only if following abnormal values |
| Hospitalization |
| Diet |
| Sips within 24 hours |
| Clear liquid diet advanced to regular diet as tolerated POD 2 |
| Activity/mobilization |
| Radial forearm donor: POD4/5 due to femoral vessels as recipients |
| Pedicled ALT: POD3/4 |
| Flap monitoring |
| Warming blanket on until mobilization |
| Flap checks Q1H for 48 hours, then Q2H for 24 hours, then Q4H until discharge |
| Discharge POD 5-7 |
| Sponge bath until return to clinic |
| Ambulate at least 3 times per day; limit hip flexion to 20 degrees on side of anastomosis |
| Keep phallus elevated in foam donuts |
| If radial forearm phalloplasty, OT makes orthoplastic splint on POD 5 when NPWT is removed |
| Outpatient OT follow-up |
| Discharge Medication Regimen |
| Acetaminophen 650 mg PO Q8H PRN |
| Ibuprofen 600 mg PO Q6H PRN |
| Gabapentin 100 mg PO TID |
| Hydrocodone-acetaminophen PO 5-325 mg Q6H PRN |
| Docusate sodium 100 mg PO BID |
| Nitrofurantoin 100 mg PO daily for 1 month |
| Aspirin 325 mg for 1 month |
| Phalloplasty Perioperative ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding hospital stay and postoperative protocol |
| Cefazolin 2 gm (weight-based dosing) |
| Heparin 5000U SC |
| Intraoperative |
| Maintain euvolemia |
| Transition from lithotomy to supine once perineal procedures are completed |
| Two-surgeon approach |
| Postoperative medication regimen |
| Pantoprazole 40 mg IV daily |
| Enoxaparin 40 mg SC daily POD1 if no concern for bleeding |
| Cefazolin 2 gm IV Q8H; switch to nitrofurantoin 100 mg PO daily on POD2 |
| Acetaminophen 650 mg PO Q8H |
| Gabapentin 100 mg PO Q8H |
| Ibuprofen 600 mg PO Q6-8H PRN |
| PCA for 2 days; transition to hydrocodone-acetaminophen 5-325 mg PO Q6H as needed POD2 |
| Senna 17.2 mg PO QHS and docusate sodium PO 100 mg BID |
| Postoperative Labs |
| Complete blood count, basic metabolic panel, BUN/creatinine, magnesium |
| Recheck only if following abnormal values |
| Hospitalization |
| Diet |
| Sips within 24 hours |
| Clear liquid diet advanced to regular diet as tolerated POD 2 |
| Activity/mobilization |
| Radial forearm donor: POD4/5 due to femoral vessels as recipients |
| Pedicled ALT: POD3/4 |
| Flap monitoring |
| Warming blanket on until mobilization |
| Flap checks Q1H for 48 hours, then Q2H for 24 hours, then Q4H until discharge |
| Discharge POD 5-7 |
| Sponge bath until return to clinic |
| Ambulate at least 3 times per day; limit hip flexion to 20 degrees on side of anastomosis |
| Keep phallus elevated in foam donuts |
| If radial forearm phalloplasty, OT makes orthoplastic splint on POD 5 when NPWT is removed |
| Outpatient OT follow-up |
| Discharge Medication Regimen |
| Acetaminophen 650 mg PO Q8H PRN |
| Ibuprofen 600 mg PO Q6H PRN |
| Gabapentin 100 mg PO TID |
| Hydrocodone-acetaminophen PO 5-325 mg Q6H PRN |
| Docusate sodium 100 mg PO BID |
| Nitrofurantoin 100 mg PO daily for 1 month |
| Aspirin 325 mg for 1 month |
ALT, anterolateral thigh; BID, 2 times a day; BUN, blood urea nitrogen; IV, intravenous; NPWT, negative-pressure wound therapy; OT, occupational therapy; PCA, patient-controlled analgesia; PO, by mouth; POD, postoperative day; PRN, as needed; Q6H, every 6 hours; QHS, at bedtime; SC, subcutaneous; TID, 3 times a day.
| Phalloplasty Perioperative ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding hospital stay and postoperative protocol |
| Cefazolin 2 gm (weight-based dosing) |
| Heparin 5000U SC |
| Intraoperative |
| Maintain euvolemia |
| Transition from lithotomy to supine once perineal procedures are completed |
| Two-surgeon approach |
| Postoperative medication regimen |
| Pantoprazole 40 mg IV daily |
| Enoxaparin 40 mg SC daily POD1 if no concern for bleeding |
| Cefazolin 2 gm IV Q8H; switch to nitrofurantoin 100 mg PO daily on POD2 |
| Acetaminophen 650 mg PO Q8H |
| Gabapentin 100 mg PO Q8H |
| Ibuprofen 600 mg PO Q6-8H PRN |
| PCA for 2 days; transition to hydrocodone-acetaminophen 5-325 mg PO Q6H as needed POD2 |
| Senna 17.2 mg PO QHS and docusate sodium PO 100 mg BID |
| Postoperative Labs |
| Complete blood count, basic metabolic panel, BUN/creatinine, magnesium |
| Recheck only if following abnormal values |
| Hospitalization |
| Diet |
| Sips within 24 hours |
| Clear liquid diet advanced to regular diet as tolerated POD 2 |
| Activity/mobilization |
| Radial forearm donor: POD4/5 due to femoral vessels as recipients |
| Pedicled ALT: POD3/4 |
| Flap monitoring |
| Warming blanket on until mobilization |
| Flap checks Q1H for 48 hours, then Q2H for 24 hours, then Q4H until discharge |
| Discharge POD 5-7 |
| Sponge bath until return to clinic |
| Ambulate at least 3 times per day; limit hip flexion to 20 degrees on side of anastomosis |
| Keep phallus elevated in foam donuts |
| If radial forearm phalloplasty, OT makes orthoplastic splint on POD 5 when NPWT is removed |
| Outpatient OT follow-up |
| Discharge Medication Regimen |
| Acetaminophen 650 mg PO Q8H PRN |
| Ibuprofen 600 mg PO Q6H PRN |
| Gabapentin 100 mg PO TID |
| Hydrocodone-acetaminophen PO 5-325 mg Q6H PRN |
| Docusate sodium 100 mg PO BID |
| Nitrofurantoin 100 mg PO daily for 1 month |
| Aspirin 325 mg for 1 month |
| Phalloplasty Perioperative ERAS Protocol . |
|---|
| Preoperative |
| Counseling and education regarding hospital stay and postoperative protocol |
| Cefazolin 2 gm (weight-based dosing) |
| Heparin 5000U SC |
| Intraoperative |
| Maintain euvolemia |
| Transition from lithotomy to supine once perineal procedures are completed |
| Two-surgeon approach |
| Postoperative medication regimen |
| Pantoprazole 40 mg IV daily |
| Enoxaparin 40 mg SC daily POD1 if no concern for bleeding |
| Cefazolin 2 gm IV Q8H; switch to nitrofurantoin 100 mg PO daily on POD2 |
| Acetaminophen 650 mg PO Q8H |
| Gabapentin 100 mg PO Q8H |
| Ibuprofen 600 mg PO Q6-8H PRN |
| PCA for 2 days; transition to hydrocodone-acetaminophen 5-325 mg PO Q6H as needed POD2 |
| Senna 17.2 mg PO QHS and docusate sodium PO 100 mg BID |
| Postoperative Labs |
| Complete blood count, basic metabolic panel, BUN/creatinine, magnesium |
| Recheck only if following abnormal values |
| Hospitalization |
| Diet |
| Sips within 24 hours |
| Clear liquid diet advanced to regular diet as tolerated POD 2 |
| Activity/mobilization |
| Radial forearm donor: POD4/5 due to femoral vessels as recipients |
| Pedicled ALT: POD3/4 |
| Flap monitoring |
| Warming blanket on until mobilization |
| Flap checks Q1H for 48 hours, then Q2H for 24 hours, then Q4H until discharge |
| Discharge POD 5-7 |
| Sponge bath until return to clinic |
| Ambulate at least 3 times per day; limit hip flexion to 20 degrees on side of anastomosis |
| Keep phallus elevated in foam donuts |
| If radial forearm phalloplasty, OT makes orthoplastic splint on POD 5 when NPWT is removed |
| Outpatient OT follow-up |
| Discharge Medication Regimen |
| Acetaminophen 650 mg PO Q8H PRN |
| Ibuprofen 600 mg PO Q6H PRN |
| Gabapentin 100 mg PO TID |
| Hydrocodone-acetaminophen PO 5-325 mg Q6H PRN |
| Docusate sodium 100 mg PO BID |
| Nitrofurantoin 100 mg PO daily for 1 month |
| Aspirin 325 mg for 1 month |
ALT, anterolateral thigh; BID, 2 times a day; BUN, blood urea nitrogen; IV, intravenous; NPWT, negative-pressure wound therapy; OT, occupational therapy; PCA, patient-controlled analgesia; PO, by mouth; POD, postoperative day; PRN, as needed; Q6H, every 6 hours; QHS, at bedtime; SC, subcutaneous; TID, 3 times a day.
DISCUSSION
The findings in this review underscore the importance of developing ERAS protocols for gender-affirming surgery, because existing literature in this area is relatively scarce. Transgender and gender-diverse patients are a marginalized patient population who face stigma, mental health challenges, limitations to accessing care, and higher rates of chronic pain and substance use disorder than their cisgender counterparts.
ERAS is a protocol-driven framework that encompasses the continuum of surgical care, with preoperative, intraoperative, and postoperative stages. While ERAS protocols have been studied and institutionalized across general surgery, there are currently no ERAS Society guidelines for GAS. A review of studies that report on investigation of the application of ERAS principles in GAS demonstrates promising outcomes, including lower opioid consumption, improved pain scores, shorter length of stay, and decreased postoperative complications.6,7,9,13,43 Many studies are limited by their retrospective nature, small sample size, and narrow scope. As an increasing number of patients seek gender-affirming surgery, the demand for standardized, GAS-specific ERAS guidelines becomes more important.50
One of the benefits of ERAS guidelines is the catalyst to analyze one's institutional outcomes and then revise and adopt data-driven protocols to “trim the fat” and remove unnecessary or ineffective policies. This audit process led us to successfully reduce our postvaginoplasty hospitalization from 7 days to 2 days.
The patient-provider therapeutic alliance is the foundation to gender-affirming care. Patients who seek gender-affirming care have often faced bias and discriminatory healthcare providers throughout their life and consequently may demonstrate considerable medical mistrust. As multidisciplinary care teams move toward the development of ERAS-GAS protocols, it is critical that team members are patient-focused and trans-competent in their care. Providing resources to obtain transgender sensitivity training is helpful to achieving this goal.
Patient regret after GAS is rare, with an incidence of 0.2% to 3%.51-53 A recent multidisciplinary working group survey study outlined 3 types of regret: true gender-related regret, social regret because of lack of acceptance by family, friends, and community, and surgical regret.53 Common themes for surgical regret include inadequate preoperative counseling, significant complications, and unmet expectations. An ERAS approach to perioperative care touches on each of these factors through an emphasis on thoroughly informing the patient, setting realistic expectations, and taking steps to minimize complications. Another key feature is the utilization of a multidisciplinary team to assess and address patient regret to help target areas of improvement.
The ERAS-GAS team should start with the providers who care for the patient in the preoperative setting, especially in the optimization of any medical or psychiatric comorbidities that may complicate the postoperative recovery. Screening patients for a history of substance use disorder will aid in the development of a pain management plan that takes individual risk factors into consideration. Preoperative counseling for both patients and their caregivers should be comprehensive and include details on potential complications and goals for each stage of care. Prophylaxis against surgical site infection and postoperative nausea and vomiting should be ubiquitous. While data suggest that estrogen therapy can safely be continued through surgery, surgeons should take into consideration patient-specific risk factors that may predispose patients to VTE.24-26 A partnership with anesthesiology is important in the development of ERAS-GAS protocols, as outlined by Aquino et al in their development of the Gender Affirming Surgical Perioperative Program at their institution.6,10 This protocol includes minimizing opioids, the use of regional anesthesia adjuncts, control of body temperature, and the maintenance of fluid balance throughout the case. In the postoperative setting, multimodal opioid-sparing pain regimens, early mobilization, and early intake of oral diet should be emphasized as important factors in preparing for a timely discharge. After implementation, ERAS guidelines should undergo an audit and evaluation process to ensure the intended outcome measures are being achieved. Institution-specific audit and evaluation of ERAS protocols may also aid in more meaningful staff and hospital-level participation and widespread adoption.
Future Research
As the field of gender-affirming surgery expands, the importance of high-quality, evidence-based ERAS protocols to optimize patient care becomes more evident. The variability of current data in this field highlights the need to further study and implement true ERAS protocols across the spectrum of gender-affirming surgical procedures. With the ongoing development of validated patient-reported outcome (PRO) tools in GAS (eg, GENDER-Q), we have an opportunity to evaluate the effectiveness of ERAS protocols in terms of PROs and the overall patient experience.54,55 In addition, our review of the present literature reveals a lack of data on ERAS protocols in the outpatient surgery setting. With a shift toward same-day gender-affirming mastectomy and facial feminization surgery, study investigators should aim to identify the impact of perioperative interventions and postoperative opioid-sparing home regimens on short-term pain scores, unexpected return visits to emergency room or clinic, and complication rates. Patients undergoing genital gender-affirming surgery experience a more extensive and at times complex hospital course with high variability in factors such as length of stay, time to ambulation, and oral intake. The charge to evaluate the protocols in various practices will help streamline, standardize, and optimize care for patients undergoing all types of gender-affirming surgeries. Last, the economic impacts of ERAS interventions in GAS have not been well-studied to date. Minimizing complications, shortening length of stay, and reducing opioid usage have all been shown to improve financial metrics in breast reconstruction.56 Providing evidence that ERAS protocols in GAS result in improved outcomes and cost savings for the health system may aid in garnering institutional support for ERAS implementation as well as expansion of gender-affirming services.
CONCLUSIONS
Gender-affirming surgery is on the rise. An emphasis on patient-centered care has proven to improve outcomes in a wide array of surgical procedures. This review emphasizes the critical need for enhanced recovery after surgery (ERAS) protocols tailored to GAS. Early studies show benefits to adopting ERAS guidelines for all types of gender-affirming surgery. A great opportunity exists to develop and implement ERAS-GAS protocols that will yield enhanced patient experience and in turn benefit health systems overall.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
Funding for this supplement was provided by Pacira BioSciences, Inc. (Tampa, FL).
REFERENCES
ERAS Society [Internet]. Accessed November 27, 2023. https://erassociety.org/
Author notes
Dr Etemad is a resident, Department of Surgery, Division of Plastic and Reconstructive Surgery, Keck Medicine of University of Southern California, Los Angeles, CA, USA.
Dr Poh is a plastic surgeon, Department of Plastic Surgery, Kaiser Permanente West Los Angeles Medical Center, Los Angeles, CA, USA.



