-
PDF
- Split View
-
Views
-
Cite
Cite
Giuseppe Catanuto, Dario Virzì, Marco Latino, Nicola Musmeci, Debora Fichera, Konstantina Balafa, Irene Cannata, Nicola Rocco, Mariagloria Marino, Gaetano Castiglione, Francesco Caruso, One-Stage Implant-Based Breast Reconstruction With Polyurethane-Coated Device: Standardized Assessment of Outcomes, Aesthetic Surgery Journal, Volume 44, Issue 5, May 2024, Pages 491–498, https://doi.org/10.1093/asj/sjad301
Close - Share Icon Share
Abstract
Nipple-sparing mastectomies (NSMs) and implant-based breast reconstructions have evolved from 2-stage reconstructions with tissue expansion and implant exchange to direct-to-implant procedures. In this study, we tested safety and efficacy of polyurethane-based implants according to standard assessment tools.
This study aimed to test safety and feasibility of polyurethane-coated implants with standardized assessment employing internationally acknowledged evaluation criteria.
Cases of NSMs followed by breast reconstruction in 1 stage with immediate prepectoral polyurethane-coated implant placement were retrospectively reviewed. Preoperative characteristics of the population have been collected. Adherence to quality assurance criteria of the Association of Breast Surgery–British Association of Plastic Reconstructive and Aesthetic Surgeons was verified. Complications were assessed with the Clavien Dindo classification, modified for the breast. Rippling, implant rotation, and malposition were also evaluated.
Sixty-three consecutive patients underwent 74 NSMs and immediate breast reconstruction with micro polyurethane foam–coated anatomic implants. In 5 cases we had unplanned readmissions with return to the operating room under general anesthesia (6.7%) and implant loss within 3 months from breast reconstruction (5 implants, 6.7%). Postoperative complications according to Clavien Dindo were grade 1 in 6 cases (8.1%), grade 2 in 3 cases (4%), and 3b in 5 cases (6.7%).
Polyurethane-coated implants may prevent rotation and malposition and capsular contracture in the short term. Unplanned readmission rates and implant loss rates in the short term may be slightly higher.
See the abstract translated into Hindi, Portuguese, Korean, German, Italian, Arabic, Chinese, and Taiwanese online here: https://doi.org/10.1093/asj/sjad301.

See the Commentary on this article here.
Technical advances in mastectomy and reconstruction have improved our ability to restore the appearance of the native breast. Nipple-skin sparing mastectomies (NSMs) and implant-based breast reconstructions (IBRs) have evolved from 2-stage reconstructions with tissue expansion (TE) to direct-to-implant (DTI) techniques often assisted by acellular dermal matrices (ADMs) or synthetic meshes.1 Prepectoral DTI breast reconstruction has demonstrated its safety, and outcomes are comparable to those of subpectoral DTI breast reconstruction, eliminating breast animation deformity, muscle weakness, and postoperative discomfort.2 This study aimed to report our experience with 74 consecutive NSMs and DTI breast reconstruction with a prepectoral, muscle-sparing technique and polyurethane foam–coated implant placement.
METHODS
Sixty-three consecutive patients underwent 74 nipple-sparing mastectomies and immediate breast reconstruction with micro polyurethane foam–coated anatomic implants (Microthane Sublime Line; Polytech, Dieburg, Germany), with therapeutic and prophylactic intent (52 unilateral, 11 bilateral) from February 2019 to March 2021 (Figure 1). Fifty-two patients underwent a unilateral mastectomy, which was followed by a contralateral symmetrization in 20 cases. Seven had a mastopexy breast reduction, and 13 an augmentation. In 4 cases contralateral surgery was performed in 1 stage, and 16 patients underwent a delayed procedure after the polyurethane implant had settled in its final position. The decision regarding a first-stage procedure without contralateral surgery was shared with the patients and considered part of the reconstruction process. When a delayed adjustment was requested cosmetic outcomes were calculated at the end of the second procedure.
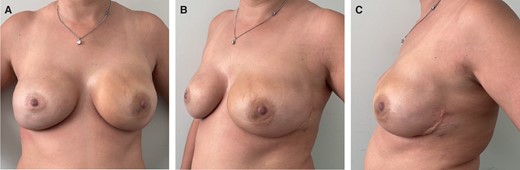
A 56-year patient who underwent bilateral nipple-sparing mastectomies (therapeutic on the left side and risk-reducing on the right side) with access at the inframammary fold and immediate direct-to-implant prepectoral reconstruction with micro polyurethane foam–coated anatomic implants (420 cc; width 13.8 cm). The patient underwent postmastectomy radiotherapy on the left side. The patient is shown 6 months postoperatively in (A) front, (B) left oblique, and (C) left side views.
Patients were informed about the risk of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) by surgeons and breast care nurses orally, with leaflets, and on the website of the Italian Ministry of Health.3 Surgery was performed by a team of 2 surgical oncologists and 2 plastic surgeons. No acellular dermal matrices or synthetic meshes were placed to create the pocket or to cover the implant.
Complications were assessed with Clavien Dindo classification, modified for the breast.6 Postoperative complications were defined as follows:7
surgical site infection, skin flap necrosis: evident soft tissue ischemia with eschar formation
wound dehiscence: full-thickness wound separation
seroma and hematoma: accumulation of fluid requiring aspiration
implant explantation: implant removal without implant replacement
Capsular contracture was graded according to Baker's classification. Infections were defined with criteria outlined by the Centers for Disease Control and Prevention for the surgical site. Quality assurance indicators of the Association of Breast Surgery–British Association of Plastic Reconstructive and Aesthetic Surgeons (ABS-BAPRAS) guidelines, numbers 9, 11, and 12, were also calculated, as follows:6
9: Implant loss at 3 months following breast reconstruction (BR). Target: complications leading to implant loss occur in <5% of cases at 3 months.
11: Unplanned return to operating room (OR) following BR/oncoplastic breast surgery (OPBS). Target: unplanned return to OR occurs in <5% of the cases.
12: Unplanned readmission is assessed and audited for BR/OPBS. Target: unplanned readmission occurs in <10% of cases within 3 months.
This study was IRB approved by the Humanitas Cancer Center in Catania, Italy, and fulfilled the Declaration of Helsinki principles. Patients signed informed consent for prospective data collection for auditing and scientific reporting.
Surgical Procedures
The mastectomy incision was planned according disease location and extent. A submammary fold or italic S incision was performed for small- to medium-size breasts, and a Wise pattern was performed for large and ptotic breasts. Intraoperative assessment of retroareolar ducts was routinely performed.
Breast reconstruction was accomplished with implants placed directly into the subcutaneous pocket; no acellular dermal matrices or synthetic meshes were placed to create the pockets or to cover the implant. In all cases, we implanted polyurethane foam–coated anatomic implants, highly cohesive, with form-stable gel.
The implant selection was based primarily on breast width. We placed medium to low projection implants to obtain a natural shape and reduce tension to the skin flaps. Extra care was taken to close the implant pocket. The serratus fascia and the rectus abdominis fascia were placed to protect the pouch laterally, medially, and inferiorly.
One suction drain was placed inside the pocket until the output was less than 50 cc and left in place for no more than 2 days. No oral antibiotics were prescribed unless clinical signs of infection were evident. Moderate compression was to be applied with a loose sports bra for 2 months. Secondary reconstructions were performed after primary failure with either submuscular placement or with other flaps for tissue coverage.
RESULTS
All patients included in this series were female, and the median age was 49 (range 30-61); the mean BMI was 22 (kg/m2). The mean volume of the implant was 375 cc, with moderate or high projection (range 220-550 cc). Patient characteristics were described according to the Ethos list of decision drivers (Table 1).4 In principle, we did not preclude diabetic patients from breast reconstruction with this technique. Smokers were also admitted (6 patients; median number of cigarettes per day, 10). All of them were told to give up smoking at the time of diagnosis.
| Patient and disease characteristics . | Value . |
|---|---|
| Age, years (range)a | 49 (21-69) |
| Mean BMI (kg/m2)a | 22 |
| Current smoking history n (%)a | 6 (9.5) |
| Diabetes n (%)a | 8 (10) |
| Axillary lymph node dissection n (%) | 18 (28.5) |
| Previous radiotherapy n (%)a | 4 (5.4) |
| Post mastectomy radiotherapy n (%) | 11 (14.8) |
| Neoadjuvant chemotherapy n (%) | 15 (23.8) |
| Adjuvant chemotherapy n (%) | 21 (33.3) |
| Previous surgery n (%)a | 4 (6.3) |
| Multicentric/multifocal disease n (%)a | 23 (33.3) |
| Hormone receptor status positive n (%)a | 51 (73.9) |
| Clinical presentation (palpable/not palpable)a | 42/32 |
| Patient and disease characteristics . | Value . |
|---|---|
| Age, years (range)a | 49 (21-69) |
| Mean BMI (kg/m2)a | 22 |
| Current smoking history n (%)a | 6 (9.5) |
| Diabetes n (%)a | 8 (10) |
| Axillary lymph node dissection n (%) | 18 (28.5) |
| Previous radiotherapy n (%)a | 4 (5.4) |
| Post mastectomy radiotherapy n (%) | 11 (14.8) |
| Neoadjuvant chemotherapy n (%) | 15 (23.8) |
| Adjuvant chemotherapy n (%) | 21 (33.3) |
| Previous surgery n (%)a | 4 (6.3) |
| Multicentric/multifocal disease n (%)a | 23 (33.3) |
| Hormone receptor status positive n (%)a | 51 (73.9) |
| Clinical presentation (palpable/not palpable)a | 42/32 |
aValues according to Ethos.
| Patient and disease characteristics . | Value . |
|---|---|
| Age, years (range)a | 49 (21-69) |
| Mean BMI (kg/m2)a | 22 |
| Current smoking history n (%)a | 6 (9.5) |
| Diabetes n (%)a | 8 (10) |
| Axillary lymph node dissection n (%) | 18 (28.5) |
| Previous radiotherapy n (%)a | 4 (5.4) |
| Post mastectomy radiotherapy n (%) | 11 (14.8) |
| Neoadjuvant chemotherapy n (%) | 15 (23.8) |
| Adjuvant chemotherapy n (%) | 21 (33.3) |
| Previous surgery n (%)a | 4 (6.3) |
| Multicentric/multifocal disease n (%)a | 23 (33.3) |
| Hormone receptor status positive n (%)a | 51 (73.9) |
| Clinical presentation (palpable/not palpable)a | 42/32 |
| Patient and disease characteristics . | Value . |
|---|---|
| Age, years (range)a | 49 (21-69) |
| Mean BMI (kg/m2)a | 22 |
| Current smoking history n (%)a | 6 (9.5) |
| Diabetes n (%)a | 8 (10) |
| Axillary lymph node dissection n (%) | 18 (28.5) |
| Previous radiotherapy n (%)a | 4 (5.4) |
| Post mastectomy radiotherapy n (%) | 11 (14.8) |
| Neoadjuvant chemotherapy n (%) | 15 (23.8) |
| Adjuvant chemotherapy n (%) | 21 (33.3) |
| Previous surgery n (%)a | 4 (6.3) |
| Multicentric/multifocal disease n (%)a | 23 (33.3) |
| Hormone receptor status positive n (%)a | 51 (73.9) |
| Clinical presentation (palpable/not palpable)a | 42/32 |
aValues according to Ethos.
Eleven patients underwent postmastectomy radiotherapy (14.8%); neoadjuvant chemotherapy was administered in 15 of 63 cases (23.8%). Ptosis was graded according to Fitoussi classification and rated as no ptosis in 38 of 74 (51.35%); minor ptosis in 28 of 74 (37.8%) and moderate ptosis in 8 of 74 (10.8%). Four patients had a history of breast conservation surgery and previous radiation (5.4%). The mean volume of the breast before mastectomy was 424 cc (calculated according to Fung’s model with digital mammography).5 Disease characteristics were as follows: 23 of 69 cases (33.3%) were multicentric or multifocal invasive or in situ breast cancer; the mean size of the index lesion was 1.8 cm. Lesions were located in the upper quadrant of the breast in 31 of 69 (44.9%). Luminal (hormone receptor status–positive) cancer was present in 51 patients out of 69 (73.9% ). The median follow-up was 12 months (range 6-19 months). The median follow-up for patients who underwent postmastectomy radiotherapy was 12 months (range 10-29 months).
In 5 cases we had an unplanned readmission with return to the OR under general anesthesia and implant loss within 3 months from breast reconstruction (5 implants; 6.7%). Explantation followed mastectomy flap necrosis only in patients with an italic S incision. Secondary breast reconstruction after implant removal was accomplished with a submuscular approach. In 2 cases we performed an autologous replacement with a thoracodorsal artery perforator flap.
Postoperative complications according to Clavien Dindo were grade 1 in 6 cases (8.1%), grade 2 in 3 cases (4%), and 3b in 5 cases (6.7%). Postoperative seroma was observed in 4 patients (5.4%); hematoma in 2 cases (2.7%); and infection in 3 (4%). All patients with postoperative seroma had received an axillary dissection. Complications are summarized in Table 2. After a median of 12 months of follow-up, we had no cases of severe capsular contracture (Baker grade 3-4). Rippling and edge visibility were present in 6 breasts (8.1%). No rotation or malposition was observed. Eleven patients underwent postmastectomy radiotherapy without developing severe capsular contracture (Figure 1).
| Complications . | Total events . | Complication rate (%) . |
|---|---|---|
| Seroma | 4 | 5.4 |
| Hematoma | 2 | 2.7 |
| Infection | 3 | 4 |
| Skin flap necrosis/wound dehiscence | 5 | 6.7 |
| Implant explantation | 5 | 6.7 |
| Capsular contracture (Baker 3-4) | 0 | 0 |
| Complications . | Total events . | Complication rate (%) . |
|---|---|---|
| Seroma | 4 | 5.4 |
| Hematoma | 2 | 2.7 |
| Infection | 3 | 4 |
| Skin flap necrosis/wound dehiscence | 5 | 6.7 |
| Implant explantation | 5 | 6.7 |
| Capsular contracture (Baker 3-4) | 0 | 0 |
| Complications . | Total events . | Complication rate (%) . |
|---|---|---|
| Seroma | 4 | 5.4 |
| Hematoma | 2 | 2.7 |
| Infection | 3 | 4 |
| Skin flap necrosis/wound dehiscence | 5 | 6.7 |
| Implant explantation | 5 | 6.7 |
| Capsular contracture (Baker 3-4) | 0 | 0 |
| Complications . | Total events . | Complication rate (%) . |
|---|---|---|
| Seroma | 4 | 5.4 |
| Hematoma | 2 | 2.7 |
| Infection | 3 | 4 |
| Skin flap necrosis/wound dehiscence | 5 | 6.7 |
| Implant explantation | 5 | 6.7 |
| Capsular contracture (Baker 3-4) | 0 | 0 |
The cosmetic outcome was assessed with the BCCT.core scale in the postoperative setting after final symmetrization. The majority of patients (89%) reported a good or excellent score (Figure 2). Major asymmetry was reported in 7 cases (11%).
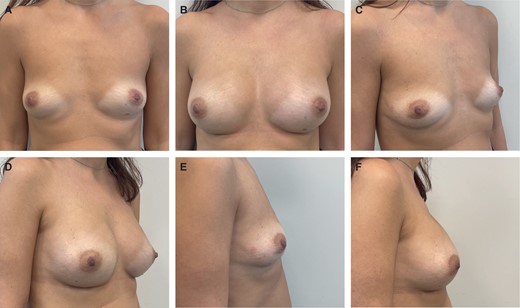
A 30-year patient who underwent risk-reducing bilateral nipple-sparing mastectomies with access at the inframammary fold and immediate direct-to-implant prepectoral reconstruction with micro polyurethane foam–coated anatomic implants (335 cc; width 12.5 cm). Preoperative (A, C, E) and 6-month postoperative (B, D, F) photographs are shown in front, right oblique, and right side views.
This study was designed to report on the safety and feasibility of polyurethane implants in the short term. Patient-reported outcome measurements were not assessed routinely. Patient symptoms were analyzed from clinical notes. Stiffness and soreness was reported in 30 (40.5%) patients during the first 8 months. In 25 (83%), complete resolution was reported. This approach was designed to obtain a natural symmetry in 1 stage. However, 16 patients (30.7%) reported unsatisfactory cosmetic results and requested a delayed procedure.
The 11 patients who underwent postmastectomy radiotherapy did not report severe capsular contracture (grades 3-4). None of the patients who had radiotherapy before the mastectomy reported complications in this series.
DISCUSSION
Nipple-sparing mastectomy followed by IBR has increased in popularity since our first report more than 15 years ago.8 Today the oncological safety and feasibility of nipple preservation has been solidly established, with great improvement in quality of life.1,9
The standard practice of implant-based breast reconstruction has recently evolved, moving from tissue expander in subpectoral pockets followed by implant exchange, to DTI prepectoral breast reconstruction. This new technique has proven to have low morbidity and to eliminate animation deformity.10,11 The prepectoral approach often relies on devices such as acellular dermal matrices for their potential of reducing capsular contracture and increasing stability of the pouch.12
In our opinion, the best candidates for nipple-sparing mastectomies and DTI reconstruction are patients with small- and moderate-size breasts, with grade 1 to 2 ptosis, who desire to maintain their breast size. In patients with large breasts a DTI procedure can be performed, but often a more complex approach with Wise pattern access and a contralateral adjustment is required to optimize final results. A higher risk of complications is expected in cases of more extended preservation of the skin envelope (including nipple-areola complex necrosis).13-16
Chatterjee and Nahabedian in their meta-analysis study reported that the most common complications following prepectoral reconstruction were flap necrosis (7.8%) and seroma (6.7%), with infection, hematoma, and dehiscence at 4.2%, 3.4%, and 3.2%, respectively.17 DTI with a prepectoral approach and micro polyurethane sponge–coated implants was first described by De Vita. That series of 34 breasts was reported to have excellent surgical results and a very low complication rate, probably due to strict exclusion criteria, such as BMI > 30 kg/m2, age > 65 years, active smoking, uncontrolled diabetes, and previous radiotherapy.18
Our series describes complications rate and surgical outcomes for DTI breast reconstructions in the setting of therapeutic and contralateral prophylactic mastectomies. Decision drivers and outcomes were reported in accordance with standardized and international scales.4,6,7
Notably, we reported an unplanned readmission rate, return to OR, and implant loss rate higher than expected when compared to targets of the quality assurance criteria for oncoplastic surgery of ABS-BAPRAS (eg, 6.7% vs 5% target).6 This can be explained by the initial learning curve and the italic S access performed in all cases with exposure and subsequent removal. We reported no wound dehiscence for the inferolateral inframammary fold and Wise pattern incisions.
Despite some qualifications (eg, no diabetic patients, few active smokers), the outcomes of our series were comparable to what has been reported in other series with ADM-assisted breast reconstructions.19-22 Seroma, for instance was present only in patients who underwent axillary lymph node dissection, without subsequent infectious sequelae.12
A higher complication rate was counterbalanced by the absence of grades 3 and 4 capsular contracture, even in patients who underwent postmastectomy radiotherapy. Similarly, we did not report implant malposition or rotation, and there was a low rate of rippling and edge visibility.
Unfortunately, this series was also too small to assess any difference in patients who received postmastectomy radiation or a secondary mastectomy after breast conservation and recurrent disease. Initially, acellular dermal matrices and synthetic meshes were utilized in combination with or without the pectoralis muscle. We hypothesized that these outcomes were due to the polyurethane–coated implants. In fact, it is well demonstrated that these devices warrant optimal short-term and long-term results with low rates of capsular contracture. Moreover, their highly adhesive surface prevents implant displacements or rotation, one of the main caveats of anatomical smooth and textured devices.23-25
Polyurethane foam–coated implants may inhibit capsular contracture and warrant a steady and firm position of the implant that can mimic effectively the function of other devices such as meshes and acellular dermal matrices.26,27 ADM-assisted breast reconstructions require 2 devices, longer OR procedures, and are more expensive.28
Correia-Pinto compared ADM-assisted to polyurethane-based reconstructions. To our knowledge theirs is the only series comparing ADM implant cover to polyurethane-coated implants in prepectoral breast reconstruction. Thirty-five patients underwent 44 mastectomies and breast reconstructions (ADM assisted in 23 cases and polyurethane implants in 21 cases). Seromas and infections occurred exclusively in the ADM cohort (seromas in 4 of 23 of cases, P = .109; infections in 3 of 23 cases, P = .234). A not statistically significant trend toward more complications was seen in the ADM group (P = .245).29
However, good outcomes are always dependent on optimal surgical practice. We recommend performing mastectomies with adequate identification and preservation of nonglandular tissue such as subdermal vascularity, chest-wall perforators, and subcutaneous tissue far from the gland.30-33
According to our results, polyurethane-coated silicone gel implants in single-stage surgery may offer a safe reconstruction immediately after mastectomy, eliminating 1 surgical step and 1 device. Less surgery also means easier access to postmastectomy radiation in patients treated with adjuvant chemotherapy, fewer outpatient consultations for tissue expander inflation, and a faster recovery to everyday activities.34,35
There are 2 types of polyurethane (PU) breast implants (those from Silimed Inc, Dallas, TX; and those from Polytech Health and Aesthetics, Dieburg, Germany). Microthane, that is Polytech polyurethane, and Silimed polyurethane do not differ from a chemical point of view. They probably behave differently because of the different silicone materials and the methods employed to embed the PU foam into the silicone shell of the implants (vulcanization for Polytech). There is no proof that both methods provide similar durable attachment of the PU to the silicone shell, however many cases of delamination have been reported with PU Silimed implants. With early delamination of the PU foam, inadequate tissue ingrowth and incomplete immobilization of the implant may occur, as could exposure to the textured surface or release of bacteria previously sequestrated in PU microcapsules.36 It is not known if these topics are relevant, but because the etiology of BIA-ALCL is still unknown, they may potentially be important. To date there is only 1 confirmed case of BIA-ALCL associated with a Polytech PU implant, and several cases associated with Silimed PU in Australia. We would like to highlight that polyurethane breast implants cannot be classified as macrotextured implants. Polyurethane foam covering the silicone breast implant provides a completely different surface, which is not a silicone elastomer, and the mechanisms of action related to tissue adhesion as well as to fibrous capsule formation differ substantially from those of smooth or textured implants. This issue reaches beyond the aim of the present study, with regard to the length of follow-up and the mean timing of BIA-ALCL development, and may be considered in future updates of our study with longer follow-up.
No information is provided in this study about the association between polyurethane-coated implants and breast implants associated with BIA-ALCL. Patients in this series have had consultations with surgeons and breast care nurses, however little is known about the risk of developing the disease when polyurethane-coated devices are placed.36
This study contributes to the current knowledge by introducing several standardized methodologies for assessment of population characteristics and final outcomes (Ethos list of features; ABS-BAPRAS quality assurance criteria; Clavien Dindo classification for reporting of complications; and BCCT.core for assessment of cosmetic outcomes). This makes the results of this work reproducible and comparable to other series with the same evaluation criteria. The absence of a comparator and the observational architecture are the main limitations of this report. Also, in this study we failed to report long-term outcomes and did not include patient-reported outcome measures for assessment of quality of life. Further studies should be designed to generate more evidence and fill these gaps.
CONCLUSIONS
Nipple-sparing mastectomy and DTI with polyurethane-coated implants can be a safe and effective strategy if surgical access and skin flaps are properly managed. The technique may warrant a single procedure, without doubling the number of devices (tissue expander or mesh-ADM) and protecting from capsular contracture and implant malposition. Future studies should identify risk factors for complications that at the moment appear to be slightly higher than standard.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
Author notes
Drs Catanuto, Virzì, and Latino are consultant oncoplastic surgeons; Drs Musmeci, Fichera, Cannata, and Marino are consultant breast surgeons; Dr Balafa is a senior research fellow; Dr Castiglione is clinical coordinator of the breast unit; and Dr Caruso is head of the oncology department, Humanitas, Istituto Clinico Catanese, Misterbianco Catania, Italy.
Dr Rocco is an assistant professor of surgery, Department of Advanced Biomedical Sciences, University of Naples Federico II, Naples, Italy.



