-
PDF
- Split View
-
Views
-
Cite
Cite
Pooja Humar, Elizabeth Moroni, Anjali Raghuram, Zainab Balogun, Xuan-Mai Nguyen, Casey Zhang, Carolyn De La Cruz, Upper Extremity Functional Outcomes After Breast Cancer Treatment: An Analysis of DASH Score in Breast Reconstruction Patients, Aesthetic Surgery Journal, Volume 44, Issue 4, April 2024, Pages 396–403, https://doi.org/10.1093/asj/sjad352
Close - Share Icon Share
Abstract
Patients undergoing postoncologic breast reconstruction can experience upper extremity (UE) functional deficits.
In this study, we utilized the disabilities of the arm, shoulder, and hand (DASH) questionnaire to identify patient factors that impacted UE functional recovery.
Patients who underwent oncologic followed by reconstructive surgery by a single surgeon from 2014 to 2019 and completed the DASH survey were included. A DASH score was calculated for each patient, with values ranging from 0 (no impairment) to 100 (severe impairment). Regression analysis was conducted to identify significant predictors for DASH score with a significance level for entry and stay set at P = .15.
Among 289 patients who underwent breast reconstruction, 157 completed the questionnaire. The average patient age was 52.6yrs ± 8.6 at the time of reconstruction. A total of 111 had implant-based reconstruction, 15 had autologous reconstruction, and 24 had a combination of both. Average DASH score was 7.7 (range 0.0-52.5), with 74.1% of patients having a score greater than 0. Regression analysis showed 5 variables associated with significantly higher DASH scores: age between 50 and 60 years (P = .13), history of radiation (P = .01), placement of a subpectoral implant (P = .06), postoperative complications (P = .10), and lymphedema (P < .01). Autologous breast reconstruction (P = .04) was associated with a significantly lower DASH score.
Implant-based reconstruction, radiation history, postoperative complications, and age at reconstruction were associated with increased UE functional impairment in patients who underwent breast reconstructive surgery. Identification of these factors can inform areas for potential practice changes and improve patient counseling regarding postoperative expectations.

Breast cancer accounts for 1 in 3 cancer diagnoses in females, with roughly 250,000 new cases diagnosed every year.1 The treatment of breast cancer is a multifaceted experience that usually involves a combination of oncologic therapy, breast surgery, and reconstructive surgery. In patients who require or choose mastectomy, plastic surgeons can utilize several reconstructive techniques to reestablish the shape and volume of the breasts after surgical intervention, including use of autologous tissue or implant-based techniques. While the method of reconstruction can vary, multiple studies have demonstrated that breast reconstruction has a positive impact on the physical and emotional health of patients and is associated with improved quality of life outcomes.2-4
While breast reconstruction offers significant advantages, there are also resulting drawbacks when combined with other common breast cancer treatment therapies. After treatment, patients are at risk for upper extremity pain, lymphedema, cording or axillary web syndrome, upper extremity impairments, and functional limitations such as decreased strength, range of motion, and tolerance for certain physical activities.5-7 Numerous existing studies focus on lymphedema care, chronic pain reduction, surgical risk reduction, patient satisfaction, and aesthetic outcomes of the chest area.8,9 However, few studies have looked at functionality of the upper extremities after surgery, particularly in the context of comparing different types of breast cancer treatment and breast reconstruction.
Upper extremity impairment can have significant effects on a patients’ physical and emotional well-being, which in turn can impact long-term patient quality of life.10 Current efforts to maximize upper extremity functional recovery after treatment include regimented physical therapy, massage techniques, and personalized garments. Despite concerted efforts to improve extremity dysfunction, there is still a paucity of data demonstrating how multiple treatment modalities in breast cancer and reconstruction impact the functionality of extremities.
In current literature, generalized upper extremity dysfunction is often quantified with the disabilities of the arm, shoulder, and hand (DASH) questionnaire. This survey is an upper-extremity specific outcome measure that was introduced by the American Academy of Orthopedic Surgeons, as a region-specific measure of disability and symptoms in people with musculoskeletal disorders of the upper limb. The questionnaire consists of a series of domains that pertain to activities of daily living and overall quality of life that would be affected by factors such as heaviness of the limb, limited range of motion, pain, numbness, or tingling.11,12 The survey consists of 30 questions, each with a Likert scale and answer choices ranging from 1 to 5. Several previous studies have been conducted with the DASH questionnaire and comparing it to other measures of upper extremity function. These studies have provided evidence of the validity, test-retest reliability, and responsiveness of the DASH questionnaire for both proximal and distal disorders, confirming its utility across the whole upper extremity.11 The DASH questionnaire is also available in several languages, with studies of reliability and validity conducted on these additional languages.13
Despite the DASH being a well-validated study of upper extremity function, breast reconstructive surgeons have not yet tapped into this resource as a method to evaluate long-term outcomes after breast cancer treatment. The primary aim of the present study was to describe upper extremity functional outcomes after breast cancer treatment and reconstruction, with a focus on comparing the differences in functional recovery of the extremities with different types of breast cancer treatment and breast reconstruction. Specifically, we sought to determine underlying patient factors associated with worsened or improved upper extremity functionality after breast cancer and reconstructive surgery.
METHODS
Patient Population
Patients diagnosed with breast cancer who underwent oncologic resection followed by breast reconstruction surgery were of interest in this study. The initial study cohort included all patients who underwent breast reconstruction surgery after mastectomy for breast cancer treatment by the principal investigator (C.D.L.C) at a single center between 2014 and 2019. These patients were all contacted to complete the DASH survey. Patients were initially contacted via email, and those who did not respond were sent a follow-up email 2 weeks later. After an additional 2 weeks, the remaining patients were contacted by phone. A second call was made a week later if there was no answer to the first phone call. A predrafted script was followed for all phone calls. After 4 contact attempts were made, the patient was excluded from the study. Patients were also excluded from the study if they had a history of shoulder surgery, preexisting cervical spinal disease, carpal tunnel syndrome, or peripheral neuropathy. All data collection, preparation, and analysis for this study took place from July 2021 to March 2023. This study was approved by the University of Pittsburgh Institutional Review Board (STUDY20110044).
Extremity Functionality Survey
The DASH questionnaire consisted of 30 questions, based on a Likert scale with answer choices ranging from 1 to 5, with 1 indicating no impairment, 2 mild impairment, 3 moderate impairment, 4 severe impairment, and 5 indicating extreme impairment or inability to perform the task in question. Twenty-one items asked about functional impairment with various routine tasks, 5 items pertained to symptoms, 3 questions asked about interference with daily activities, and 1 question pertained to effect on self-confidence. Patients were administered the survey by email, phone, or at an outpatient clinic visit. Informed consent was obtained from patients at the time of questionnaire administration. DASH scores were calculated according to the scoring formula: ([(sum of n responses)/n] −1* (25), where n represented the number of completed items.14 This was the standardized scoring formula validated for the DASH questionnaire. The cumulative DASH score calculated for each patient ranged from 0 (no impairment) to 100 (severe impairment). Our study included an additional 5 questions asking patients about postoperative exercise, physical therapy, and lymphedema therapy. These questions were not included in DASH score calculations.
Predictor and Outcome Variables
A retrospective review was conducted of electronic health records to extract patient demographics and medical, oncologic, and surgical history. Demographics included age at time of surgery, BMI, and race. Pertinent medical history included diabetes, hypertension, and smoking status. Data on history and timing of chemotherapy or radiation therapy were also obtained. Operative reports were reviewed for intraoperative details, including axillary lymph node dissection (ALND), type and timing (immediate vs delayed) of reconstruction, and size and location of any implant. Postoperative clinic notes were reviewed to identify complications, including seroma or hematoma formation, infection, skin necrosis, or dehiscence. Management of the complication was documented, particularly if it required return to the operating room. If the complication required removal of the implant, this was noted as well. Outpatient clinic visit notes were reviewed to determine whether patients had postoperative lymphedema. Lymphedema diagnosis was made based on a comprehensive breast and upper extremity exam, including assessment of timing and nature of symptoms, posture, flexibility, and range of motion in the upper extremity. Scoring was performed to determine evidence of clinical or subclinical lymphedema.
Statistical Analysis
Descriptive statistics were performed to characterize the patient cohort. A univariate analysis between mean DASH score and categorical variables was conducted. Significance level was set at P < .05. Multivariate regression analysis was conducted with all covariables included in a single model to identify significant predictor variables for the DASH score. Stepwise regression analysis was then performed with a significance level for entry and stay at P = .15, which was the accepted statistical cut-off for determining variable significance for inclusion/exclusion in a stepwise regression. Conventional P = .15 was also the default value in SAS (SAS Institute, Cary, NC) for stepwise regression. Among 16 variables (BMI, age, race, diabetes, hypertension, tobacco usage, ALND, chemotherapy, radiation, immediate reconstruction, autologous implant, subpectoral implant, postoperative complications, lymphedema, referral to physical therapy, and postoperative exercises) we identified a regression model for DASH score prediction. The final model was based on a stepwise variable selection procedure with iterations between forward and backward steps. All statistical analyses were conducted with SAS 9.4.
RESULTS
A total of 289 patients met the inclusion criteria, having undergone surgical intervention for breast cancer followed by breast reconstruction surgery by the principal investigator (C.D.L.C) between 2014 and 2019. After 2 email attempts, 72 patients responded to the survey. The remaining patients were then contacted by phone, resulting in an additional 42 patients completing the survey. A total of 175 patients were unreachable after 2 phone attempts or were not interested in participating in the study. The remaining 43 patients were given the survey in the outpatient clinic at a regularly scheduled follow-up appointment. This resulted in a cohort of 157 patients, all of whom completed the DASH survey (Figure 1).

In this patient cohort, the average age at the time of reconstruction surgery was 52.6 years ± 8.6. Ninety-seven percent of patients were White and the remaining 3% were African American. Average BMI was 27.8 ± 5.7. Patient comorbidities of interest are outlined in Table 1. Review of oncologic history found that 68 patients (43.3%) underwent chemotherapy (35 adjuvant, 33 neoadjuvant) and 34 patients (21.7%) had history of radiation therapy for breast cancer treatment. Forty-six patients (29.3%) underwent intraoperative axillary lymph node dissection (Table 1). All patients underwent breast reconstruction, of which 145 were immediate (92.4%) and the remaining were delayed. A total of 111 had implant-based reconstruction, 15 had autologous reconstruction, and 24 had a combination of both (eg, latissimus flap with implant placement).
| Variable . | n (%) . |
|---|---|
| Patient demographics | |
| Age, mean ± SD (years) | 52.6 ± 8.6 |
| Race (White) | 152 (97%) |
| BMI | 27.8 ± 5.7 |
| Medical comorbidities | |
| Diabetes | 18 (11.5%) |
| Hypertension | 41 (26.1%) |
| Tobacco | 25 (15.9%) |
| Oncologic history | |
| Chemotherapy | 68 (43.3%) |
| Adjuvant | 35 (22.3%) |
| Neoadjuvant | 33 (21.0%) |
| Radiation | 34 (21.7%) |
| Mastectomy laterality | |
| Unilateral | 51 (32.5%) |
| Bilateral | 106 (67.3%) |
| Axillary lymph node dissection | 46 (29.3%) |
| Reconstruction History | |
| Timing (immediate) | 145 (92.4%) |
| Type | |
| Implant | 111 (70.7%) |
| Autologous | 15 (9.6%) |
| Both | 24 (15.3%) |
| Variable . | n (%) . |
|---|---|
| Patient demographics | |
| Age, mean ± SD (years) | 52.6 ± 8.6 |
| Race (White) | 152 (97%) |
| BMI | 27.8 ± 5.7 |
| Medical comorbidities | |
| Diabetes | 18 (11.5%) |
| Hypertension | 41 (26.1%) |
| Tobacco | 25 (15.9%) |
| Oncologic history | |
| Chemotherapy | 68 (43.3%) |
| Adjuvant | 35 (22.3%) |
| Neoadjuvant | 33 (21.0%) |
| Radiation | 34 (21.7%) |
| Mastectomy laterality | |
| Unilateral | 51 (32.5%) |
| Bilateral | 106 (67.3%) |
| Axillary lymph node dissection | 46 (29.3%) |
| Reconstruction History | |
| Timing (immediate) | 145 (92.4%) |
| Type | |
| Implant | 111 (70.7%) |
| Autologous | 15 (9.6%) |
| Both | 24 (15.3%) |
BMI, body mass index; SD, standard deviation.
| Variable . | n (%) . |
|---|---|
| Patient demographics | |
| Age, mean ± SD (years) | 52.6 ± 8.6 |
| Race (White) | 152 (97%) |
| BMI | 27.8 ± 5.7 |
| Medical comorbidities | |
| Diabetes | 18 (11.5%) |
| Hypertension | 41 (26.1%) |
| Tobacco | 25 (15.9%) |
| Oncologic history | |
| Chemotherapy | 68 (43.3%) |
| Adjuvant | 35 (22.3%) |
| Neoadjuvant | 33 (21.0%) |
| Radiation | 34 (21.7%) |
| Mastectomy laterality | |
| Unilateral | 51 (32.5%) |
| Bilateral | 106 (67.3%) |
| Axillary lymph node dissection | 46 (29.3%) |
| Reconstruction History | |
| Timing (immediate) | 145 (92.4%) |
| Type | |
| Implant | 111 (70.7%) |
| Autologous | 15 (9.6%) |
| Both | 24 (15.3%) |
| Variable . | n (%) . |
|---|---|
| Patient demographics | |
| Age, mean ± SD (years) | 52.6 ± 8.6 |
| Race (White) | 152 (97%) |
| BMI | 27.8 ± 5.7 |
| Medical comorbidities | |
| Diabetes | 18 (11.5%) |
| Hypertension | 41 (26.1%) |
| Tobacco | 25 (15.9%) |
| Oncologic history | |
| Chemotherapy | 68 (43.3%) |
| Adjuvant | 35 (22.3%) |
| Neoadjuvant | 33 (21.0%) |
| Radiation | 34 (21.7%) |
| Mastectomy laterality | |
| Unilateral | 51 (32.5%) |
| Bilateral | 106 (67.3%) |
| Axillary lymph node dissection | 46 (29.3%) |
| Reconstruction History | |
| Timing (immediate) | 145 (92.4%) |
| Type | |
| Implant | 111 (70.7%) |
| Autologous | 15 (9.6%) |
| Both | 24 (15.3%) |
BMI, body mass index; SD, standard deviation.
Regarding postoperative outcomes, responses to additional survey questions indicated that 116 patients (73.9%) completed some form of postoperative exercises, and 46 patients (29.3%) completed physical therapy sessions. Postoperative complications occurred in 43 patients (27.4%), with skin necrosis being the most common among all complications (30.2%), followed by seroma (23.3%) (Table 2). Of these patients, 23 required operative management and 15 had a removal of the tissue expander or implant as part of the intervention. Thirty-one patients (19.7%) had diagnosed postoperative lymphedema; 77.4% of these patients reported participating in lymphedema therapy, including use of compression garments and lymphatic massage.
| Variable . | n (%) . |
|---|---|
| Postoperative complications | 43 (27.4%) |
| Hematoma | 8 |
| Seroma | 10 |
| Infection | 6 |
| Skin/nipple necrosis | 13 |
| Implant rupture | 1 |
| Other | 5 |
| Reintervention | 23 (53.4%) |
| Implant or expander removal | 15 |
| Lymphedema | 31 (19.7%) |
| Variable . | n (%) . |
|---|---|
| Postoperative complications | 43 (27.4%) |
| Hematoma | 8 |
| Seroma | 10 |
| Infection | 6 |
| Skin/nipple necrosis | 13 |
| Implant rupture | 1 |
| Other | 5 |
| Reintervention | 23 (53.4%) |
| Implant or expander removal | 15 |
| Lymphedema | 31 (19.7%) |
| Variable . | n (%) . |
|---|---|
| Postoperative complications | 43 (27.4%) |
| Hematoma | 8 |
| Seroma | 10 |
| Infection | 6 |
| Skin/nipple necrosis | 13 |
| Implant rupture | 1 |
| Other | 5 |
| Reintervention | 23 (53.4%) |
| Implant or expander removal | 15 |
| Lymphedema | 31 (19.7%) |
| Variable . | n (%) . |
|---|---|
| Postoperative complications | 43 (27.4%) |
| Hematoma | 8 |
| Seroma | 10 |
| Infection | 6 |
| Skin/nipple necrosis | 13 |
| Implant rupture | 1 |
| Other | 5 |
| Reintervention | 23 (53.4%) |
| Implant or expander removal | 15 |
| Lymphedema | 31 (19.7%) |
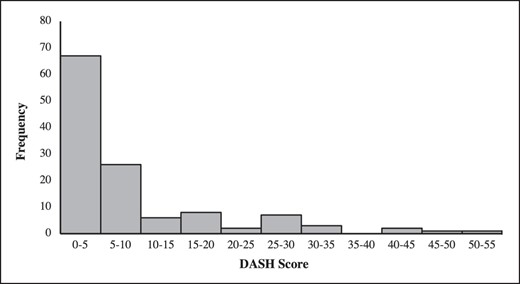
DASH surveys were completed an average of 4.3 years ± 2.1 years after the patient's final reconstructive surgery. The average DASH score was 7.7 (range 0.0-52.5), with 74.1% of patients having a score greater than 0, suggesting that these patients had some level of upper extremity (UE) impairment. A total of 57.9% (n = 91) of patients had a DASH score between 0 and 5; 19.1% (n = 30) had a score ranging from 5 to 10; 11.5% (n = 18) of patients had DASH scores between 10 and 20; and 11.5% (n = 18) had DASH scores greater than 20 (Figure 2). Survey questions were grouped into 3 main categories, including assessment of degree of functional impairment; upper extremity symptoms such as pain, numbness, or tingling; and interference of impairment with daily activities. A total of 20.8% of patients reported some degree of UE functional impairment (Table 3). Activities with the highest scores were “pushing open a heavy door,” “placing an object on a shelf above your head,” and “carrying a heavy bag or briefcase.” Some level of difficulty with recreational activities (golf, tennis, frisbee) was reported by 40.5% of patients after their breast surgery. Some degree of continued symptoms including pain, weakness, tingling, or stiffness in the UE was reported by 30.7% of patients (Table 3). When asked about regular limitations, 17.4% of female patients reported that during the past week they felt limited at work or in other regular daily activities because of their upper extremity dysfunction. Functional impairment resulted in 25.5% of females feeling less confident.
| . | Severity of upper extremity impairment, n (%) . | |||||
|---|---|---|---|---|---|---|
| DASH question category . | None . | Mild . | Moderate . | Severe . | Extreme/unable . | Total no. of patient responsesa . |
| Functional (21 questions) | 2612 (79.2%) | 488 (14.8%) | 158 (4.8%) | 22 (0.7%) | 17 (0.5%) | 3297 |
| Symptomatic (5 questions) | 544 (69.3%) | 171 (21.8%) | 63 (8.0%) | 6 (0.8%) | 1 (0.1%) | 785 |
| Interference with daily activity (4 questions) | 483 (76.9%) | 97 (15.5%) | 37 (6.9%) | 8 (1.3%) | 3 (0.5%) | 628 |
| . | Severity of upper extremity impairment, n (%) . | |||||
|---|---|---|---|---|---|---|
| DASH question category . | None . | Mild . | Moderate . | Severe . | Extreme/unable . | Total no. of patient responsesa . |
| Functional (21 questions) | 2612 (79.2%) | 488 (14.8%) | 158 (4.8%) | 22 (0.7%) | 17 (0.5%) | 3297 |
| Symptomatic (5 questions) | 544 (69.3%) | 171 (21.8%) | 63 (8.0%) | 6 (0.8%) | 1 (0.1%) | 785 |
| Interference with daily activity (4 questions) | 483 (76.9%) | 97 (15.5%) | 37 (6.9%) | 8 (1.3%) | 3 (0.5%) | 628 |
aNumber of patient responses in each category of functional impairment severity are reported. For example, with our 157 total patients, there were 21 questions × 157 = 3297 responses in the functional question category. DASH, disabilities of the arm, shoulder, and hand.
| . | Severity of upper extremity impairment, n (%) . | |||||
|---|---|---|---|---|---|---|
| DASH question category . | None . | Mild . | Moderate . | Severe . | Extreme/unable . | Total no. of patient responsesa . |
| Functional (21 questions) | 2612 (79.2%) | 488 (14.8%) | 158 (4.8%) | 22 (0.7%) | 17 (0.5%) | 3297 |
| Symptomatic (5 questions) | 544 (69.3%) | 171 (21.8%) | 63 (8.0%) | 6 (0.8%) | 1 (0.1%) | 785 |
| Interference with daily activity (4 questions) | 483 (76.9%) | 97 (15.5%) | 37 (6.9%) | 8 (1.3%) | 3 (0.5%) | 628 |
| . | Severity of upper extremity impairment, n (%) . | |||||
|---|---|---|---|---|---|---|
| DASH question category . | None . | Mild . | Moderate . | Severe . | Extreme/unable . | Total no. of patient responsesa . |
| Functional (21 questions) | 2612 (79.2%) | 488 (14.8%) | 158 (4.8%) | 22 (0.7%) | 17 (0.5%) | 3297 |
| Symptomatic (5 questions) | 544 (69.3%) | 171 (21.8%) | 63 (8.0%) | 6 (0.8%) | 1 (0.1%) | 785 |
| Interference with daily activity (4 questions) | 483 (76.9%) | 97 (15.5%) | 37 (6.9%) | 8 (1.3%) | 3 (0.5%) | 628 |
aNumber of patient responses in each category of functional impairment severity are reported. For example, with our 157 total patients, there were 21 questions × 157 = 3297 responses in the functional question category. DASH, disabilities of the arm, shoulder, and hand.
In a univariate analysis, 6 variables were associated with a significantly higher DASH score, including age greater than 50 years (P = .03); BMI greater than 25 (P = .04); implant-based reconstruction (P = .03); radiation (P < .01); postoperative complications (P = .02); and lymphedema (P < .0001). Patients completing postoperative physical therapy exercises had a lower average DASH score, however this was not statistically significant (10.0 vs 6.9, P = .07). Multivariate regression analysis resulted in 6 variables of significance at P = .15 (Table 4). Variables identified by regression analysis which were associated with a significantly higher DASH score included age between 50 and 60 years (P = .10); history of radiation to the chest wall (P = .01); placement of a subpectoral implant (P = .10); postoperative complications (P = .05); and lymphedema (P = .001). Autologous breast reconstruction (P = .03) was associated with a significantly lower DASH score. Stepwise regression, regardless of forward or backward iteration, identified the same 6 significant variables.
| Variable . | Parameter estimate . | Standard error . | F value . | P value . |
|---|---|---|---|---|
| Age (50-60 years) | 4.15 | 1.58 | 6.9 | .10 |
| Radiation | 5.32 | 2.07 | 6.6 | .01 |
| Autologous reconstruction | −4.43 | 1.92 | 5.3 | .03 |
| Subpectoral implant | 3.26 | 1.99 | 2.7 | .10 |
| Postoperative complication | 4.02 | 2.01 | 4.0 | .05 |
| Lymphedema | 7.21 | 2.03 | 12.5 | .001 |
| Variable . | Parameter estimate . | Standard error . | F value . | P value . |
|---|---|---|---|---|
| Age (50-60 years) | 4.15 | 1.58 | 6.9 | .10 |
| Radiation | 5.32 | 2.07 | 6.6 | .01 |
| Autologous reconstruction | −4.43 | 1.92 | 5.3 | .03 |
| Subpectoral implant | 3.26 | 1.99 | 2.7 | .10 |
| Postoperative complication | 4.02 | 2.01 | 4.0 | .05 |
| Lymphedema | 7.21 | 2.03 | 12.5 | .001 |
| Variable . | Parameter estimate . | Standard error . | F value . | P value . |
|---|---|---|---|---|
| Age (50-60 years) | 4.15 | 1.58 | 6.9 | .10 |
| Radiation | 5.32 | 2.07 | 6.6 | .01 |
| Autologous reconstruction | −4.43 | 1.92 | 5.3 | .03 |
| Subpectoral implant | 3.26 | 1.99 | 2.7 | .10 |
| Postoperative complication | 4.02 | 2.01 | 4.0 | .05 |
| Lymphedema | 7.21 | 2.03 | 12.5 | .001 |
| Variable . | Parameter estimate . | Standard error . | F value . | P value . |
|---|---|---|---|---|
| Age (50-60 years) | 4.15 | 1.58 | 6.9 | .10 |
| Radiation | 5.32 | 2.07 | 6.6 | .01 |
| Autologous reconstruction | −4.43 | 1.92 | 5.3 | .03 |
| Subpectoral implant | 3.26 | 1.99 | 2.7 | .10 |
| Postoperative complication | 4.02 | 2.01 | 4.0 | .05 |
| Lymphedema | 7.21 | 2.03 | 12.5 | .001 |
To examine meaningful clinical association, we conducted logistic regression analyses to determine which variables were associated with at least a 20-point change in DASH score. For this analysis, White race and diabetes variables were removed, given no cases of DASH ≥ 20 or DASH < 20. In the model with no selection and all variables included, we observed 5 variables that were significant: age of 50 to 60, radiation, autologous implant, subpectoral implant, and lymphedema. Stepwise regression identified only 4 variables significantly associated with DASH ≥ 20, which included the 50 to 60 age group (odds ratio = 3.97); autologous implant (odds ratio = 0.22); radiation (odds ratio = 4.13); and lymphedema (odds ratio = 4.16) (Table 5). These findings indicate that patients who develop lymphedema, have a history of radiation, or are above the age of 50 are each approximately 4 times more likely to have a high DASH score, indicative of significant UE dysfunction.
| Analysis of maximum likelihood estimates . | Odds ratio estimates . | |||||
|---|---|---|---|---|---|---|
| Parameter . | Estimate . | Standard error . | Chi-square . | P value . | Odds ratio . | 95% CI . |
| Intercept | −3.21 | 0.51 | 39.47 | <.0001 | ||
| Age (50-60 years) | 1.38 | 0.57 | 5.96 | .01 | 3.97 | 1.31-12.0 |
| Radiation | 1.42 | 0.66 | 4.62 | .03 | 4.13 | 1.13-15.1 |
| Autologous reconstruction | −1.52 | 0.78 | 3.77 | .05 | 0.22 | 0.05-1.02 |
| Lymphedema | 1.43 | 0.61 | 5.41 | .02 | 4.16 | 1.25-13.8 |
| Analysis of maximum likelihood estimates . | Odds ratio estimates . | |||||
|---|---|---|---|---|---|---|
| Parameter . | Estimate . | Standard error . | Chi-square . | P value . | Odds ratio . | 95% CI . |
| Intercept | −3.21 | 0.51 | 39.47 | <.0001 | ||
| Age (50-60 years) | 1.38 | 0.57 | 5.96 | .01 | 3.97 | 1.31-12.0 |
| Radiation | 1.42 | 0.66 | 4.62 | .03 | 4.13 | 1.13-15.1 |
| Autologous reconstruction | −1.52 | 0.78 | 3.77 | .05 | 0.22 | 0.05-1.02 |
| Lymphedema | 1.43 | 0.61 | 5.41 | .02 | 4.16 | 1.25-13.8 |
CI, confidence interval; DASH, disabilities of the arm, shoulder, and hand.
| Analysis of maximum likelihood estimates . | Odds ratio estimates . | |||||
|---|---|---|---|---|---|---|
| Parameter . | Estimate . | Standard error . | Chi-square . | P value . | Odds ratio . | 95% CI . |
| Intercept | −3.21 | 0.51 | 39.47 | <.0001 | ||
| Age (50-60 years) | 1.38 | 0.57 | 5.96 | .01 | 3.97 | 1.31-12.0 |
| Radiation | 1.42 | 0.66 | 4.62 | .03 | 4.13 | 1.13-15.1 |
| Autologous reconstruction | −1.52 | 0.78 | 3.77 | .05 | 0.22 | 0.05-1.02 |
| Lymphedema | 1.43 | 0.61 | 5.41 | .02 | 4.16 | 1.25-13.8 |
| Analysis of maximum likelihood estimates . | Odds ratio estimates . | |||||
|---|---|---|---|---|---|---|
| Parameter . | Estimate . | Standard error . | Chi-square . | P value . | Odds ratio . | 95% CI . |
| Intercept | −3.21 | 0.51 | 39.47 | <.0001 | ||
| Age (50-60 years) | 1.38 | 0.57 | 5.96 | .01 | 3.97 | 1.31-12.0 |
| Radiation | 1.42 | 0.66 | 4.62 | .03 | 4.13 | 1.13-15.1 |
| Autologous reconstruction | −1.52 | 0.78 | 3.77 | .05 | 0.22 | 0.05-1.02 |
| Lymphedema | 1.43 | 0.61 | 5.41 | .02 | 4.16 | 1.25-13.8 |
CI, confidence interval; DASH, disabilities of the arm, shoulder, and hand.
DISCUSSION
The primary aim of the present study was to determine the effect of patient demographic factors, oncologic history, and breast reconstruction type on a patient's upper extremity functional recovery after breast surgery by evaluating postoperative DASH scores. DASH has been shown to be valuable for orthopedic patients, specifically with shoulder, arm, or hand impairments, however little is known about how DASH scores apply to plastic and breast surgery. The DASH survey was selected as opposed to the abbreviated, 11-question QuickDASH to obtain granular data regarding specific daily activities and motions that may be difficult for patients. The DASH questionnaire assessed functional impairment, difficulty with activities of daily living, and residual numbness, pain, or tingling in the upper extremity. Most patients had some degree of functional impairment or physical symptoms at or after the 1-year postoperative time point. Significant preoperative risk factors for UE dysfunction included patient age and BMI, oncologic and reconstructive factors included type of reconstruction and history of radiation, and postoperative factors included complications and lymphedema development.
Upper extremity dysfunction (UED) after breast cancer treatment is a well-documented phenomenon, and findings from this study support the lasting effects that these interventions can have on patients. Many patients reported difficulty with common activities such as lifting arms above the head or carrying heavy objects. Radiation and lymphedema have been noted in previous literature for being associated with UED, which were found to be the most significant variables in this model. Lymphedema, which is a chronic and debilitating condition, can occur in up to 40% of patients after breast cancer treatment. Patients often report UE physical symptoms, including pain, stiffness, weakness, and overall decreased mobility.15 Results from this study support such conclusions; patients with lymphedema had a 4-fold increased risk of having a DASH score increase of 20 points or more compared to those without lymphedema. Radiation has often been regarded as a cause of lymphedema. Postmastectomy radiation, which includes treatment to the infraclavicular and supraclavicular areas, chest wall, posterior axilla, and internal mammary nodes, is associated with significant potential for postradiation fibrosis in the involved muscle and soft tissue.16 Given this, it is likely that radiation is an independent risk factor for UED and higher DASH scores, which is supported by the findings in this study.
An increasing number of female patients elect to undergo breast reconstruction after mastectomy, with an estimated prevalence of over 40% in 2021.17,18 In previous literature comparing long-term patient-reported outcomes by type of reconstruction, researchers have shown that patients with autologous reconstruction (AR) had higher levels of physical well-being of the chest, in addition to improved psychosocial and sexual well-being.17 The BREAST-Q physical well-being scale specifically measured for severity of chest muscle pain and other symptoms, including tightness, pulling, and tenderness. Additional items asked about activity limitations and sleep problems due to discomfort. Findings in our study showed that patients with autologous reconstruction had lower DASH scores than those with implant-based reconstruction, indicative of less UE impairment. Many studies looking at patient outcomes have reported similar findings of improved long-term quality of life and well-being with AR. Despite these findings, in the United States, the majority of patients elect to have implant-based reconstruction (estimated 78% to 82%); autologous reconstruction only represents about 18% to 20% of breast reconstruction procedures.19
Given the high prevalence of breast cancer, with an increasing number of patients seeking curative treatment and reconstructive surgery, greater emphasis is needed on mitigating upper extremity dysfunction after treatment. Advances in surgical treatment have allowed for more sentinel lymph node procedures and fewer patients requiring axillary lymph node dissection, a known risk factor for lymphedema and UED.20-22 On the reconstructive front, this study shows improved upper extremity outcomes in patients with autologous reconstruction. While the anatomy of every patient may not allow for flap-based autologous reconstruction, the use of fat grafting is a viable option, with a low rate of postoperative complications, even with multiple rounds of lipoaugmentation.23,24 Despite this, implant-based reconstruction continues to account for 75% to 85% of breast reconstruction after cancer treatment. The shift from subpectoral implants to prepectoral implants within the last 5 years has preliminarily shown promise in improving overall patient satisfaction.25,26 However, longitudinal studies are needed to compare long-term UE outcomes.
In addition to operative advancements, postoperative care is also a potential target for improving upper extremity outcomes. Previous retrospective studies show that there is significant benefit to targeted UE physical therapy after breast surgery for improving range of motion and decreasing pain.27,28 Findings from this study were consistent with this, showing a correlation between postoperative physical therapy and lower DASH scores. However, in a study surveying practice patterns across the United States, there was found to be a discrepancy between early vs late (>3 weeks) postoperative physical therapy.28 Due to the nature of the survey, time point and duration were not obtained. However, given that an association between time to PT and outcomes may exist, there is a need for surgeons and physical therapists to work together to standardize practice patterns that maximize functional outcomes after surgical intervention for breast cancer.
Limitations
There were several limitations to this study. Roughly 54% of the original cohort completed the DASH and were included in the study. While several attempts were made to recruit as many patients as possible, this cohort did not include all patients in the identified study period. Another limitation of this study was response bias. For example, respondents with particularly positive or negative experiences with the surgeon or with their reconstructive outcomes may have been more likely to respond to the survey. The study was also subject to recall bias, in that some participants might not remember if they had completed postoperative exercises or physical therapy. Last, due to the retrospective nature of this study, we did not have baseline DASH scores for patients. Having preintervention DASH scores would have allowed us to examine upper extremity functionality before and after surgical intervention, radiation therapy, and in some cases, placement of implants. This would be particularly helpful to know about in patients with higher-than-average DASH scores to determine the degree to which surgical intervention affected upper extremity functionality.
CONCLUSIONS
Breast cancer treatment can result in lasting upper extremity physical and functional effects that have the potential to disrupt activities of daily living and hinder patients from returning to their normal lives. Our study described upper extremity impairment in the setting of patients undergoing surgical intervention for breast cancer treatment. The identified patient demographic and intraoperative and postoperative factors that were significantly associated with worse or improved upper extremity outcomes in this study were consistent with what is described in existing studies. However, in this study we were able to quantify the frequency and severity with which these symptoms occurred in the patient cohort, which is not readily available in current literature. Given that this study provides information regarding specific functional and symptomatic impairment, it can be adapted by rehabilitation professionals for targeted UE physical therapy. Furthermore, these findings should encourage surgeons to adopt new surgical practices and improve patient education to reduce postoperative UE dysfunction.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
Author notes
Ms Humar, Ms Balogun, and Ms Zhang are medical students, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Dr Moroni and Dr Raghuram are residents, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Dr Nguyen is a medical student, Carle Illinois College of Medicine, University of Illinois Urbana-Champaign, Champaign-Urbana, IL, USA.
Dr De La Cruz is an associate professor, Department of Plastic Surgery, University of Pittsburgh, Pittsburgh, PA, USA.



