-
PDF
- Split View
-
Views
-
Cite
Cite
Alexander Rivkin, Jeremy B Green, Suzanne Bruce, Sue Ellen Cox, Oscar Hevia, Smita Chawla, Marta Sartor, Safe and Effective Restoration of Jawline Definition With Hyaluronic Acid Injectable Gel VYC-25L: Results From a Randomized Controlled Study, Aesthetic Surgery Journal, Volume 44, Issue 12, December 2024, Pages 1341–1349, https://doi.org/10.1093/asj/sjae147
Close - Share Icon Share
Abstract
A well-defined jawline improves overall facial aesthetics, motivating patients to seek jawline augmentation.
In this study we evaluated the safety and effectiveness of the hyaluronic acid injectable gel VYC-25L for restoring jawline definition.
In a US multicenter, evaluator-blinded study adults with grade 2 (moderate) or 3 (severe) Allergan Loss of Jawline Definition Scale (ALJDS) scores were randomized. Participants were randomized to the VYC-25L treatment group or control group at study onset, with 12-month follow-up. The control group had the option to receive treatment after 6 months (primary endpoint completion). Effectiveness measures included Month 6 ALJDS responders rate (proportion of participants with ≥1-grade improvement from baseline on both sides), FACE-Q Satisfaction With Lower Face and Jawline scores, and Global Aesthetic Improvement Scale (GAIS) responders (improved/much improved) as assessed by the investigator and participants. Injection site responses (ISRs) and adverse events (AEs) were monitored.
At Month 6, ALJDS responder rates were 69.0% vs 38.0% in the VYC-25L treatment (n = 157) and control (n = 49) groups, respectively (P = .0001). In the VYC-25L treatment group, FACE-Q scores improved by a mean of 45.9 points from baseline at Month 6 (P < .0001). Furthermore, 88.4% and 89.0% of participants in the VYC-25L treatment group were GAIS responders at Month 6 by participant and investigator assessment, respectively. Most ISRs were mild or moderate and resolved within 2 weeks. Most treatment-related AEs were mild and resolved within 1 week.
VYC-25L safely and effectively restores jawline definition through 1 year.

A balanced approach to facial rejuvenation requires attention to the lower face, including the jawline.1 With age, the jawline loses definition and volume as a result of mandibular bone remodeling and skin laxity, as well as descent and atrophy of facial fat and soft tissue.2-4 Restoring a strong and defined jawline has been recognized as an aesthetic imperative in a wide range of patients for many years. Jawline contour plays an important role in gender- and culture-related ideals of beauty.1,4 Preferred jawline angles and shapes differ between cultures globally, ranging from a bulky, square jawline to a soft and elegant yet defined jawline curvature.4,5
Injectable fillers, including hyaluronic acid (HA), are a widely administered, nonsurgical option for reshaping the jawline and projecting soft tissue, resulting in improvements to facial contour and jawline aesthetics.4,6 VYC-25L (Juvéderm Volux XC; Allergan Aesthetics, an AbbVie company, Irvine, CA) is an injectable HA filler that features a comparatively higher G′ (elastic modulus, a measure of resistance to deformation), cohesivity, and HA concentration than other HA fillers, as well as novel cross-linking technology.7 These characteristics are expected to provide advanced volume, lift, and moldability after injection and facilitate sculpting, contouring, and shaping, particularly in high-mobility areas such as the chin and jaw.7-9 A European study reported the safety and effectiveness of VYC-25L for restoring and creating facial volume in the chin by injections to the pogonion, mentum, prejowl sulci, and sublabial crease.8,10 The present study was the first to consider the effectiveness of VYC-25L in restoring definition and volume to the entirety of the jawline, including the angle and body of the mandible, the postjowl and prejowl sulci, as well as the chin and marionette line areas. Effectiveness was measured with a new, validated photonumeric rating scale to assess jawline definition: the Allergan Loss of Jawline Definition Scale (ALJDS). This complete correction was the basis of the US Food and Drug Administration’s approval of VYC-25L for the indication of improving definition and replacing lost volume in the jawline.
METHODS
Allergan Loss of Jawline Definition Scale
The ALJDS is a proprietary, validated 5-grade photonumeric scale (0 = none; 1 = mild; 2 = moderate; 3 = severe; 4 = extreme) in which a 1-grade difference is considered clinically significant.
Image Capture
Images were captured with a VECTRA M3 Lift camera system (Canfield Scientific, Inc., Parsipanny, NJ) with standardized lighting conditions following a predefined image capture protocol. The photographers at each study site were trained on the camera system, and the images were reviewed for photographic quality.
Study Design
This randomized, controlled, evaluator-blinded, 12-month pivotal study (Supplemental Figure 1, located online at www.aestheticsurgeryjournal.com) was conducted from November 2018 to January 2021 across 19 centers in the US (ClinicalTrials.gov ID number: NCT03712137). Participants were randomized 3:1 to either the VYC-25L treatment or control groups. Randomization was performed with an interactive web response system, in which the participants were assigned unique identification numbers. The VYC-25L treatment group received treatment to the jawline at study onset, and the control group did not receive treatment for 6 months. Treatment consisted of VYC-25L on Day 1 with optional touch-up at Day 30. After a 12-month follow-up period, participants in the VYC-25L treatment group had the option of receiving maintenance treatment with no touch-up followed by an additional 3-month follow-up period (VYC-25L maintenance treatment group). Optional touch-up was recommended for participants who, in the opinion of the treating investigator (TI), had not achieved at least a 1-grade improvement in scores for each side of the jawline on the ALJDS.
After 6 months without treatment, control group participants could either complete the study or opt to receive treatment with VYC-25L (optional treatment control group). Initial treatment with optional touch-up treatment 30 days later was followed by a 12-month follow-up with no optional maintenance treatment.
The study was approved by an institutional review board (Copernicus Group IRB, Cary, NC) and conducted in compliance with the Declaration of Helsinki and guidelines on good clinical practice. Participants provided written informed consent.
Participants
Eligible participants were ages 22 years or older with an ALJDS grade of moderate (grade 2) or severe (grade 3) loss of jawline definition on each side of the jawline (both sides of the jaw had to be eligible but did not need to have the same score), deemed by the TI to be amenable to at least a 1-grade ALJDS improvement. Exclusion criteria included any previous or planned facial implants, laser procedures, chemical peels, mesotherapy, liposuction, or lower two-thirds facial fat injections; semipermanent dermal filler treatment (eg, calcium hydroxyapatite, poly-L-lactic acid) within 36 months or temporary filler treatment below the subnasale within 12 months of enrollment; botulinum toxin injections in the lower face or deoxycholic acid treatment in the submental region in the 6 months before enrollment; severe midface volume deficit, prominent submental fullness, extreme skin laxity, significant facial asymmetry, a history of trauma to the chin and jaw area within 6 months of enrollment with residual deficiencies, deformities, or scarring; and allergy to lidocaine, HA products, or streptococcal protein.
Treatment Administration Procedures
VYC-25L was administered with supplied 27-gauge 1/2-inch needles for injection and/or a 25-gauge, 1-1/2-inch cannula. The choice of needle, cannula, or a combination of both was based on the TI's preference. Treatment guidance was given to inject the prejowl sulci, body of the mandible/postjowl sulci, and marionette lines (optional) to help restore jawline definition. Injections were also allowed in the chin and angle of the mandible to further enhance jawline definition and provide aesthetic continuity for the facial lower third (Supplemental Figure 2, located online at www.aestheticsurgeryjournal.com).11 As a safety precaution, injections were avoided above the permitted areas or in the antegonial notch, parotid gland, temporomandibular joint, and in or below the jowl fat pad. The total volume of VYC-25L for initial and touch-up treatments combined was not to exceed 8 mL based on the VYC-25L Investigational Instructions for Use. The total volume for maintenance treatment had the same volume limitation.
Safety and Effectiveness Assessments
Assessments occurred at months 1, 3, 6 (the primary endpoint), 9, and 12 after the last treatment (initial or touch-up) and at months 1 and 3 after optional maintenance treatment.
Effectiveness
The following outcome measurements were assessed throughout the study: ALJDS responder rate, FACE-Q Satisfaction with Lower Face and Jawline overall scores, and Global Aesthetic Improvement Scale (GAIS) responder rates.
The primary effectiveness endpoint was the ALJDS responder rate, defined as the proportion of participants with ≥1-grade improvement from their baseline ALJDS score on both sides of the jawline at Month 6. This endpoint was assessed by the blinded evaluating investigator (EI) based on photographs.
Secondary effectiveness endpoints included the mean change from baseline in overall participant satisfaction with the jawline at Month 6 with the validated FACE-Q Satisfaction with Lower Face and Jawline questionnaire. This questionnaire consisted of 5 items (jawline prominence, jawline definition, facial profile, lower face smoothness, and lower facial appearance) evaluated on a 4-point scale (1 = very dissatisfied; 2 = somewhat dissatisfied; 3 = somewhat satisfied; and 4 = very satisfied).
Another secondary effectiveness endpoint was the GAIS responder rate at Month 6, as assessed by the EI (for both treatment and control groups) and participants (treatment group alone) noncollaboratively with photographs. The GAIS responder rate was defined as the proportion of participants with a rating of “improved” or “much improved” in overall jawline area aesthetics with the 5-point GAIS (2 = much improved; 1 = improved; 0 = no change; −1 = worse; −2 = much worse).
Safety
Safety assessments included analysis of injection site responses (ISRs), adverse events (AEs), procedural pain score (11-point scale), jaw function (20-item Jaw Functional Limitation Scale), facial sensation, pronunciation video recordings, and vision tests (Snellen visual acuity, confrontational visual fields, and ocular motility). Participants were provided with a daily e-diary to record the presence and severity of ISRs for up to 30 consecutive days after treatment starting on the day of treatment. AEs of special interest were vision-related AEs.
Subgroup Analyses
The relationship between method of injection (ie, needle alone vs cannula with or without a needle) and ALJDS responder rate as well as the incidences of ISRs and AEs were analyzed.
Statistical Methods
The statistical criteria for achieving the primary endpoint (≥1-grade improvement from baseline to Month 6 on the ALJDS) were a 50% or higher responder rate for the VYC-25L treatment group and a significant difference from the control group of P < .025 on a 1-sided Fisher exact test. The last observation carried forward method was employed to impute missing values at Month 6 (ie, last observed score was the Month 6 value). Analysis of secondary endpoints included EI- and participant-assessed GAIS responder rates with a 95% CI for the VYC-25L treatment group. FACE-Q raw individual and total scores were transformed by Rasch unidimensional methods to create a score ranging from 0 to 100, with higher scores reflecting a better outcome. Data were analyzed with the 2-sided paired t test at the 5% level (Month 6 score vs baseline). Safety assessments were descriptively summarized, and ISRs and AEs were summarized by incidence, severity, and duration.
Effectiveness assessments were conducted in the modified intent-to-treat (mITT) population, which included all randomized participants. The VYC-25L safety population included all VYC-25L treatment group participants who received at least 1 study treatment, plus all participants in the control group.
RESULTS
Participants
A total of 206 participants were randomized and evaluated (mITT population; VYC-25L treatment group, n = 157; control group, n = 49). The median (range) age of the mITT population was 59 (26–81) years, and participants were mainly female, White, and non-Hispanic or Latino (Supplemental Table 1, located online at www.aestheticsurgeryjournal.com). At baseline, loss of jawline definition based on the side with the highest ALJDS scores was moderate (grade 2), severe (grade 3), or extreme (grade 4) in 22.8%, 72.8%, and 4.4% of participants, respectively. The percentages of participants with moderate (grade 2) or severe (grade 3) baseline ALJDS scores were comparable between groups. Due to a protocol deviation, 13 participants were randomized despite having at least 1 ALJDS score outside of grades 2 or 3. Four patients (2 in the VYC-25L treatment group and 2 in the control group) had at least 1 side of the jaw with ALJDS grade 1 (mild), and 9 patients (all in the VYC-25L treatment group) had at least 1 side with ALJDS grade 4 (severe). Sensitivity analysis confirmed consistency of outcomes between these excluded participants and the rest of the study population (data not shown). A total of 27 of 206 participants (13.1%) in the mITT population discontinued the study (VYC-25L treatment group, n = 18; control group, n = 9). The most common reasons for discontinuation were withdrawal by participant and loss to follow-up. The safety population was comprised of 206 participants (VYC-25L treatment group, n = 156; control group, n = 50).
Of 157 participants randomized to the VYC-25L treatment group, all but 1 participant received initial treatment. Of the 156 participants who received initial treatment, 129 participants (82.7%) received touch-up treatment 30 days later, and 87 participants (55.8%) received maintenance treatment at the 12-month follow-up visit. The most common reasons for not receiving maintenance treatment were full jawline correction or COVID-19 pandemic restrictions or concerns.
Treatments Administered
In the VYC-25L treatment group, 52.6% (82/156) of participants were injected by needle only for initial and touch-up treatments vs 47.4% (74/156) by cannula (with or without a needle). The maintenance treatment group showed a similar distribution, with a slightly higher percentage of participants treated with needle alone. Initial treatments included injections in most areas of the jawline, with a higher frequency in the right and left prejowl (n = 154, 98.7%) and postjowl areas (n = 148, 94.9%) compared with the chin (n = 108, 69.2%), right and left marionette lines (n = 139, 89.1%), and right and left angles of the mandible (n = 135, 86.5%). The average overall treatment volume (initial and touch-up combined) was 6.22 mL (n = 156) for the VYC-25L treatment group and 3.40 mL (n = 87) for maintenance treatment. In the VYC-25L treatment group, the average volume for touch-up was approximately one-half of the average volume for initial treatment at 2.04 mL (n = 129) vs 4.53 mL (n = 156), respectively. Injection volumes were similar for the left and right sides.
Among control group participants, 42 of 49 participants (85.7%) chose to receive optional VYC-25L treatment at the end of the 6-month no-treatment control period (optional treatment control group). The average injection volumes, injection sites, and injection modes were similar to those in the VYC-25L treatment group.
Primary Effectiveness Outcome
The primary endpoint was met. ALJDS responder rates were higher than 50% at Month 6 in the VYC-25L treatment group and significantly higher than the control group (69.0% vs 38.0%, respectively; P = .0001; 95% CI, 15.33-46.54; Figure 1). High responder rates in the VYC-25L treatment group persisted throughout the study. ALJDS responder rates at Month 6 were similar whether treated by needle alone or cannula (with or without a needle): 73.5% (n = 68) vs 66.7% (n = 78), respectively.
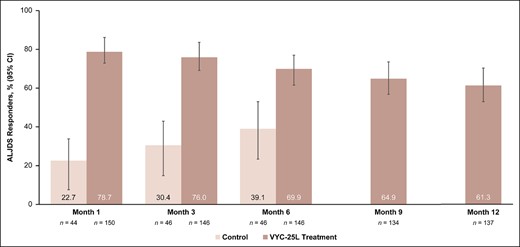
ALJDS responder rates at all time points. Based on observed data (no imputation). ALJDS responders were participants with ≥1-grade improvement from baseline on the ALJDS, as assessed by the evaluating investigator with photographs. ALJDS, Allergan Loss of Jawline Definition Scale; CI, confidence interval.
Secondary Effectiveness Outcomes
EI- and participant-rated GAIS responder rates at Month 6 for the treatment group (Figure 2) were >88% and remained high at ≥74% up to Month 12, with similar results observed after maintenance treatment.
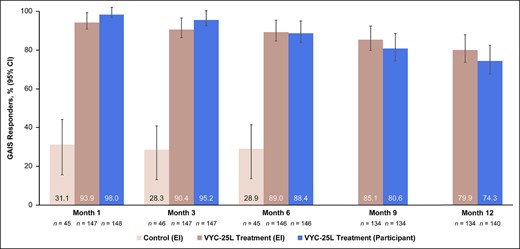
GAIS responder rates, as assessed by both EI and participant. GAIS responders were those who scored “improved” or “much improved.” CI, confidence interval; EI, evaluating investigator; GAIS, Global Aesthetic Improvement Scale.
Participants receiving VYC-25L treatment had a significantly higher mean FACE-Q satisfaction score of 67.8 at Month 6 compared with 21.9 at baseline (P < .0001), representing a mean (SD) improvement of 45.9 (33.1; Supplemental Figure 2, located online at www.aestheticsurgeryjournal.com). Mean improvement scores for the VYC-25L treatment group exceeded 30 points through 12 months. After maintenance treatment, results were similar, with mean satisfaction scores above 72%.
Representative examples of a participant showing improvements in jawline definition at Month 6 are presented in Figure 3.
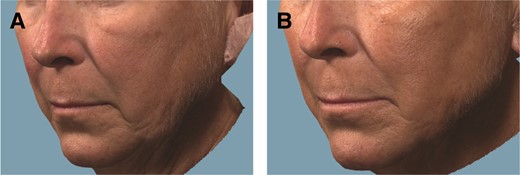
Representative photographs of a 60-year-old white female patient who underwent jawline definition with VYC-25L treatment. The patient received a total injection volume of 4.0 mL at initial treatment and 2.8 mL at touch-up treatment by cannula (with or without a needle): 2.1 mL total for the prejowl area, 1.9 mL for the postjowl area, 1.0 mL for marionette lines, 0.9 mL for the angle of the mandible, and 0.9 mL for the chin. (A) Pretreatment, ALJDS grade 3 (severe); (B) 6 months posttreatment, ALJDS grade 1 (mild). ALJDS, Allergan Loss of Jawline Definition Scale.
Safety
Of 196 participants in the VYC-25L safety population (ie, all treated participants) who completed diary entries, 167 (85.2%) reported at least 1 ISR after initial treatment, with the most common being tenderness to touch (80.1%), lumps/bumps (79.1%), and pain after injection (78.1%); incidences were similar between the treatment group and the optional treatment control group. Most ISRs were mild or moderate in severity (Supplemental Table 2, located online at www.aestheticsurgeryjournal.com) and resolved within 2 weeks of injection. The incidence rates of ISRs were lower for touch-up (77.0%) and maintenance treatments (76.3%) than for initial treatment.
Among 198 participants in the VYC-25L safety population, 73 participants (36.9%) experienced a treatment-emergent AE (TEAE), and 16 participants (8.1%) experienced a treatment-related TEAE (Supplemental Table 3, located online at www.aestheticsurgeryjournal.com). The most common TEAEs after initial/touch up treatment were headache (n = 10, 5.1%) and nasopharyngitis (n = 6, 3.0%); all other TEAEs occurred in fewer than 3% of VYC-25L–treated participants. The most common treatment-related TEAE after initial/touch-up treatment was mastication disorder (n = 4, 2.0%; AE terms included “difficulty chewing,” “trouble chewing,” and “pain chewing”), with all the others occurring in less than 2% of the VYC-25L safety population (Supplemental Table 4, located online at www.aestheticsurgeryjournal.com). Most of the treatment-related TEAEs were mild in severity and resolved within 1 week, with all mastication disorders resolved within 3 days. All treatment-related TEAEs resolved without sequelae.
Of the 87 participants from the VYC-25L treatment group who received maintenance treatment, 14 participants (16.1%) experienced a TEAE, and 3 participants (3.4%) experienced a treatment-related TEAE (Supplemental Table 3). The TEAEs included injection site nodule (n = 2, 2.3%) and vitreous detachment (n = 2, 2.3%), with all other TEAEs occurring in fewer than 2% of the maintenance population. The 3 participants with treatment-related TEAEs included the participants who reported injection site nodules (n = 2) and another participant who reported mastication disorder (n = 1) (Supplemental Table 4). Most of the events were mild.
In the VYC-25L safety population (198 participants), treatment-related serious AEs (SAEs) were experienced by 3 participants (1.5%) after initial/touch-up treatment (injection site infection, injection site nodule, and injection site hypersensitivity) and by 2 participants (injection site nodules) after maintenance treatment (Supplemental Table 3). Among the participants with a treatment-related SAE, 3 were treated with hyaluronidase (2 participants after initial/touch-up and 1 participant after maintenance treatment). Participants who received hyaluronidase treatment were withdrawn from the study per protocol. No unanticipated adverse device effects were reported.
Of the 3 incidences of injection site nodules occurring in 3 participants, 1 resolved in 30 days upon treatment with subcutaneous steroids and hyaluronidase; another resolved in 48 days with oral antibiotics and steroids plus abscess drainage; and the third resolved in 80 days with treatment that included oral antibiotics, oral steroids, intralesional steroids, and hyaluronidase. The single case of injection site hypersensitivity presented as erythema and swelling with various states of intensity, occurred 17 days after initial treatment, was moderate in severity, and resolved in 118 days with oral antibiotics and steroids in combination with hyaluronidase. The participant later recalled a medical history of silicone treatment in the forehead not reported at the time of enrollment. Permanent facial implants, such as silicone implants, were an exclusion criterion in this study.
By Method of Administration
Participants had similar rates of ISRs reported ≤30 days after initial treatment, whether they were injected by needle only (87.7% [93/106]) or by cannula (82.2% [74/90]). The majority of ISRs were mild or moderate regardless of method of administration. There was a higher number of severe ISRs for needle only (n = 14) vs cannula (n = 6). Among 106 participants injected by needle alone, 10 (9.4%) had at least 1 treatment-related TEAE, and among 92 participants injected with a cannula, 6 (6.5%) had at least 1 treatment-related TEAE.
Other Safety Assessments
Participants reported minimal procedural pain during injection (mean score of 2.2 for initial and 2.1 for touch-up for the treatment group). Jaw Functional Limitation Scale scores did not change between baseline and posttreatment follow-up visits, nor did those for the articulation measures, diadochokinetic rate, diadochokinetic accuracy, spoken paragraph naturalness, and facial sensation tests. Snellen visual acuity measures did not demonstrate significant changes in participants’ vision due to treatment as assessed by the principal investigator. No changes were observed in confrontational visual fields or ocular motility.
DISCUSSION
The jawline is a key defining feature of the lower face.12 Irregular contour or loss of definition in this area, which develops with age-related facial structure changes, negatively impacts facial aesthetics and, consequently, psychological well-being, self-confidence, and behavior.3,13-16 Awareness of just how critical a well-defined jawline is for perception of attractiveness and youth has increased steadily with the advent of social media, with a concomitant increase in demand for safe and effective nonsurgical treatments for jawline restoration.17
In this study we demonstrated the safety and effectiveness of VYC-25L for improving jawline definition and restoring jawline volume in approximately 200 adults with moderate to severe loss of jawline definition. The primary endpoint was met; at Month 6 the ALJDS responder rate for the VYC-25L treatment group was greater than the 50% statistical criteria and significantly higher than the ALJDS responder rate for the control group. Secondary endpoints were also met, with maintained high ALJDS responder rates and improvement in FACE-Q and GAIS scores (EI and participant) through this 12-month study. The unique strengths of this study compared with previous studies involving HA filler augmentation of the jawline included a large study population, validated rating scales (eg, photonumeric scale assessments), robust patient-reported outcomes specific to the jawline area, and its 12-month duration.6,9,10,17
Similar proportions of participants achieved the primary effectiveness endpoint whether injected by cannula or by needle only, providing insights into comparative outcomes by injection method. Maintenance treatments involved needles more frequently than cannulas, potentially because maintenance treatments may include smaller treatment areas, and needles may be chosen for greater precision.
There was minimal procedural pain and no compromise to vision, jaw function, sensation, or pronunciation with VYC-25L treatment. Most ISRs were mild or moderate in intensity and resolved within 2 weeks. There were no unanticipated AEs, and most treatment-related TEAEs were mild in severity and consistent with previous studies.1,4,6,9,17 Although the rates of ISRs and treatment-related TEAEs with needle only vs cannula were similar, the incidence of severe ISRs with needle was numerically higher than with cannula. The incidence of ISRs was lower for touch-up and maintenance treatments than for initial treatment, possibly because of the lower average volumes injected for maintenance.
This study aligns with previous studies utilizing VYC-25L for jawline and chin contouring and restoration. In a retrospective, 6-month, single-center analysis of data from 30 adults treated with VYC-25L in the chin and jawline, 96.7% of participants rated their appearance at 20 days posttreatment as “much improved” or “very much improved” on the GAIS, and participant satisfaction was very high.17 In a prospective, single-blind, controlled European study of VYC-25L injectable gel for the treatment of chin retrusion in 119 adults, significant improvements in the glabella-subnasale-pogonion angle followed initial VYC-25L treatment at Day 30 and generally lasted through 18 months. Similar to the present study, subjective improvements in the GAIS and FACE-Q Satisfaction with Lower Face and Jawline scores remained high throughout the study.8 A similar safety profile was also observed, with the majority of participants (96.6%) reporting at least 1 ISR for initial and touch-up treatment combined. Fewer participants reported treatment-related TEAEs in the current US study compared with the European VYC-25L study (8.1% vs 25.2%, respectively), and 3 participants reported treatment-related serious TEAEs in the US study compared with none in the European study (1.5% vs 0%).10
The filler injection volumes in this study were based on the VYC-25L Investigational Instructions for Use, a document that permits a maximum total volume of 8.0 mL per person for initial and touch-up treatments combined to both sides of the jawline. The average total volume of filler per participant was approximately 6 mL, which is more than would likely be administered in the clinical setting. However, the study volume was injected across up to 9 treatment sites (chin, left and right prejowl areas, left and right postjowl areas, left and right marionette lines, left and right angles of the mandible). This 1-session treatment approach is considerably more comprehensive than what would likely be performed in the clinical setting. Another reason for the higher injection volumes in the study compared with in the clinic involved the study objective of assessing long-term safety. With the fairly high volumes, the safety of any volume below this maximum volume is also characterized.
This study included participants with a broad range of characteristics, such as age and skin type, to draw conclusions across a diverse population; a post hoc subgroup analysis comparing different participant populations is reported elsewhere.18 Assessing the jawline area is a complex task because it involves multiple subunits of the lower face, including the prejowl and postjowl sulci, mandible, marionette lines, and chin. A scale that precisely measures all of these areas may be too complex to manage. The scale we have developed primarily focuses on prejowl and postjowl areas as the key determinants of jawline definition and relies on analysis of photographs. Although photographic assessment is an objective and standardized approach in clinical trials of aesthetic treatments, it depends on consistent image collection factors, including maintaining neutral facial expressions, appropriate head positions, and consistent lighting conditions.19,20 Maintaining a neutral and static facial expression relies heavily on the ability of the participants to follow instructions, which may be challenging to implement. In contrast, live assessments permit investigators to examine the overall effects of treatment on the entire face from multiple angles and avoid potentially confounding influences of specific participant microexpressions. The present study results emphasize the importance of EI blinding, the combination of objective and subjective effectiveness measurements, and live assessments when possible. For the present study, objective jawline measurements with 3-dimensional imaging are reported elsewhere.18
Similar responder rate profiles for the treatment and untreated control groups have been observed in previous pivotal filler studies with a delayed treatment control group that utilized a photonumeric scale as the primary endpoint measure.21,22 In a VYC-20L (Juvéderm Voluma XC; Allergan Aesthetics, an AbbVie company) study for treatment of midface volume deficits, the responder rates (≥1-point improvement in the validated Mid-Face Volume Deficit Scale) for the treatment and untreated control groups were 85.6% (178/208) vs 38.9% (14/36), respectively.21 Similar patterns of treatment vs untreated or control group responder rates were observed in a study of Restylane (Galderma Laboratories, LP, Fort Worth, TX) for lip fullness (92.6% [125/135] vs 28.9% [13/45]), VYC-20L for chin retrusion (56.3% [71/126] vs 27.5% [11/40]), and Restylane Lyft (Galderma Laboratories) with lidocaine for hand rejuvenation (85.9% [73/85] vs 21.2% [18/85]).22-24
CONCLUSIONS
VYC-25L is a safe and effective nonsurgical treatment for improving jawline definition and restoring jawline volume up to 12 months. Maintenance treatment was safe and effective, and resulted in similar participant satisfaction levels as initial and touch-up treatment while administering less than half the injected volume.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Acknowledgments
Allergan Aesthetics (Irvine, CA), an AbbVie company (North Chicago, IL), and the authors thank the participants, study sites, and investigators who participated in this clinical trial, as well as Shradda Mehta, PhD, a former employee of AbbVie, and Rowena Bastero, PhD, an AbbVie employee, for their support as study statisticians. Clinical trial data associated with this article can be requested online by qualified researchers via Vivli (Burlington, MA; https://vivli.org/ourmember/abbvie) following review and approval of a research proposal, statistical analysis plan (SAP), and execution of a data sharing agreement (DSA). The data will be accessible for 12 months, with possible extensions considered.
Disclosures
Dr Rivkin is an investigator, consultant, and speaker for Allergan Aesthetics (Irvine, CA), an AbbVie company (North Chicago, IL). Dr Green is a clinical researcher, advisory board member, and speaker for Allergan Aesthetics. Dr Bruce is an investigator and expert witness for Allergan Aesthetics and a speaker for Revance Therapeutics, Inc. (Nashville, TN). Dr Cox is an investigator, consultant, and advisory board member for Allergan Aesthetics, Evolus (Newport Beach, CA), Galderma (Lausanne, Switzerland), Revance, and Zeltiq Aesthetics (Pleasanton, CA); a consultant and advisory board member for Merz Pharma GmbH & Co. KGaA (Frankfurt, Germany) and Solta Medical International, Inc. (Bridgewater, NJ); an investigator and consultant for Croma-Pharma GmbH (Leobendorf, Austria); a consultant for Cearna Aesthetics, Inc. (San Diego, CA) and Pulse BioSciences (Hayward, CA); and an advisory board member for Hugel Aesthetics (Newport Beach, CA) and Suneva Medical (San Diego, CA). Dr Hevia is a clinical researcher and advisory board member for Allergan Aesthetics. Dr Chawla and Dr Sartor are full-time employees of AbbVie.
Funding
Allergan Aesthetics, an AbbVie company (Irvine, CA), funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. Medical writing support was provided to the authors by Regina Kelly, MA, and Maria Lim, PhD, of Peloton Advantage LLC, an OPEN Health company (Parsippany, NJ) and funded by Allergan Aesthetics.
REFERENCES
Author notes
Dr Rivkin is a facial cosmetic surgeon in private practice, Los Angeles, CA, USA.
Dr Green is a dermatologist in private practice, Coral Gables, FL, USA.
Dr Bruce is a dermatologist in private practice, Houston, TX, USA.
Dr Cox is a dermatologist in private practice, Chapel Hill, NC, USA.
Dr Hevia is a dermatologist in private practice, Coral Gables, FL, USA.
Dr Chawla and Dr Sartor are directors of clinical development, Allergan Aesthetics (an AbbVie Company), Irvine, CA, USA.
Presented at: American Academy of Dermatology Association Annual Meeting, Boston, MA, in March 2022.



