-
PDF
- Split View
-
Views
-
Cite
Cite
Karie Villanueva, Nisha Gupta, Tahera Alnaseri, Andrew L Da Lio, Jason Roostaeian, Michael DeLong, Plastic Surgeons’ Perspective on the FDA Breast Implant Regulatory Mandates, Aesthetic Surgery Journal, Volume 44, Issue 10, October 2024, Pages NP676–NP683, https://doi.org/10.1093/asj/sjae106
Close - Share Icon Share
Abstract
In 2021, the US FDA issued a new checklist, labeling, and rupture-screening recommendations for breast implants to improve the decision-making process.
The aim of this study was to understand plastic surgeons' perspective on these changes and their perceived impact on clinical practice.
In September 2023, a 27-question multiple-choice cross-sectional survey was distributed to 4352 active members of the American Society of Plastic Surgeons to evaluate attitudes on the FDA's black-box warning, informed decision checklist, and updated rupture-screening recommendations.
A total of 591 responses were collected (13.6%). The majority of respondents were between the ages of 45 and 64 years (58%) and had been in practice for more than 20 years (52%). Surgeons felt that some additions were appropriate; however, the majority (57%) stated that the informed decision checklist did not have a positive impact on workflow; 66% were also neutral or disagreed with the reported incidence rates related to complications and cancer. Nearly half of respondents (47%) did not feel the black-box warning improved their patients’ understanding of the risks and benefits. Additionally, 47% of respondents also believed these requirements, in combination, did not improve the overall patient experience with implants.
Respondents had an overall positive response towards the addition of risk information provided by the FDA-issued guidance and updates to rupture-screening recommendations. However, they remained divided as to whether the black-box warning and patient decision checklist had an overall positive impact on clinical practice patterns.

See the Commentary on this article here.
Breast implants have been routinely used for breast augmentation and reconstruction for several decades.1-3 However, implant devices have been increasingly scrutinized recently due to growing evidence of long-term implant complications, such as breast implant associated–anaplastic large cell lymphoma (BIA-ALCL) and systemic symptoms reported by women with implants (also referred to as breast implant illness [BII]). In 2021, the US FDA took several actions to improve communication of the risks associated with breast implants and breast implant surgery to help patients make informed decisions.4-7 First, the agency issued warning letters to implant manufacturers as a response to inadequate follow-up from postapproval safety studies. In addition, it issued updated guidance mandating new labeling requirements that must now include a black-box warning, a patient informed decision checklist, a description listing materials used in manufacturing implants, a patient implant card, and updated silicone implant rupture-screening recommendations. These requirements were specifically applied to breast implants; other surgical devices used in orthopedic and vascular surgery are not subject to such restrictions.8,9
These requirements were implemented as a result of the testimony provided by patients and experts at the 2019 General and Plastic Surgery Devices Advisory Committee Meeting. Briefly, the black-box warning clarifies that breast implants are not lifetime devices and informs patients of the risks of BIA-ALCL and systemic symptoms. The informed decision checklist was partitioned into several sections, which include information on incidence rates for device complications, BII, and BIA-ALCL. It also details updated screening recommendations for detecting silent ruptures of silicone implants. The checklist must be reviewed with patients and signed by both patient and provider to ensure they understand the risks, benefits, and information related to breast implants. These major regulatory changes were intended to improve patients’ experiences for those considering breast implants. However, it remains unclear how stakeholder groups view such changes.
A few previous studies have provided survey data focused on public perceptions related to the recent changes implemented by the FDA. From their cross-sectional survey of 494 women, Hyland et al demonstrated that 75% of respondents were less likely to receive breast implants after reviewing the cancer risk, with greater odds among Hispanics and lower odds among higher-income individuals.10 More than half of participants in a similar study conducted at Johns Hopkins Hospital stated they were less likely to receive implants.11
Although these studies have attempted to understand the public's views of the recent FDA mandate, there are few studies, to our knowledge, that have assessed the experience of plastic surgeons. Plastic surgeons are important stakeholders in breast implant safety and utility; therefore, their perspectives on improving implant labeling and risk communication should be investigated. In this study, we aimed to evaluate their opinions on the patient decision checklist, the black-box warning, and the updated screening recommendations. Our secondary aim was to review each of these components and their impact on current practice patterns.
METHODS
An anonymous, voluntary survey (SurveyMonkey Inc., San Mateo, CA) was distributed to a cohort of 4352 active American Society of Plastic Surgeons (ASPS) members. The survey was distributed on September 26, 2023, and was followed by 3 email reminders prior to closure on November 1, 2023. The survey consisted of 27 multiple-choice questions. Respondent demographics, including age and years in practice, were collected. Preferences for implant shell, shape, and fill type were also documented. A series of statements were presented to elicit the respondent's opinions on the black-box warning, the informed decision checklist, and the updated rupture-screening recommendations. Respondents were then asked to provide their opinion of the statement based on a 5-point Likert scale (ie, not applicable, neutral, disagree, agree, strongly disagree, and strongly agree). The survey statements can be found in the Appendix. Figures were created with Microsoft Excel software.
RESULTS
Demographics
A total of 591 responses were collected (13.6% response rate). Of these, the majority of respondents were between the ages of 45 and 64 years (n = 343, 58%). More than half of respondents reported having been in practice for more than 20 years (n = 307, 52%). Approximately half of respondents reported that implant procedures represented 25% to 50% of their clinical practice (n = 278, 47%). The majority preferred smooth (n = 579, 98%), round (n = 579, 98%), and silicone (n = 550, 93%) implants. Surgeon characteristics are summarized in Table 1.
| Demographic data . | n (%) . |
|---|---|
| Age group (years) | |
| Under 35 | 10 (2%) |
| 35-44 | 120 (20%) |
| 45-54 | 169 (29%) |
| 55-64 | 171 (29%) |
| 65 and over | 121 (20%) |
| Years in practice | |
| 5 | 64 (11%) |
| 5-9 | 64 (11%) |
| 10-14 | 87 (15%) |
| 15-19 | 69 (11%) |
| 20-24 years | 94 (16%) |
| 25 or more years | 213 (36%) |
| Implant preferences | |
| Textured vs smooth shell | 13 (2%) vs 565 (98%) |
| Anatomic vs round shape | 11 (2%) vs 567 (98%) |
| Saline vs silicone fill | 41 (7%) vs 537 (93%) |
| Demographic data . | n (%) . |
|---|---|
| Age group (years) | |
| Under 35 | 10 (2%) |
| 35-44 | 120 (20%) |
| 45-54 | 169 (29%) |
| 55-64 | 171 (29%) |
| 65 and over | 121 (20%) |
| Years in practice | |
| 5 | 64 (11%) |
| 5-9 | 64 (11%) |
| 10-14 | 87 (15%) |
| 15-19 | 69 (11%) |
| 20-24 years | 94 (16%) |
| 25 or more years | 213 (36%) |
| Implant preferences | |
| Textured vs smooth shell | 13 (2%) vs 565 (98%) |
| Anatomic vs round shape | 11 (2%) vs 567 (98%) |
| Saline vs silicone fill | 41 (7%) vs 537 (93%) |
| Demographic data . | n (%) . |
|---|---|
| Age group (years) | |
| Under 35 | 10 (2%) |
| 35-44 | 120 (20%) |
| 45-54 | 169 (29%) |
| 55-64 | 171 (29%) |
| 65 and over | 121 (20%) |
| Years in practice | |
| 5 | 64 (11%) |
| 5-9 | 64 (11%) |
| 10-14 | 87 (15%) |
| 15-19 | 69 (11%) |
| 20-24 years | 94 (16%) |
| 25 or more years | 213 (36%) |
| Implant preferences | |
| Textured vs smooth shell | 13 (2%) vs 565 (98%) |
| Anatomic vs round shape | 11 (2%) vs 567 (98%) |
| Saline vs silicone fill | 41 (7%) vs 537 (93%) |
| Demographic data . | n (%) . |
|---|---|
| Age group (years) | |
| Under 35 | 10 (2%) |
| 35-44 | 120 (20%) |
| 45-54 | 169 (29%) |
| 55-64 | 171 (29%) |
| 65 and over | 121 (20%) |
| Years in practice | |
| 5 | 64 (11%) |
| 5-9 | 64 (11%) |
| 10-14 | 87 (15%) |
| 15-19 | 69 (11%) |
| 20-24 years | 94 (16%) |
| 25 or more years | 213 (36%) |
| Implant preferences | |
| Textured vs smooth shell | 13 (2%) vs 565 (98%) |
| Anatomic vs round shape | 11 (2%) vs 567 (98%) |
| Saline vs silicone fill | 41 (7%) vs 537 (93%) |
Black-Box Warning
Nearly half of respondents (n = 242, 41%) either agreed or strongly agreed that the black-box warning was an appropriate part of implant labeling, with the majority in favor of the inclusion of the information regarding BIA-ALCL (n = 420, 71% agree or strongly agree). In contrast, a greater number of respondents were not in favor of the information presented about BII (n = 254, 43% disagree or strongly disagree). When asked about its effect on patient communication, 47% of respondents stated it did not improve their patients’ understanding of the risks and benefits of breast implants (n = 277, 47% disagree or strongly disagree). According to the surveyed surgeons, the black-box warning has increased the amount of time spent discussing breast implant–related questions during clinic visits, and the majority of respondents (n = 455, 77% disagree or strongly disagree) believed it did not reduce the number of questions from patients. However, the addition of the black-box warning did not appear to dissuade patients from receiving breast implants: most respondents did not experience a reduction in the number of patients interested in cosmetic augmentation (n = 290, 49% disagree or strongly disagree) or reconstruction (n = 272%, 46% disagree or strongly disagree) with implants. Thirty-four percent of respondents did not experience an increase in patients transitioning to autologous reconstruction as result of the black-box warning (n = 201, 34% disagree or strongly disagree) (Figure 1).
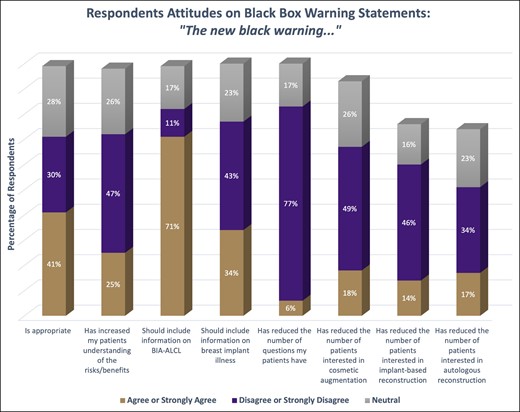
The respondents’ attitudes on the questions related to the black-box warning. Each column compares the percentage who either strongly agreed/agreed, strongly disagreed/disagreed, or were neutral on the statement.
Informed Decision Checklist
Similar to their opinions on the black-box warning, respondents had mixed opinions on whether the informed decision checklist had increased patient understanding of the risks of breast implants (39% disagree or strongly disagree vs 37% agree or strongly agree), but generally felt that it did not reduce the number of implant-related questions (n = 420, 71% disagree or strongly disagree). Respondents were also relatively evenly split on whether systemic symptoms should be included in the informed decision checklist (41% agree or strongly agree vs 35% disagree or strongly disagree). More than half of respondents were neutral or disagreed with the surgical incidence rates related to breast implants stated in the informed decision checklist (n = 390; 33% disagree or strongly disagree, 33% neutral). With regards to impact on clinical practice, most respondents did not feel that the informed decision checklist had a positive impact on clinical workflow or patient experience (n = 337, 57% disagree or strongly disagree). The checklist also appeared to increase the amount of time allotted for discussion about breast implants (n = 402, 68% agree or strongly agree). Lastly, according to the surveyed surgeons, the checklist did not appear to reduce the number of patients interested in cosmetic (n = 272, 46% disagree or strongly disagree) or reconstructive (n = 242, 41% disagree or strongly disagree) procedures with implants. Nor did they see a greater number of patients undergoing autologous reconstruction (n = 195, 33% disagree or strongly disagree vs 14% agree or strongly agree) (Figure 2).
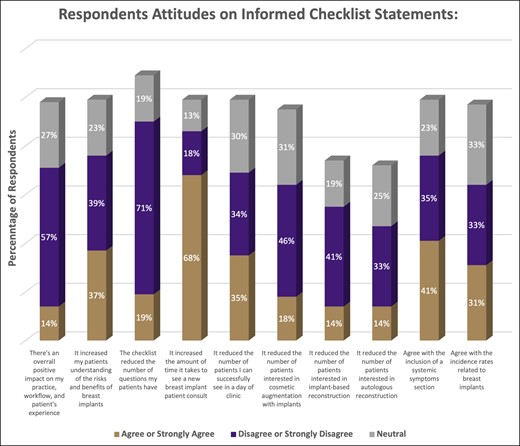
The respondents’ attitudes on the questions related to the informed checklist. Each column compares the percentage who either strongly agreed/agreed, strongly disagreed/disagreed, or were neutral on the statement.
Rupture-Screening Updates and Other Considerations
The majority of respondents felt that the changes to implant rupture-screening recommendations were appropriate (n = 420, 71% agree or strongly agree). However, opinions were evenly distributed regarding whether such changes will increase compliance overall, with a slight increase of those in favor (n = 202, 38%, agree and strongly agree; n = 174, 33% disagree and strongly disagree) (Figure 3).
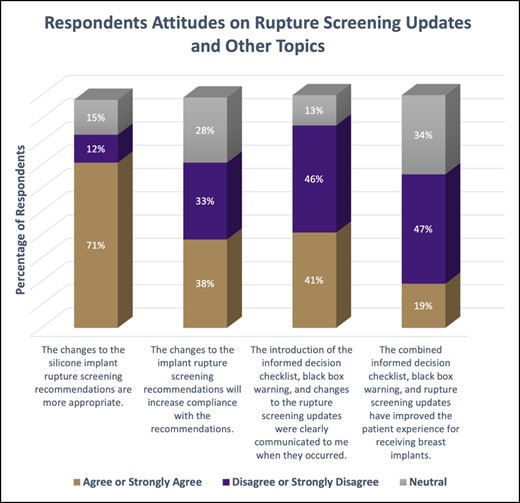
The respondents’ attitudes on the questions related to the rupture-screening updates or other topics in the mandate. Each column compares the percentage who either strongly agreed/agreed, strongly disagreed/disagreed, or were neutral on the statement.
Respondents were also asked how effectively the new mandates were communicated to them when they were introduced. Nearly half of respondents felt they were not appropriately informed about the new requirements (n = 272, 46% disagree or strongly disagree). A higher number of respondents also believed these requirements, in combination, did not improve the overall patient experience with implants (n = 278, 47% disagree or strongly disagree) (Figure 3).
DISCUSSION
We conducted a survey of current ASPS members to provide an understanding of plastic surgeons’ experience with the recent regulatory mandates, including their views on the informed decision checklist, black-box warning, and updated implant-rupture surveillance. The FDA implemented new guidelines for manufacturers to ensure patients considering breast implants receive a comprehensive overview of associated risks to enhance the decision-making process. These changes were largely driven by a growing body of epidemiologic evidence demonstrating an association between breast implants and cancer. BIA-ALCL remains an uncommon yet emerging type of non-Hodgkin T cell lymphoma that is associated with textured implants.12-15 Patients with breast implants have also reported developing systemic symptoms, including arthralgia, anxiety, fatigue, skin changes, lethargy, and breast pain. These symptoms are poorly understood and are collectively described as BII.12,14,15 Plastic surgeons, as a result, are required to review such risks with prospective breast implant patients by means of both a black-box warning and an informed decision checklist.
It is also important to note that the FDA released a safety communication that discussed reports of breast implant–associated squamous cell carcinoma (BIA-SCC) and other lymphomas in the surrounding capsule that develops. Although these incidents came after the mandated black-box warning and do not change the current recommendations, patients should be aware of these risks and understand that they are separate disease processes than those described as BIA-ACL. As of March 2023, the FDA reported approximately 19 cases of SCC from the published literature. One systematic review found the risk of BIA-SCC to be 180 times lower than that of BIA-ALCL. Although rare, these cancers can carry significant mortality and morbidity. Ongoing vigilance and research are imperative to better understand these rare breast implant–associated malignancies. However, these safety communications were released after the introduction of the new labeling mandates, and information related to these new concerns is not currently included in the black-box warning or the informed decision checklist.16-20
In our study, all respondents performed breast implant procedures and included surgeons of different age groups who have been in practice between 5 and 25 or more years. Most respondents agreed that the black-box warning had a positive impact on the consenting process, particularly related to the information about BIA-ALCL. However, a minority of respondents viewed the inclusion of information regarding BII in the black-box warning less favorably. Such opinions could reflect the status of evidence-based research and knowledge gaps surrounding systemic symptoms and breast implants.15,21-24 Some providers may not be comfortable with stating diagnoses of this nature being the consequence of high-profile regulatory action such as a black-box warning.
With regards to the patient decision checklist, respondents were relatively more split on the inclusion of BII-related information. Respondents were also divided in their opinion of the local complications portion of the checklist. These findings are consistent with a recent study that indicated that plastic surgeons may find the checklist’s comprehensiveness to be overstated. The Aesthetic Society conducted a study in 2022 based on responses from 206 of its members regarding their views of the checklist. Most respondents expressed concern about the accuracy of the data provided in the checklist. Some reported the stated risk for most complications to be inaccurate compared to their anecdotal outcomes and what had been previously reported in the scientific literature.25 Respondents also reported that the new mandates increased appointment duration for new patient consultations. In addition to the individualized patient counseling traditionally provided during these sessions, separate comprehensive checklists for each manufacturer outlining all potential risks of implants and requiring numerous signatures inevitably introduces additional questions and documentation time. Patient care and safety is of utmost importance and should not be compromised; however, it is also important to acknowledge factors that can have an impact on workflow. Additionally, some studies found that the overall readability of the patient checklist materials correlates with college reading level,8,9 whereas the general recommendation for all public documents is eighth-grade reading level. This suggests that patients who lack college-level education may have difficulty understanding the language of the materials. These potential barriers to understanding the information presented may result in further questions and/or misunderstandings.
Implant-rupture surveillance was recently modified to include either ultrasound or MRI. Implant rupture is a concern for patients and surgeons, and occurs in 10% to 20% of patients with implants within 10 years of the procedure.2,5 Patients with implants are now recommended to undergo a screening test at 5 or 6 years after implant placement, and then every 2 to 3 years afterwards. This is in contrast to historical recommendations that advised the first screening MRI to be performed at 3 years after implantation, and then every 2 years thereafter. Such change was largely driven by findings from well-powered studies, including the continuation of the original premarket core studies, demonstrating the risk of implant rupture to be quite low for the first 4 to 6 years following implant placement across manufacturers.8,9,26 From a multicentered study of nearly 100,000 patients, surgeons also reported low compliance rates, with 5 percent of patients undergoing their first screening MRI based on historical recommendations.7 In a recent survey of ASPS members, more than half of respondents stated they did not adhere to previous guidelines for breast implant surveillance. The consensus was that imaging was not typically indicated in the clinical setting. Most surgeons elected to obtain imaging for symptomatic patients.4,27 In contrast, most respondents in our study found the recent changes in surveillance to be appropriate and many believed it would increase patient compliance. This may be explained by the wider availability and lower cost of ultrasound imaging. Patients may also be more inclined to undergo either screening method at 5 years compared to 3 years and may be more aware of these recommendations due to their inclusion in the mandatory informed decision checklist.
The breast implant population is generally satisfied with their implants despite the significant rate of complications and revisional surgeries.8,15,25,28 However, the increasing scrutiny of breast implant safety may have the potential to impact perceptions and receptivity. Overall, many respondents in the current study did not experience a reduction in patients undergoing breast implant procedures. However, most surgeons believed the combined checklist, black-box warning, and rupture-screening updates did not have a positive impact on patients’ experiences with implants. Labeling of materials is generally used to supplement rather than replace the surgeon's discussion of the risks and benefits. Most respondents appeared to believe the labels provide sufficient information to help guide decision-making. Furthermore, most surgeons reported they failed to receive clear communication about these updates when the mandate was released, which may translate in an inability to disseminate implant information to current and prospective patients. As breast implant labeling continues to undergo a number of regulatory changes, surgeons are essential in facilitating discussions that are focused on the most pertinent benefits and risks associated with implants. Our study highlights the importance of understanding the surgeon's perspective to ensure communication between patient and surgeon is effective and based on well-supported scientific literature.
Although this is the largest study identifying plastic surgeons’ perception of the recent FDA recommendations regarding breast implant safety, we acknowledge it has limitations. As with any survey methodology, we must consider the possibility of response bias. The response rate, 13.6%, was relatively low. However, this is comparable with rates in recently published studies.5 Questions pertaining to the new mandates may also limit the number of respondents who may not be familiar with this topic, contributing to a lower response rate. The generalizability of our results may be limited because not all surgeons who perform breast implant procedures are ASPS members. Future investigations are recommended to evaluate proposed implant labeling recommendations in the clinical setting.
CONCLUSIONS
The recent changes to breast implant labeling and distribution have been a subject of discussion, with research focused on understanding the patient's standpoint. We conducted a study from active ASPS members to better understand the surgeon's perspective. Overall, respondents in our study believed patients should be provided with the risk information associated with implants and had positive opinions of many of the changes. Most surgeons felt the updated rupture-screening recommendations to be appropriate and may increase compliance. However, respondents were not as optimistic toward the impact of the black-box warning and patient decision checklist on clinical practice.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
Author notes
From the Division of Plastic & Reconstructive Surgery, UCLA David Geffen School of Medicine, Los Angeles, CA, USA.



