-
PDF
- Split View
-
Views
-
Cite
Cite
Yanping Guo, Wuhan Wei, Haoyu Wang, Qiang Li, Changzheng Wei, Jingyu Zhang, Peisheng Jin, Effect of a New Hyaluronic Acid Hydrogel Dermal Filler Cross-Linked With Lysine Amino Acid for Skin Augmentation and Rejuvenation, Aesthetic Surgery Journal, Volume 44, Issue 1, January 2024, Pages NP87–NP97, https://doi.org/10.1093/asj/sjad169
Close - Share Icon Share
Abstract
Hyaluronic acid (HA) fillers are the most popular filler agents for skin rejuvenation. Although 1,4-butanediol diglycidyl ether is regarded as a relatively safe cross-linker, it still exhibits certain cytotoxicity.
We presented here an amino acid–cross-linked HA (ACHA) which was obtained by an amidation reaction with lysine and HA. This study aimed to investigate ACHA's efficacy and safety for skin augmentation and rejuvenation.
Rheology, compressive tests, and swelling experiments were conducted to investigate ACHA's mechanical and viscoelastic properties. The effects of ACHA on the human keratinocytes (HaCaT) cells and the human dermal fibroblast (HDF) were investigated by Transwell and wound healing assays. Its impacts on the epithelial thickness and collagen synthesis were further examined in a mouse experimental model. We recruited 50 patients with moderate to severe nasolabial folds (NLFs). The patients were randomly allocated to receive ACHA or Restylane injections. The resulting retention rates of HA and the Wrinkle Severity Rating Scale and Global Aesthetic Improvement Scale outcomes were evaluated and compared.
ACHA exhibited good viscoelasticity. It not only promoted migration and proliferation of HaCat and HDF and secretion of various growth factors but also increased skin thickness and promoted the generation of collagen. Patients who received ACHA had more residual volume 12 months after treatment. ACHA exhibited a promising augmentation effect in NLF correction with few adverse reactions.
ACHA has shown promise as a biomaterial with excellent biocompatibility and viscoelastic characteristics in both research and the clinic.
See the abstract translated into Hindi, Portuguese, Korean, German, Italian, Arabic, Chinese, and Taiwanese online here: https://doi.org/10.1093/asj/sjad169.

Hyaluronic acid (HA) is a natural polysaccharide, a naturally degraded and absorbable biomedical material.1 It is easily biodegradable, which leads to a short residence time in the human body.2 In addition, its poor mechanical strength and stiffness limits its application as a dermal filler in soft tissue. Therefore, various cross-linking methods were applied to improve the stability of HA and broaden its application in biomedicine-related fields.3,4 HA is usually cross-linked by chemically reactive linker molecules, but most of the cross-linkers are toxic chemical reagents.5,6
Currently, 1,4-butanediol diglycidyl ether (BDDE) cross-linking is a widely used approach.7 In in vivo toxicity studies, research has found that BDDE had low or no toxicity in rats.6 Some researchers pointed out that chemical cross-linkers promote cytotoxicity by reactive oxygen and inflammatory response.8,9 Also, highly cytotoxic cross-linkers may impair the biocompatibility of cross-linked HA.10 If the concentration of cross-linkers decreases, so does the therapeutic effect. Genipin and procyanidin are known as naturally occurring nontoxic cross-linkers with good biocompatibility and sufficient mechanical properties.11,12 These natural cross-linkers overcame the shortcomings of traditional chemical cross-linkers and have been employed successfully for biomaterials. However, the cross-linked product was slightly dark and not suitable dermal filler application.
Here we present an amino acid cross-linked HA (ACHA). The carboxyl groups of HA can undergo an amidation reaction with the amino groups of lysine to form an amino amide, achieving a nontoxic cross-linked HA.
METHODS
Materials
The ACHA filler (18 mg/ml HA) was supplied by Qisheng Biological Preparation Co. Ltd. (Shanghai, China). Restylane was purchased from Q-Med (Uppsala, Sweden).
Toluidine Blue Staining
For toluidine blue staining, the HA fillers were stained with 2% toluidine blue solution (Sigma Aldrich, St. Louis, MO) for 10 minutes. They were then diluted with distilled water and incubated at 4°C overnight. The HA morphology was observed and photographed under the microscope (Olympus, Tokyo, Japan).
Scanning Electron Microscopy
The dehydrated HA specimens were sputter-coated with gold, and the surface morphology of the HA was observed using a field-emission scanning electron microscope (SEM; FEI, Hillsboro, OR).
Rheological Properties Measurements
The rheological characterization of HA fillers was evaluated using a rheometer (Kinexus Pro, Malvern, UK) equipped with parallel plate geometry (gap, 1.0 mm). Frequency sweeps were performed using 2% strain amplitude in the linear viscoelastic region and a frequency range of 0.1-10 Hz at 25°C.
Physicochemical Properties Measurements
Cohesion index was determined by the dripping method. The HA fillers were loaded into a 1-mL syringe tipped with an 18-gauge stainless steel needle. A mechanical tester (ESM303; Mark-10 Corp, Copiague, NY) was used to extrude the HA fillers at a speed of 7.5 mm/minute. When the increase in force was stable, the extruded gel was collected and weighed. Swelling rate measurements were performed using a previously validated method. HA (Wd), 500 mg, was added to 6 mL of phosphate-buffered saline (PBS) and left at room temperature for 3 to 5 hours. The resulting mixture was then centrifuged, and the supernatant was removed. The precipitate was collected and weighed (Ws). The swelling rate was calculated with the following formula: swelling rate (%) = [(Ws – Wd)/Wd] × 100.
Cell Culture
The cell lines required for the experiments, the human keratinocytes (HaCaT) cells, and the human dermal fibroblasts (HDF) were purchased from the cell bank of the Chinese Academy of Science. The cells were grown in Dulbecco’s Modified Eagle medium (Gibco, Billings, MT) containing 10% fetal bovine serum (Clark Bioscience, Webster, TX) and 5% CO2 at 37°C. Cells were assigned to the control group (cells cultured with normal medium), ACHA group (cells cultured with medium contained 10% AHCA), and Restylane group (cells cultured with medium contained 10% Restylane).
Cell Viability Assessment
The cell viability was determined using the trypan blue exclusion method. A sample of cells was stained with trypan blue (Sigma Aldrich, St. Louis, MO) and counted using a cell counter (Countess; Thermo Fisher Scientific, Waltham, MA).
Scratch Wound Healing Assay
When cells were grown to confluence on plates, a linear scratched cell free area was obtained using a 200-μL tip. At 0 and 24 hours after the scratch, images of the scratch area were captured by microscope. The remaining wound area was measured using ImageJ software (National Institutes of Health, Bethesda, MD), compared to the initial scratch area, and the percentage of wound healing was calculated.
Transwell Assay
Cells were collected and resuspended in 200 μL of serum-free culture medium and then placed in the upper chamber of the culture system. The lower chamber was filled with normal medium, medium with 10% Restylane, or medium with 10% ACHA. The migrated cells were fixed with 4% formaldehyde after 24 hours of incubation, and 0.1% crystal violet solution was used to stain the target cells. The cells that migrated into the lower chamber were captured using a microscope and calculated.
Enzyme-Linked Immunoassay (ELISA) Assay
The amounts of secreted epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) in the cell culture medium were measured using an ELISA kit (Proteintech, Wuhan, China). The optical density value of each well was recorded with the automatic microplate reader (BioTek, Winooski, VT).
Animal Experiment Design
All animal studies were approved by the Animal Ethics Committee of Xuzhou Medical University (Xuzhou, China) and conducted according to the guidelines of the National Health and Medical Research Council of China. Six-week-old male BALB/c mice were maintained in individually ventilated cages with ad lib access to food and water, in a room with controlled 12-hour light and dark cycles. Each mouse received a subcutaneous injection of 0.3 mL of saline, Restylane, or ACHA filler on the back. Four weeks after the HA injection, 5 mice from each group were sacrificed and the fresh back skin of each mouse was harvested.
Hematoxylin and Eosin and Masson Staining
The harvested fresh skin samples were embedded in paraffin and cut into 5-μm serial paraffin sections. Then they were stained with hematoxylin and eosin (HE) and Masson staining. Morphological examination was performed using a light microscope.
Immunohistochemical Staining
Immunohistochemical staining was used to determine the expression of collagen 1 and collagen 3. The sections of each group were incubated in 3% hydrogen peroxide (H2O2) to block endogenous peroxidase activity and incubated with primary antibody against collagen 1 (1:50; Abcam, Cambridge, UK), and collagen 3 (1:50) at 4 °C overnight. The sections were then incubated with corresponding secondary antibodies and labeled with horseradish peroxidase after being washed with PBS. Staining was achieved by DAB after washing with PBS and counterstaining with hematoxylin. All images were obtained under a light microscope. The staining intensity was analyzed using ImageJ.
Western Blot Analysis
The protein was extracted from skin tissue and subjected to 10% sodium dodecyl-sulfate–polyacrylamide gels. After separation, the proteins were transferred onto nitrocellulose membranes and blocked with 5% skim milk powder. The membranes then were probed with collagen 1 (1:1000; Proteintech) and collagen 3 primary antibodies overnight at 4°C. Following washing twice with tris-buffered saline with 0.1% Tween 20 detergent (Sigma Aldrich) and incubation with horseradish peroxidase–conjugated secondary antibodies for 1 hour at room temperature, the blots were detected using an enhanced chemiluminescent substrate kit (New Cell and Molecular Biotech, Suzhou, China) and quantified using ImageJ.
Clinical Study Design
We performed a clinical study to evaluate the efficacy and safety of ACHA fillers compared to Restylane for the correction of nasolabial folds (NLFs). From June 2021 and September 2022, 50 patients with an NLF rated as 3 (moderate) or 4 (severe), as assessed by the treating investigator and based on the Wrinkle Severity Rating Scale (WSRS), were recruited from the Department of Plastic Surgery, Xuzhou Medical University's Affiliated Hospital (Ethical approval no. XYFY2020-QL-144-12). The study protocol complied with the Declaration of Helsinki and was approved by the ethics committee of the Affiliated Hospital of Xuzhou Medical University. All patients provided written informed consent. After being screened for eligibility, patients were randomly allocated to the ACHA group or the Restylane group at a 1:1 ratio by computer-generated randomization.
The HA was injected with a 27-gauge, 13-mm needle into the periosteal layer in the upper part of the NLF. About 0.2 to 0.4 mL of HA was applied for deep support. Then the HA was injected using a 25-gauge, 40-mm cannula into the NLF with superficial and deep fan-shaped linear injection technique. A maximum volume of 3.0 mL was injected at the initial treatment. If necessary, at the week 2 follow-up visit, a touch-up treatment could be performed with a maximum volume of 1.0 mL, using the same product received during the initial treatment.
Three-dimensional scanning (Artec 3D, Santa Clara, CA) and WSRS evaluation were performed for all patients at baseline (pretreatment) and follow-up. The retention rate of HA was calculated using the methods described.13 The patients were instructed to complete the Global Aesthetic Improvement Scale (GAIS) at each follow-up visit. All adverse events were recorded each day by patient self-report for 15 days after injection.
Statistical Analysis
Data were presented as mean ± SD. Comparisons between groups were determined using unpaired t test and 1-way analysis of variance (ANOVA) with Dunnett's multiple comparisons post-hoc testing. Values of P < .05 were defined as statistically significant.
RESULTS
Preparation Procedure for Acid–Cross-Linked Hyaluronic Acid (ACHA) Fillers
The cross-linking process of ACHA is presented in Figure 1A. In this study, sodium hyaluronate was transformed to acidic and low concentration HA by strongly acidic ion exchange resin. After the addition of phase-transfer catalyst, it was transformed into HA solution. Subsequently, HA reacted with the amino groups of the N-terminus and side-chains of lysine by amidation reaction, leading to cross-linked HA.
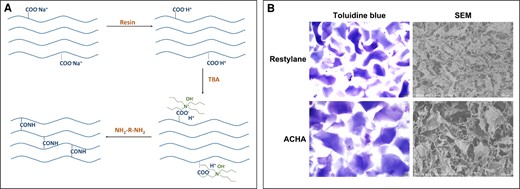
Preparation and characterization of amino acid–cross-linked hyaluronic acid (ACHA). (A) The cross-linking pattern of ACHA. (B) Photographs of ACHA and Restylane taken after staining with 1% toluidine blue under light microscope (scale bar = 200 μm) and scanning electron microscopy (SEM) images of ACHA and Restylane (scale bar = 500 μm). TBA, tert-butyl alcohol.
Characterization of ACHA Fillers
After being dissolved in distilled water, the HA had an irregular shape and varied in size; both giant and small particles were observed (Supplemental Figure 1, available online at www.aestheticsurgeryjournal.com). The bright field images showed that the Restylane particles were rectangular with relatively regular shapes; ACHA particles showed square shapes and had larger diameters than Restylane (Figure 1B). Scanning electron microscopy images showed the differences in microscopic structure of the 2 HAs (Figure 1B). The observations confirmed both of HA’s particle properties.
Rheological Properties and Physicochemical Properties of ACHA Fillers
We evaluated the rheological properties of ACHA and Restylane to investigate the changes in storage modulus (G′), loss modulus (G′′), complex modulus (G*), and loss tangent as the elastic part and viscous part of gels (Table 1). The results validated that ACHA fillers had superior elasticity and viscosity to Restylane. The gel cohesion forces showed that ACHA contained more HA per drop than Restylane; however, ACHA a had lower swelling ratio than Restylane (Table 1).
| . | HA (mg/ml) . | G′ (Pa) . | G′′ (Pa) . | G* (Pa) . | Tanδ . | Gel cohesion (mg/drop) . | Swelling rate (%) . |
|---|---|---|---|---|---|---|---|
| Restylane | 20 | 322 | 90 | 334 | 0.28 | 24 ± 1 | 110 ± 10 |
| ACHA | 18 | 647 | 138 | 662 | .21 | 27 ± 1 | 82 ± 9 |
| . | HA (mg/ml) . | G′ (Pa) . | G′′ (Pa) . | G* (Pa) . | Tanδ . | Gel cohesion (mg/drop) . | Swelling rate (%) . |
|---|---|---|---|---|---|---|---|
| Restylane | 20 | 322 | 90 | 334 | 0.28 | 24 ± 1 | 110 ± 10 |
| ACHA | 18 | 647 | 138 | 662 | .21 | 27 ± 1 | 82 ± 9 |
Storage modulus (G′), loss modulus (G′′), complex modulus (G*), and loss tangent (tan δ = G′′/G′) measured at a frequency of 0.1 to 10 Hz at 2% strain amplitude, 25°C. The gel cohesion was measured using a dripping method to obtain the average gel drop weight. The swelling rate was applied to measure the water absorbability of HA fillers. ACHA, amino acid–cross-linked hyaluronic acid; HA, hyaluronic acid.
| . | HA (mg/ml) . | G′ (Pa) . | G′′ (Pa) . | G* (Pa) . | Tanδ . | Gel cohesion (mg/drop) . | Swelling rate (%) . |
|---|---|---|---|---|---|---|---|
| Restylane | 20 | 322 | 90 | 334 | 0.28 | 24 ± 1 | 110 ± 10 |
| ACHA | 18 | 647 | 138 | 662 | .21 | 27 ± 1 | 82 ± 9 |
| . | HA (mg/ml) . | G′ (Pa) . | G′′ (Pa) . | G* (Pa) . | Tanδ . | Gel cohesion (mg/drop) . | Swelling rate (%) . |
|---|---|---|---|---|---|---|---|
| Restylane | 20 | 322 | 90 | 334 | 0.28 | 24 ± 1 | 110 ± 10 |
| ACHA | 18 | 647 | 138 | 662 | .21 | 27 ± 1 | 82 ± 9 |
Storage modulus (G′), loss modulus (G′′), complex modulus (G*), and loss tangent (tan δ = G′′/G′) measured at a frequency of 0.1 to 10 Hz at 2% strain amplitude, 25°C. The gel cohesion was measured using a dripping method to obtain the average gel drop weight. The swelling rate was applied to measure the water absorbability of HA fillers. ACHA, amino acid–cross-linked hyaluronic acid; HA, hyaluronic acid.
ACHA Promoted Cellular Proliferation, Migration, and Secretion of Growth Factors in Vitro
The HaCaT and HDF were grown for 72 hours in media containing ACHA and Restylane and counted. The numbers of HaCaT in the ACHA group and Restylane group at 0, 24, and 72 hours were (12.05 ± 0.91) × 105 cells/mL, (54.58 ± 4.98) × 105 cells/mL, (79.17 ± 4.73) × 105 cells/mL; and (11.60 ± 0.78) × 105 cells/mL, (36.62 ± 3.57) × 105 cells/mL, (54.87 ± 5.16) × 105 cells/mL, respectively. The numbers of HDF in the ACHA group and Restylane group at 0, 24, and 72 hours were (13.45 ± 0.91) × 105 cells/mL, (73.8 ± 2.98) × 105 cells/mL, (126.79 ± 3.73) × 105 cells/mL; and (12.06 ± 3.78) × 105 cells/mL, (49.62 ± 9.57) × 105 cells/mL, (97.87 ± 2.16) × 105 cells/mL, respectively (Figure 2A). The analysis suggested that ACHA could significantly increase the viability of HaCaT and HDF. The capacity of cell migration was determined by the scratch wound healing and Transwell assays. The wound healing rates of HaCaT for the ACHA group and Restylane group were 63.78 ± 5.86% and 41.82 ± 2.17%, respectively; the wound healing rates of HDF for the ACHA group and Restylane group were 51.79 ± 5.09% and 35.26 ± 2.51%, respectively (Figure 2B, C). The numbers of migrated HaCaT for the ACHA group and Restylane group were 701.00 ± 37.21% and 559.00 ± 26.18%, respectively; the numbers of migrated HDF for the ACHA group and Restylane group were 628.00 ± 35.20% and 487.00 ± 26.42%, respectively (Figure 2D, E). Therefore, the migratory ability of HaCaT and HDF cells were enhanced by HA treatment, and more cells in the ACHA group migrated cells than in the Restylane and control groups. ELISA was performed, and the cell mediums of HaCaT and HDF were used to quantify EFG and VEGF. The EGF and VEGF expressions of HaCaT for the ACHA group and Restylane group were 324.80 ± 12.67 and 223.51 ± 18.26; and 570.23 ± 12.05 and 406.50 ± 16.42, respectively; The EGF and VEGF expressions of HDF for the ACHA group and Restylane group were 403.81 ± 17.67 and 273.53 ± 16.26; and 583.23 ± 15.5 and 436.49 ± 18.42, respectively (Figure 2F). The results indicated that ACHA could significantly increase the secretion of EGF and VEGF in HaCaT and HDF cells.
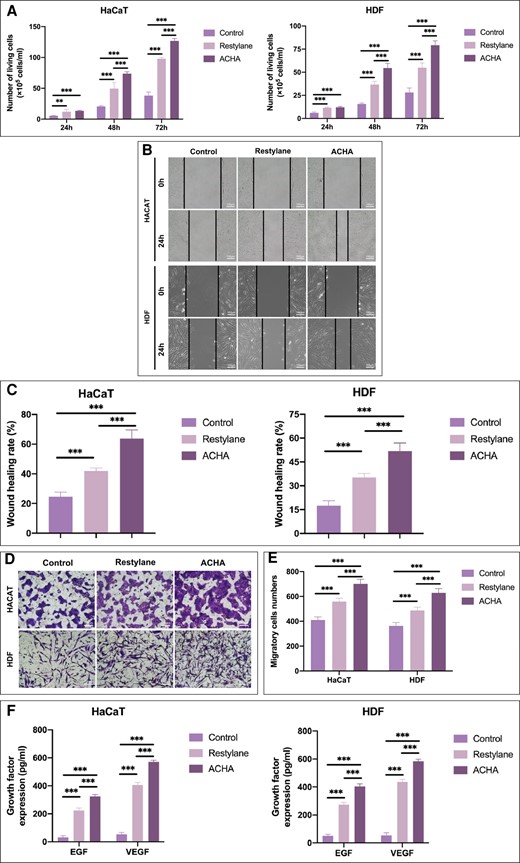
Comparison of cell viability and proliferation and secretion of growth factors after amino acid–cross-linked hyaluronic acid (ACHA) and Restylane treatment. (A) Human keratinocyte (HaCaT) and human dermal fibroblast (HDF) viability analysis in response to Restylane and ACHA as determined by Trypan blue staining. (B, C) Scratch wound assay analyzed the effects of ACHA and Restylane on the migration of HaCaT and HDF (scale bar = 100 μm). (D, E) Transwell experiments evaluated the effects of ACHA and Restylane on cell migratory ability (scale bar = 100 μm). (F) Enzyme-linked immunoassay (ELISA) of secreted EGF and VEGF in HaCaT and HDF cells. **P < .01, ***P < .001. h, hours. pg/ml, picograms/milliliter.
ACHA Increased Epithelial Thickness and Collagen Synthesis of Mice in Vivo
ACHA and Restylane were injected subcutaneously in nude mice and evaluated at 4 weeks. HE staining showed that the epidermal thickness for the ACHA group and Restylane group was 18.74 ± 2.53 μm and 14.65 ± 1.96 μm, respectively; the skin thickness for the ACHA group and Restylane group was 909.27 ± 49.38 μm and 659.10 ± 87.17 μm, respectively (Figure 3A, B). When compared with the untreated control group, treatment with ACHA and Restylane significantly increased the epidermal thickness and skin thickness. In addition, after treatment, little obvious inflammatory cell infiltration was observed in HE staining of the ACHA and Restylane groups (Figure 3A). Masson staining showed that the ACHA-treated group exhibited increased collagen fibers and more neatly arranged collagen fibers than the Restylane and control groups (Figure 3A). Immunohistochemistry staining showed positive expression of type 1 and type 3 collagen in the epithelial tissue (Figure 3C). The positive expression rates of type 1 collagen for the ACHA group and Restylane group were 53.18 ± 4.29% and 46.80 ± 3.68%, respectively; the positive expression rates of type 3 collagen for the ACHA group and Restylane group were 63.37 ± 5.23% and 47.73 ± 6.24%, respectively (Figure 3D). Similarly, the increased expression of collagen 1 and collagen 3 in ACHA group was confirmed by Western blot analysis (Figure 3E, F). These results suggest that ACHA could significantly promote the secretion and deposition of collagen and achieve increased skin thickness.
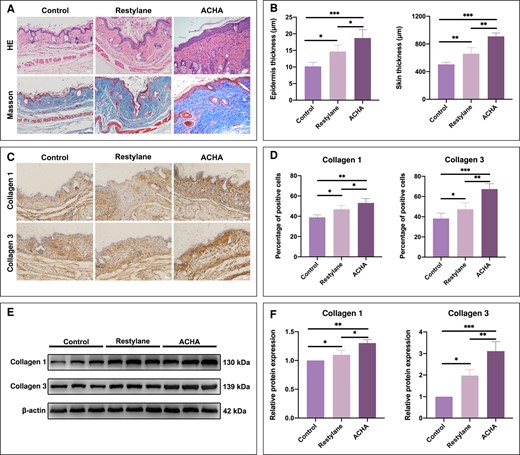
Amino acid–cross-linked hyaluronic acid (ACHA) could enhance epithelial hyperplasia and collagen synthesis. (A, B) HE staining was performed to evaluate the epidermis thickness and skin thickness and Masson staining was performed to evaluate collagen deposition 4 weeks after injecting hyaluronic acid into the dorsal skin of nude mice (scale bar = 200 μm). (C, D) Immunohistochemistry detecting the staining of collagen 1 and collagen 3 proteins in the dorsal skin of nude mice (scale bar = 200 μm). (E, F) Western blot analysis was performed to detect collagen 1 and collagen 3 protein expression in the dorsal skin of nude mice. *P < .05, **P < .01, ***P < .001. HE, hematoxylin and eosin; kDa, kilodalton.
Treatment Efficacy and Safety of ACHA
Among the 50 patients, 3 were male (6%) and 47 were female (94%). The mean baseline WSRS of patients in the ACHA group and Restylane group were 3.32 ± 0.47 and 3.29 ± 0.46, respectively; the difference between groups was not significant (P = .83). All patients completed the study, without dropouts. The HA volume of ACHA was 2.77 ± 0.33 mL, whereas the HA volume of Restylane was 2.65 ± 0.48 mL. There were no statistical differences in the volume of HA between the 2 groups (P = .10). Representative cases are shown in Figure 4A.
Twelve months after injection of HA into the NLF of the patients, the volume of each type of HA filler had decreased significantly (Figure 4B). From 3-dimensional graphs, it was evident that the residual HA of the ACHA group did not undergo distinct gel shift and diffusion. In addition, the augmented volume rates for the ACHA group and Restylane group at 1, 3, 6, and 12 months after injection were 90.87 ± 4.23%, 80.37 ± 5.22%, 45.04 ± 5.91%, 18.87 ± 5.69%; and 91.85 ± 3.49%, 78.98 ± 4.88%, 38.97 ± 4.96%, 11.81 ± 3.80%, respectively (Figure 4C). The rate of augmented volume in the ACHA group was higher than that in the Restylane group at month 6 and month 12 (P < .05). The mean WSRS scores of the ACHA group and Restylane group at baseline and 1, 3, 6, and 12 months after injection were 3.32 ± 0.47, 1.29 ± 0.46, 1.63 ± 0.67, 1.86 ± .72, 2.4 ± 0.55; and 3.29 ± 0.46, 1.19 ± 0.48, 1.83 ± 0.78, 2.26 ± 0.73, 2.66 ± 0.49, respectively (Figure 4D). The WSRS of the ACHA group was lower than that of the Restylane group at months 6 and 12 (P = .02 at month 6; P = .04 at month 12). The mean GAIS scores of the ACHA group and Restylane group at 1, 3, 6, and 12 months after injection were 1.59 ± 0.74, 1.83 ± 0.76, 2.37 ± 0.67, 2.94 ± 0.85; and 1.86 ± 0.82, 1.93 ± 0.87, 2.87 ± 0.69, 3.4 ± 0.73, respectively (Figure 4E). Regarding adverse events, injection site swelling, induration, and tenderness were the most common adverse events reported postoperatively. However, there was no significant difference in overall adverse events between the 2 groups (Figure 4F). Therefore, ACHA improved the therapeutic efficacy and did not cause more side effects.
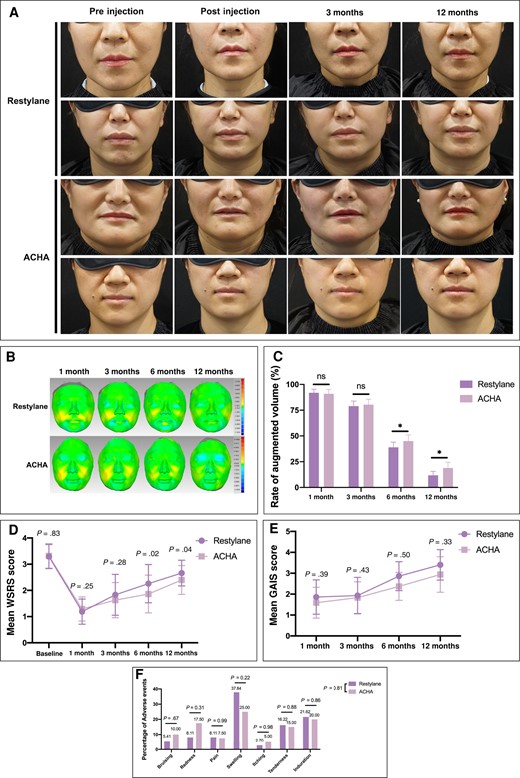
The clinical evaluation of amino acid–cross-linked hyaluronic acid (ACHA) compared to Restylane. (A) Representative photographs of patients’ nasolabial folds obtained before injection, immediately after injection, 3 months postinjection, and 12 months postinjection. (B) Three-dimensional visualizations illustrated the hyaluronic acid filler retention in the ACHA and Restylane groups via Geomagic software. (C) Quantitative statistics of the mean volume changes in the augmented volume at follow-up visits compared to immediately after surgery. (D) Comparison of mean Wrinkle Severity Rating Scale (WSRS) scores between groups. (E) Comparison of Global Aesthetic Improvement Scale (GAIS) scores between groups. (F) Comparison of adverse events rates between groups. *P < .05. ns, not significant.
DISCUSSION
In this study, we prepared an HA that was cross-linked with lysine, and then investigated the efficacy and safety of ACHA at the cellular, animal, and clinical experimental levels. The results indicate that ACHA had the higher viscoelastic properties at relatively low concentrations of HA. It also promoted the proliferation of epidermal cells and enhanced their collagen-secreting ability. When applied clinically, ACHA prolonged the retention rate of HA, resulting in a better therapeutic effect for skin wrinkles.
BDDE, a low-toxicity cross-linker, has been widely used as a chemical cross-linker in cross-linking HA. However, several reports have shown that BDDE had a cytotoxic content, and that residual BDDE could cause inflammation.8,9 Therefore, we provided a reasonable approach to improving cross-linking with HA. Because 2 amino groups of lysine could react with HA through an amide cross-linking reaction, amidation achieved nontoxic cross-linked HA. The ACHA was covalently cross-linked by lysine, induing the coupling of the carboxyl and amino groups to form stable amide bonds, which in turn increased the stability and mechanical properties.
Lysine is well known to be one of the most important essential amino acids in human body, and can promote human development, enhance immune function, and improve the function of central nervous tissue.14,15 Several studies have demonstrated that lysine can help control and treat viral pathologies.16 And lysine can prevent bone loss and promote calcium absorption.17 Combined with other amino acids, it can increase collagen production and prevent osteoporosis.18
Main features of skin aging are thinning of the dermal layer, with decreased collagen leading to wrinkles.19 HDF and HaCaT are the major components of reepithelization and the epithelial barrier.20,21 A product containing collagen was shown to effectively alleviate skin aging.22 Previous study has reported that cross-linking hyaluronic acid and poly-lysine hydrogel matrix could improve the proliferation of fibroblasts and enhance the secretion of various growth factors.23 As we found in our experiments, the ACHA facilitated cell proliferation and migration of HaCaT and HDF. Not only that, but more collagen production and increased skin thickness were seen in the ACHA-treated groups than the Restylane group in animal experiments. In clinical study, the mean WSRS in the ACHA group was lower than that in the Restylane group at 6 and 12 months. No serious adverse events were observed, and ACHA treatment did not increase the rate of adverse events when compared with the Restylane group. This indicates that ACHA substantially promoted skin thickness, collagen synthesis, and delayed wrinkle formation in aging skin.
Comparing the rheological and physicochemical properties demonstrated that ACHA exhibited higher G″ than Restylane. The higher the elastic modulus (G′) is, the stiffer the resulting HA becomes. It suggests that ACHA is suitable for deep injection into the periosteal plane to provide support. Higher G″″ implies that ACHA has a viscous appearance and poor fluidity. As shown in 3-dimensional (3D) visualization images, the displacement and diffusion of HA are not significantly apparent in the ACHA group. Therefore, we recommend ACHA for the nose, chin, brow bone, suborbital hollows, nasal base, and other areas.
To evaluate the actual volumetric changes of HA in a quantitative manner, 3D scanning analysis was applied with the Geomagic software (3D Systems, Rock Hill, SC) as has been previously described.13 The results revealed that patients who received treatment with ACHA had a higher retention rate of augmented volume. We speculated that the augmented volume in patients’ faces may have been influenced by HA retention and increased skin thickness. ACHA exhibits higher viscoelasticity and anti-hyaluronidase ability, resulting in better performance results for duration and efficacy. The carboxylic group of ACHA is covalently linked, shielding the recognition site from hyaluronidase. Moreover, ACHA can stimulate synthesis of collagen in human skin, contributing to the maintenance of the augmented volume. In summary, due to the above points ACHA has good capacity for cross-linking, leading to its brilliant skin augmentation effect.
The duration of ACHA was slightly longer than that of Restylane; this means that, to some extent, the dissolution time of hyaluronidase was significantly longer with a low dissolution rate, which would make the treatment of complications more difficult. More attention should have been given to the complications to avoid delaying treatment.
In addition, ACHA had a lower swelling rate than Restylane. The volume of HA increases dramatically during swelling, which is essentially caused by water absorption. In this case, the stronger water absorption capacity of HA would lead to acute postoperative swelling, and increased discomfort during the postoperative recovery phase. The results of the clinical trial revealed that although a lower adverse events rate of swelling was observed in ACHA group, no differences were found between the groups.
In this study, a novel lysine cross-linked injectable HA was designed for skin augmentation. Compared to conventional cross-linking techniques, the ACHA may have several advantages. First, unlike other cross-linkers, lysine is a natural protein and is non-toxic. This novel modification provided new ideas for further modification and cross-linking. Second, the amide bond was formed between the carboxyl groups of HA and the amino groups of lysine, thus the hyaluronidase recognition site has been concealed. This indicated ACHA could withstand constant heat and resist degradation at relatively low concentrations of HA. As an injectable filler, ACHA might prolong its resident time in vivo. Third, ACHA possessed higher viscoelasticity and lower fluidity. The progress of ACHA expanded the application for cosmetic skin augmentation.
Despite the strength of our findings, there of course remain certain limitations. First, we evaluated the inflammatory cell infiltration in HE-stained sections, but the proinflammatory and anti-inflammatory markers were not assessed in in vitro or in vivo experiments. Second, in the mice experiments, increased skin thickness is likely due to increased collagen production. We did not perform assessments specific to HA retention. It is possible that neocollagenesis increases the risk of hypertrophic scars or keloids in the area of injection. Although there are no related adverse events reported in our clinical trial, the ACHA is not recommended for people at risk of hypertrophic scars or keloids. Third, a clinical study was conducted to validate ACHA's clinical effectiveness and safe use for skin augmentation. There were no patient satisfaction data in this study, so changes in patient satisfaction related to ACHA treatment cannot be discerned. For ethical reasons, we did not explore a split face, non-inferiority design. To eliminate the baseline differences between the ACHA group and the Restylane group as much as possible, we examined the variance of the baseline WSRS and the volume of injected HA, and no significant variations were observed between the 2 groups. It is noted that these results may be specific to the NLF, and further studies involving other human face wrinkles will need to be undertaken. Also, because this was a single-center feasibility study, a larger multicenter study is needed to confirm our results.
CONCLUSION
ACHA possessed outstanding viscoelasticity and antienzymatic characteristics. It can promote the proliferation of keratinocytes and fibroblasts and enhance the growth factors, and therefore promote collagen synthesis. Additionally, ACHA exhibited a promising augmentation effect in NLF correction with few adverse reactions.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Acknowledgments
Drs Guo, W. Wei, Wang, and Li made an equal contribution to this work as co-first authors.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
This research was supported by the Scientific Research Project of the Affiliated Hospital of Xuzhou Medical University (Xuzhou, Jiangsu, China), grant no. 2021ZA07.
REFERENCES
Author notes
Dr Guo is an attending plastic surgeon, Department of Plastic Surgery, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China.
Dr W. Wei is a residents, Department of Plastic Surgery, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China.
Dr Wang is a residents, Department of Plastic Surgery, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China.
Dr Zhang is a residents, Department of Plastic Surgery, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China.
Dr Li is a professor, Department of Plastic Surgery, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China.
Dr Jin is the chief plastic surgeon, Department of Plastic Surgery, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China.
Dr C. Wei is a vice general manager, Shanghai Qisheng Biological Preparation Co., Ltd., Shanghai, China.
- edema
- amino acids
- epithelium
- biomaterials
- bodily secretions
- butylene glycols
- collagen
- esthetics
- ether, ethyl
- ethers
- growth factor
- hyaluronic acid
- hydrogel
- keratinocytes
- lysine
- rejuvenation
- residual volume
- rheology
- safety
- wound healing
- mice
- skin
- cytotoxicity
- adverse effects
- restylane
- levels of evidence
- dermal fillers
- collagen synthesis
- nasolabial folds
- skin fibroblasts
- viscoelasticity
- hindi
- portuguese
- taiwanese
- biocompatibility



