-
PDF
- Split View
-
Views
-
Cite
Cite
Ayaka Nishikawa, Yoshiyuki Aikawa, Taro Kono, Current Status of Early Complications Caused by Hyaluronic Acid Fillers: Insights From a Descriptive, Observational Study of 41,775 Cases, Aesthetic Surgery Journal, Volume 43, Issue 8, August 2023, Pages 893–904, https://doi.org/10.1093/asj/sjad039
Close - Share Icon Share
Abstract
The number of hyaluronic acid (HA) filler treatments has increased in recent years. Although extremely rare, serious complications associated with these treatments, such as skin necrosis, blindness, and stroke caused by vascular compromise, have been reported.
To evaluate the specific details related to early complications caused by HA filler injection in our group, understand the current status, and gain further insights from the findings.
A nationwide, observational, descriptive, multicenter, retrospective study was conducted. Of the 41,775 cases (58,533 sites of injection), 29 cases of early complications (onset of less than 14 days after injection) were included in the study.
The injection site with the highest rate of early complications was the upper eyelids (0.41%; n = 1/241 sites). The most commonly injected site was the nasolabial fold (n = 13/29 cases), and the most common early complication was vascular compromise (n = 18/29 cases). The average experience of the injectors was 28.7 ± 31.9 months.
All 13 patients injected in the nasolabial fold experienced vascular compromise, potentially related to the anatomical feature of a facial artery running parallel to the nasolabial fold, which is commonly found in Asian populations. Regardless of the injection site, accurate anatomical knowledge and knowledge and experience regarding HA fillers, including appropriate patient selection and injection techniques, are strictly required for injectors to anticipate early complications. Therefore, it is important to establish original guidelines based on experience and ensure their thorough implementation in our facilities.

See the Commentary on this article here.
Injectable hyaluronic acid (HA) fillers are one of the most widely employed treatments for facial rejuvenation in cosmetic dermatology.1 According to an annual nationwide survey conducted by the Japan Society of Aesthetic Plastic Surgery (JSAPS), treatment with injectable HA fillers was very frequently performed in Japan in 2017 (n = 149,324, excluding cases of breast augmentation), 2018 (n = 124,845), 2019 (n = 126,921), 2020 (n = 66,712), and 2021 (n = 106,613; Figure 1).2-5 The observed decrease in the number of cases between 2019 and 2020 can be attributed to COVID-19 pandemic–related factors.
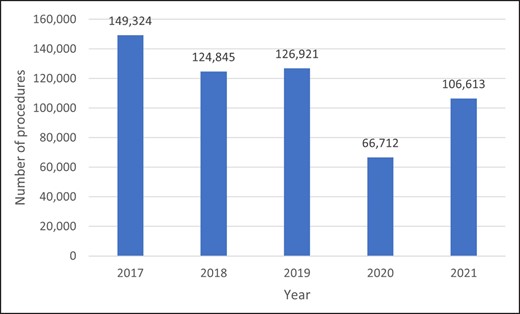
Number of annual injectable hyaluronic acid filler treatments nationwide from 2017 through 2021. JSAPS (Japan Society of Aesthetic Plastic Surgery) annual nationwide surveys from 2019 to 2022.2-5
The majority of complications associated with HA filler treatment are minor, such as redness, swelling, tenderness, firmness, lumps/bumps, discoloration, and bruising at the injection site.6 However, serious complications, such as skin necrosis, transient or irreversible blindness, and stroke (cerebral ischemia, cerebral hemorrhage, or cerebral infarction) have been reported in rare cases due to erroneous injection into the blood vessels or tissue compression.7
HA injection into a blood vessel causes vascular embolization and impairs blood flow. For instance, skin necrosis can occur in cases where the skin is the site of vascular compromise.7 However, in cases where the eye is the site of vascular compromise, there may be serious complications, including decreased vision and blindness.8
In 2021, the Shonan Beauty Clinic Medical Group, which has over 120 clinics nationwide, performed HA filler treatments in 41,775 cases (58,533 sites; some patients were injected at multiple sites). To understand the current status of HA filler injection treatment, we investigated these 41,775 cases and retrospectively selected patients who experienced serious complications. The aim of this study was to evaluate the specific details related to early complications caused by HA filler injection treatment and gain further insights from the findings.
Among these complications, vascular compromise was the primary focus of this study, because it can cause serious side effects that can compromise patients' safety and well-being.9
METHODS
Study Design
This was an observational, descriptive, multicenter, retrospective study. We retrospectively evaluated the medical records of 41,775 patients treated with HA filler injections for facial rejuvenation between January and December 2021. The study was approved by the Medical Corporation Shoubikai Ethics Committee (approval no. 4-004) and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from the patients with a written consent form (comprising consent explanation, consent document, and consent withdrawal document. The documentation did not authorize use of patient photos so case photographs were not included.)
The following 5 individuals were involved in the study during the cohort period (from January 2021 to October 2022) and the period required for the actual review of the large amount of data in this study: principal author AN, 2 staff members in the department to whom adverse events or complications must be reported, and 2 staff members in the department responsible for data management of this study.
Injection Products
The injectable products utilized involved the Juvéderm Vista Series (Ultra Plus XC, Volbella XC, Volift XC, Voluma XC, and Volux XC; manufactured by Allergan Aesthetics, Abbvie, Irvine, CA), which has been approved by the Ministry of Health, Labour and Welfare in Japan.
The distribution of the injectable products by injection site is presented in Table 1. Juvéderm Vista Voluma XC was the most common product (n = 20,177/58,533 sites; 34.47%); followed by Juvéderm Vista Ultra Plus XC (n = 17,172; 29.34%). In addition, the nasolabial fold was the most common injection site, (n = 23,047/58,533; 39.37%); followed by the cheek (n = 10,417; 17.81%). In our group, the glabella and tip of the nose are contraindicated and are not injected.
Filler Brand Distribution (Number of Injection Sites) of the 41,775 Cases (58,533 Sites) Treated With Hyaluronic Acid Filler Injection
| . | Filler . | |||||
|---|---|---|---|---|---|---|
| Injection site . | Juvéderm Vista Ultra Plus XC . | Juvéderm Vista Volbella XC . | Juvéderm Vista Volift XC . | Juvéderm Vista Voluma XC . | Juvéderm Vista Volux XC . | TOTAL (sites) . |
| Nasolabial fold | 9,422 (54.9%) | 283 (4.8%) | 7,404 (57.5%) | 5,900 (29.2%) | 38 (1.6%) | 23,047 (39.4%) |
| Cheek | 2,543 (14.8%) | 64 (1.1%) | 1,292 (10.0%) | 6,235 (30.9%) | 283 (12.0%) | 10,417 (17.8%) |
| Chin | 2,217 (12.9%) | 152 (2.6%) | 2,089 (35.1%) | 2,524 (12.5%) | 1,368 (57.8%) | 8,350 (14.3%) |
| Midcheek groove | 1,517 (8.8%) | 150 (2.5%) | 849 (6.6%) | 2,128 (10.5%) | 77 (3.3%) | 4,721 (8.1%) |
| Temple | 733 (4.3%) | 53 (0.89%) | 230 (1.8%) | 1,887 (9.3%) | 59 (2.5%) | 2,962 (5.1%) |
| Periorbital puffiness | 25 (0.15%) | 2,201 (37.0%) | 24 (0.19%) | 4 (0.02%) | 2,254 (3.9%) | |
| Forehead | 356 (0.15%) | 125 (2.1%) | 544 (4.2%) | 683 (3.4%) | 11 (0.46%) | 1,719 (2.9%) |
| Lips | 35 (0.20%) | 1,551 (26.1%) | 30 (0.23%) | 14 (0.07%) | 13 (0.55%) | 1,643 (2.8%) |
| Tear trough | 82 (0.48%) | 1,107 (18.6%) | 208 (1.6%) | 176 (0.87%) | 1 (0.04%) | 1,574 (2.7%) |
| Jowls | 168 (0.98%) | 37 (0.29%) | 540 (2.7%) | 425 (18.0%) | 1,170 (2.0%) | |
| Marionette line | 53 (0.31%) | 1 (0.02%) | 152 (1.2%) | 52 (0.26%) | 6 (0.25%) | 264 (0.45%) |
| Upper eyelids | 1 (0.01%) | 235 (4.0%) | 3 (0.02%) | 2 (0.01%) | 241 (0.41%) | |
| Nose | 8 (0.05%) | 3 (0.05%) | 1 (0.01%) | 16 (0.08%) | 85 (3.6%) | 113 (0.19%) |
| Lateral Orbital | 2 (0.01%) | 15 (0.25%) | 1 (0.01%) | 5 (0.02%) | 23 (0.04%) | |
| Eyebrow tail | 7 (0.04%) | 1 (0.02%) | 8 (0.04%) | 16 (0.03%) | ||
| Hand | 2 (0.01%) | 3 (0.05%) | 6 (0.05%) | 2 (0.01%) | 13 (0.02%) | |
| Neck | 1 (0.01%) | 4 (0.07%) | 1 (0.005%) | 6 (0.01%) | ||
| TOTAL | 17,172 | 5,948 | 12,870 | 20,177 | 2,366 | 58,533 |
| . | Filler . | |||||
|---|---|---|---|---|---|---|
| Injection site . | Juvéderm Vista Ultra Plus XC . | Juvéderm Vista Volbella XC . | Juvéderm Vista Volift XC . | Juvéderm Vista Voluma XC . | Juvéderm Vista Volux XC . | TOTAL (sites) . |
| Nasolabial fold | 9,422 (54.9%) | 283 (4.8%) | 7,404 (57.5%) | 5,900 (29.2%) | 38 (1.6%) | 23,047 (39.4%) |
| Cheek | 2,543 (14.8%) | 64 (1.1%) | 1,292 (10.0%) | 6,235 (30.9%) | 283 (12.0%) | 10,417 (17.8%) |
| Chin | 2,217 (12.9%) | 152 (2.6%) | 2,089 (35.1%) | 2,524 (12.5%) | 1,368 (57.8%) | 8,350 (14.3%) |
| Midcheek groove | 1,517 (8.8%) | 150 (2.5%) | 849 (6.6%) | 2,128 (10.5%) | 77 (3.3%) | 4,721 (8.1%) |
| Temple | 733 (4.3%) | 53 (0.89%) | 230 (1.8%) | 1,887 (9.3%) | 59 (2.5%) | 2,962 (5.1%) |
| Periorbital puffiness | 25 (0.15%) | 2,201 (37.0%) | 24 (0.19%) | 4 (0.02%) | 2,254 (3.9%) | |
| Forehead | 356 (0.15%) | 125 (2.1%) | 544 (4.2%) | 683 (3.4%) | 11 (0.46%) | 1,719 (2.9%) |
| Lips | 35 (0.20%) | 1,551 (26.1%) | 30 (0.23%) | 14 (0.07%) | 13 (0.55%) | 1,643 (2.8%) |
| Tear trough | 82 (0.48%) | 1,107 (18.6%) | 208 (1.6%) | 176 (0.87%) | 1 (0.04%) | 1,574 (2.7%) |
| Jowls | 168 (0.98%) | 37 (0.29%) | 540 (2.7%) | 425 (18.0%) | 1,170 (2.0%) | |
| Marionette line | 53 (0.31%) | 1 (0.02%) | 152 (1.2%) | 52 (0.26%) | 6 (0.25%) | 264 (0.45%) |
| Upper eyelids | 1 (0.01%) | 235 (4.0%) | 3 (0.02%) | 2 (0.01%) | 241 (0.41%) | |
| Nose | 8 (0.05%) | 3 (0.05%) | 1 (0.01%) | 16 (0.08%) | 85 (3.6%) | 113 (0.19%) |
| Lateral Orbital | 2 (0.01%) | 15 (0.25%) | 1 (0.01%) | 5 (0.02%) | 23 (0.04%) | |
| Eyebrow tail | 7 (0.04%) | 1 (0.02%) | 8 (0.04%) | 16 (0.03%) | ||
| Hand | 2 (0.01%) | 3 (0.05%) | 6 (0.05%) | 2 (0.01%) | 13 (0.02%) | |
| Neck | 1 (0.01%) | 4 (0.07%) | 1 (0.005%) | 6 (0.01%) | ||
| TOTAL | 17,172 | 5,948 | 12,870 | 20,177 | 2,366 | 58,533 |
Filler Brand Distribution (Number of Injection Sites) of the 41,775 Cases (58,533 Sites) Treated With Hyaluronic Acid Filler Injection
| . | Filler . | |||||
|---|---|---|---|---|---|---|
| Injection site . | Juvéderm Vista Ultra Plus XC . | Juvéderm Vista Volbella XC . | Juvéderm Vista Volift XC . | Juvéderm Vista Voluma XC . | Juvéderm Vista Volux XC . | TOTAL (sites) . |
| Nasolabial fold | 9,422 (54.9%) | 283 (4.8%) | 7,404 (57.5%) | 5,900 (29.2%) | 38 (1.6%) | 23,047 (39.4%) |
| Cheek | 2,543 (14.8%) | 64 (1.1%) | 1,292 (10.0%) | 6,235 (30.9%) | 283 (12.0%) | 10,417 (17.8%) |
| Chin | 2,217 (12.9%) | 152 (2.6%) | 2,089 (35.1%) | 2,524 (12.5%) | 1,368 (57.8%) | 8,350 (14.3%) |
| Midcheek groove | 1,517 (8.8%) | 150 (2.5%) | 849 (6.6%) | 2,128 (10.5%) | 77 (3.3%) | 4,721 (8.1%) |
| Temple | 733 (4.3%) | 53 (0.89%) | 230 (1.8%) | 1,887 (9.3%) | 59 (2.5%) | 2,962 (5.1%) |
| Periorbital puffiness | 25 (0.15%) | 2,201 (37.0%) | 24 (0.19%) | 4 (0.02%) | 2,254 (3.9%) | |
| Forehead | 356 (0.15%) | 125 (2.1%) | 544 (4.2%) | 683 (3.4%) | 11 (0.46%) | 1,719 (2.9%) |
| Lips | 35 (0.20%) | 1,551 (26.1%) | 30 (0.23%) | 14 (0.07%) | 13 (0.55%) | 1,643 (2.8%) |
| Tear trough | 82 (0.48%) | 1,107 (18.6%) | 208 (1.6%) | 176 (0.87%) | 1 (0.04%) | 1,574 (2.7%) |
| Jowls | 168 (0.98%) | 37 (0.29%) | 540 (2.7%) | 425 (18.0%) | 1,170 (2.0%) | |
| Marionette line | 53 (0.31%) | 1 (0.02%) | 152 (1.2%) | 52 (0.26%) | 6 (0.25%) | 264 (0.45%) |
| Upper eyelids | 1 (0.01%) | 235 (4.0%) | 3 (0.02%) | 2 (0.01%) | 241 (0.41%) | |
| Nose | 8 (0.05%) | 3 (0.05%) | 1 (0.01%) | 16 (0.08%) | 85 (3.6%) | 113 (0.19%) |
| Lateral Orbital | 2 (0.01%) | 15 (0.25%) | 1 (0.01%) | 5 (0.02%) | 23 (0.04%) | |
| Eyebrow tail | 7 (0.04%) | 1 (0.02%) | 8 (0.04%) | 16 (0.03%) | ||
| Hand | 2 (0.01%) | 3 (0.05%) | 6 (0.05%) | 2 (0.01%) | 13 (0.02%) | |
| Neck | 1 (0.01%) | 4 (0.07%) | 1 (0.005%) | 6 (0.01%) | ||
| TOTAL | 17,172 | 5,948 | 12,870 | 20,177 | 2,366 | 58,533 |
| . | Filler . | |||||
|---|---|---|---|---|---|---|
| Injection site . | Juvéderm Vista Ultra Plus XC . | Juvéderm Vista Volbella XC . | Juvéderm Vista Volift XC . | Juvéderm Vista Voluma XC . | Juvéderm Vista Volux XC . | TOTAL (sites) . |
| Nasolabial fold | 9,422 (54.9%) | 283 (4.8%) | 7,404 (57.5%) | 5,900 (29.2%) | 38 (1.6%) | 23,047 (39.4%) |
| Cheek | 2,543 (14.8%) | 64 (1.1%) | 1,292 (10.0%) | 6,235 (30.9%) | 283 (12.0%) | 10,417 (17.8%) |
| Chin | 2,217 (12.9%) | 152 (2.6%) | 2,089 (35.1%) | 2,524 (12.5%) | 1,368 (57.8%) | 8,350 (14.3%) |
| Midcheek groove | 1,517 (8.8%) | 150 (2.5%) | 849 (6.6%) | 2,128 (10.5%) | 77 (3.3%) | 4,721 (8.1%) |
| Temple | 733 (4.3%) | 53 (0.89%) | 230 (1.8%) | 1,887 (9.3%) | 59 (2.5%) | 2,962 (5.1%) |
| Periorbital puffiness | 25 (0.15%) | 2,201 (37.0%) | 24 (0.19%) | 4 (0.02%) | 2,254 (3.9%) | |
| Forehead | 356 (0.15%) | 125 (2.1%) | 544 (4.2%) | 683 (3.4%) | 11 (0.46%) | 1,719 (2.9%) |
| Lips | 35 (0.20%) | 1,551 (26.1%) | 30 (0.23%) | 14 (0.07%) | 13 (0.55%) | 1,643 (2.8%) |
| Tear trough | 82 (0.48%) | 1,107 (18.6%) | 208 (1.6%) | 176 (0.87%) | 1 (0.04%) | 1,574 (2.7%) |
| Jowls | 168 (0.98%) | 37 (0.29%) | 540 (2.7%) | 425 (18.0%) | 1,170 (2.0%) | |
| Marionette line | 53 (0.31%) | 1 (0.02%) | 152 (1.2%) | 52 (0.26%) | 6 (0.25%) | 264 (0.45%) |
| Upper eyelids | 1 (0.01%) | 235 (4.0%) | 3 (0.02%) | 2 (0.01%) | 241 (0.41%) | |
| Nose | 8 (0.05%) | 3 (0.05%) | 1 (0.01%) | 16 (0.08%) | 85 (3.6%) | 113 (0.19%) |
| Lateral Orbital | 2 (0.01%) | 15 (0.25%) | 1 (0.01%) | 5 (0.02%) | 23 (0.04%) | |
| Eyebrow tail | 7 (0.04%) | 1 (0.02%) | 8 (0.04%) | 16 (0.03%) | ||
| Hand | 2 (0.01%) | 3 (0.05%) | 6 (0.05%) | 2 (0.01%) | 13 (0.02%) | |
| Neck | 1 (0.01%) | 4 (0.07%) | 1 (0.005%) | 6 (0.01%) | ||
| TOTAL | 17,172 | 5,948 | 12,870 | 20,177 | 2,366 | 58,533 |
Inclusion and Exclusion Criteria
Figure 2 shows the inclusion and exclusion criteria. Of the 41,775 cases, we included 37 cases listed as “serious complications caused by HA filler treatment,” which qualified as adverse events or complications and required a report. Minor complications (redness, swelling, tenderness, firmness, lumps/bumps, discoloration, and bruising) were excluded. Based on the complication categorization by Rohrich et al (2019), of these 37 cases, 8 cases that qualified as late complications (onset between 14 days to one year after injection) or delayed complications (onset more than 1 year after injection) were excluded.9,10 The remaining 29 cases with early complications (onset less than 14 days after injection) were included.10 The results of our study on late and delayed complications will be published in the future.
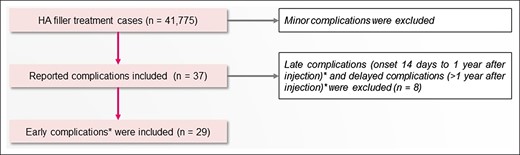
Inclusion and exclusion criteria. *Rohrich et al (2019).10 HA, hyaluronic acid.
Statistical Analysis
Using the comparing two independent population proportions with Microsoft® Excel® MSO (Version 2209 Build 16.0.15629.20196) 32-bit to assess statistical significances with respect to the rate of early complications by age group, injected site, and injectors’ experience. A P-value <.05 was considered statistically significant.
RESULTS
Patient Demographics
Of the total study population, 2,908 were males (6.96%) and 38,867 were females (93.04%). The age of patients ranged from 15 to 90 years, the majority of whom were age 40 to 49 years (n = 12,931; 30.95%), followed by patients age 50 to 59 years (n = 9,806; 23.47%). The age distribution of the patients is shown in Figure 3.
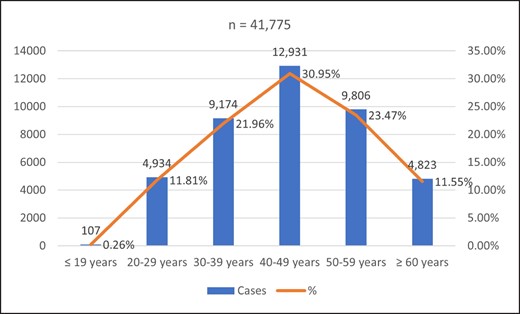
Age distribution of patients treated with HA filler injections in 2021. Range, 15-90. The chart indicates the age of patients on the day of treatment.
The ethnic distribution of patients is shown in Figure 4. The overwhelming majority were Japanese (n = 40,098; 96.00%), followed by Chinese (n = 959; 2.30%), Korean (n = 262; 0.63%), Taiwanese (n = 73; 0.17%), and Filipinos (n = 65; 0.16%). Overall, 99.24% of the study population (n = 41,457) was of Asian ethnicity.
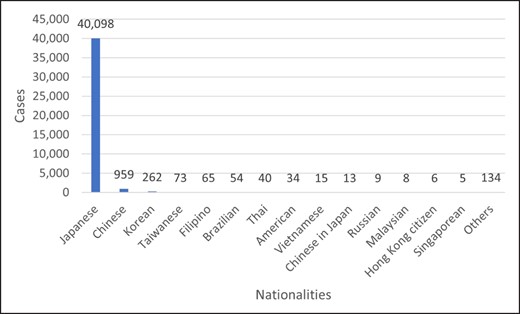
Nationalities of patients who received HA filler injections in 2021.
The 29 cases with early complications associated with HA filler injections are summarized in Table 2. Patients included 2 males (6.90%) and 27 females (93.10%), all Japanese. The average age of patients was 42.24 ± 12.34 years (ranging from 23 to 74 years). The age groups included in the study were 40 to 49 years (n = 8; 27.59%), 30 to 39 years (n = 8; 27.59%), 50 to 59 years (n = 6; 20.69%), 20 to 29 years (n = 5; 17.24%), and 60 years or older (n = 2; 6.90%).
Summary of the 29 Cases of Early Complications Associated With Hyaluronic Acid Filler Injections (Patients Injected in 2021)
| Case no. . | Agea /sex . | Date of injection (2021) . | Date of onset (2021) . | Filler brand . | Injection site . | Injection type (Gauge) . | Complication . | Management . | Injector Proficiencyb . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38F | 1/4 | 1/4 | Juvéderm Vista Volbella XC | Periorbital puffiness | Needle | Infection | − Antibiotics, drainage several times | 1 yr 9 mo MD, plastic surgeon | Resolved |
| 2 | 56F | 3/2 | 3/2 | Juvéderm Vista Ultra Plus XC | Forehead | Needle | Vascular compromise | − Hyaluronidase, prostaglandin + antibiotics | 2 yr 11 mo MD, dermatologist | Incomplete resolution |
| 3 | 47F | 3/7 | 3/10 | Juvéderm Vista Volux XC | Chin | Needle | Infection (possibility of injection into a vein) | − Hyaluronidase | 1 yr 2 mo MD, dermatologist | Resolved |
| 4 | 44F | 3/17 | 3/18 | Juvéderm Vista Voluma XC | Nasolabial fold | Needle | Vascular compromise (pain, numbness, epidermis peeling on nose tip and right wing of nose) | − Hyaluronidase, corticosteroid, antibiotics, prostaglandin + antibiotics, NSAIDs | 5 yr 5 mo MD, aesthetic surgeon | Resolved |
| 5 | 25F | 3/17 | 3/25 | Juvéderm Vista Volbella XC | Forehead | Cannula (18G) | Infection | − Hyaluronidase, antibiotics, antihistamine, synthetic antibacterial, H2-receptor antagonist | 0 yr 11 mo MD, plastic surgeon | Resolved |
| 6 | 37F | 4/14 | 4/15 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, antibiotics + normal saline − antibiotics, prostaglandin | 2 yr 0 mo MD, aesthetic surgeon | Resolved |
| 7 | 28F | 4/21 | 4/21 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase | 2 yr 2 mo MD, PhD, plastic surgeon | Resolved |
| 8 | 37F | 4/24 | 4/25 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, antibiotics, NSAIDs, prostaglandin | 1 yr 1 mo MD, aesthetic surgeon | Resolved |
| 9 | 23F | 4/26 | 5/9 | Juvéderm Vista Volbella XC | Lips | Needle | Allergy | − Hyaluronidase | 2 yr 1 mo MD, dermatologist | Resolved |
| 10 | 54F | 5/6 | 5/6 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (23G) | Vascular compromise | − Hyaluronidase, prostaglandin | 7 yr 4 mo MD, aesthetic surgeon | Resolved |
| 11 | 62M | 5/31 | 5/31 | Juvéderm Vista Voluma XC + Volift XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − Hyaluronidase, prostaglandin, NSAIDs | 1 yr 1 mo MD, aesthetic surgeon | Resolved |
| 12 | 36F | 6/19 | 6/19 | Juvéderm Vista Ultra Plus XC | Midcheek groove | Needle | Vascular compromise | − Corticosteroid + normal saline − antibiotics + normal saline − hyaluronidase − prostaglandin, antibiotics, synthetic antibacterial, H2-receptor antagonist | 2 yr 2 mo MD, dermatologist | Resolved |
| 13 | 44F | 6/25 | 6/25 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Prostaglandin, antibiotics, NSAIDs − prostaglandin + petroleum jelly | 0 yr 2 mo MD, plastic surgeon | Resolved |
| 14 | 48F | 7/1 | 7/2 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − NSAIDs, antiplasmin, antibiotics, − hyaluronidase + normal saline | 2 yr 6 mo MD, aesthetic surgeon | Resolved |
| 15 | 33F | 7/13 | 7/13 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase − levofloxacin hydration, NSAIDs, corticosteroid, prostaglandin, antibiotics | 0 yr 10 mo MD, PhD, aesthetic surgeon | Resolved |
| 16 | 41F | 7/28 | 7/29 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (25G) | Vascular compromise | − Hyaluronidase, prostaglandin | 2 yr 3 mo MD, aesthetic surgeon | Resolved |
| 17 | 49F | 8/25 | 8/25 | Juvéderm Vista Volift XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − Clinic visit because of redness that did not disappear -> hyaluronidase on the right nasolabial fold | 0 yr 4 mo MD, plastic surgeon | Resolved |
| 18 | 31F | 9/19 | 9/23 | Juvéderm Vista Voluma XC + Volux XC | Midcheek groove | Cannula (25G) | Allergy | − Levofloxacin hydration, synthetic antibacterial | 1 yr 5 mo MD, dermatologist | Resolved |
| 19 | 55F | 10/11 | 10/12 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase | 1 yr 7 mo MD, dermatologist | Resolved |
| 20 | 31F | 10/13 | 10/13 | Juvéderm Vista Volbella XC | Periorbital puffiness and lips | Needle | Allergy | − Hyaluronidase, corticosteroid + normal saline, antihistamine, synthetic antibacterial | 1 yr 2 mo MD, dermatologist | Resolved |
| 21 | 45F | 10/17 | 10/18 | Juvéderm Vista Volbella XC + Juvéderm Vista Volift XC | Forehead | Needle | Neuropathy | − Vitamin B12 preparations | 2 yr 7 mo MD, dermatologist | Resolved |
| 22 | 40F | 10/18 | 10/18 | Juvéderm Vista Volbella XC | Upper eyelids | Needle | Acute conjunctivitis | − Caused by HA injection into the conjunctiva of the eyeball; − removal of HA at another ophthalmological center | 14 yr 6 mo MD, aesthetic surgeon | Lost to follow-up |
| 23 | 25M | 11/3 | 11/5 | Juvéderm Vista Volbella XC | Lips | Needle | Infection | − Hyaluronidase, antibiotics | 0 yr 7 mo MD, dermatologist | Resolved |
| 24 | 55F | 11/4 | 11/6 | Juvéderm Vista Voluma XC | Midcheek groove | Needle | Vascular compromise | − Corticosteroid + antibiotics − prostaglandin, hyaluronidase, acetaminophen, levofloxacin hydration | 1 yr 2 mo MD, plastic surgeon | Resolved |
| 25 | 24F | 11/6 | 11/10 | Juvéderm Vista Volbella XC | Lips | Needle | Infection | − Hyaluronidase | 1 yr 7 mo MD, PhD, aesthetic surgeon | Resolved |
| 26 | 59F | 12/10 | 12/10 | Juvéderm Vista Volbella XC | Forehead | Cannula (25G) | Vascular compromise (Pulley vein layer extensive pain/poor circulation) | − Hyaluronidase | 2 yr 8 mo MD, aesthetic surgeon | Resolved |
| 27 | 51F | 12/9 | 12/9 | Juvéderm Vista Volift XC | Temple | Needle | Vascular compromise (redness along the right coronary artery) | − Hyaluronidase | 2 yr 6 mo MD, plastic surgeon | Resolved |
| 28 | 33F | 12/12 | 12/12 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, synthetic antibacterial, hydroquinone | 2 yr 9 mo MD, plastic surgeon | Resolved |
| 29 | 74F | 12/16 | 12/16 | Juvéderm Vista Volift XC | Forehead | Cannula | Neuropathy | − Vitamin B12 preparations, synthetic antibacterial | 0 yr 8 mo MD, aesthetic surgeon | Resolved |
| Case no. . | Agea /sex . | Date of injection (2021) . | Date of onset (2021) . | Filler brand . | Injection site . | Injection type (Gauge) . | Complication . | Management . | Injector Proficiencyb . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38F | 1/4 | 1/4 | Juvéderm Vista Volbella XC | Periorbital puffiness | Needle | Infection | − Antibiotics, drainage several times | 1 yr 9 mo MD, plastic surgeon | Resolved |
| 2 | 56F | 3/2 | 3/2 | Juvéderm Vista Ultra Plus XC | Forehead | Needle | Vascular compromise | − Hyaluronidase, prostaglandin + antibiotics | 2 yr 11 mo MD, dermatologist | Incomplete resolution |
| 3 | 47F | 3/7 | 3/10 | Juvéderm Vista Volux XC | Chin | Needle | Infection (possibility of injection into a vein) | − Hyaluronidase | 1 yr 2 mo MD, dermatologist | Resolved |
| 4 | 44F | 3/17 | 3/18 | Juvéderm Vista Voluma XC | Nasolabial fold | Needle | Vascular compromise (pain, numbness, epidermis peeling on nose tip and right wing of nose) | − Hyaluronidase, corticosteroid, antibiotics, prostaglandin + antibiotics, NSAIDs | 5 yr 5 mo MD, aesthetic surgeon | Resolved |
| 5 | 25F | 3/17 | 3/25 | Juvéderm Vista Volbella XC | Forehead | Cannula (18G) | Infection | − Hyaluronidase, antibiotics, antihistamine, synthetic antibacterial, H2-receptor antagonist | 0 yr 11 mo MD, plastic surgeon | Resolved |
| 6 | 37F | 4/14 | 4/15 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, antibiotics + normal saline − antibiotics, prostaglandin | 2 yr 0 mo MD, aesthetic surgeon | Resolved |
| 7 | 28F | 4/21 | 4/21 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase | 2 yr 2 mo MD, PhD, plastic surgeon | Resolved |
| 8 | 37F | 4/24 | 4/25 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, antibiotics, NSAIDs, prostaglandin | 1 yr 1 mo MD, aesthetic surgeon | Resolved |
| 9 | 23F | 4/26 | 5/9 | Juvéderm Vista Volbella XC | Lips | Needle | Allergy | − Hyaluronidase | 2 yr 1 mo MD, dermatologist | Resolved |
| 10 | 54F | 5/6 | 5/6 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (23G) | Vascular compromise | − Hyaluronidase, prostaglandin | 7 yr 4 mo MD, aesthetic surgeon | Resolved |
| 11 | 62M | 5/31 | 5/31 | Juvéderm Vista Voluma XC + Volift XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − Hyaluronidase, prostaglandin, NSAIDs | 1 yr 1 mo MD, aesthetic surgeon | Resolved |
| 12 | 36F | 6/19 | 6/19 | Juvéderm Vista Ultra Plus XC | Midcheek groove | Needle | Vascular compromise | − Corticosteroid + normal saline − antibiotics + normal saline − hyaluronidase − prostaglandin, antibiotics, synthetic antibacterial, H2-receptor antagonist | 2 yr 2 mo MD, dermatologist | Resolved |
| 13 | 44F | 6/25 | 6/25 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Prostaglandin, antibiotics, NSAIDs − prostaglandin + petroleum jelly | 0 yr 2 mo MD, plastic surgeon | Resolved |
| 14 | 48F | 7/1 | 7/2 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − NSAIDs, antiplasmin, antibiotics, − hyaluronidase + normal saline | 2 yr 6 mo MD, aesthetic surgeon | Resolved |
| 15 | 33F | 7/13 | 7/13 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase − levofloxacin hydration, NSAIDs, corticosteroid, prostaglandin, antibiotics | 0 yr 10 mo MD, PhD, aesthetic surgeon | Resolved |
| 16 | 41F | 7/28 | 7/29 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (25G) | Vascular compromise | − Hyaluronidase, prostaglandin | 2 yr 3 mo MD, aesthetic surgeon | Resolved |
| 17 | 49F | 8/25 | 8/25 | Juvéderm Vista Volift XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − Clinic visit because of redness that did not disappear -> hyaluronidase on the right nasolabial fold | 0 yr 4 mo MD, plastic surgeon | Resolved |
| 18 | 31F | 9/19 | 9/23 | Juvéderm Vista Voluma XC + Volux XC | Midcheek groove | Cannula (25G) | Allergy | − Levofloxacin hydration, synthetic antibacterial | 1 yr 5 mo MD, dermatologist | Resolved |
| 19 | 55F | 10/11 | 10/12 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase | 1 yr 7 mo MD, dermatologist | Resolved |
| 20 | 31F | 10/13 | 10/13 | Juvéderm Vista Volbella XC | Periorbital puffiness and lips | Needle | Allergy | − Hyaluronidase, corticosteroid + normal saline, antihistamine, synthetic antibacterial | 1 yr 2 mo MD, dermatologist | Resolved |
| 21 | 45F | 10/17 | 10/18 | Juvéderm Vista Volbella XC + Juvéderm Vista Volift XC | Forehead | Needle | Neuropathy | − Vitamin B12 preparations | 2 yr 7 mo MD, dermatologist | Resolved |
| 22 | 40F | 10/18 | 10/18 | Juvéderm Vista Volbella XC | Upper eyelids | Needle | Acute conjunctivitis | − Caused by HA injection into the conjunctiva of the eyeball; − removal of HA at another ophthalmological center | 14 yr 6 mo MD, aesthetic surgeon | Lost to follow-up |
| 23 | 25M | 11/3 | 11/5 | Juvéderm Vista Volbella XC | Lips | Needle | Infection | − Hyaluronidase, antibiotics | 0 yr 7 mo MD, dermatologist | Resolved |
| 24 | 55F | 11/4 | 11/6 | Juvéderm Vista Voluma XC | Midcheek groove | Needle | Vascular compromise | − Corticosteroid + antibiotics − prostaglandin, hyaluronidase, acetaminophen, levofloxacin hydration | 1 yr 2 mo MD, plastic surgeon | Resolved |
| 25 | 24F | 11/6 | 11/10 | Juvéderm Vista Volbella XC | Lips | Needle | Infection | − Hyaluronidase | 1 yr 7 mo MD, PhD, aesthetic surgeon | Resolved |
| 26 | 59F | 12/10 | 12/10 | Juvéderm Vista Volbella XC | Forehead | Cannula (25G) | Vascular compromise (Pulley vein layer extensive pain/poor circulation) | − Hyaluronidase | 2 yr 8 mo MD, aesthetic surgeon | Resolved |
| 27 | 51F | 12/9 | 12/9 | Juvéderm Vista Volift XC | Temple | Needle | Vascular compromise (redness along the right coronary artery) | − Hyaluronidase | 2 yr 6 mo MD, plastic surgeon | Resolved |
| 28 | 33F | 12/12 | 12/12 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, synthetic antibacterial, hydroquinone | 2 yr 9 mo MD, plastic surgeon | Resolved |
| 29 | 74F | 12/16 | 12/16 | Juvéderm Vista Volift XC | Forehead | Cannula | Neuropathy | − Vitamin B12 preparations, synthetic antibacterial | 0 yr 8 mo MD, aesthetic surgeon | Resolved |
G, gauge; HA, hyaluronic acid. aAge of patients at the time of treatment. The average age was 42.24 ± 12.34 years (range: 23-74 years). bYear and month of injector’s experience at the time of injection. The average experience of injectors at the Shonan Beauty Clinic Medical Group was 28.69 ± 31.88 months (range: 2-174 months).
Summary of the 29 Cases of Early Complications Associated With Hyaluronic Acid Filler Injections (Patients Injected in 2021)
| Case no. . | Agea /sex . | Date of injection (2021) . | Date of onset (2021) . | Filler brand . | Injection site . | Injection type (Gauge) . | Complication . | Management . | Injector Proficiencyb . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38F | 1/4 | 1/4 | Juvéderm Vista Volbella XC | Periorbital puffiness | Needle | Infection | − Antibiotics, drainage several times | 1 yr 9 mo MD, plastic surgeon | Resolved |
| 2 | 56F | 3/2 | 3/2 | Juvéderm Vista Ultra Plus XC | Forehead | Needle | Vascular compromise | − Hyaluronidase, prostaglandin + antibiotics | 2 yr 11 mo MD, dermatologist | Incomplete resolution |
| 3 | 47F | 3/7 | 3/10 | Juvéderm Vista Volux XC | Chin | Needle | Infection (possibility of injection into a vein) | − Hyaluronidase | 1 yr 2 mo MD, dermatologist | Resolved |
| 4 | 44F | 3/17 | 3/18 | Juvéderm Vista Voluma XC | Nasolabial fold | Needle | Vascular compromise (pain, numbness, epidermis peeling on nose tip and right wing of nose) | − Hyaluronidase, corticosteroid, antibiotics, prostaglandin + antibiotics, NSAIDs | 5 yr 5 mo MD, aesthetic surgeon | Resolved |
| 5 | 25F | 3/17 | 3/25 | Juvéderm Vista Volbella XC | Forehead | Cannula (18G) | Infection | − Hyaluronidase, antibiotics, antihistamine, synthetic antibacterial, H2-receptor antagonist | 0 yr 11 mo MD, plastic surgeon | Resolved |
| 6 | 37F | 4/14 | 4/15 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, antibiotics + normal saline − antibiotics, prostaglandin | 2 yr 0 mo MD, aesthetic surgeon | Resolved |
| 7 | 28F | 4/21 | 4/21 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase | 2 yr 2 mo MD, PhD, plastic surgeon | Resolved |
| 8 | 37F | 4/24 | 4/25 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, antibiotics, NSAIDs, prostaglandin | 1 yr 1 mo MD, aesthetic surgeon | Resolved |
| 9 | 23F | 4/26 | 5/9 | Juvéderm Vista Volbella XC | Lips | Needle | Allergy | − Hyaluronidase | 2 yr 1 mo MD, dermatologist | Resolved |
| 10 | 54F | 5/6 | 5/6 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (23G) | Vascular compromise | − Hyaluronidase, prostaglandin | 7 yr 4 mo MD, aesthetic surgeon | Resolved |
| 11 | 62M | 5/31 | 5/31 | Juvéderm Vista Voluma XC + Volift XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − Hyaluronidase, prostaglandin, NSAIDs | 1 yr 1 mo MD, aesthetic surgeon | Resolved |
| 12 | 36F | 6/19 | 6/19 | Juvéderm Vista Ultra Plus XC | Midcheek groove | Needle | Vascular compromise | − Corticosteroid + normal saline − antibiotics + normal saline − hyaluronidase − prostaglandin, antibiotics, synthetic antibacterial, H2-receptor antagonist | 2 yr 2 mo MD, dermatologist | Resolved |
| 13 | 44F | 6/25 | 6/25 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Prostaglandin, antibiotics, NSAIDs − prostaglandin + petroleum jelly | 0 yr 2 mo MD, plastic surgeon | Resolved |
| 14 | 48F | 7/1 | 7/2 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − NSAIDs, antiplasmin, antibiotics, − hyaluronidase + normal saline | 2 yr 6 mo MD, aesthetic surgeon | Resolved |
| 15 | 33F | 7/13 | 7/13 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase − levofloxacin hydration, NSAIDs, corticosteroid, prostaglandin, antibiotics | 0 yr 10 mo MD, PhD, aesthetic surgeon | Resolved |
| 16 | 41F | 7/28 | 7/29 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (25G) | Vascular compromise | − Hyaluronidase, prostaglandin | 2 yr 3 mo MD, aesthetic surgeon | Resolved |
| 17 | 49F | 8/25 | 8/25 | Juvéderm Vista Volift XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − Clinic visit because of redness that did not disappear -> hyaluronidase on the right nasolabial fold | 0 yr 4 mo MD, plastic surgeon | Resolved |
| 18 | 31F | 9/19 | 9/23 | Juvéderm Vista Voluma XC + Volux XC | Midcheek groove | Cannula (25G) | Allergy | − Levofloxacin hydration, synthetic antibacterial | 1 yr 5 mo MD, dermatologist | Resolved |
| 19 | 55F | 10/11 | 10/12 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase | 1 yr 7 mo MD, dermatologist | Resolved |
| 20 | 31F | 10/13 | 10/13 | Juvéderm Vista Volbella XC | Periorbital puffiness and lips | Needle | Allergy | − Hyaluronidase, corticosteroid + normal saline, antihistamine, synthetic antibacterial | 1 yr 2 mo MD, dermatologist | Resolved |
| 21 | 45F | 10/17 | 10/18 | Juvéderm Vista Volbella XC + Juvéderm Vista Volift XC | Forehead | Needle | Neuropathy | − Vitamin B12 preparations | 2 yr 7 mo MD, dermatologist | Resolved |
| 22 | 40F | 10/18 | 10/18 | Juvéderm Vista Volbella XC | Upper eyelids | Needle | Acute conjunctivitis | − Caused by HA injection into the conjunctiva of the eyeball; − removal of HA at another ophthalmological center | 14 yr 6 mo MD, aesthetic surgeon | Lost to follow-up |
| 23 | 25M | 11/3 | 11/5 | Juvéderm Vista Volbella XC | Lips | Needle | Infection | − Hyaluronidase, antibiotics | 0 yr 7 mo MD, dermatologist | Resolved |
| 24 | 55F | 11/4 | 11/6 | Juvéderm Vista Voluma XC | Midcheek groove | Needle | Vascular compromise | − Corticosteroid + antibiotics − prostaglandin, hyaluronidase, acetaminophen, levofloxacin hydration | 1 yr 2 mo MD, plastic surgeon | Resolved |
| 25 | 24F | 11/6 | 11/10 | Juvéderm Vista Volbella XC | Lips | Needle | Infection | − Hyaluronidase | 1 yr 7 mo MD, PhD, aesthetic surgeon | Resolved |
| 26 | 59F | 12/10 | 12/10 | Juvéderm Vista Volbella XC | Forehead | Cannula (25G) | Vascular compromise (Pulley vein layer extensive pain/poor circulation) | − Hyaluronidase | 2 yr 8 mo MD, aesthetic surgeon | Resolved |
| 27 | 51F | 12/9 | 12/9 | Juvéderm Vista Volift XC | Temple | Needle | Vascular compromise (redness along the right coronary artery) | − Hyaluronidase | 2 yr 6 mo MD, plastic surgeon | Resolved |
| 28 | 33F | 12/12 | 12/12 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, synthetic antibacterial, hydroquinone | 2 yr 9 mo MD, plastic surgeon | Resolved |
| 29 | 74F | 12/16 | 12/16 | Juvéderm Vista Volift XC | Forehead | Cannula | Neuropathy | − Vitamin B12 preparations, synthetic antibacterial | 0 yr 8 mo MD, aesthetic surgeon | Resolved |
| Case no. . | Agea /sex . | Date of injection (2021) . | Date of onset (2021) . | Filler brand . | Injection site . | Injection type (Gauge) . | Complication . | Management . | Injector Proficiencyb . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38F | 1/4 | 1/4 | Juvéderm Vista Volbella XC | Periorbital puffiness | Needle | Infection | − Antibiotics, drainage several times | 1 yr 9 mo MD, plastic surgeon | Resolved |
| 2 | 56F | 3/2 | 3/2 | Juvéderm Vista Ultra Plus XC | Forehead | Needle | Vascular compromise | − Hyaluronidase, prostaglandin + antibiotics | 2 yr 11 mo MD, dermatologist | Incomplete resolution |
| 3 | 47F | 3/7 | 3/10 | Juvéderm Vista Volux XC | Chin | Needle | Infection (possibility of injection into a vein) | − Hyaluronidase | 1 yr 2 mo MD, dermatologist | Resolved |
| 4 | 44F | 3/17 | 3/18 | Juvéderm Vista Voluma XC | Nasolabial fold | Needle | Vascular compromise (pain, numbness, epidermis peeling on nose tip and right wing of nose) | − Hyaluronidase, corticosteroid, antibiotics, prostaglandin + antibiotics, NSAIDs | 5 yr 5 mo MD, aesthetic surgeon | Resolved |
| 5 | 25F | 3/17 | 3/25 | Juvéderm Vista Volbella XC | Forehead | Cannula (18G) | Infection | − Hyaluronidase, antibiotics, antihistamine, synthetic antibacterial, H2-receptor antagonist | 0 yr 11 mo MD, plastic surgeon | Resolved |
| 6 | 37F | 4/14 | 4/15 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, antibiotics + normal saline − antibiotics, prostaglandin | 2 yr 0 mo MD, aesthetic surgeon | Resolved |
| 7 | 28F | 4/21 | 4/21 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase | 2 yr 2 mo MD, PhD, plastic surgeon | Resolved |
| 8 | 37F | 4/24 | 4/25 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, antibiotics, NSAIDs, prostaglandin | 1 yr 1 mo MD, aesthetic surgeon | Resolved |
| 9 | 23F | 4/26 | 5/9 | Juvéderm Vista Volbella XC | Lips | Needle | Allergy | − Hyaluronidase | 2 yr 1 mo MD, dermatologist | Resolved |
| 10 | 54F | 5/6 | 5/6 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (23G) | Vascular compromise | − Hyaluronidase, prostaglandin | 7 yr 4 mo MD, aesthetic surgeon | Resolved |
| 11 | 62M | 5/31 | 5/31 | Juvéderm Vista Voluma XC + Volift XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − Hyaluronidase, prostaglandin, NSAIDs | 1 yr 1 mo MD, aesthetic surgeon | Resolved |
| 12 | 36F | 6/19 | 6/19 | Juvéderm Vista Ultra Plus XC | Midcheek groove | Needle | Vascular compromise | − Corticosteroid + normal saline − antibiotics + normal saline − hyaluronidase − prostaglandin, antibiotics, synthetic antibacterial, H2-receptor antagonist | 2 yr 2 mo MD, dermatologist | Resolved |
| 13 | 44F | 6/25 | 6/25 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Prostaglandin, antibiotics, NSAIDs − prostaglandin + petroleum jelly | 0 yr 2 mo MD, plastic surgeon | Resolved |
| 14 | 48F | 7/1 | 7/2 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − NSAIDs, antiplasmin, antibiotics, − hyaluronidase + normal saline | 2 yr 6 mo MD, aesthetic surgeon | Resolved |
| 15 | 33F | 7/13 | 7/13 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase − levofloxacin hydration, NSAIDs, corticosteroid, prostaglandin, antibiotics | 0 yr 10 mo MD, PhD, aesthetic surgeon | Resolved |
| 16 | 41F | 7/28 | 7/29 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Cannula (25G) | Vascular compromise | − Hyaluronidase, prostaglandin | 2 yr 3 mo MD, aesthetic surgeon | Resolved |
| 17 | 49F | 8/25 | 8/25 | Juvéderm Vista Volift XC | Nasolabial fold | Cannula (27G) | Vascular compromise | − Clinic visit because of redness that did not disappear -> hyaluronidase on the right nasolabial fold | 0 yr 4 mo MD, plastic surgeon | Resolved |
| 18 | 31F | 9/19 | 9/23 | Juvéderm Vista Voluma XC + Volux XC | Midcheek groove | Cannula (25G) | Allergy | − Levofloxacin hydration, synthetic antibacterial | 1 yr 5 mo MD, dermatologist | Resolved |
| 19 | 55F | 10/11 | 10/12 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase | 1 yr 7 mo MD, dermatologist | Resolved |
| 20 | 31F | 10/13 | 10/13 | Juvéderm Vista Volbella XC | Periorbital puffiness and lips | Needle | Allergy | − Hyaluronidase, corticosteroid + normal saline, antihistamine, synthetic antibacterial | 1 yr 2 mo MD, dermatologist | Resolved |
| 21 | 45F | 10/17 | 10/18 | Juvéderm Vista Volbella XC + Juvéderm Vista Volift XC | Forehead | Needle | Neuropathy | − Vitamin B12 preparations | 2 yr 7 mo MD, dermatologist | Resolved |
| 22 | 40F | 10/18 | 10/18 | Juvéderm Vista Volbella XC | Upper eyelids | Needle | Acute conjunctivitis | − Caused by HA injection into the conjunctiva of the eyeball; − removal of HA at another ophthalmological center | 14 yr 6 mo MD, aesthetic surgeon | Lost to follow-up |
| 23 | 25M | 11/3 | 11/5 | Juvéderm Vista Volbella XC | Lips | Needle | Infection | − Hyaluronidase, antibiotics | 0 yr 7 mo MD, dermatologist | Resolved |
| 24 | 55F | 11/4 | 11/6 | Juvéderm Vista Voluma XC | Midcheek groove | Needle | Vascular compromise | − Corticosteroid + antibiotics − prostaglandin, hyaluronidase, acetaminophen, levofloxacin hydration | 1 yr 2 mo MD, plastic surgeon | Resolved |
| 25 | 24F | 11/6 | 11/10 | Juvéderm Vista Volbella XC | Lips | Needle | Infection | − Hyaluronidase | 1 yr 7 mo MD, PhD, aesthetic surgeon | Resolved |
| 26 | 59F | 12/10 | 12/10 | Juvéderm Vista Volbella XC | Forehead | Cannula (25G) | Vascular compromise (Pulley vein layer extensive pain/poor circulation) | − Hyaluronidase | 2 yr 8 mo MD, aesthetic surgeon | Resolved |
| 27 | 51F | 12/9 | 12/9 | Juvéderm Vista Volift XC | Temple | Needle | Vascular compromise (redness along the right coronary artery) | − Hyaluronidase | 2 yr 6 mo MD, plastic surgeon | Resolved |
| 28 | 33F | 12/12 | 12/12 | Juvéderm Vista Ultra Plus XC | Nasolabial fold | Needle | Vascular compromise | − Hyaluronidase, synthetic antibacterial, hydroquinone | 2 yr 9 mo MD, plastic surgeon | Resolved |
| 29 | 74F | 12/16 | 12/16 | Juvéderm Vista Volift XC | Forehead | Cannula | Neuropathy | − Vitamin B12 preparations, synthetic antibacterial | 0 yr 8 mo MD, aesthetic surgeon | Resolved |
G, gauge; HA, hyaluronic acid. aAge of patients at the time of treatment. The average age was 42.24 ± 12.34 years (range: 23-74 years). bYear and month of injector’s experience at the time of injection. The average experience of injectors at the Shonan Beauty Clinic Medical Group was 28.69 ± 31.88 months (range: 2-174 months).
The early complication rate of total HA fillers was 0.07% (n = 29/41,775) in our group (Figure 5). Our results showed that the younger the age group, the higher the complication rate, starting with the age 20 to 29 group (0.10%; n = 5/4,934). However, no statistically significant differences were observed among the groups (P = .2695; Figure 5). The details of the excluded 8 cases with late or delayed complications are summarized in Supplemental Table 1, available online at www.aestheticsurgeryjournal.com.
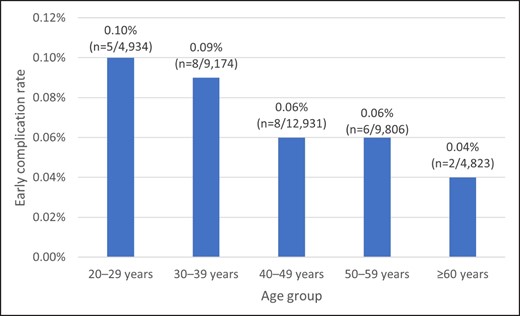
Early complication rates according to different patients age groups. Total early complication rate of total cases was 0.07% (n = 29/41,775). Average age was 42.24 ± 12.34 years (range, 23-74 years). No statistically significant differences were observed pertaining to the early complication rate among different age groups (P = .2695).
Early Complication Rate by Injection Site
The early complication rates by injection site are shown in Table 3. The most common site was the upper eyelids (0.41%; n = 1/241), followed by the forehead (0.29%; n = 5/1,719), lips (0.18%; n = 3/1,643), nasolabial fold (0.06%; n = 13/23,047), periorbital puffiness (0.04%; n = 2,254), midcheek groove (0.04%; 3/4,721), periorbital puffiness and lips (0.03%; 1/3,897), temple (0.03%; 1/2,962), and chin (0.01%; 1/8,350). No statistically significant differences were observed between the upper eyelids and forehead (P = .744), lips (P = .4643), and periorbital puffiness (P = .0534). In contrast, statistically significant differences were observed between the upper eyelids and the nasolabial fold,* periorbital puffiness,* midcheek groove,* periorbital puffiness and lips,* temple,* and chin** (*P < .05, **P < .001).
| Injection site . | Early complication rate (cases/injection sites) . | Complication . |
|---|---|---|
| Upper eyelids | 0.41% (1/241) | − Acute conjunctivitis (n = 1) |
| Forehead | 0.29% (5/1719) | − Vascular compromise (n = 2) − Infection (n = 1) − Neuropathy (n = 2) |
| Lips | 0.18% (3/1643) | − Infection (n = 2) − Allergy (n = 1) |
| Nasolabial fold* | 0.06% (13/23,047) | − Vascular compromise (n = 13) |
| Periorbital puffiness | 0.04% (1/2254) | − Infection (n = 1) |
| Midcheek groove* | 0.04% (3/4721) | − Vascular compromise (n = 2) − Allergy (n = 1) |
| Periorbital puffiness and lips* | 0.03% (1/3897) | − Allergy (n = 1) |
| Temple* | 0.03% (1/2962) | − Vascular compromise (n = 1) |
| Chin** | 0.01% (1/8350) | − Infection (n = 1) |
| Injection site . | Early complication rate (cases/injection sites) . | Complication . |
|---|---|---|
| Upper eyelids | 0.41% (1/241) | − Acute conjunctivitis (n = 1) |
| Forehead | 0.29% (5/1719) | − Vascular compromise (n = 2) − Infection (n = 1) − Neuropathy (n = 2) |
| Lips | 0.18% (3/1643) | − Infection (n = 2) − Allergy (n = 1) |
| Nasolabial fold* | 0.06% (13/23,047) | − Vascular compromise (n = 13) |
| Periorbital puffiness | 0.04% (1/2254) | − Infection (n = 1) |
| Midcheek groove* | 0.04% (3/4721) | − Vascular compromise (n = 2) − Allergy (n = 1) |
| Periorbital puffiness and lips* | 0.03% (1/3897) | − Allergy (n = 1) |
| Temple* | 0.03% (1/2962) | − Vascular compromise (n = 1) |
| Chin** | 0.01% (1/8350) | − Infection (n = 1) |
No statistically significant difference in early complication rates was observed between the upper eyelids and forehead (P = .744), lips (P = .4643), and periorbital puffiness (P = .0534). In contrast, statistically significant differences were observed between the upper eyelids and the nasolabial fold,* midcheek groove,* periorbital puffiness and lips,* temple,* and chin** (*P < .05, **P < .001; comparing two independent population proportions).
| Injection site . | Early complication rate (cases/injection sites) . | Complication . |
|---|---|---|
| Upper eyelids | 0.41% (1/241) | − Acute conjunctivitis (n = 1) |
| Forehead | 0.29% (5/1719) | − Vascular compromise (n = 2) − Infection (n = 1) − Neuropathy (n = 2) |
| Lips | 0.18% (3/1643) | − Infection (n = 2) − Allergy (n = 1) |
| Nasolabial fold* | 0.06% (13/23,047) | − Vascular compromise (n = 13) |
| Periorbital puffiness | 0.04% (1/2254) | − Infection (n = 1) |
| Midcheek groove* | 0.04% (3/4721) | − Vascular compromise (n = 2) − Allergy (n = 1) |
| Periorbital puffiness and lips* | 0.03% (1/3897) | − Allergy (n = 1) |
| Temple* | 0.03% (1/2962) | − Vascular compromise (n = 1) |
| Chin** | 0.01% (1/8350) | − Infection (n = 1) |
| Injection site . | Early complication rate (cases/injection sites) . | Complication . |
|---|---|---|
| Upper eyelids | 0.41% (1/241) | − Acute conjunctivitis (n = 1) |
| Forehead | 0.29% (5/1719) | − Vascular compromise (n = 2) − Infection (n = 1) − Neuropathy (n = 2) |
| Lips | 0.18% (3/1643) | − Infection (n = 2) − Allergy (n = 1) |
| Nasolabial fold* | 0.06% (13/23,047) | − Vascular compromise (n = 13) |
| Periorbital puffiness | 0.04% (1/2254) | − Infection (n = 1) |
| Midcheek groove* | 0.04% (3/4721) | − Vascular compromise (n = 2) − Allergy (n = 1) |
| Periorbital puffiness and lips* | 0.03% (1/3897) | − Allergy (n = 1) |
| Temple* | 0.03% (1/2962) | − Vascular compromise (n = 1) |
| Chin** | 0.01% (1/8350) | − Infection (n = 1) |
No statistically significant difference in early complication rates was observed between the upper eyelids and forehead (P = .744), lips (P = .4643), and periorbital puffiness (P = .0534). In contrast, statistically significant differences were observed between the upper eyelids and the nasolabial fold,* midcheek groove,* periorbital puffiness and lips,* temple,* and chin** (*P < .05, **P < .001; comparing two independent population proportions).
Early Complication Rate of Vascular Compromise
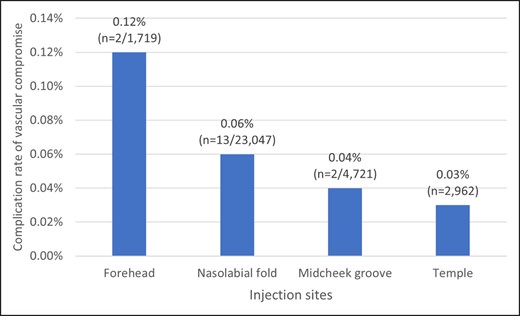
The early complication types (Tables 2, 3) included vascular compromise (n = 18), infection (n = 5), allergy (n = 3), neuropathy (n = 2), and acute conjunctivitis (n = 1). No skin necrosis was observed in our study sample. Figure 6 shows the complication rate of vascular compromise by injection site. The forehead was the most frequent site of vascular compromise (0.12%; n = 2/1,719), and the nasolabial fold was the most popular site for cosmetic treatment (n = 13/23,047; 0.06%).
Time of Onset
The average time of onset of early complications post injection was 1.4 ± 2.7 days. In 16 patients, the onset of early complication symptoms appeared on the same day as the injection. Among the early complications occurring in more than one case, vascular compromise was the earliest to appear (0.4 ± 0.6 days), and allergy was the latest one (5.7 ± 4.7 days). The average time of onset of each complication is presented in Supplemental Table 2, available online at www.aestheticsurgeryjournal.com. In cases with vascular compromise, the rate of cases for which the injector confirmed the presence of vascular compromise during or immediately after the injection, which was then treated immediately, was 33.33% (n = 6/18).
Early Complication Rate by Filler Type
The early complication rate by filler type is shown in Supplemental Table 3, available online at www.aestheticsurgeryjournal.com. It was found that the highest early complication rate for vascular compromise was with filler Juvéderm Vista Ultra Plus XC (0.07%; n = 12/17,172 sites), and for infection was with filler Juvéderm Vista Volbella XC (0.07%; n = 4/5,948).
Injector Experience
The average experience of injectors involved with the 29 early complication cases was 28.7 ± 32.0 months (range: 2-174 months; Table 2). The injectors who performed these 29 cases were divided into 3 groups: less than 1 year, 1 to 5 years, and more than 5 years of experience. The respective distributions are presented in Table 4.
| Injector experiencea . | No. of injectors with early complications (%) . | No. of injectors without early complications . | Total . |
|---|---|---|---|
| >5 years | 3 (4.62%) | 62 | 65 |
| 1-5 years | 20 (13.61%) | 127 | 147 |
| <1 year | 6 (9.38%) | 58 | 64 |
| TOTAL | 29 (10.50%) | 247 | 276 |
| Injector experiencea . | No. of injectors with early complications (%) . | No. of injectors without early complications . | Total . |
|---|---|---|---|
| >5 years | 3 (4.62%) | 62 | 65 |
| 1-5 years | 20 (13.61%) | 127 | 147 |
| <1 year | 6 (9.38%) | 58 | 64 |
| TOTAL | 29 (10.50%) | 247 | 276 |
“Experience” refers to experience at the Shonan Beauty Clinic Medical Group. The average experience was 28.69 ± 31.88 months (range: 2-174 months).
No statistically significant difference was observed in the early complication rates among these groups (>5 years vs 1-5 years, P = .0523; >5 years vs <1 year, P = .2887; 1-5 years vs <1 year, P = .3901). aInjector experience until December 31, 2012.
| Injector experiencea . | No. of injectors with early complications (%) . | No. of injectors without early complications . | Total . |
|---|---|---|---|
| >5 years | 3 (4.62%) | 62 | 65 |
| 1-5 years | 20 (13.61%) | 127 | 147 |
| <1 year | 6 (9.38%) | 58 | 64 |
| TOTAL | 29 (10.50%) | 247 | 276 |
| Injector experiencea . | No. of injectors with early complications (%) . | No. of injectors without early complications . | Total . |
|---|---|---|---|
| >5 years | 3 (4.62%) | 62 | 65 |
| 1-5 years | 20 (13.61%) | 127 | 147 |
| <1 year | 6 (9.38%) | 58 | 64 |
| TOTAL | 29 (10.50%) | 247 | 276 |
“Experience” refers to experience at the Shonan Beauty Clinic Medical Group. The average experience was 28.69 ± 31.88 months (range: 2-174 months).
No statistically significant difference was observed in the early complication rates among these groups (>5 years vs 1-5 years, P = .0523; >5 years vs <1 year, P = .2887; 1-5 years vs <1 year, P = .3901). aInjector experience until December 31, 2012.
Pertaining to injectors belonging to our group, the percentage of injectors treating patients who experienced early complications during the study period was the highest in the 1 to 5 years group (13.61%; 20/147), followed by the less than 1 year group (9.38%; 6/64), and the lowest was in the more than 5 years group (4.62%; 3/65). However, no statistically significant differences were observed among these groups (>5 years vs 1-5 years, P = .0523; >5 years vs <1 year, P = .2887; 1-5 years vs<1 year, P = .3901).
Treatment and Outcomes
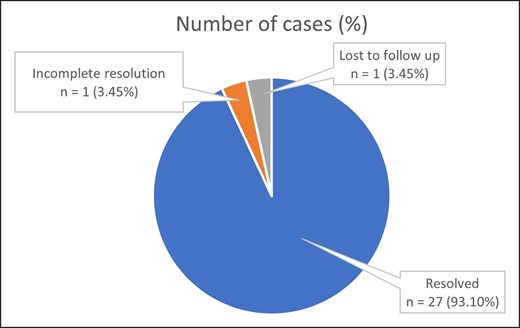
A summary of the treatment modalities employed to manage early complications is presented in Table 5, and the respective outcomes are shown in Figure 7. Of the 29 cases, one case was lost to follow-up, one case had incomplete resolution, and the remaining 27 cases (93.10%) were successfully treated at our facilities.
| . | Complications (no. of cases) . | ||||
|---|---|---|---|---|---|
| Treatment: no. of cases (%) for each complication . | Vascular compromise (n = 18) . | Infection (n = 5) . | Allergies (n = 3) . | Neuropathy (n = 2) . | Acute conjunctivitisa (n = 1) . |
| Hyaluronidase | 17 (94.44%) | 4 (80.00%) | 2 (66.67%) | ||
| Prostaglandin | 9 (50.00%) | ||||
| NSAIDs | 6 (33.33%) | ||||
| Antibiotics | 5 (27.78%) | 5 (100.00%) | |||
| Corticosteroid | 3 (16.67%) | 1 (20.00%) | 2 (66.67%) | 1 (50.00%) | |
| Vitamin B12 preparations | 2 (100.00%) | ||||
| Synthetic antibacterial | 1 (20.00%) | ||||
| Antihistamine | 1 (20.00%) | 1 (33.33%) | |||
| H2-receptor antagonist | 1 (20.00%) | ||||
| . | Complications (no. of cases) . | ||||
|---|---|---|---|---|---|
| Treatment: no. of cases (%) for each complication . | Vascular compromise (n = 18) . | Infection (n = 5) . | Allergies (n = 3) . | Neuropathy (n = 2) . | Acute conjunctivitisa (n = 1) . |
| Hyaluronidase | 17 (94.44%) | 4 (80.00%) | 2 (66.67%) | ||
| Prostaglandin | 9 (50.00%) | ||||
| NSAIDs | 6 (33.33%) | ||||
| Antibiotics | 5 (27.78%) | 5 (100.00%) | |||
| Corticosteroid | 3 (16.67%) | 1 (20.00%) | 2 (66.67%) | 1 (50.00%) | |
| Vitamin B12 preparations | 2 (100.00%) | ||||
| Synthetic antibacterial | 1 (20.00%) | ||||
| Antihistamine | 1 (20.00%) | 1 (33.33%) | |||
| H2-receptor antagonist | 1 (20.00%) | ||||
Removal of hyaluronic acid by another ophthalmologist.
| . | Complications (no. of cases) . | ||||
|---|---|---|---|---|---|
| Treatment: no. of cases (%) for each complication . | Vascular compromise (n = 18) . | Infection (n = 5) . | Allergies (n = 3) . | Neuropathy (n = 2) . | Acute conjunctivitisa (n = 1) . |
| Hyaluronidase | 17 (94.44%) | 4 (80.00%) | 2 (66.67%) | ||
| Prostaglandin | 9 (50.00%) | ||||
| NSAIDs | 6 (33.33%) | ||||
| Antibiotics | 5 (27.78%) | 5 (100.00%) | |||
| Corticosteroid | 3 (16.67%) | 1 (20.00%) | 2 (66.67%) | 1 (50.00%) | |
| Vitamin B12 preparations | 2 (100.00%) | ||||
| Synthetic antibacterial | 1 (20.00%) | ||||
| Antihistamine | 1 (20.00%) | 1 (33.33%) | |||
| H2-receptor antagonist | 1 (20.00%) | ||||
| . | Complications (no. of cases) . | ||||
|---|---|---|---|---|---|
| Treatment: no. of cases (%) for each complication . | Vascular compromise (n = 18) . | Infection (n = 5) . | Allergies (n = 3) . | Neuropathy (n = 2) . | Acute conjunctivitisa (n = 1) . |
| Hyaluronidase | 17 (94.44%) | 4 (80.00%) | 2 (66.67%) | ||
| Prostaglandin | 9 (50.00%) | ||||
| NSAIDs | 6 (33.33%) | ||||
| Antibiotics | 5 (27.78%) | 5 (100.00%) | |||
| Corticosteroid | 3 (16.67%) | 1 (20.00%) | 2 (66.67%) | 1 (50.00%) | |
| Vitamin B12 preparations | 2 (100.00%) | ||||
| Synthetic antibacterial | 1 (20.00%) | ||||
| Antihistamine | 1 (20.00%) | 1 (33.33%) | |||
| H2-receptor antagonist | 1 (20.00%) | ||||
Removal of hyaluronic acid by another ophthalmologist.
DISCUSSION
A Case of Acute Conjunctivitis Following Injection to the Upper Eyelids
The upper eyelid was the injection site with the highest early complication rate. However, only one patient with this type of early complication was included because of the extremely low number of patients treated at this site compared to other sites. A case of acute conjunctivitis caused by injection in the upper eyelids is therefore discussed (Table 2, case 22).
In case 22, the patient experienced subjective symptoms of edema in the inner white area of the left eye after injection. Consequently, the patient went to an ophthalmologist and was diagnosed with “acute conjunctivitis caused by the injection of HA into the left eyelid (left bulbar conjunctiva).” The patient underwent emergency surgery, and consultation by an ophthalmologist after a few days confirmed that the patient had recovered (last visit to our clinic).
A retrospective observational study of 17 patients who developed upper eyelid edema 6 to 24 months after HA filler injection in the supraorbital area was previously reported.11 Although there is no significant evidence associating complications of HA filler injection and anatomical sites, especially injections to the upper eyelid, injections to areas associated with severe complications should be avoided due to the anatomy of the periorbital region.12,13
Two Cases of Neuropathy Caused by Injection to the Forehead
The forehead was the second most common complication site (0.29%; n = 5/1,719; Table 3). Our guidelines dictate that “an 18-gauge must be used when using a cannula to inject HA into the forehead as a countermeasure against vascular compromise.” An injector did not follow this guideline, causing vascular compromise to one patient (case 26). However, if we exclude this exceptional case, the rate of early complications in the forehead is almost the same (0.23%; n = 4/1,719) as that in the lips (0.18%; n = 3/1,643). This is further confirmed by the absence of a statistically significant difference between the early complication rates associated with HA filler injected into the forehead and lips (P = .5195). The outline of the 2 complications of neuropathy (cases 21 and 29) caused by injection into the forehead is as follows:
These patients wanted designs that would round out the forehead by injecting HA into the entire forehead area, so the injectors used cannulas imagining injection technique as for fat grafting. In our group, it is a regulation to utilize an 18-gauge cannula, and the injectors believed that the patients would be in pain without anesthesia and performed nerve blocks before the injection. Because the injection layer cannot be accurately identified without diagnostic imaging, the injectors aimed the injection onto the periosteum.
Case 21: After blocking the frontal nerve, 2.7 mL of filler was injected into the periosteum with an 18-gauge cannula. This in turn rendered the lateral branch area of the left supratrochlear nerve numb.
Case 29: After blocking the frontal nerve, 4.0 mL of filler was injected into the periosteum with an 18-gauge cannula. This in turn rendered the left supratrochlear nerve area numb.
Consequently, it is reasonable to believe that the nerve damage in both registered cases was caused by the nerve blocking procedure or the cannula injection rather than nerve compression by HA.
Nasolabial Fold and Vascular Compromise
In 13 patients, HA fillers were injected into the nasolabial fold (Tables 2, 3). Interestingly, vascular compromise was observed in all 13 patients. The injection site was the nasolabial fold in 13 of 18 cases with vascular compromise. The forehead, midcheek groove, and temple were the injection sites in the remaining 5 cases. According to the above findings, one of the factors that may have caused vascular compromise in all 13 patients injected into the nasolabial fold is the anatomy of the facial artery running parallel to the nasolabial fold in Asian populations.14-16
Time of Onset in Cases With Vascular Compromise
Supplemental Table 4, available online at www.aestheticsurgeryjournal.com, provides the symptoms noticed by the injectors, such as discoloration, pain, swelling, and numbness, which were associated with vascular compromise during or immediately after the injection. All 6 cases were immediately treated.
Cannula Method
In cases 16 and 26, vascular compromise occurred even when a 25-gauge cannula was employed. In cases 11, 14, and 17, vascular compromise occurred even with a 27-gauge cannula (Table 2). This finding is in accordance with current literature confirming that the use of a cannula does not guarantee safety.17,18
In principle, early complications are caused by inappropriate patient selection and issues associated with injection technique. Therefore, it is critical for every HA injector to obtain an accurate medical history of the patient and also to have significant knowledge of and experience with HA fillers, enabling appropriate patient selection and injection techniques.19,20
Rheological Properties of HA Fillers
Supplemental Table 5, available online at www.aestheticsurgeryjournal.com, shows the rheological properties of the HA fillers utilized in this study.21,22 Juvéderm Vista Ultra Plus XC had the highest swelling factor, and all 12 complications were related to vascular compromises.
Injector Experience
In this study, we found that the experience of the injector was not a reliable predictor of complications because complications were caused by even well-experienced injectors. Among the 29 cases, 6 cases of early complications were associated with HA injection performed by injectors with less than 1 year of experience in our group, whereas 3 cases of early complications associated with HA injection were performed by injectors with more than 5 years of experience (Table 4), including an injector with 14 years and 6 months of experience. Moreover, as previously mentioned, no statistically significant differences were observed among these groups. Therefore, the injector’s experience was not considered an absolute indicator of treatment outcomes, including the occurrence of complications.23
Our Guideline for Complication Management With HA Injectable Treatments
Our group has agreed upon and thoroughly implemented the procedures according to “The Guideline for Complications Associated With HA Injectable Treatments” by the group, which is updated as needed. In our group, reexamination following injection is not routinely performed; however, we do encourage patients to revisit our clinic if they experience any of the following symptoms: numbness, malaise, headache, pain, itching, swelling, fever, cough, cold sweat, anaphylactic shock, dyspnea, or left-right asymmetry.
The 29 cases of early complications in this study were all treated and managed according to these guidelines, as shown in Table 5. Figure 7 shows that optimal outcomes were observed in most patients. Our group’s guidelines with regard to HA filler injection are as follows. Contraindicated sites are the glabella and tip of the nose; caution sites include the base of the nose (sharp needle, on the periosteum, only a bolus injection can be used, otherwise contraindicated) and forehead (sharp needle or only 18-gauge cannula can be used). There is a worldwide consensus that the glabella and tip of the nose are high risk sites for HA filler injection, and based on this evidence our group has strictly maintained its internal guidelines.19,24-26 For this reason the overall early complication rate with HA filler treatment was significantly low.
Limitations
We are aware that our study may have some limitations. First, it is possible that some patients in our group who received HA filler injections may have visited other hospitals when complications occurred. Consequently, these patients were not included in this study and the actual complication rate may be higher than the one reported.
Second, we intentionally selected narrow inclusion criteria in this study because we aimed to focus on serious early complications caused by HA filler injection and perform detailed analyses. Therefore, it was not possible to study cases of late- and delayed-onset complications (onset ≥14 days after injection).
Third, we excluded lumps as one of the minor complications at the beginning of the study so that we could focus on serious early complications. Finally, we were unable to include case photographs as consent had not been obtained from patients.
CONCLUSIONS
Regardless of the injection site, such as the upper eyelids (only one case included but with the highest rate of early complications) or the nasolabial fold (which had the highest number of injections and early complications in this study), accurate anatomical knowledge and knowledge and experience regarding HA fillers (including appropriate patient selection and injection techniques) are strictly required for injectors to anticipate early complications. Therefore, it is important to establish original guidelines based on experience to ensure their thorough implementation in our facilities.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
Author notes
Dr Nishikawa is the director of Dermatology Department, Shonan Beauty Clinic (SBC) Medical Group, Medical Corporation Shoubikai, Tokyo, Japan.
Dr Aikawa is the director general, SBC Medical Group, Tokyo, Japan.
Dr Kono is a professor of Department of Plastic Surgery, Tokai University School of Medicine, Kanagawa, Japan.



