-
PDF
- Split View
-
Views
-
Cite
Cite
Sachin M Shridharani, Grace M Tisch, MacKenzie L Kennedy, Injection Adipocytolysis for Body and Jawline Contouring: Real-World Experience and Treatment Considerations, Aesthetic Surgery Journal, Volume 43, Issue 4, April 2023, Pages 470–483, https://doi.org/10.1093/asj/sjac285
Close - Share Icon Share
Abstract
The role of ATX-101 in submental fat reduction has been well documented; however, its applicability across multiple anatomic areas is to be explored.
The authors sought to describe the experience with ATX-101 subcutaneous injections for body and jawline contouring and evaluate its safety.
This single-arm, single-center observational study included 201 patients who underwent injection adipocytolysis with ATX-101 (area-adjusted dose of 2 mg/cm2) in the jowl, abdomen (upper/lower), thigh (inner/outer/banana roll), arm, anterior periaxillary fat, back (lower/upper/nape/lipoma), knee (anterior/medial), chest, and/or neck. The number of treatment sessions, treatment volumes, doses, injections required for each anatomic area, and associated adverse events were recorded.
The mean number of treatment sessions conducted was 1.8. Multiple sessions were common for the jowl (mean: 2.0 and mean volume administered varied significantly between persons receiving 1 or multiple sessions [P = 0.005]). The mean volume and mean number of injections per session were highest in the chest (84.7 mL and 423.5, respectively) and lowest in the jowl (0.8 mL and 4.6, respectively). The chest (0.2 mL) and nape (0.2 mL) received the highest mean ATX-101 dose per injection site per session, whereas the inner thigh (0.11 mL) and upper back (0.11 mL) received the least. Adverse events observed were localized to the injection site. All patients experienced edema after each session, whereas numbness, tenderness, bruising, and paresis were experienced by 99.6%, 94.2%, 33.1%, and 2.6% of patients, respectively. Alopecia was not observed.
ATX-101 was well tolerated for body and jawline contouring.

Modern society has witnessed a widespread trend to lose weight, reduce fat, and rejuvenate the skin, which is fueled by the desire to attain an aesthetically improved figure.1 Fat reduction was one of the top surgical and nonsurgical cosmetic procedures in the United States in 2021, with more than $1.6 billion spent on injectables alone.2 The inherent shortcomings of surgical body contouring, including pain, prolonged recovery, scarring, hematoma, or infection, have made noninvasive alternatives, such as injectable adipolysis, cryolipolysis, lasers, high-intensity focused ultrasound, and radiofrequency devices, increasingly popular.1,3,4 However, noncytolytic body-contouring modalities are purported to produce transient sculpting effects.5
The minimally invasive method of injection adipolysis has sparked interest because it offers a more targeted approach attuned to patients’ needs.6 ATX-101 (Kybella; Allergan, Inc.; Irvine, CA), an adipocytolytic agent, is the first injectable therapy for submental fat reduction to obtain US FDA approval. It can target pockets of adipose tissue that would be difficult to access with other modalities, entails minimal downtime, and can be easily administered compared with other modalities.6,7 As a patented synthetic replicate of in vivo deoxycholic acid, which functions as a fat emulsifier, ATX-101 effectuates preferential cytolysis of fat cells due to its accentuated affinity for adipose tissue membranes over other tissues.7,8 Adipocytolysis induces a local inflammatory response that clears cell membrane fragments and improves collagen deposition by recruiting fibroblasts, leading to soft-tissue tightening.9 Patients may develop edema along with pain, numbness, and bruising at the injection area; however, the pharmacological and safety profiles of ATX-101 appear favorable, and only mild to moderate and transient adverse events (AEs) have been reported, thereby encouraging the further exploration of its efficacy and safety in body contouring.7,9,10
The Reduction of Localized Subcutaneous Fat in the Submental Area (REFINE 1) phase 3 trial found that ATX-101 significantly improved the psychological impact of submental fat fullness (SMF) and satisfaction with treatment compared with placebo.11 Of the 256 patients treated with ATX-101, 55% and 75% of patients reported a 1-grade improvement in clinician-assessed SMF after 2 and 4 treatments, respectively. The AEs reported were mostly mild or moderate, localized to the injection site, and transient.11 Moreover, improvements in submental contouring achieved with ATX-101 during the REFINE trials were maintained for up to 3 years in most patients.12
The utilization of ATX-101 injections for fat reduction has been primarily focused on SMF; moreover, there is a paucity of data on its suitability for other areas with subcutaneous fat. Hence, this study aimed to describe the experience with ATX-101 subcutaneous injections for facial and body contouring in clinical practice and evaluate the safety profile of ATX-101 for each anatomic area.
METHODS
Study Design and Patient Selection
This single-center, single-arm, observational study was conducted at a private clinical practice in New York between January 2018 and December 2020. It comprised a retrospective chart review of 201 patients between 18 and 80 years of age who were seeking a targeted reduction in subcutaneous fat deposited in anatomic areas, such as the jowl, abdomen (upper/lower), thigh (inner/outer/banana roll), arm, anterior periaxillary fat (APAF), back (lower/upper/nape/lipoma), knee (anterior/medial), chest, and neck, other than the SMF.
Patients were enrolled for treatment with ATX-101 based on the consensus between the patient and the clinician regarding the presence of conspicuous fullness/convexity in an anatomic area. Patients were counseled prior to the procedure on the need for the procedure, the possibility of undergoing multiple rounds of treatment, and the risks and benefits of alternative therapies. The study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization Tripartite Guidelines for Good Clinical Practice. No IRB approval was sought based on the nature of the study. Patients provided written consent to the treatment procedure and the utilization of their deidentified photographs in this manuscript.
Fullness/convexity resulting from other underlying conditions (ie, cervical adenopathy, excessive skin laxity, submandibular ptosis, and thyromegaly), injection site infections, pregnancy, previous utilization of injectable lipolytic agents (phosphatidylcholine and/or deoxycholate) in the treatment area, or anticoagulants were grounds for exclusion. Patients presenting with changed anatomy or landmarks or with scar tissue that could hinder either the safe administration of ATX-101 injections or the desired aesthetic result were cautioned.
Treatment Procedure
The lateral and medial borders of each treatment area were marked with a surgical pen, and a 1-cm injection grid was positioned over the treatment area. An area-adjusted dose (2 mg/cm2) of ATX-101 was injected adjacent to the grid marking employing a 1-mL syringe with a 32-gauge 0.5-inch needle (in the jowl) or a 27-gauge 0.5-inch needle (in all body areas except the jowl) up to a depth of 6 to 10 mm while carefully avoiding anatomic structures.
During each treatment session, patients received a set of injections administered 1 cm apart. The maximum volume administered per injection was 0.2 mL in any anatomic area. Considering that ATX-101 is a surface area–based treatment, the volume to be administered in each session was decided after an FDA-approved grid-based assessment of the area of concern. The number of injections and the need for multiple treatment sessions were determined by the clinician based on the amount and distribution of excess subcutaneous fat at the treatment area and the surface area of the anatomic site, patient willingness, and aesthetic improvement.
In addition to pre- and post-injection ice packs (applied intermittently for 48 hours after treatment), local anesthesia (lidocaine + epinephrine 15 minutes before treatment) and an analgesic (acetaminophen) were provided to alleviate any procedure-related discomfort before and after the treatment, if needed. No changes in methodology were required to adjust for different anatomic areas.
Outcomes
Treatment success was evaluated based on the analysis of standard photography of patients documented before and after treatment, patient satisfaction, and the clinical practitioner's aesthetic assessment.13,14
The number of treatment sessions, treatment intervals between subsequent visits, treatment volumes, doses, and injections required for patients opting for ATX-101 were documented. Multiple anatomic areas could be treated in a single session, depending on the suitability of treatment and patient willingness. Patients underwent physical/local examination immediately posttreatment, at the follow-up visit 1 day after the procedure, and subsequent treatment sessions (if any). At each follow-up visit, the safety of the treatment was assessed through the incidence and duration of solicited AEs, such as edema, numbness, tenderness, bruising, alopecia, and paresis. AEs were also reported by patients via telephone.
Statistical Analysis
Cross-evaluation of data was performed with R version 4.1.1 (RStudio; Boston, MA). Categorical variables are depicted in counts and percentages, and continuous variables are summarized as total data count, mean, standard deviation (SD), median, and interquartile range (minimum and maximum). The Mann–Whitney U test was employed to compare continuous variables.
RESULTS
Demographic and Baseline Characteristics
Overall, 201 patients underwent subcutaneous fat reduction treatment with ATX-101, of whom 155 (77.1%) were female and 46 (22.9%) were male (Table 1). Patients were aged between 20.3 and 87.6 years. The mean ± SD age of the cohort was 43.4 ± 12.7 years, and the mean ± SD BMI was 24.9 ± 3.9 kg/m2. Of the 201 patients, 58.7% had undergone prior nonsurgical procedures. Injectables, such as botulinum toxin (41.3%) and dermal filler (34.3%), were the most common nonsurgical cosmetic procedures that patients had previously received (Table 1). These injectables were also the most common subsequent nonsurgical procedures (botulinum toxin, 32.3%; dermal filler, 29.8%). In this cohort, 55.2% of patients reported a history of surgical procedures prior to ATX-101 treatment. Liposuction (32.8%) and rhinoplasty (14.4%) were the most common surgical treatments previously undergone by patients (Table 1).
| Characteristics . | Patients receiving ATX-101 (N = 201) . |
|---|---|
| Age, mean ± SD, y | 43.4 ± 12.7 |
| Gender, n (%) | |
| Female | 155 (77.1) |
| Male | 46 (22.9) |
| Racea, n (%) | |
| White | 155 (77.1) |
| Asian | 28 (13.9) |
| Hispanic | 10 (5.0) |
| Black or African American | 7 (3.5) |
| Height, mean ± SD, in | 66.0 ± 3.5 |
| Weight, mean ± SD, lb | 155.6 ± 33.4 |
| BMI, mean ± SD, kg/m2 | 24.9 ± 3.9 |
| Prior nonsurgical procedures, n (%) | |
| Botulinum toxin | 83 (41.3) |
| Dermal filler | 69 (34.3) |
| ATX-101 (SMF) | 14 (7.0) |
| CoolSculpting | 11 (5.5) |
| Laser | 5 (2.5) |
| Microneedling | 5 (2.5) |
| PRP injections | 5 (2.5) |
| Othersb | ≤1.5% |
| Surgical history, n (%) | |
| Liposuction | 66 (32.8) |
| Rhinoplasty | 29 (14.4) |
| Breast augmentation | 24 (11.9) |
| Blepharoplasty | 24 (11.9) |
| Abdominoplasty | 17 (8.5) |
| Facelift | 11 (5.5) |
| Breast implant exchange | 9 (4.5) |
| Mastopexy/breast lift | 8 (4.0) |
| Platysmaplasty/neck lift | 6 (3.0) |
| Breast reduction | 6 (3.0) |
| Hair transplant | 5 (2.5) |
| Fat grafting | 5 (2.5) |
| Brachioplasty/arm lift | 4 (2.0) |
| Othersc | ≤1.5% |
| Characteristics . | Patients receiving ATX-101 (N = 201) . |
|---|---|
| Age, mean ± SD, y | 43.4 ± 12.7 |
| Gender, n (%) | |
| Female | 155 (77.1) |
| Male | 46 (22.9) |
| Racea, n (%) | |
| White | 155 (77.1) |
| Asian | 28 (13.9) |
| Hispanic | 10 (5.0) |
| Black or African American | 7 (3.5) |
| Height, mean ± SD, in | 66.0 ± 3.5 |
| Weight, mean ± SD, lb | 155.6 ± 33.4 |
| BMI, mean ± SD, kg/m2 | 24.9 ± 3.9 |
| Prior nonsurgical procedures, n (%) | |
| Botulinum toxin | 83 (41.3) |
| Dermal filler | 69 (34.3) |
| ATX-101 (SMF) | 14 (7.0) |
| CoolSculpting | 11 (5.5) |
| Laser | 5 (2.5) |
| Microneedling | 5 (2.5) |
| PRP injections | 5 (2.5) |
| Othersb | ≤1.5% |
| Surgical history, n (%) | |
| Liposuction | 66 (32.8) |
| Rhinoplasty | 29 (14.4) |
| Breast augmentation | 24 (11.9) |
| Blepharoplasty | 24 (11.9) |
| Abdominoplasty | 17 (8.5) |
| Facelift | 11 (5.5) |
| Breast implant exchange | 9 (4.5) |
| Mastopexy/breast lift | 8 (4.0) |
| Platysmaplasty/neck lift | 6 (3.0) |
| Breast reduction | 6 (3.0) |
| Hair transplant | 5 (2.5) |
| Fat grafting | 5 (2.5) |
| Brachioplasty/arm lift | 4 (2.0) |
| Othersc | ≤1.5% |
BMI, body mass index; PRP, platelet-rich plasma; SD, standard deviation; SMF, submental fat. aRacial data of 1 patient were missing. bIncludes prior nonsurgical procedures such as ultherapy, InstaLift, hair transplant, SculpSure, injectables, HydraFacial, lipodissolve, and mesotherapy each undergone by ≤1.5% of patients. cIncludes surgical procedures such as bilateral sagittal split osteotomy, chin implant, buccal fat pad excision, brow lift, thigh lift, cheek implant, chest reduction, chin augmentation, lip lift, lip reduction, otoplasty, septoplasty, and anterior periaxillary skin excision each undergone by ≤1.5% of patients.
| Characteristics . | Patients receiving ATX-101 (N = 201) . |
|---|---|
| Age, mean ± SD, y | 43.4 ± 12.7 |
| Gender, n (%) | |
| Female | 155 (77.1) |
| Male | 46 (22.9) |
| Racea, n (%) | |
| White | 155 (77.1) |
| Asian | 28 (13.9) |
| Hispanic | 10 (5.0) |
| Black or African American | 7 (3.5) |
| Height, mean ± SD, in | 66.0 ± 3.5 |
| Weight, mean ± SD, lb | 155.6 ± 33.4 |
| BMI, mean ± SD, kg/m2 | 24.9 ± 3.9 |
| Prior nonsurgical procedures, n (%) | |
| Botulinum toxin | 83 (41.3) |
| Dermal filler | 69 (34.3) |
| ATX-101 (SMF) | 14 (7.0) |
| CoolSculpting | 11 (5.5) |
| Laser | 5 (2.5) |
| Microneedling | 5 (2.5) |
| PRP injections | 5 (2.5) |
| Othersb | ≤1.5% |
| Surgical history, n (%) | |
| Liposuction | 66 (32.8) |
| Rhinoplasty | 29 (14.4) |
| Breast augmentation | 24 (11.9) |
| Blepharoplasty | 24 (11.9) |
| Abdominoplasty | 17 (8.5) |
| Facelift | 11 (5.5) |
| Breast implant exchange | 9 (4.5) |
| Mastopexy/breast lift | 8 (4.0) |
| Platysmaplasty/neck lift | 6 (3.0) |
| Breast reduction | 6 (3.0) |
| Hair transplant | 5 (2.5) |
| Fat grafting | 5 (2.5) |
| Brachioplasty/arm lift | 4 (2.0) |
| Othersc | ≤1.5% |
| Characteristics . | Patients receiving ATX-101 (N = 201) . |
|---|---|
| Age, mean ± SD, y | 43.4 ± 12.7 |
| Gender, n (%) | |
| Female | 155 (77.1) |
| Male | 46 (22.9) |
| Racea, n (%) | |
| White | 155 (77.1) |
| Asian | 28 (13.9) |
| Hispanic | 10 (5.0) |
| Black or African American | 7 (3.5) |
| Height, mean ± SD, in | 66.0 ± 3.5 |
| Weight, mean ± SD, lb | 155.6 ± 33.4 |
| BMI, mean ± SD, kg/m2 | 24.9 ± 3.9 |
| Prior nonsurgical procedures, n (%) | |
| Botulinum toxin | 83 (41.3) |
| Dermal filler | 69 (34.3) |
| ATX-101 (SMF) | 14 (7.0) |
| CoolSculpting | 11 (5.5) |
| Laser | 5 (2.5) |
| Microneedling | 5 (2.5) |
| PRP injections | 5 (2.5) |
| Othersb | ≤1.5% |
| Surgical history, n (%) | |
| Liposuction | 66 (32.8) |
| Rhinoplasty | 29 (14.4) |
| Breast augmentation | 24 (11.9) |
| Blepharoplasty | 24 (11.9) |
| Abdominoplasty | 17 (8.5) |
| Facelift | 11 (5.5) |
| Breast implant exchange | 9 (4.5) |
| Mastopexy/breast lift | 8 (4.0) |
| Platysmaplasty/neck lift | 6 (3.0) |
| Breast reduction | 6 (3.0) |
| Hair transplant | 5 (2.5) |
| Fat grafting | 5 (2.5) |
| Brachioplasty/arm lift | 4 (2.0) |
| Othersc | ≤1.5% |
BMI, body mass index; PRP, platelet-rich plasma; SD, standard deviation; SMF, submental fat. aRacial data of 1 patient were missing. bIncludes prior nonsurgical procedures such as ultherapy, InstaLift, hair transplant, SculpSure, injectables, HydraFacial, lipodissolve, and mesotherapy each undergone by ≤1.5% of patients. cIncludes surgical procedures such as bilateral sagittal split osteotomy, chin implant, buccal fat pad excision, brow lift, thigh lift, cheek implant, chest reduction, chin augmentation, lip lift, lip reduction, otoplasty, septoplasty, and anterior periaxillary skin excision each undergone by ≤1.5% of patients.
Treatment Parameters
Overall, the cohort had a total of 370 ATX-101 treatment sessions with a mean of 1.8 treatment sessions. Figures 1, 2 show the abdominal contouring achieved after a single treatment session with ATX-101 in a female and male patient, respectively. Figure 3 compares the appearance of the jowl pretreatment and posttreatment with ATX-101. The highest mean number of treatment sessions was noted in the jowl (2.0), followed by APAF (1.9) and anterior knee (1.7) as shown in Table 2. Supplemental Table 1, available online at www.aestheticsurgeryjournal.com, presents the time intervals between consecutive treatment sessions. According to the distribution of ATX-101 treatment by anatomic location, only women underwent treatment in the thigh, arm, APAF, knee, and neck, whereas only men received treatment in the chest. Supplemental Figure 1, available online at www.aestheticsurgeryjournal.com, demonstrates the markings made by the clinical practitioner prior to ATX-101 treatment.
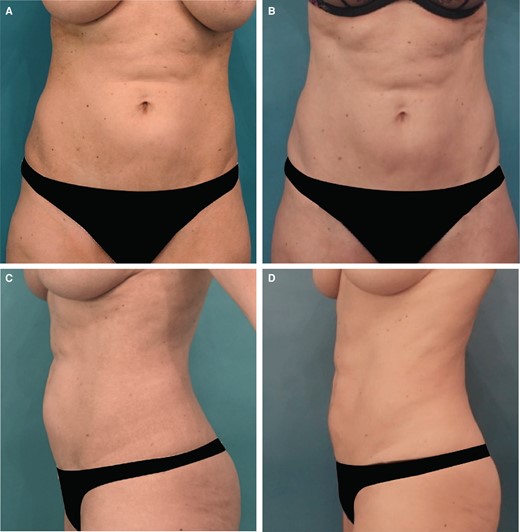
Images of this 47-year-old female patient who received 1 treatment with 36 mL of ATX-101 in the lower abdomen. (A, C) Front and lateral views of the abdomen prior to ATX-101 injections and (B, D) front and lateral views of the abdomen 3 months after treatment.
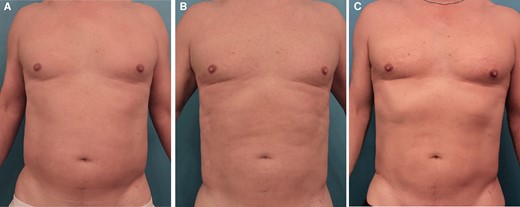
Images of this 52-year-old male patient who received 1 treatment with ATX-101 in the lower and upper abdomen (47 cc to the right and 47 cc to the left). (A) Abdomen prior to ATX-101 injections, (B) abdomen 3 months after treatment, and (C) abdomen 5 months after treatment.
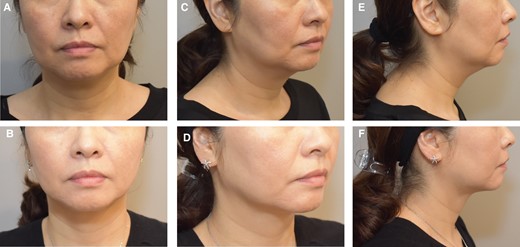
Images of this 44-year-old female patient who received 3 treatments with ATX-101 in the jowl. (A, C, E) Front, oblique, and lateral views of the jowl prior to ATX-101 injections, and (B, D, F) front, oblique, and lateral views of the jowl 3 months after her third treatment.
Distribution of Patients Undergoing ≥ 1 Treatment Sessions With ATX-101 for Each Anatomic Area
| Anatomic area . | Patients, n . | Total sessions . | No. of patients based on treatment session . | Mean sessions (per body area) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| First session . | Second session . | Third session . | Fourth session . | Fifth session . | Sixth session . | . | |||
| All areas | 201 | 370 | 99 | 60 | 26 | 10 | 3 | 3 | 1.8 |
| Nonbilateral sites | |||||||||
| Abdomen (upper) | 19 | 26 | 13 | 5 | 1 | 0 | 0 | 0 | 1.4 |
| Abdomen (lower) | 19 | 26 | 13 | 5 | 1 | 0 | 0 | 0 | 1.4 |
| Back (nape) | 2 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 1.5 |
| Back (lipoma) | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Neck | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Bilateral sites | |||||||||
| Jowl | 135 | 270 | 60 | 38 | 23 | 8 | 3 | 3 | 2.0 |
| Chest | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Back (lower) | 9 | 11 | 7 | 2 | 0 | 0 | 0 | 0 | 1.2 |
| Back (upper) | 5 | 8 | 3 | 1 | 1 | 0 | 0 | 0 | 1.6 |
| Thigh (inner) | 10 | 13 | 7 | 3 | 0 | 0 | 0 | 0 | 1.3 |
| Thigh (outer) | 13 | 17 | 9 | 4 | 0 | 0 | 0 | 0 | 1.3 |
| Thigh (banana roll) | 6 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Arm | 16 | 21 | 12 | 3 | 1 | 0 | 0 | 0 | 1.3 |
| APAF | 15 | 29 | 7 | 4 | 2 | 2 | 0 | 0 | 1.9 |
| Knee (anterior) | 9 | 15 | 3 | 6 | 0 | 0 | 0 | 0 | 1.7 |
| Knee (medial) | 8 | 11 | 5 | 3 | 0 | 0 | 0 | 0 | 1.4 |
| Anatomic area . | Patients, n . | Total sessions . | No. of patients based on treatment session . | Mean sessions (per body area) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| First session . | Second session . | Third session . | Fourth session . | Fifth session . | Sixth session . | . | |||
| All areas | 201 | 370 | 99 | 60 | 26 | 10 | 3 | 3 | 1.8 |
| Nonbilateral sites | |||||||||
| Abdomen (upper) | 19 | 26 | 13 | 5 | 1 | 0 | 0 | 0 | 1.4 |
| Abdomen (lower) | 19 | 26 | 13 | 5 | 1 | 0 | 0 | 0 | 1.4 |
| Back (nape) | 2 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 1.5 |
| Back (lipoma) | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Neck | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Bilateral sites | |||||||||
| Jowl | 135 | 270 | 60 | 38 | 23 | 8 | 3 | 3 | 2.0 |
| Chest | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Back (lower) | 9 | 11 | 7 | 2 | 0 | 0 | 0 | 0 | 1.2 |
| Back (upper) | 5 | 8 | 3 | 1 | 1 | 0 | 0 | 0 | 1.6 |
| Thigh (inner) | 10 | 13 | 7 | 3 | 0 | 0 | 0 | 0 | 1.3 |
| Thigh (outer) | 13 | 17 | 9 | 4 | 0 | 0 | 0 | 0 | 1.3 |
| Thigh (banana roll) | 6 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Arm | 16 | 21 | 12 | 3 | 1 | 0 | 0 | 0 | 1.3 |
| APAF | 15 | 29 | 7 | 4 | 2 | 2 | 0 | 0 | 1.9 |
| Knee (anterior) | 9 | 15 | 3 | 6 | 0 | 0 | 0 | 0 | 1.7 |
| Knee (medial) | 8 | 11 | 5 | 3 | 0 | 0 | 0 | 0 | 1.4 |
APAF, anterior periaxillary fat.
Distribution of Patients Undergoing ≥ 1 Treatment Sessions With ATX-101 for Each Anatomic Area
| Anatomic area . | Patients, n . | Total sessions . | No. of patients based on treatment session . | Mean sessions (per body area) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| First session . | Second session . | Third session . | Fourth session . | Fifth session . | Sixth session . | . | |||
| All areas | 201 | 370 | 99 | 60 | 26 | 10 | 3 | 3 | 1.8 |
| Nonbilateral sites | |||||||||
| Abdomen (upper) | 19 | 26 | 13 | 5 | 1 | 0 | 0 | 0 | 1.4 |
| Abdomen (lower) | 19 | 26 | 13 | 5 | 1 | 0 | 0 | 0 | 1.4 |
| Back (nape) | 2 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 1.5 |
| Back (lipoma) | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Neck | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Bilateral sites | |||||||||
| Jowl | 135 | 270 | 60 | 38 | 23 | 8 | 3 | 3 | 2.0 |
| Chest | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Back (lower) | 9 | 11 | 7 | 2 | 0 | 0 | 0 | 0 | 1.2 |
| Back (upper) | 5 | 8 | 3 | 1 | 1 | 0 | 0 | 0 | 1.6 |
| Thigh (inner) | 10 | 13 | 7 | 3 | 0 | 0 | 0 | 0 | 1.3 |
| Thigh (outer) | 13 | 17 | 9 | 4 | 0 | 0 | 0 | 0 | 1.3 |
| Thigh (banana roll) | 6 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Arm | 16 | 21 | 12 | 3 | 1 | 0 | 0 | 0 | 1.3 |
| APAF | 15 | 29 | 7 | 4 | 2 | 2 | 0 | 0 | 1.9 |
| Knee (anterior) | 9 | 15 | 3 | 6 | 0 | 0 | 0 | 0 | 1.7 |
| Knee (medial) | 8 | 11 | 5 | 3 | 0 | 0 | 0 | 0 | 1.4 |
| Anatomic area . | Patients, n . | Total sessions . | No. of patients based on treatment session . | Mean sessions (per body area) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| First session . | Second session . | Third session . | Fourth session . | Fifth session . | Sixth session . | . | |||
| All areas | 201 | 370 | 99 | 60 | 26 | 10 | 3 | 3 | 1.8 |
| Nonbilateral sites | |||||||||
| Abdomen (upper) | 19 | 26 | 13 | 5 | 1 | 0 | 0 | 0 | 1.4 |
| Abdomen (lower) | 19 | 26 | 13 | 5 | 1 | 0 | 0 | 0 | 1.4 |
| Back (nape) | 2 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 1.5 |
| Back (lipoma) | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Neck | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Bilateral sites | |||||||||
| Jowl | 135 | 270 | 60 | 38 | 23 | 8 | 3 | 3 | 2.0 |
| Chest | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Back (lower) | 9 | 11 | 7 | 2 | 0 | 0 | 0 | 0 | 1.2 |
| Back (upper) | 5 | 8 | 3 | 1 | 1 | 0 | 0 | 0 | 1.6 |
| Thigh (inner) | 10 | 13 | 7 | 3 | 0 | 0 | 0 | 0 | 1.3 |
| Thigh (outer) | 13 | 17 | 9 | 4 | 0 | 0 | 0 | 0 | 1.3 |
| Thigh (banana roll) | 6 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Arm | 16 | 21 | 12 | 3 | 1 | 0 | 0 | 0 | 1.3 |
| APAF | 15 | 29 | 7 | 4 | 2 | 2 | 0 | 0 | 1.9 |
| Knee (anterior) | 9 | 15 | 3 | 6 | 0 | 0 | 0 | 0 | 1.7 |
| Knee (medial) | 8 | 11 | 5 | 3 | 0 | 0 | 0 | 0 | 1.4 |
APAF, anterior periaxillary fat.
The mean total volume of ATX-101 injected per patient was 18.9 mL, and the mean volume injected per patient per session was 12.3 mL (Table 3). Among the nonbilateral sites, the mean volume was highest in the upper abdomen (20.2 mL) and lowest in the back lipoma (4.2 mL). Among the bilateral sites, the mean volume of ATX-101 injected per site was highest in the chest (84.7 mL) and lowest in the jowl (0.8 mL; Table 3). The mean volume of ATX-101 administered per session to the jowl of patients who had 1 session and ≥2 sessions was 1.8 and 1.6 mL, respectively, showing significant variation (P = .005; Table 3) likely due to the small treatment area. However, the variation in mean volume of ATX-101 administered to patients in other anatomic areas was not significant (P > .05). In general, the mean volume of ATX-101 (11.2 mL) injected per session was higher in patients undergoing a single treatment session compared with those who underwent multiple treatments sessions (2 sessions, 8.8 mL; 3 sessions, 7.6 mL; 4 sessions, 2.9 mL; 5 sessions, 3.2 mL; 6 sessions, 1.7 mL; see Supplemental Table 2, available online at www.aestheticsurgeryjournal.com).
Difference in Volume of ATX-101 Administered to Patients in 1 Session Vs ≥2 Sessions
| Anatomic area . | Volume of ATX-101 administered/session, mL . | P . | |||||
|---|---|---|---|---|---|---|---|
| . | Total sessions . | Mean ± SD . | Median . | IQR . | Minimum . | Maximum . | . |
| All areas (per person per session) | 370 | 12.3 ± 19.5 | 2.2 | 13.4 | 0.4 | 92.6 | NA |
| Nonbilateral sites | |||||||
| Abdomen (upper), n = 19 | |||||||
| Overall | 26 | 20.2 ± 21.9 | 14.0 | 23.3 | 0.6 | 56.0 | NA |
| Single session only | 13 | 17.4 ± 17.1 | 9.2 | 20 | 0.6 | 56 | .143 |
| Multiple sessions | 13 | 23.0 ± 10.0 | 22.0 | 22.8 | 12 | 36.6 | |
| Abdomen (lower), n = 19 | |||||||
| Overall | 26 | 17.7 ± 11.8 | 18.0 | 15.2 | 0.4 | 50.0 | NA |
| Single session only | 13 | 17.0 ± 13.7 | 17 | 18.0 | 0.4 | 50 | .589 |
| Multiple sessions | 13 | 18.3 ± 10.1 | 21 | 15.2 | 7.8 | 42 | |
| Back (nape), n = 2 | |||||||
| Overall | 3 | 7.0 ± 1.7 | 8.0 | 1.5 | 5.0 | 8.0 | NA |
| Single session only | 1 | 5.0 ± NA | 5.0 | 0 | 5 | 5 | .479 |
| Multiple sessions | 2 | 8.0 ± 0 | 8.0 | 0 | 8 | 8 | |
| Bilateral sites | |||||||
| Jowl, n = 135 | |||||||
| Overall | 270 | 0.8 ± 0.4 | 1.0 | 0.4 | 0.1 | 3.3 | NA |
| Single session only | 60 | 1.8 ± 0.6 | 2.0 | 1 | 0.4 | 3.0 | .005a |
| Multiple sessions | 210 | 1.6 ± 0.7 | 1.9 | 1 | 0.2 | 6.6 | |
| Back (lower), n = 9 | |||||||
| Overall | 11 | 28.7 ± 9.2 | 30.0 | 14.5 | 14.0 | 42.0 | NA |
| Single session only | 7 | 28.8 ± 8.4 | 30 | 12.2 | 20 | 42 | 1.000 |
| Multiple sessions | 4 | 28.5 ± 11.8 | 30 | 15.5 | 14 | 40 | |
| Back (upper), n = 5 | |||||||
| Overall | 8 | 15.9 ± 3.3 | 16.0 | 0.7 | 10.0 | 22.0 | NA |
| Single session only | 3 | 16.0 ± 6.0 | 16.0 | 6 | 10 | 22 | 1.000 |
| Multiple sessions | 5 | 15.8 ± 1.1 | 16.0 | 0 | 14 | 17 | |
| Thigh (inner), n = 10 | |||||||
| Overall | 13 | 8.2 ± 6.2 | 5.2 | 7.4 | 2.0 | 24.0 | NA |
| Single session only | 7 | 20.1 ± 14.5 | 21 | 17.2 | 2 | 44 | .063 |
| Multiple sessions | 6 | 6.5 ± 3.2 | 6.5 | 4.7 | 2 | 10 | |
| Thigh (outer), n = 13 | |||||||
| Overall | 17 | 9.2 ± 2.9 | 9.0 | 2.0 | 3.0 | 17.0 | NA |
| Single session only | 9 | 14.9 ± 4.4 | 16 | 8 | 9 | 21 | .923 |
| Multiple sessions | 8 | 15.6 ± 10.1 | 14.5 | 13.7 | 3 | 30 | |
| Arm, n = 16 | |||||||
| Overall | 21 | 16.1 ± 8.1 | 17.0 | 6.0 | 0.8 | 32.0 | NA |
| Single session only | 12 | 33.9 ± 12.0 | 36 | 7.5 | 6 | 52 | .240 |
| Multiple sessions | 9 | 29.8 ± 21.4 | 32 | 21 | 2.8 | 62 | |
| APAF, n = 15 | |||||||
| Overall | 29 | 4.2 ± 1.6 | 4.0 | 2.0 | 1.4 | 7.5 | NA |
| Single session only | 7 | 8.0 ± 2.9 | 8.0 | 4 | 3.8 | 12 | .797 |
| Multiple sessions | 22 | 8.4 ± 3.1 | 8.4 | 4 | 4 | 14 | |
| Knee (anterior), n = 9 | |||||||
| Overall | 15 | 6.1 ± 3.0 | 5.2 | 5.0 | 1.0 | 12.0 | NA |
| Single session only | 3 | 12.8 ± 7.4 | 10.5 | 6 | 8 | 20 | .561 |
| Multiple sessions | 12 | 11.0 ± 5.7 | 8.5 | 9 | 2 | 22 | |
| Knee (medial), n = 8 | |||||||
| Overall | 11 | 4.8 ± 2.0 | 5.0 | 3.0 | 1.0 | 7.5 | NA |
| Single session only | 5 | 6.9 ± 3.5 | 6 | 4 | 2 | 10.5 | .358 |
| Multiple sessions | 6 | 9.7 ± 4.3 | 11 | 5.7 | 4 | 15 | |
| Anatomic area . | Volume of ATX-101 administered/session, mL . | P . | |||||
|---|---|---|---|---|---|---|---|
| . | Total sessions . | Mean ± SD . | Median . | IQR . | Minimum . | Maximum . | . |
| All areas (per person per session) | 370 | 12.3 ± 19.5 | 2.2 | 13.4 | 0.4 | 92.6 | NA |
| Nonbilateral sites | |||||||
| Abdomen (upper), n = 19 | |||||||
| Overall | 26 | 20.2 ± 21.9 | 14.0 | 23.3 | 0.6 | 56.0 | NA |
| Single session only | 13 | 17.4 ± 17.1 | 9.2 | 20 | 0.6 | 56 | .143 |
| Multiple sessions | 13 | 23.0 ± 10.0 | 22.0 | 22.8 | 12 | 36.6 | |
| Abdomen (lower), n = 19 | |||||||
| Overall | 26 | 17.7 ± 11.8 | 18.0 | 15.2 | 0.4 | 50.0 | NA |
| Single session only | 13 | 17.0 ± 13.7 | 17 | 18.0 | 0.4 | 50 | .589 |
| Multiple sessions | 13 | 18.3 ± 10.1 | 21 | 15.2 | 7.8 | 42 | |
| Back (nape), n = 2 | |||||||
| Overall | 3 | 7.0 ± 1.7 | 8.0 | 1.5 | 5.0 | 8.0 | NA |
| Single session only | 1 | 5.0 ± NA | 5.0 | 0 | 5 | 5 | .479 |
| Multiple sessions | 2 | 8.0 ± 0 | 8.0 | 0 | 8 | 8 | |
| Bilateral sites | |||||||
| Jowl, n = 135 | |||||||
| Overall | 270 | 0.8 ± 0.4 | 1.0 | 0.4 | 0.1 | 3.3 | NA |
| Single session only | 60 | 1.8 ± 0.6 | 2.0 | 1 | 0.4 | 3.0 | .005a |
| Multiple sessions | 210 | 1.6 ± 0.7 | 1.9 | 1 | 0.2 | 6.6 | |
| Back (lower), n = 9 | |||||||
| Overall | 11 | 28.7 ± 9.2 | 30.0 | 14.5 | 14.0 | 42.0 | NA |
| Single session only | 7 | 28.8 ± 8.4 | 30 | 12.2 | 20 | 42 | 1.000 |
| Multiple sessions | 4 | 28.5 ± 11.8 | 30 | 15.5 | 14 | 40 | |
| Back (upper), n = 5 | |||||||
| Overall | 8 | 15.9 ± 3.3 | 16.0 | 0.7 | 10.0 | 22.0 | NA |
| Single session only | 3 | 16.0 ± 6.0 | 16.0 | 6 | 10 | 22 | 1.000 |
| Multiple sessions | 5 | 15.8 ± 1.1 | 16.0 | 0 | 14 | 17 | |
| Thigh (inner), n = 10 | |||||||
| Overall | 13 | 8.2 ± 6.2 | 5.2 | 7.4 | 2.0 | 24.0 | NA |
| Single session only | 7 | 20.1 ± 14.5 | 21 | 17.2 | 2 | 44 | .063 |
| Multiple sessions | 6 | 6.5 ± 3.2 | 6.5 | 4.7 | 2 | 10 | |
| Thigh (outer), n = 13 | |||||||
| Overall | 17 | 9.2 ± 2.9 | 9.0 | 2.0 | 3.0 | 17.0 | NA |
| Single session only | 9 | 14.9 ± 4.4 | 16 | 8 | 9 | 21 | .923 |
| Multiple sessions | 8 | 15.6 ± 10.1 | 14.5 | 13.7 | 3 | 30 | |
| Arm, n = 16 | |||||||
| Overall | 21 | 16.1 ± 8.1 | 17.0 | 6.0 | 0.8 | 32.0 | NA |
| Single session only | 12 | 33.9 ± 12.0 | 36 | 7.5 | 6 | 52 | .240 |
| Multiple sessions | 9 | 29.8 ± 21.4 | 32 | 21 | 2.8 | 62 | |
| APAF, n = 15 | |||||||
| Overall | 29 | 4.2 ± 1.6 | 4.0 | 2.0 | 1.4 | 7.5 | NA |
| Single session only | 7 | 8.0 ± 2.9 | 8.0 | 4 | 3.8 | 12 | .797 |
| Multiple sessions | 22 | 8.4 ± 3.1 | 8.4 | 4 | 4 | 14 | |
| Knee (anterior), n = 9 | |||||||
| Overall | 15 | 6.1 ± 3.0 | 5.2 | 5.0 | 1.0 | 12.0 | NA |
| Single session only | 3 | 12.8 ± 7.4 | 10.5 | 6 | 8 | 20 | .561 |
| Multiple sessions | 12 | 11.0 ± 5.7 | 8.5 | 9 | 2 | 22 | |
| Knee (medial), n = 8 | |||||||
| Overall | 11 | 4.8 ± 2.0 | 5.0 | 3.0 | 1.0 | 7.5 | NA |
| Single session only | 5 | 6.9 ± 3.5 | 6 | 4 | 2 | 10.5 | .358 |
| Multiple sessions | 6 | 9.7 ± 4.3 | 11 | 5.7 | 4 | 15 | |
Multiple sessions refers to treatment with ATX-101 in 2 or more sessions. Number of sites injected in each bilateral anatomic area were as follows: jowl, 537; thigh (inner), 22; thigh (outer), 28; arm, 42; APAF, 57; knee (anterior), 28; knee (medial), 20. P values were calculated employing the Mann–Whitney U test; Overall volumes injected for back lipoma, chest, neck, and thigh banana roll have not been presented here as patients underwent a single session for these anatomic areas. APAF, anterior periaxillary fat; IQR, interquartile range; n, patients who received ATX-101 injections at a particular anatomic area; NA, not applicable; SD, standard deviation. aP < .05 was considered significant.
Difference in Volume of ATX-101 Administered to Patients in 1 Session Vs ≥2 Sessions
| Anatomic area . | Volume of ATX-101 administered/session, mL . | P . | |||||
|---|---|---|---|---|---|---|---|
| . | Total sessions . | Mean ± SD . | Median . | IQR . | Minimum . | Maximum . | . |
| All areas (per person per session) | 370 | 12.3 ± 19.5 | 2.2 | 13.4 | 0.4 | 92.6 | NA |
| Nonbilateral sites | |||||||
| Abdomen (upper), n = 19 | |||||||
| Overall | 26 | 20.2 ± 21.9 | 14.0 | 23.3 | 0.6 | 56.0 | NA |
| Single session only | 13 | 17.4 ± 17.1 | 9.2 | 20 | 0.6 | 56 | .143 |
| Multiple sessions | 13 | 23.0 ± 10.0 | 22.0 | 22.8 | 12 | 36.6 | |
| Abdomen (lower), n = 19 | |||||||
| Overall | 26 | 17.7 ± 11.8 | 18.0 | 15.2 | 0.4 | 50.0 | NA |
| Single session only | 13 | 17.0 ± 13.7 | 17 | 18.0 | 0.4 | 50 | .589 |
| Multiple sessions | 13 | 18.3 ± 10.1 | 21 | 15.2 | 7.8 | 42 | |
| Back (nape), n = 2 | |||||||
| Overall | 3 | 7.0 ± 1.7 | 8.0 | 1.5 | 5.0 | 8.0 | NA |
| Single session only | 1 | 5.0 ± NA | 5.0 | 0 | 5 | 5 | .479 |
| Multiple sessions | 2 | 8.0 ± 0 | 8.0 | 0 | 8 | 8 | |
| Bilateral sites | |||||||
| Jowl, n = 135 | |||||||
| Overall | 270 | 0.8 ± 0.4 | 1.0 | 0.4 | 0.1 | 3.3 | NA |
| Single session only | 60 | 1.8 ± 0.6 | 2.0 | 1 | 0.4 | 3.0 | .005a |
| Multiple sessions | 210 | 1.6 ± 0.7 | 1.9 | 1 | 0.2 | 6.6 | |
| Back (lower), n = 9 | |||||||
| Overall | 11 | 28.7 ± 9.2 | 30.0 | 14.5 | 14.0 | 42.0 | NA |
| Single session only | 7 | 28.8 ± 8.4 | 30 | 12.2 | 20 | 42 | 1.000 |
| Multiple sessions | 4 | 28.5 ± 11.8 | 30 | 15.5 | 14 | 40 | |
| Back (upper), n = 5 | |||||||
| Overall | 8 | 15.9 ± 3.3 | 16.0 | 0.7 | 10.0 | 22.0 | NA |
| Single session only | 3 | 16.0 ± 6.0 | 16.0 | 6 | 10 | 22 | 1.000 |
| Multiple sessions | 5 | 15.8 ± 1.1 | 16.0 | 0 | 14 | 17 | |
| Thigh (inner), n = 10 | |||||||
| Overall | 13 | 8.2 ± 6.2 | 5.2 | 7.4 | 2.0 | 24.0 | NA |
| Single session only | 7 | 20.1 ± 14.5 | 21 | 17.2 | 2 | 44 | .063 |
| Multiple sessions | 6 | 6.5 ± 3.2 | 6.5 | 4.7 | 2 | 10 | |
| Thigh (outer), n = 13 | |||||||
| Overall | 17 | 9.2 ± 2.9 | 9.0 | 2.0 | 3.0 | 17.0 | NA |
| Single session only | 9 | 14.9 ± 4.4 | 16 | 8 | 9 | 21 | .923 |
| Multiple sessions | 8 | 15.6 ± 10.1 | 14.5 | 13.7 | 3 | 30 | |
| Arm, n = 16 | |||||||
| Overall | 21 | 16.1 ± 8.1 | 17.0 | 6.0 | 0.8 | 32.0 | NA |
| Single session only | 12 | 33.9 ± 12.0 | 36 | 7.5 | 6 | 52 | .240 |
| Multiple sessions | 9 | 29.8 ± 21.4 | 32 | 21 | 2.8 | 62 | |
| APAF, n = 15 | |||||||
| Overall | 29 | 4.2 ± 1.6 | 4.0 | 2.0 | 1.4 | 7.5 | NA |
| Single session only | 7 | 8.0 ± 2.9 | 8.0 | 4 | 3.8 | 12 | .797 |
| Multiple sessions | 22 | 8.4 ± 3.1 | 8.4 | 4 | 4 | 14 | |
| Knee (anterior), n = 9 | |||||||
| Overall | 15 | 6.1 ± 3.0 | 5.2 | 5.0 | 1.0 | 12.0 | NA |
| Single session only | 3 | 12.8 ± 7.4 | 10.5 | 6 | 8 | 20 | .561 |
| Multiple sessions | 12 | 11.0 ± 5.7 | 8.5 | 9 | 2 | 22 | |
| Knee (medial), n = 8 | |||||||
| Overall | 11 | 4.8 ± 2.0 | 5.0 | 3.0 | 1.0 | 7.5 | NA |
| Single session only | 5 | 6.9 ± 3.5 | 6 | 4 | 2 | 10.5 | .358 |
| Multiple sessions | 6 | 9.7 ± 4.3 | 11 | 5.7 | 4 | 15 | |
| Anatomic area . | Volume of ATX-101 administered/session, mL . | P . | |||||
|---|---|---|---|---|---|---|---|
| . | Total sessions . | Mean ± SD . | Median . | IQR . | Minimum . | Maximum . | . |
| All areas (per person per session) | 370 | 12.3 ± 19.5 | 2.2 | 13.4 | 0.4 | 92.6 | NA |
| Nonbilateral sites | |||||||
| Abdomen (upper), n = 19 | |||||||
| Overall | 26 | 20.2 ± 21.9 | 14.0 | 23.3 | 0.6 | 56.0 | NA |
| Single session only | 13 | 17.4 ± 17.1 | 9.2 | 20 | 0.6 | 56 | .143 |
| Multiple sessions | 13 | 23.0 ± 10.0 | 22.0 | 22.8 | 12 | 36.6 | |
| Abdomen (lower), n = 19 | |||||||
| Overall | 26 | 17.7 ± 11.8 | 18.0 | 15.2 | 0.4 | 50.0 | NA |
| Single session only | 13 | 17.0 ± 13.7 | 17 | 18.0 | 0.4 | 50 | .589 |
| Multiple sessions | 13 | 18.3 ± 10.1 | 21 | 15.2 | 7.8 | 42 | |
| Back (nape), n = 2 | |||||||
| Overall | 3 | 7.0 ± 1.7 | 8.0 | 1.5 | 5.0 | 8.0 | NA |
| Single session only | 1 | 5.0 ± NA | 5.0 | 0 | 5 | 5 | .479 |
| Multiple sessions | 2 | 8.0 ± 0 | 8.0 | 0 | 8 | 8 | |
| Bilateral sites | |||||||
| Jowl, n = 135 | |||||||
| Overall | 270 | 0.8 ± 0.4 | 1.0 | 0.4 | 0.1 | 3.3 | NA |
| Single session only | 60 | 1.8 ± 0.6 | 2.0 | 1 | 0.4 | 3.0 | .005a |
| Multiple sessions | 210 | 1.6 ± 0.7 | 1.9 | 1 | 0.2 | 6.6 | |
| Back (lower), n = 9 | |||||||
| Overall | 11 | 28.7 ± 9.2 | 30.0 | 14.5 | 14.0 | 42.0 | NA |
| Single session only | 7 | 28.8 ± 8.4 | 30 | 12.2 | 20 | 42 | 1.000 |
| Multiple sessions | 4 | 28.5 ± 11.8 | 30 | 15.5 | 14 | 40 | |
| Back (upper), n = 5 | |||||||
| Overall | 8 | 15.9 ± 3.3 | 16.0 | 0.7 | 10.0 | 22.0 | NA |
| Single session only | 3 | 16.0 ± 6.0 | 16.0 | 6 | 10 | 22 | 1.000 |
| Multiple sessions | 5 | 15.8 ± 1.1 | 16.0 | 0 | 14 | 17 | |
| Thigh (inner), n = 10 | |||||||
| Overall | 13 | 8.2 ± 6.2 | 5.2 | 7.4 | 2.0 | 24.0 | NA |
| Single session only | 7 | 20.1 ± 14.5 | 21 | 17.2 | 2 | 44 | .063 |
| Multiple sessions | 6 | 6.5 ± 3.2 | 6.5 | 4.7 | 2 | 10 | |
| Thigh (outer), n = 13 | |||||||
| Overall | 17 | 9.2 ± 2.9 | 9.0 | 2.0 | 3.0 | 17.0 | NA |
| Single session only | 9 | 14.9 ± 4.4 | 16 | 8 | 9 | 21 | .923 |
| Multiple sessions | 8 | 15.6 ± 10.1 | 14.5 | 13.7 | 3 | 30 | |
| Arm, n = 16 | |||||||
| Overall | 21 | 16.1 ± 8.1 | 17.0 | 6.0 | 0.8 | 32.0 | NA |
| Single session only | 12 | 33.9 ± 12.0 | 36 | 7.5 | 6 | 52 | .240 |
| Multiple sessions | 9 | 29.8 ± 21.4 | 32 | 21 | 2.8 | 62 | |
| APAF, n = 15 | |||||||
| Overall | 29 | 4.2 ± 1.6 | 4.0 | 2.0 | 1.4 | 7.5 | NA |
| Single session only | 7 | 8.0 ± 2.9 | 8.0 | 4 | 3.8 | 12 | .797 |
| Multiple sessions | 22 | 8.4 ± 3.1 | 8.4 | 4 | 4 | 14 | |
| Knee (anterior), n = 9 | |||||||
| Overall | 15 | 6.1 ± 3.0 | 5.2 | 5.0 | 1.0 | 12.0 | NA |
| Single session only | 3 | 12.8 ± 7.4 | 10.5 | 6 | 8 | 20 | .561 |
| Multiple sessions | 12 | 11.0 ± 5.7 | 8.5 | 9 | 2 | 22 | |
| Knee (medial), n = 8 | |||||||
| Overall | 11 | 4.8 ± 2.0 | 5.0 | 3.0 | 1.0 | 7.5 | NA |
| Single session only | 5 | 6.9 ± 3.5 | 6 | 4 | 2 | 10.5 | .358 |
| Multiple sessions | 6 | 9.7 ± 4.3 | 11 | 5.7 | 4 | 15 | |
Multiple sessions refers to treatment with ATX-101 in 2 or more sessions. Number of sites injected in each bilateral anatomic area were as follows: jowl, 537; thigh (inner), 22; thigh (outer), 28; arm, 42; APAF, 57; knee (anterior), 28; knee (medial), 20. P values were calculated employing the Mann–Whitney U test; Overall volumes injected for back lipoma, chest, neck, and thigh banana roll have not been presented here as patients underwent a single session for these anatomic areas. APAF, anterior periaxillary fat; IQR, interquartile range; n, patients who received ATX-101 injections at a particular anatomic area; NA, not applicable; SD, standard deviation. aP < .05 was considered significant.
The mean number of ATX-101 injections per session per site was lowest in the jowl (4.6) and highest in the chest (423.5), followed by that in the lower back (229.2) and upper abdomen (171.9), as shown in Table 4. The mean ATX-101 dose per injection site per session was highest in the chest (0.2 mL) and nape (0.2 mL) and lowest in the inner thigh (0.11 mL) and upper back (0.11 mL; Table 4). The chest required the highest mean volume of local anesthesia per treatment session (55.0 mL), whereas the jowl required the least (1.7 mL; Table 4).
ATX-101 Injections, Doses, and Local Anesthesia Administered to Patients in Each Anatomic Area
| Anatomic area . | Patients, n . | No. of injections per session per site, mean ± SD . | Dose per injection site per session, mean ± SD, mL . | Local anesthesia volume per session, mean ± SD, mL . |
|---|---|---|---|---|
| Nonbilateral sites | ||||
| Abdomen (upper) | 19 | 171.9 ± 124.2 | 0.13 ± 0.04 | 18.2 ± 12.5 |
| Abdomen (lower) | 19 | 144.2 ± 85.2 | 0.13 ± 0.04 | 13 ± 9.3 |
| Back (nape) | 2 | 35.0 ± 8.7 | 0.20 ± 0.00 | 5.7 ± 2.1 |
| Back (lipoma) | 2 | 32.0 ± 17.0 | 0.15 ± 0.07 | 11.5 ± 4.9 |
| Neck | 2 | 56.5 ± 33.2 | 0.15 ± 0.07 | 6.5 ± 2.1 |
| Bilateral sites | ||||
| Jowl | 135 | 4.6 ± 1.7 | 0.18 ± 0.04 | 1.7 ± 0.7 |
| Chest | 2 | 423.5 ± 23.3 | 0.20 ± 0.00 | 55.0 ± 7.1 |
| Back (lower) | 9 | 229.2 ± 85.8 | 0.13 ± 0.04 | 19.1 ± 11.1 |
| Back (upper) | 5 | 145.0 ± 26.2 | 0.11 ± 0.03 | 11.3 ± 7.1 |
| Thigh (inner) | 10 | 76.0 ± 62.4 | 0.11 ± 0.03 | 15.9 ± 13.0 |
| Thigh (outer) | 13 | 89.3 ± 31.2 | 0.12 ± 0.03 | 22.5 ± 11.2 |
| Thigh (banana roll) | 6 | 54.6 ± 19.5 | 0.13 ± 0.05 | 18.7 ± 8.3 |
| Arm | 16 | 143.1 ± 80.0 | 0.12 ± 0.04 | 22.2 ± 14.4 |
| APAF | 15 | 23.2 ± 10.3 | 0.19 ± 0.03 | 10.1 ± 3.8 |
| Knee (anterior) | 9 | 43.1 ± 26.9 | 0.15 ± 0.05 | 14.0 ± 7.6 |
| Knee (medial) | 8 | 33.5 ± 14.0 | 0.14 ± 0.04 | 12.4 ± 5.9 |
| Anatomic area . | Patients, n . | No. of injections per session per site, mean ± SD . | Dose per injection site per session, mean ± SD, mL . | Local anesthesia volume per session, mean ± SD, mL . |
|---|---|---|---|---|
| Nonbilateral sites | ||||
| Abdomen (upper) | 19 | 171.9 ± 124.2 | 0.13 ± 0.04 | 18.2 ± 12.5 |
| Abdomen (lower) | 19 | 144.2 ± 85.2 | 0.13 ± 0.04 | 13 ± 9.3 |
| Back (nape) | 2 | 35.0 ± 8.7 | 0.20 ± 0.00 | 5.7 ± 2.1 |
| Back (lipoma) | 2 | 32.0 ± 17.0 | 0.15 ± 0.07 | 11.5 ± 4.9 |
| Neck | 2 | 56.5 ± 33.2 | 0.15 ± 0.07 | 6.5 ± 2.1 |
| Bilateral sites | ||||
| Jowl | 135 | 4.6 ± 1.7 | 0.18 ± 0.04 | 1.7 ± 0.7 |
| Chest | 2 | 423.5 ± 23.3 | 0.20 ± 0.00 | 55.0 ± 7.1 |
| Back (lower) | 9 | 229.2 ± 85.8 | 0.13 ± 0.04 | 19.1 ± 11.1 |
| Back (upper) | 5 | 145.0 ± 26.2 | 0.11 ± 0.03 | 11.3 ± 7.1 |
| Thigh (inner) | 10 | 76.0 ± 62.4 | 0.11 ± 0.03 | 15.9 ± 13.0 |
| Thigh (outer) | 13 | 89.3 ± 31.2 | 0.12 ± 0.03 | 22.5 ± 11.2 |
| Thigh (banana roll) | 6 | 54.6 ± 19.5 | 0.13 ± 0.05 | 18.7 ± 8.3 |
| Arm | 16 | 143.1 ± 80.0 | 0.12 ± 0.04 | 22.2 ± 14.4 |
| APAF | 15 | 23.2 ± 10.3 | 0.19 ± 0.03 | 10.1 ± 3.8 |
| Knee (anterior) | 9 | 43.1 ± 26.9 | 0.15 ± 0.05 | 14.0 ± 7.6 |
| Knee (medial) | 8 | 33.5 ± 14.0 | 0.14 ± 0.04 | 12.4 ± 5.9 |
APAF, anterior periaxillary fat; n, patients who received ATX-101 injections at a particular anatomic area; SD, standard deviation.
ATX-101 Injections, Doses, and Local Anesthesia Administered to Patients in Each Anatomic Area
| Anatomic area . | Patients, n . | No. of injections per session per site, mean ± SD . | Dose per injection site per session, mean ± SD, mL . | Local anesthesia volume per session, mean ± SD, mL . |
|---|---|---|---|---|
| Nonbilateral sites | ||||
| Abdomen (upper) | 19 | 171.9 ± 124.2 | 0.13 ± 0.04 | 18.2 ± 12.5 |
| Abdomen (lower) | 19 | 144.2 ± 85.2 | 0.13 ± 0.04 | 13 ± 9.3 |
| Back (nape) | 2 | 35.0 ± 8.7 | 0.20 ± 0.00 | 5.7 ± 2.1 |
| Back (lipoma) | 2 | 32.0 ± 17.0 | 0.15 ± 0.07 | 11.5 ± 4.9 |
| Neck | 2 | 56.5 ± 33.2 | 0.15 ± 0.07 | 6.5 ± 2.1 |
| Bilateral sites | ||||
| Jowl | 135 | 4.6 ± 1.7 | 0.18 ± 0.04 | 1.7 ± 0.7 |
| Chest | 2 | 423.5 ± 23.3 | 0.20 ± 0.00 | 55.0 ± 7.1 |
| Back (lower) | 9 | 229.2 ± 85.8 | 0.13 ± 0.04 | 19.1 ± 11.1 |
| Back (upper) | 5 | 145.0 ± 26.2 | 0.11 ± 0.03 | 11.3 ± 7.1 |
| Thigh (inner) | 10 | 76.0 ± 62.4 | 0.11 ± 0.03 | 15.9 ± 13.0 |
| Thigh (outer) | 13 | 89.3 ± 31.2 | 0.12 ± 0.03 | 22.5 ± 11.2 |
| Thigh (banana roll) | 6 | 54.6 ± 19.5 | 0.13 ± 0.05 | 18.7 ± 8.3 |
| Arm | 16 | 143.1 ± 80.0 | 0.12 ± 0.04 | 22.2 ± 14.4 |
| APAF | 15 | 23.2 ± 10.3 | 0.19 ± 0.03 | 10.1 ± 3.8 |
| Knee (anterior) | 9 | 43.1 ± 26.9 | 0.15 ± 0.05 | 14.0 ± 7.6 |
| Knee (medial) | 8 | 33.5 ± 14.0 | 0.14 ± 0.04 | 12.4 ± 5.9 |
| Anatomic area . | Patients, n . | No. of injections per session per site, mean ± SD . | Dose per injection site per session, mean ± SD, mL . | Local anesthesia volume per session, mean ± SD, mL . |
|---|---|---|---|---|
| Nonbilateral sites | ||||
| Abdomen (upper) | 19 | 171.9 ± 124.2 | 0.13 ± 0.04 | 18.2 ± 12.5 |
| Abdomen (lower) | 19 | 144.2 ± 85.2 | 0.13 ± 0.04 | 13 ± 9.3 |
| Back (nape) | 2 | 35.0 ± 8.7 | 0.20 ± 0.00 | 5.7 ± 2.1 |
| Back (lipoma) | 2 | 32.0 ± 17.0 | 0.15 ± 0.07 | 11.5 ± 4.9 |
| Neck | 2 | 56.5 ± 33.2 | 0.15 ± 0.07 | 6.5 ± 2.1 |
| Bilateral sites | ||||
| Jowl | 135 | 4.6 ± 1.7 | 0.18 ± 0.04 | 1.7 ± 0.7 |
| Chest | 2 | 423.5 ± 23.3 | 0.20 ± 0.00 | 55.0 ± 7.1 |
| Back (lower) | 9 | 229.2 ± 85.8 | 0.13 ± 0.04 | 19.1 ± 11.1 |
| Back (upper) | 5 | 145.0 ± 26.2 | 0.11 ± 0.03 | 11.3 ± 7.1 |
| Thigh (inner) | 10 | 76.0 ± 62.4 | 0.11 ± 0.03 | 15.9 ± 13.0 |
| Thigh (outer) | 13 | 89.3 ± 31.2 | 0.12 ± 0.03 | 22.5 ± 11.2 |
| Thigh (banana roll) | 6 | 54.6 ± 19.5 | 0.13 ± 0.05 | 18.7 ± 8.3 |
| Arm | 16 | 143.1 ± 80.0 | 0.12 ± 0.04 | 22.2 ± 14.4 |
| APAF | 15 | 23.2 ± 10.3 | 0.19 ± 0.03 | 10.1 ± 3.8 |
| Knee (anterior) | 9 | 43.1 ± 26.9 | 0.15 ± 0.05 | 14.0 ± 7.6 |
| Knee (medial) | 8 | 33.5 ± 14.0 | 0.14 ± 0.04 | 12.4 ± 5.9 |
APAF, anterior periaxillary fat; n, patients who received ATX-101 injections at a particular anatomic area; SD, standard deviation.
Safety
Patients were visually examined for AEs immediately after treatment at the 24-hour follow-up and at subsequent follow-ups (typically 1, 4, and 12 weeks after treatment with ATX-101). Some may have received ATX-101 treatments within these intervals, but the initial purpose of the follow-ups was to assess any emerging adverse events with a shift in focus to include efficacy during later follow-up visits. Table 5 presents the incidence and duration of solicited AEs observed among patients in this study. All AEs observed in this study were localized to the injection site. Figure 4 shows the occurrence of edema, erythema, and bruising of the APAF the day after receiving treatment with ATX-101.
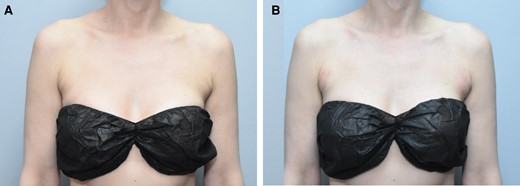
Images of this 38-year-old female who received ATX-101 to her right and left anterior periaxillary fat. (A) Anterior periaxillary fat before her first treatment and (B) anterior periaxillary fat 1 day after her first treatment showing signs of edema, erythema, and bruising.
| Anatomic area . | Adverse events . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Edema . | Numbness . | Tenderness . | Paresis . | Bruising . | |||||
| Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | |
| All areas, N = 201 | 462 (100) | 7.4 ± 4.0 | 460 (99.6) | 23.1 ± 9.8 | 435 (94.2) | 5.5 ± 3.7 | 12 (2.6) | 8.6 ± 7.6 | 153 (33.1) | 9.8 ± 3.5 |
| Jowl, n = 135 | 270 (100) | 5.7 ± 3.5 | 270(100) | 22.5 ± 11.0 | 247 (91.5) | 3.8 ± 2.7 | 10 (3.7) | 8.5 ± 8.4 | 9 (3.3) | 4.7 ± 3.4 |
| Abdomen (upper), n = 19 | 26 (100) | 10.8 ± 3.2 | 26 (100) | 25.3 ± 8.7 | 26 (100) | 7.5 ± 2.9 | 0 (0) | NA | 23 (88.5) | 10.4 ± 3.2 |
| Abdomen (lower), n = 19 | 26 (100) | 11.0 ± 3.2 | 26 (100) | 25.5 ± 8.9 | 26 (100) | 8.0 ± 3.1 | 0 (0) | NA | 22 (84.6) | 9.8 ± 2.7 |
| Thigh (inner), n = 10 | 13 (100) | 11.9 ± 5.2 | 13 (100) | 23.8 ± 11.4 | 13 (100) | 7.8 ± 4.2 | 0 (0) | NA | 7 (53.8) | 13.4 ± 3.9 |
| Thigh (outer), n = 13 | 17 (100) | 10.6 ± 3.1 | 17 (100) | 21.0 ± 8.3 | 17 (100) | 9.3 ± 4.5 | 0 (0) | NA | 16 (94.1) | 10.1 ± 3.4 |
| Thigh (banana roll), n = 6 | 6 (100) | 11.2 ± 2.2 | 6 (100) | 20.0 ± 2.8 | 6 (100) | 9.3 ± 3.1 | 0 (0) | NA | 5 (83.3) | 10.6 ± 2.9 |
| Arm, n = 16 | 21 (100) | 8.9 ± 3.1 | 19 (90.5) | 26.8 ± 5.6 | 19 (90.5) | 9.2 ± 4.6 | 1 (4.8) | 9.0 ± NA | 17 (81.0) | 11.9 ± 3.1 |
| APAF, n = 15 | 29 (100) | 6.6 ± 2.7 | 29 (100) | 21.2 ± 4.9 | 29 (100) | 6.1 ± 3.0 | 1 (3.4) | 9.0 ± NA | 16 (55.2) | 6.8 ± 2.1 |
| Back (lower), n = 9 | 11 (100) | 11.4 ± 2.3 | 11 (100) | 27.0 ± 9.2 | 11 (100) | 7.9 ± 3.3 | 0 (0) | NA | 8 (72.7) | 9.5 ± 1.8 |
| Back (upper), n = 5 | 8 (100) | 9.2 ± 3.2 | 8 (100) | 27.9 ± 10.3 | 8 (100) | 7.5 ± 4.3 | 0 (0) | NA | 2 (25) | 10.5 ± 0.7 |
| Back (nape), n = 2 | 3 (100) | 6.3 ± 3.5 | 3 (100) | 16.7 ± 12.7 | 3 (100) | 6.7 ± 4.5 | 0 (0) | NA | 2 (66.7) | 12.0 ± 0 |
| Back (lipoma), n = 2 | 2 (100) | 12.5 ± 4.9 | 2 (100) | 22.5 ± 0.7 | 2 (100) | 7.0 ± NA | 0 (0) | NA | 1 (50) | 9.0 ± NA |
| Knee (anterior), n = 9 | 15 (100) | 8.9 ± 2.9 | 15 (100) | 23.9 ± 4.9 | 13 (86.7) | 8.0 ± 2.8 | 0 (0) | NA | 12 (80) | 11.2 ± 3.5 |
| Knee (medial), n = 8 | 11 (100) | 10.3 ± 2.7 | 11 (100) | 23.1 ± 5.7 | 11 (100) | 7.2 ± 3.1 | 0 (0) | NA | 9 (81.8) | 9.2 ± 1.6 |
| Chest, n = 2 | 2 (100) | 11.0 ± 1.4 | 2 (100) | 21.5 ± 3.5 | 2 (100) | 7.5 ± 0.7 | 0 (0) | NA | 2 (100) | 8.0 ± 4.2 |
| Neck, n = 2 | 2 (100) | 8.0 ± 1.4 | 2 (100) | 20.5 ± 2.1 | 2 (100) | 5.0 ± 1.4 | 0 (0) | NA | 2 (100) | 6.0 ± 1.4 |
| Anatomic area . | Adverse events . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Edema . | Numbness . | Tenderness . | Paresis . | Bruising . | |||||
| Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | |
| All areas, N = 201 | 462 (100) | 7.4 ± 4.0 | 460 (99.6) | 23.1 ± 9.8 | 435 (94.2) | 5.5 ± 3.7 | 12 (2.6) | 8.6 ± 7.6 | 153 (33.1) | 9.8 ± 3.5 |
| Jowl, n = 135 | 270 (100) | 5.7 ± 3.5 | 270(100) | 22.5 ± 11.0 | 247 (91.5) | 3.8 ± 2.7 | 10 (3.7) | 8.5 ± 8.4 | 9 (3.3) | 4.7 ± 3.4 |
| Abdomen (upper), n = 19 | 26 (100) | 10.8 ± 3.2 | 26 (100) | 25.3 ± 8.7 | 26 (100) | 7.5 ± 2.9 | 0 (0) | NA | 23 (88.5) | 10.4 ± 3.2 |
| Abdomen (lower), n = 19 | 26 (100) | 11.0 ± 3.2 | 26 (100) | 25.5 ± 8.9 | 26 (100) | 8.0 ± 3.1 | 0 (0) | NA | 22 (84.6) | 9.8 ± 2.7 |
| Thigh (inner), n = 10 | 13 (100) | 11.9 ± 5.2 | 13 (100) | 23.8 ± 11.4 | 13 (100) | 7.8 ± 4.2 | 0 (0) | NA | 7 (53.8) | 13.4 ± 3.9 |
| Thigh (outer), n = 13 | 17 (100) | 10.6 ± 3.1 | 17 (100) | 21.0 ± 8.3 | 17 (100) | 9.3 ± 4.5 | 0 (0) | NA | 16 (94.1) | 10.1 ± 3.4 |
| Thigh (banana roll), n = 6 | 6 (100) | 11.2 ± 2.2 | 6 (100) | 20.0 ± 2.8 | 6 (100) | 9.3 ± 3.1 | 0 (0) | NA | 5 (83.3) | 10.6 ± 2.9 |
| Arm, n = 16 | 21 (100) | 8.9 ± 3.1 | 19 (90.5) | 26.8 ± 5.6 | 19 (90.5) | 9.2 ± 4.6 | 1 (4.8) | 9.0 ± NA | 17 (81.0) | 11.9 ± 3.1 |
| APAF, n = 15 | 29 (100) | 6.6 ± 2.7 | 29 (100) | 21.2 ± 4.9 | 29 (100) | 6.1 ± 3.0 | 1 (3.4) | 9.0 ± NA | 16 (55.2) | 6.8 ± 2.1 |
| Back (lower), n = 9 | 11 (100) | 11.4 ± 2.3 | 11 (100) | 27.0 ± 9.2 | 11 (100) | 7.9 ± 3.3 | 0 (0) | NA | 8 (72.7) | 9.5 ± 1.8 |
| Back (upper), n = 5 | 8 (100) | 9.2 ± 3.2 | 8 (100) | 27.9 ± 10.3 | 8 (100) | 7.5 ± 4.3 | 0 (0) | NA | 2 (25) | 10.5 ± 0.7 |
| Back (nape), n = 2 | 3 (100) | 6.3 ± 3.5 | 3 (100) | 16.7 ± 12.7 | 3 (100) | 6.7 ± 4.5 | 0 (0) | NA | 2 (66.7) | 12.0 ± 0 |
| Back (lipoma), n = 2 | 2 (100) | 12.5 ± 4.9 | 2 (100) | 22.5 ± 0.7 | 2 (100) | 7.0 ± NA | 0 (0) | NA | 1 (50) | 9.0 ± NA |
| Knee (anterior), n = 9 | 15 (100) | 8.9 ± 2.9 | 15 (100) | 23.9 ± 4.9 | 13 (86.7) | 8.0 ± 2.8 | 0 (0) | NA | 12 (80) | 11.2 ± 3.5 |
| Knee (medial), n = 8 | 11 (100) | 10.3 ± 2.7 | 11 (100) | 23.1 ± 5.7 | 11 (100) | 7.2 ± 3.1 | 0 (0) | NA | 9 (81.8) | 9.2 ± 1.6 |
| Chest, n = 2 | 2 (100) | 11.0 ± 1.4 | 2 (100) | 21.5 ± 3.5 | 2 (100) | 7.5 ± 0.7 | 0 (0) | NA | 2 (100) | 8.0 ± 4.2 |
| Neck, n = 2 | 2 (100) | 8.0 ± 1.4 | 2 (100) | 20.5 ± 2.1 | 2 (100) | 5.0 ± 1.4 | 0 (0) | NA | 2 (100) | 6.0 ± 1.4 |
APAF, anterior periaxillary fat; N, total number of patients enrolled in the study; n, patients who received ATX-101 injections at a particular anatomic area; NA, not applicable; SD, standard deviation.
| Anatomic area . | Adverse events . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Edema . | Numbness . | Tenderness . | Paresis . | Bruising . | |||||
| Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | |
| All areas, N = 201 | 462 (100) | 7.4 ± 4.0 | 460 (99.6) | 23.1 ± 9.8 | 435 (94.2) | 5.5 ± 3.7 | 12 (2.6) | 8.6 ± 7.6 | 153 (33.1) | 9.8 ± 3.5 |
| Jowl, n = 135 | 270 (100) | 5.7 ± 3.5 | 270(100) | 22.5 ± 11.0 | 247 (91.5) | 3.8 ± 2.7 | 10 (3.7) | 8.5 ± 8.4 | 9 (3.3) | 4.7 ± 3.4 |
| Abdomen (upper), n = 19 | 26 (100) | 10.8 ± 3.2 | 26 (100) | 25.3 ± 8.7 | 26 (100) | 7.5 ± 2.9 | 0 (0) | NA | 23 (88.5) | 10.4 ± 3.2 |
| Abdomen (lower), n = 19 | 26 (100) | 11.0 ± 3.2 | 26 (100) | 25.5 ± 8.9 | 26 (100) | 8.0 ± 3.1 | 0 (0) | NA | 22 (84.6) | 9.8 ± 2.7 |
| Thigh (inner), n = 10 | 13 (100) | 11.9 ± 5.2 | 13 (100) | 23.8 ± 11.4 | 13 (100) | 7.8 ± 4.2 | 0 (0) | NA | 7 (53.8) | 13.4 ± 3.9 |
| Thigh (outer), n = 13 | 17 (100) | 10.6 ± 3.1 | 17 (100) | 21.0 ± 8.3 | 17 (100) | 9.3 ± 4.5 | 0 (0) | NA | 16 (94.1) | 10.1 ± 3.4 |
| Thigh (banana roll), n = 6 | 6 (100) | 11.2 ± 2.2 | 6 (100) | 20.0 ± 2.8 | 6 (100) | 9.3 ± 3.1 | 0 (0) | NA | 5 (83.3) | 10.6 ± 2.9 |
| Arm, n = 16 | 21 (100) | 8.9 ± 3.1 | 19 (90.5) | 26.8 ± 5.6 | 19 (90.5) | 9.2 ± 4.6 | 1 (4.8) | 9.0 ± NA | 17 (81.0) | 11.9 ± 3.1 |
| APAF, n = 15 | 29 (100) | 6.6 ± 2.7 | 29 (100) | 21.2 ± 4.9 | 29 (100) | 6.1 ± 3.0 | 1 (3.4) | 9.0 ± NA | 16 (55.2) | 6.8 ± 2.1 |
| Back (lower), n = 9 | 11 (100) | 11.4 ± 2.3 | 11 (100) | 27.0 ± 9.2 | 11 (100) | 7.9 ± 3.3 | 0 (0) | NA | 8 (72.7) | 9.5 ± 1.8 |
| Back (upper), n = 5 | 8 (100) | 9.2 ± 3.2 | 8 (100) | 27.9 ± 10.3 | 8 (100) | 7.5 ± 4.3 | 0 (0) | NA | 2 (25) | 10.5 ± 0.7 |
| Back (nape), n = 2 | 3 (100) | 6.3 ± 3.5 | 3 (100) | 16.7 ± 12.7 | 3 (100) | 6.7 ± 4.5 | 0 (0) | NA | 2 (66.7) | 12.0 ± 0 |
| Back (lipoma), n = 2 | 2 (100) | 12.5 ± 4.9 | 2 (100) | 22.5 ± 0.7 | 2 (100) | 7.0 ± NA | 0 (0) | NA | 1 (50) | 9.0 ± NA |
| Knee (anterior), n = 9 | 15 (100) | 8.9 ± 2.9 | 15 (100) | 23.9 ± 4.9 | 13 (86.7) | 8.0 ± 2.8 | 0 (0) | NA | 12 (80) | 11.2 ± 3.5 |
| Knee (medial), n = 8 | 11 (100) | 10.3 ± 2.7 | 11 (100) | 23.1 ± 5.7 | 11 (100) | 7.2 ± 3.1 | 0 (0) | NA | 9 (81.8) | 9.2 ± 1.6 |
| Chest, n = 2 | 2 (100) | 11.0 ± 1.4 | 2 (100) | 21.5 ± 3.5 | 2 (100) | 7.5 ± 0.7 | 0 (0) | NA | 2 (100) | 8.0 ± 4.2 |
| Neck, n = 2 | 2 (100) | 8.0 ± 1.4 | 2 (100) | 20.5 ± 2.1 | 2 (100) | 5.0 ± 1.4 | 0 (0) | NA | 2 (100) | 6.0 ± 1.4 |
| Anatomic area . | Adverse events . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Edema . | Numbness . | Tenderness . | Paresis . | Bruising . | |||||
| Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | Incidence, no. of sites (%) . | Duration (days)/session, mean ± SD . | |
| All areas, N = 201 | 462 (100) | 7.4 ± 4.0 | 460 (99.6) | 23.1 ± 9.8 | 435 (94.2) | 5.5 ± 3.7 | 12 (2.6) | 8.6 ± 7.6 | 153 (33.1) | 9.8 ± 3.5 |
| Jowl, n = 135 | 270 (100) | 5.7 ± 3.5 | 270(100) | 22.5 ± 11.0 | 247 (91.5) | 3.8 ± 2.7 | 10 (3.7) | 8.5 ± 8.4 | 9 (3.3) | 4.7 ± 3.4 |
| Abdomen (upper), n = 19 | 26 (100) | 10.8 ± 3.2 | 26 (100) | 25.3 ± 8.7 | 26 (100) | 7.5 ± 2.9 | 0 (0) | NA | 23 (88.5) | 10.4 ± 3.2 |
| Abdomen (lower), n = 19 | 26 (100) | 11.0 ± 3.2 | 26 (100) | 25.5 ± 8.9 | 26 (100) | 8.0 ± 3.1 | 0 (0) | NA | 22 (84.6) | 9.8 ± 2.7 |
| Thigh (inner), n = 10 | 13 (100) | 11.9 ± 5.2 | 13 (100) | 23.8 ± 11.4 | 13 (100) | 7.8 ± 4.2 | 0 (0) | NA | 7 (53.8) | 13.4 ± 3.9 |
| Thigh (outer), n = 13 | 17 (100) | 10.6 ± 3.1 | 17 (100) | 21.0 ± 8.3 | 17 (100) | 9.3 ± 4.5 | 0 (0) | NA | 16 (94.1) | 10.1 ± 3.4 |
| Thigh (banana roll), n = 6 | 6 (100) | 11.2 ± 2.2 | 6 (100) | 20.0 ± 2.8 | 6 (100) | 9.3 ± 3.1 | 0 (0) | NA | 5 (83.3) | 10.6 ± 2.9 |
| Arm, n = 16 | 21 (100) | 8.9 ± 3.1 | 19 (90.5) | 26.8 ± 5.6 | 19 (90.5) | 9.2 ± 4.6 | 1 (4.8) | 9.0 ± NA | 17 (81.0) | 11.9 ± 3.1 |
| APAF, n = 15 | 29 (100) | 6.6 ± 2.7 | 29 (100) | 21.2 ± 4.9 | 29 (100) | 6.1 ± 3.0 | 1 (3.4) | 9.0 ± NA | 16 (55.2) | 6.8 ± 2.1 |
| Back (lower), n = 9 | 11 (100) | 11.4 ± 2.3 | 11 (100) | 27.0 ± 9.2 | 11 (100) | 7.9 ± 3.3 | 0 (0) | NA | 8 (72.7) | 9.5 ± 1.8 |
| Back (upper), n = 5 | 8 (100) | 9.2 ± 3.2 | 8 (100) | 27.9 ± 10.3 | 8 (100) | 7.5 ± 4.3 | 0 (0) | NA | 2 (25) | 10.5 ± 0.7 |
| Back (nape), n = 2 | 3 (100) | 6.3 ± 3.5 | 3 (100) | 16.7 ± 12.7 | 3 (100) | 6.7 ± 4.5 | 0 (0) | NA | 2 (66.7) | 12.0 ± 0 |
| Back (lipoma), n = 2 | 2 (100) | 12.5 ± 4.9 | 2 (100) | 22.5 ± 0.7 | 2 (100) | 7.0 ± NA | 0 (0) | NA | 1 (50) | 9.0 ± NA |
| Knee (anterior), n = 9 | 15 (100) | 8.9 ± 2.9 | 15 (100) | 23.9 ± 4.9 | 13 (86.7) | 8.0 ± 2.8 | 0 (0) | NA | 12 (80) | 11.2 ± 3.5 |
| Knee (medial), n = 8 | 11 (100) | 10.3 ± 2.7 | 11 (100) | 23.1 ± 5.7 | 11 (100) | 7.2 ± 3.1 | 0 (0) | NA | 9 (81.8) | 9.2 ± 1.6 |
| Chest, n = 2 | 2 (100) | 11.0 ± 1.4 | 2 (100) | 21.5 ± 3.5 | 2 (100) | 7.5 ± 0.7 | 0 (0) | NA | 2 (100) | 8.0 ± 4.2 |
| Neck, n = 2 | 2 (100) | 8.0 ± 1.4 | 2 (100) | 20.5 ± 2.1 | 2 (100) | 5.0 ± 1.4 | 0 (0) | NA | 2 (100) | 6.0 ± 1.4 |
APAF, anterior periaxillary fat; N, total number of patients enrolled in the study; n, patients who received ATX-101 injections at a particular anatomic area; NA, not applicable; SD, standard deviation.
Edema was experienced by all patients after each treatment session with ATX-101 and lasted for a mean of 16.9 days per patient. The mean duration of edema per session per site was 7.4 days. The mean duration of edema was the longest for the treatment of back lipoma (12.5 days) and the shortest for the jowl (5.7 days). Numbness was experienced after 99.6% of sessions with ATX-101. Numbness was noted after every treatment session in the jowl, abdomen (upper and lower), thigh (inner, outer, and banana roll), APAF, back (upper, lower, nape, and lipoma), knee (anterior and medial), chest, and neck. Numbness occurred the least in the arm. The mean total duration of numbness experienced per patient was 52.8 days, and the mean duration of numbness per session per site was 23.1 days. Tenderness was experienced after 94.2% of sessions with ATX-101. Tenderness occurred after all treatment sessions in the abdomen (upper and lower), thigh (inner, outer, and banana roll), APAF, back (upper, lower, nape, and lipoma), knee (medial), chest, and neck and was the least in the knee (anterior). The mean total duration of tenderness experienced per patient was 12.3 days, and the mean duration of tenderness per session per site was 5.5 days. Bruising was experienced after 33.1% of sessions with ATX-101. Bruising was most observed in the chest and neck and least observed in the jowl. The mean total duration of bruising experienced per patient was 19.6 days, and the mean duration of bruising per session per site was 9.8 days. Supplemental Figure 2, available online at www.aestheticsurgeryjournal.com, shows the presence of mild bruising along with edema 7 days after treatment with ATX-101. Paresis was experienced after 2.6% of sessions with ATX-101 and was only observed in the arm, jowl, and APAF (least observed). The mean total duration of paresis experienced per patient was 11.4 days and the mean duration of paresis per session per site was 8.6 days. No paresis occurred after treatment in the abdomen, thigh, back, knee, chest, or neck (Table 5).
None of the patients experienced alopecia after any session of ATX-101. Mild hemosiderin staining at the outer thigh or knee was observed in 3 patients and was assessed to be nonserious and related to the treatment.
DISCUSSION
In the past decade, nonsurgical body contouring has become one of the fastest growing domains of aesthetic medicine due to the prioritization of well-being and lifestyle.3,15 This study reports the treatment and safety data of 201 patients who opted for nonsurgical subcutaneous fat reduction via adipocytolysis with ATX-101 injections. Although the mean number of treatment sessions was similar for most anatomic sites, the volume of ATX-101 injected and the time interval between consecutive treatment sessions varied with each anatomic site. Overall, jawline and body contouring with ATX-101 was a well-tolerated treatment option for patients seeking reduction of excess subcutaneous fat, because all AEs were mostly mild and resolved within a mean of 4 to 28 days depending on the anatomic areas treated.
Weight reduction is not restricted to a certain body area. Although region-specific exercise can improve the musculature of the area, it may not specifically eliminate excess fat.16 Following the success of targeted SMF reduction with ATX-101 and the consequent improvement in psychological factors of patients, exploration of ATX-101 utilization in various areas of the face and body has provided the opportunity to address region-specific fat deposits.7,17,18,19 Especially since some individuals associate skin laxity or the presence of excess subcutaneous fat in conspicuous areas with a loss of social visibility, there has been an increased interest in undergoing multiple treatment sessions to perfect the jowl, considering it is a facial feature that can show prominent improvement and entails lower treatment costs due to its smaller surface area, which would require the least amount of ATX-101 and fewer injections.20,21 Multiple treatment sessions were more common among patients receiving ATX-101 injections for jowl fat reduction compared with those required for other anatomic sites. While patients overall underwent 1.8 treatment sessions on average, treatment of the chest, a much larger body surface area needing large quantities of ATX-101, comprised only 1 session per patient. This could be attributed to the large surface area treated entailing higher costs, high number of injections required per session, satisfaction with the results, and reluctance among men to undergo multiple cosmetic treatment sessions.22 In addition, it might simply be a reflection of the small number of patients undergoing chest treatment with ATX-101. Nonetheless, it is imperative to consider the fat distribution and thickness and treatment goals of the patients while deciding the number of treatment session and injections per session required.23 Our results were reflected in the REFINE trials, where approximately 20% of ATX-101–treated patients received <6 treatment sessions because of patient satisfaction or insufficient SMF, thereby underscoring the importance of tailoring treatment for patients opting for ATX-101 from the outset.24 Furthermore, because ATX-101 therapy is designed to eliminate fat cells, retreatment is often not required. Additionally, it offers the advantage of maintaining improved appearance for up to 3 years after the initial treatment in most patients.12
Individual patient attitudes to ATX-101 are contingent on the level of results achieved and the number of treatments required.23,25 Therefore, careful counseling of patients on the number of treatment sessions potentially required for each anatomic area is vital to address their concerns and manage expectations, which in turn can augment patient compliance and their willingness to participate in or repeat treatment with ATX-101.23 Upon successful treatment of an anatomic area, patients may also initiate or repeat a procedure in other anatomic areas. In this study, some patients were administered treatment with ATX-101 on several anatomic areas in a single day and therefore underwent more ATX-101 treatments in fewer treatment sessions. To permit patient recovery, resolution of the anticipated inflammatory response, and remodeling of treatment area tissues following the destruction of fat cells, ATX-101 treatments are typically spaced 6 to 8 weeks apart.23 Although all patients are only re-treated after a minimum of 6 weeks, patients targeting tissue containing mature adipocytes may see visible results much sooner than those targeting protein-rich cells, because the former are more susceptible to the cytolytic action of ATX-101 cells.26 Hence, time intervals between consecutive sessions may vary depending on patient preference, prolonged downtime, and costs incurred, which in turn depend on the treated area and the corresponding volume of ATX-101 required.
Treatment volumes administered to patients in each anatomic area may differ based on the size of the treatment area, the remaining volume of fat, and its distribution following consecutive sessions.23 As the surface area diminishes, lower volumes of ATX-101 will be required. For example, in the REFINE trials, 5.4 mL of ATX-101 was administered per treatment session, but this volume decreased with subsequent treatment sessions. Conversely, in the CONTOUR trial, 3.2 to 3.5 mL of ATX-101 was administered per treatment session, but this volume remained relatively unchanged over time.23,24,27 In this study, the increase in the mean volume of ATX-101 administered to the abdomen (upper) with each consecutive session may be attributed to the heterogeneity in fat distribution of patients, wherein few patients with less fat required fewer sessions and lower mean doses compared with those with a higher fat composition who required multiple sessions with higher mean doses of ATX-101. For instance, although subcutaneous adipose tissue tends to accumulate in the gluteal, femoral, and abdominal regions, it may be distributed differently among patients depending on an individual's physiological and hormonal factors.28 In addition, with increasing age, the proportion of adipose tissue increases; in men, this increase occurs primarily above the waist due to declining testosterone levels.29 However, certain anatomic regions with smaller surface areas, such as the jowl, can resist changes induced by diet and exercise and are thus prime targets for injection adipocytolysis.30
Additional care should be taken in terms of injection volume depending on the area of the targeted body region. A significant reduction in the volume of ATX-101 administered to the patients’ jowls vs other anatomic areas can be due to the smaller surface area of the jowl, which requires careful titration that is not too diluted by the local anesthetic. Smaller injection volumes are advisable for the treatment of small surface areas, such as the jowl, to avoid damage to the marginal mandibular nerve superficial to the facial artery and vein.31
In addition to demonstrating promising results in terms of visible fat reduction, ATX-101 was well tolerated. In this study, most AEs across anatomic areas related to ATX-101 were mild and occurred at the treatment site within a short period after treatment as observed previously with SMF.32 As expected, the safety profile of ATX-101 resembled aspects of subcutaneous administration, mechanism of action, and local tissue response.17,24 This included all patients experiencing edema, albeit for a short duration. This transient AE is indicative of treatment progress being in line with previously reported rapid absorption and elimination of ATX-101 by protein binding.23,33 Contrary to the findings in the REFINE and CONTOUR trials, which have reported incidence rates of 0.4% and 0.2% for alopecia, respectively, alopecia was not observed in any patient in the current study.23
Because the last date of follow-up for each patient was not recorded, the average patient follow-up time could not be determined. Other limitations of this study include the missed opportunity of documenting patient satisfaction after the procedure as well as the high costs that may result in selection bias among patients who have the means and are keener to lose the remaining subcutaneous fat reserves. Furthermore, patients opting for treatment of certain anatomic areas, such as the back, chest, and neck, were fewer compared with those opting for treatment of other anatomic regions. The strength of this study lies in its inclusion of a diverse patient population while also providing clinical insights into a comprehensive list of anatomic areas that can be subjected to fat reduction employing ATX-101. To our knowledge, this is the first study that presents a consolidated analysis of undergoing treatment with ATX-101 across several anatomic areas. The treatment parameters such as number of treatment sessions, injections, volumes, and time intervals reported in this study can guide clinical practitioners in safely administering ATX-101 treatments for facial and body contouring. Nonetheless, additional studies are needed to validate the efficacy of ATX-101 injections for reducing excess fat across multiple areas of the face and body.
CONCLUSIONS
ATX-101 is a nonsurgical, in-office procedure that allows physicians to customize the treatment paradigm for each patient in terms of treatment sessions, dose, volume administered, and time intervals based on the distribution of excess subcutaneous fat, patient and physician satisfaction with aesthetic improvements, and achievement of treatment goals. Furthermore, ATX-101 was found to be a well-tolerated alternative to contemporary noninvasive treatment modalities for subcutaneous fat reduction in several anatomic areas, apart from SMF.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Disclosures
Dr Shridharani serves as an advisory board member and consultant for Allergan, Inc. (Irvine, CA) and has received payment from Allergan, Inc. for writing and reviewing the manuscript. The other authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
Medical writing support was provided by Nidhi Kona, MSc, of Cactus Life Sciences (part of Cactus Communications; Mumbai, Maharashtra, India) and funded by Allergan, Inc (Irvine, CA). Medical writing services were supported by Allergan Aesthetics, an AbbVie company. No honoraria or payments were made for authorship. The data that support the findings of this study are available from the corresponding author, Dr Shridharani, upon reasonable request.
REFERENCES
Author notes
Dr Shridharani is an associate clinical professor of plastic surgery, Washington University—St. Louis, School of Medicine, St. Louis, MO, USA and is a Cosmetic Medicine section editor for Aesthetic Surgery Journal.
Ms Tisch is an intern at a private practice in New York, NY, USA.
Ms Kennedy is the director of clinical research at a private practice in New York, NY, USA.



