-
PDF
- Split View
-
Views
-
Cite
Cite
A Bert Chabot, Salomon Puyana, John T Lindsey, The Use of Mean Gray Value (MGV) as a Guide to Tension-Reducing Strategies in Body Contouring Surgery Reduces Wound-Related Morbidity, Aesthetic Surgery Journal, Volume 43, Issue 2, February 2023, Pages NP122–NP130, https://doi.org/10.1093/asj/sjac223
Close - Share Icon Share
Abstract
Currently there are no known structural parameters of the integument that can be measured noninvasively which are used in the planning of body contouring surgery.
The aim of this study was to see if mean gray value (MGV), when taken into account preoperatively, can reduce wound-related morbidity.
This project was a prospective cohort study. Ultrasound imaging of the subcutaneous tissue was performed prospectively on patients undergoing body contouring surgery to quantify the superficial fascial system so that average MGV could be calculated over the proposed surgical sites. Patients with average to poor MGV (≤0.127) were identified preoperatively for tension-reducing procedures. Wound complication rates were compared with rates in a retrospective cohort which did not undergo preoperative imaging.
There were 115 patients in each of the 2 cohorts. There were 3 exclusions due to loss of ultrasound images, leaving 112 patients available for analysis in the prospective cohort. The cohorts were similar except for a higher incidence of patients with diabetes in the retrospective group (1 vs 9, P = 0.026). The wound complication rate was significantly reduced in the prospective group (5/112, 4.4%) when compared with the retrospective group (20/115, 17%, P = 0.0062). The revision and infection rates were also significantly reduced in the prospective group (1/112, 0.9%; 3/112, 2.6%) when compared with the retrospective group (8/115, 7%, P = 0.019; 10/115 8.6%, P = 0.051).
MGV is a unique, patient- and area-specific structural parameter of the integument, and its measurement may be useful in reducing wound-related morbidity in body contouring surgery.

See the Commentary on this article here.
Wound complication rates remain persistently high in body contouring surgery despite advances in surgical technique and patient care.1-4 Body contouring procedures are the third most frequent source of litigation in plastic surgery, and wound complications account for 40% of all claims in the field.5 Current predictors of patient morbidity are indirect, physiologic parameters such as smoking status, diabetes, BMI, anemia, vitamin deficiency, and nutritional status, all of which should be assessed and modified. preoperatively during the medical optimization process.1,6-8 Here we report on patients who have all been medically optimized and screened, and who present as good candidates for body contouring surgery.
Plastic surgeons must always balance patient desires for maximal improvement in body contour and aesthetic enhancement with patient safety and avoidance of morbidity. Until now, this has been a subjective risk assessment by the surgeon, balancing safe tissue resection and tension with contour improvement.
Repair of the superficial fascial system (SFS), originally espoused by Ted Lockwood, continues to be a fundamental part of body contouring surgery.6,8 It is also known that the SFS waxes and wanes throughout the body and is variable within the same patient and among different patients, and this variability can be detected by computed tomography scanning, MRI, ultrasound, and from histologic sections.9-14 Our research group has previously reported that new, portable, high-resolution ultrasound can also image this variability.15 It is our hypothesis that the ability to preoperatively quantify patient- and area-specific SFS (or collagen content) may be a useful tool in the planning of body contouring surgery.
Our research group has previously reported ultrasonographic SFS patterns, and has demonstrated that MGV correlates positively with subcutaneous tissue collagen content and suture pullout strength.16 In a retrospective study of 36 patients undergoing body contouring, all patients experiencing wound complications had an MGV of <0.127.17 In this current study, MGV was used to identify patients at risk for wound morbidity and to prospectively plan for tension-reducing procedures accordingly.
METHODS
This project was a prospective cohort study. The East Jefferson General Hospital (Metairie, LA) IRB approved this study (EJ-JL-2102). Ultrasound imaging of the subcutaneous tissue was performed prospectively on all patients undergoing body contouring surgery. All imaging was done by the senior author in B mode (gray scale) with the Lumify app (Philips, Amsterdam, The Netherlands) on a Samsung Galaxy Tab A tablet (Samsung Electronics, Suwon-si, South Korea) on the superficial setting, gain 54, and with depth settings between 1.5 and 4 cm, depending on the thickness of the subcutaneous tissue. A Linear Array 12-4 probe (Philips) was used for all ultrasound imaging. For patients undergoing abdominoplasty, 4 images of subcutaneous tissues were taken: periumbilical, suprapubic, and right and left lower quadrants. For patients undergoing brachioplasty, 3 images were taken over the tissue to be resected in the upper, mid-, and lower arm areas. For patients undergoing thigh lifts, 3 images were taken over the tissue to be resected in the anterior, mid- and posterior upper inner thigh areas. For patients having combined thigh lifts and reductions, additional images were taken in the mid- and lower inner thigh areas. Images were cropped with XnView (xnview.com, XnSoft; Reims, France) to exclude overlying dermis and underlying muscle and muscle fascia and then analyzed with CellProfiler (cellprofiler.org, Broad Institute; Cambridge, MA) to obtain a preoperative MGV in designated areas over the proposed surgical site. The MGVs of all areas imaged were then averaged to give an average MGV for the body area to be contoured. Patients with average to poor MGV (≤0.127) were identified preoperatively for tension-reducing procedures. All adult patients deemed medically and mentally appropriate for plastic surgery were included in this study which was conducted between May 2016 and September 2021. There were no exclusion criteria.
Tension-Reducing Strategies
Clinical maneuvers undertaken to reduce tension closure in body contouring surgery were as follows: (1) removal of skin excess such that cut skin flaps lay in apposition prior to final closure (Figure 1); (2) avoidance of pleating to make up differences in skin length mismatch (Figure 2); and (3) avoidance of postural body changes to facilitate wound closure (Figures 3, 4). Incisions were lengthened to minimize pleating. For patients undergoing abdominoplasty, flexion at the waist was limited to no more than 10° as part of “beach chair” positioning. For patients with MGV > 0.127, waist flexion was as much as 40°. For patients undergoing thigh lift closure, skin resection was done with skin apposition at 30° of thigh abduction rather than with the thighs fully adducted.
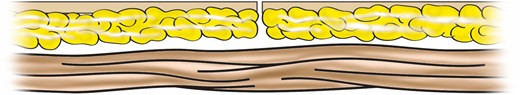
Skin flap margins in apposition after measured resection prior to final layered wound closure for patients with mean gray value <0.127.
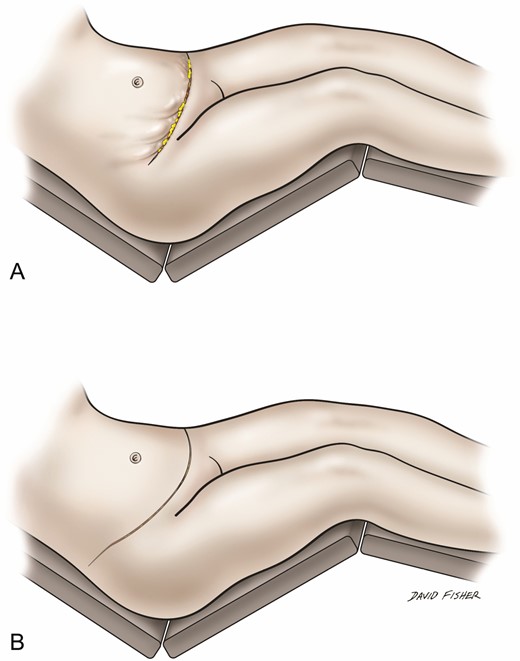
Avoidance of pleating: (A) pleating along lateral abdominoplasty incision and (B) incision extended laterally to make up skin length mismatch and avoid pleating.
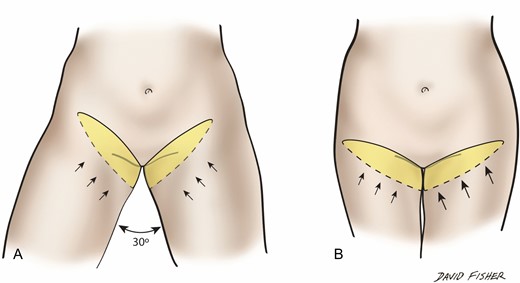
Thigh lift skin excision is planned and measured (A) with thighs at 30° of abduction rather than (B) with the thighs fully adducted.
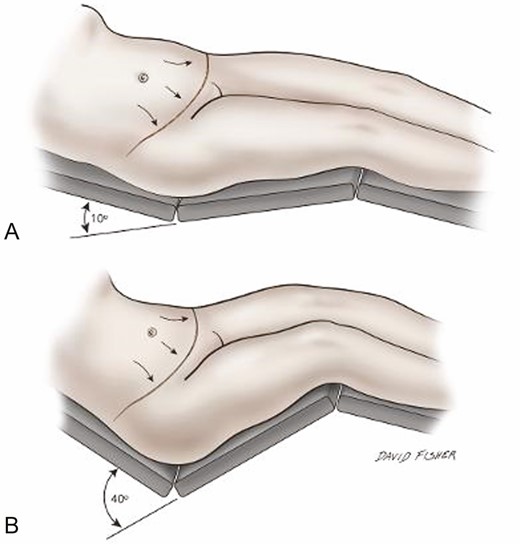
Abdominoplasty skin excision is planned and measured (A) with waist flexed at 10° or less rather than (B) with typical waist flexion.
Wound complication rates were compared with those of a retrospective cohort. Wounds were classified as shown in Table 1 and stratified according to the Dindo-Clavien grading system.18 Major wound complications required return to the operating room for management (Dindo-Clavien IIIb). Wound complications were defined as any wound healing problem that was managed in an outpatient office setting but still required an intervention such as incision and drainage, packing, or minor bedside debridement (Dindo-Clavien I). Wound healing imperfections were identified as small areas of scabbing, eschar, or dehiscence that required nothing more than observation or topical antibiotic treatment and did not interfere with the planned patient recovery (no complication according to Dindo-Clavien due to no deviation from the normal postoperative course). Infections were defined as any wound requiring antibiotics in the postoperative period.
| Degree of complication . | Classification . |
|---|---|
| Major complication | (Dindo-Clavien IIIb) Return to operating room |
| Complication | (Dindo-Clavien I) Managed in an office setting >1.5 cm × 3 cm externally and 1 cm deep or Packing required or Requiring bedside incision and drainage or minor office debridement |
| Would healing imperfection | (Not considered complication) Managed in an office setting <1.5 cm × 3 cm externally and 1 cm deep or Small eschar not requiring debridement or Managed with observation alone or topical antibiotic ointment |
| Degree of complication . | Classification . |
|---|---|
| Major complication | (Dindo-Clavien IIIb) Return to operating room |
| Complication | (Dindo-Clavien I) Managed in an office setting >1.5 cm × 3 cm externally and 1 cm deep or Packing required or Requiring bedside incision and drainage or minor office debridement |
| Would healing imperfection | (Not considered complication) Managed in an office setting <1.5 cm × 3 cm externally and 1 cm deep or Small eschar not requiring debridement or Managed with observation alone or topical antibiotic ointment |
| Degree of complication . | Classification . |
|---|---|
| Major complication | (Dindo-Clavien IIIb) Return to operating room |
| Complication | (Dindo-Clavien I) Managed in an office setting >1.5 cm × 3 cm externally and 1 cm deep or Packing required or Requiring bedside incision and drainage or minor office debridement |
| Would healing imperfection | (Not considered complication) Managed in an office setting <1.5 cm × 3 cm externally and 1 cm deep or Small eschar not requiring debridement or Managed with observation alone or topical antibiotic ointment |
| Degree of complication . | Classification . |
|---|---|
| Major complication | (Dindo-Clavien IIIb) Return to operating room |
| Complication | (Dindo-Clavien I) Managed in an office setting >1.5 cm × 3 cm externally and 1 cm deep or Packing required or Requiring bedside incision and drainage or minor office debridement |
| Would healing imperfection | (Not considered complication) Managed in an office setting <1.5 cm × 3 cm externally and 1 cm deep or Small eschar not requiring debridement or Managed with observation alone or topical antibiotic ointment |
Patients selected for inclusion in this study had abdominoplasties, brachioplasties, or thigh lifts with or without thigh reduction. Procedures were all performed by the senior author, who used the same suture materials and techniques for each type of procedure. All wounds were closed in a similar 3-layered fashion. For abdominoplasties, 2-0 Vicryl (Ethicon, Inc., New Brunswick, NJ) was used in the subcutaneous layer followed by 0 Quill (Ethicon, Inc.) in the dermis and 2-0 Quill in the subcuticular layer. Progressive tension sutures with 2-0 Vicryl were routinely used for all abdominoplasties. Abdominoplasty technique included supraumbilical undermining, umbilical transposition, rectus abdominis plication, and liposuction of the flanks. For brachioplasties, 3-0 Vicryl was used in the subcutaneous layer followed by 2-0 Quill in the dermis and 3-0 Quill in the subcuticular layer. For the thigh lifts, 0 Vicryl was used in the subcutaneous layer fixed to the periosteum of the inferior pubic ramus followed by 2-0 Vicryl in the dermis followed by a running simple 2-0 Proline (Ethicon, Inc.) superficially. The primary endpoint for this study was wound healing. Continuous variables were compared by t test. Categorical variables were compared by Fisher’s exact test.
RESULTS
In total, 230 patients underwent major body contouring surgery under general anesthesia at the same accredited ambulatory surgery facility between May 26, 2016 and September 20, 2021 by the senior author. The average age was 46.75 years (range, 22-75 years). There were 222 females and 8 males. All patients who received major body contouring surgery between these dates were included. Patients had the option to opt out of the study if they were not interested in participating, but none decided to do so. There were no changes in surgical techniques or suture materials during the study period. All patients after August 15, 2018 received ultrasound imaging of the subcutaneous tissues followed by image processing and calculation of MGV. There were 115 patients in each of the 2 cohorts. There were 3 exclusions in the prospective abdominoplasty group due to loss of ultrasound images, leaving 112 patients available for analysis. None of the excluded patients experienced wound complications.
All patients were available for follow-up until healed and discharged. The average follow-up for the prospective and retrospective groups was 233.5 days (range, 33-1069 days) and 253 days (43-1782 days), respectively. The cohorts were similar except for a higher incidence of patients with diabetes in the retrospective group (1 vs 9, P = 0.026). The patient with diabetes identified in the prospective group was non–insulin dependent controlled on an oral agent and did not suffer a wound complication. Two of the 9 patients with diabetes identified in the retrospective group suffered a wound complication. Of the 9 patients with diabetes in the retrospective group, 7 were non–insulin dependent controlled on an oral agent, 1 was a diet-controlled, and 1 was type 1 insulin-dependent. There was 1 patient in the prospective group who failed to meet the criteria of “nonsmoker,” meaning either never-smoker or completely abstinent for a minimum of 7 weeks preoperatively, and this patient did not suffer a wound complication. The types of procedures (abdominoplasty, brachioplasty, and thigh lift) were symmetrically distributed between the 2 groups. There was no statistically significant difference between the 2 groups in patients who had received previous bariatric surgery. No nutritional deficiency or anemia was identified at the time of surgery in either group. No patient was deemed in need of a medically necessary procedure related to body contouring, meaning that no patient had a history of panniculitis, furunculosis, or intractable intertrigo that failed medical therapy; thus, all patients were self-pay, cosmetic patients. A summary of group comparisons is shown in Table 2.
| . | Prospective . | Retrospective . | P-value . |
|---|---|---|---|
| Age (years) | 45.9 (24-72) | 47.6 (22-75) | 0.313 |
| BMI (kg/m2) | 29.2 (17.6-50.3) | 28.1 (19.08-39.29) | 0.083 |
| Weight resected (g) | 1045.6 (50-6900) | 1180.4 (30-13,154) | 0.450 |
| Diabetes | 1 | 9 | 0.026 |
| Smoking | 1 | 0 | — |
| History of massive weight lossa | 4 | 8 | 0.254 |
| History of bariatric surgery | 23 | 30 | 0.323 |
| Abdominoplasty | 82 | 85 | — |
| Brachioplasty | 22 | 22 | — |
| Thigh lift/reduction | 8 | 8 | — |
| Healing imperfection | 18 | 15 | 0.647 |
| Wound complication | 5 | 19 | 0.006 |
| Major wound complication | 0 | 1 | 0.978 |
| Revision rates | 1 | 8 | 0.019 |
| Seromas | 1 | 2 | 0.576 |
| Pulmonary embolism | 0 | 1 | — |
| Hematoma | 1 | 0 | — |
| Wound infection | 3 | 10 | 0.051 |
| Follow-up (days) | 233.5 (35-1069) | 253 (43-1782) | |
| Total patients | 112 | 115 |
| . | Prospective . | Retrospective . | P-value . |
|---|---|---|---|
| Age (years) | 45.9 (24-72) | 47.6 (22-75) | 0.313 |
| BMI (kg/m2) | 29.2 (17.6-50.3) | 28.1 (19.08-39.29) | 0.083 |
| Weight resected (g) | 1045.6 (50-6900) | 1180.4 (30-13,154) | 0.450 |
| Diabetes | 1 | 9 | 0.026 |
| Smoking | 1 | 0 | — |
| History of massive weight lossa | 4 | 8 | 0.254 |
| History of bariatric surgery | 23 | 30 | 0.323 |
| Abdominoplasty | 82 | 85 | — |
| Brachioplasty | 22 | 22 | — |
| Thigh lift/reduction | 8 | 8 | — |
| Healing imperfection | 18 | 15 | 0.647 |
| Wound complication | 5 | 19 | 0.006 |
| Major wound complication | 0 | 1 | 0.978 |
| Revision rates | 1 | 8 | 0.019 |
| Seromas | 1 | 2 | 0.576 |
| Pulmonary embolism | 0 | 1 | — |
| Hematoma | 1 | 0 | — |
| Wound infection | 3 | 10 | 0.051 |
| Follow-up (days) | 233.5 (35-1069) | 253 (43-1782) | |
| Total patients | 112 | 115 |
Values are mean (range) or number.
aDefined as greater than 50-pound (22.7-kg) weight loss by nonsurgical means.
| . | Prospective . | Retrospective . | P-value . |
|---|---|---|---|
| Age (years) | 45.9 (24-72) | 47.6 (22-75) | 0.313 |
| BMI (kg/m2) | 29.2 (17.6-50.3) | 28.1 (19.08-39.29) | 0.083 |
| Weight resected (g) | 1045.6 (50-6900) | 1180.4 (30-13,154) | 0.450 |
| Diabetes | 1 | 9 | 0.026 |
| Smoking | 1 | 0 | — |
| History of massive weight lossa | 4 | 8 | 0.254 |
| History of bariatric surgery | 23 | 30 | 0.323 |
| Abdominoplasty | 82 | 85 | — |
| Brachioplasty | 22 | 22 | — |
| Thigh lift/reduction | 8 | 8 | — |
| Healing imperfection | 18 | 15 | 0.647 |
| Wound complication | 5 | 19 | 0.006 |
| Major wound complication | 0 | 1 | 0.978 |
| Revision rates | 1 | 8 | 0.019 |
| Seromas | 1 | 2 | 0.576 |
| Pulmonary embolism | 0 | 1 | — |
| Hematoma | 1 | 0 | — |
| Wound infection | 3 | 10 | 0.051 |
| Follow-up (days) | 233.5 (35-1069) | 253 (43-1782) | |
| Total patients | 112 | 115 |
| . | Prospective . | Retrospective . | P-value . |
|---|---|---|---|
| Age (years) | 45.9 (24-72) | 47.6 (22-75) | 0.313 |
| BMI (kg/m2) | 29.2 (17.6-50.3) | 28.1 (19.08-39.29) | 0.083 |
| Weight resected (g) | 1045.6 (50-6900) | 1180.4 (30-13,154) | 0.450 |
| Diabetes | 1 | 9 | 0.026 |
| Smoking | 1 | 0 | — |
| History of massive weight lossa | 4 | 8 | 0.254 |
| History of bariatric surgery | 23 | 30 | 0.323 |
| Abdominoplasty | 82 | 85 | — |
| Brachioplasty | 22 | 22 | — |
| Thigh lift/reduction | 8 | 8 | — |
| Healing imperfection | 18 | 15 | 0.647 |
| Wound complication | 5 | 19 | 0.006 |
| Major wound complication | 0 | 1 | 0.978 |
| Revision rates | 1 | 8 | 0.019 |
| Seromas | 1 | 2 | 0.576 |
| Pulmonary embolism | 0 | 1 | — |
| Hematoma | 1 | 0 | — |
| Wound infection | 3 | 10 | 0.051 |
| Follow-up (days) | 233.5 (35-1069) | 253 (43-1782) | |
| Total patients | 112 | 115 |
Values are mean (range) or number.
aDefined as greater than 50-pound (22.7-kg) weight loss by nonsurgical means.
The wound complication rate was significantly reduced in the prospective group (5/112, 4.4%) compared with the retrospective group (20/115, 17%, P = 0.0062). The incidence of wound healing imperfections was not statistically significantly different between the 2 groups (16% vs 13%, P = 0.647). Among all patients, there was only 1 major wound complication, and this occurred in the retrospective group.
The revision rate was significantly reduced in the prospective group (1/112, 0.9%) compared with the retrospective group (8/115, 7%, P = 0.019). All revisions were performed in the office setting under local anesthesia and were related to unsatisfactory scarring attributable to wound complications.
The wound infection rate was significantly reduced in the prospective cohort (3/112, 2.6%) compared with the retrospective group (10/115, 8.6%, P = 0.051). All wound infections were managed in the office setting with oral antibiotics in both groups.
Low MGV (≤0.127) was identified in 55 of 112 (49%) patients in the prospective group, and this finding resulted in a preoperatively planned tension-reducing procedure. The most common morbidity in this study was related to wound healing. In this study there were only 3 seromas (3/227 patients, 1.3%), all managed nonoperatively with serial aspirations or placement of a Seromacath (Summit Medical, Berkeley Heights, NJ). There was 1 pulmonary embolism (1/227,0.44%), managed with outpatient subcutaneous Lovenox, and 1 hematoma (1/227, 0.44%), managed with office incision and drainage. There were no admissions for cellulitis, pneumonia, or any other procedure-related complication. There was no mortality.
A subanalysis of patients undergoing abdominoplasty was also performed. Comparisons between the prospective and retrospective groups showed more homogeneity (Table 3). When analyzing abdominoplasty alone, the wound complication rate was also significantly reduced in the prospective group (2/82, 2.4%) compared with the retrospective group (11/85, 12.9%, P = 0.025).
| . | Prospective . | Retrospective . | P-value . |
|---|---|---|---|
| Number of patients | 82 | 85 | |
| Age (years) | 43.96 (24-72) | 43.97 (22-70) | 0.99 |
| BMI (kg/m2) | 28.2 (17.6-50.3) | 27.81 (19.08-39.29) | 0.577 |
| Weight resected (g) | 1355.9 (50-6900) | 1532 (154-13,154) | 0.401 |
| Diabetes | 1 | 5 | 0.22 |
| Smoking | 1 | 0 | — |
| History of massive weight lossa | 2 | 4 | 0.71 |
| History of bariatric Surgery | 12 | 12 | 0.887 |
| Healing imperfection | 8 | 10 | 0.86 |
| Wound complications | 2 | 11 | 0.025 |
| Major wound complication | 0 | 1 | — |
| Follow-up (days) | 218 (40-1069) | 267 (43-1782) |
| . | Prospective . | Retrospective . | P-value . |
|---|---|---|---|
| Number of patients | 82 | 85 | |
| Age (years) | 43.96 (24-72) | 43.97 (22-70) | 0.99 |
| BMI (kg/m2) | 28.2 (17.6-50.3) | 27.81 (19.08-39.29) | 0.577 |
| Weight resected (g) | 1355.9 (50-6900) | 1532 (154-13,154) | 0.401 |
| Diabetes | 1 | 5 | 0.22 |
| Smoking | 1 | 0 | — |
| History of massive weight lossa | 2 | 4 | 0.71 |
| History of bariatric Surgery | 12 | 12 | 0.887 |
| Healing imperfection | 8 | 10 | 0.86 |
| Wound complications | 2 | 11 | 0.025 |
| Major wound complication | 0 | 1 | — |
| Follow-up (days) | 218 (40-1069) | 267 (43-1782) |
Values are mean (range) or number.
aDefined as greater than 50-pound (22.7-kg) weight loss by nonsurgical means.
| . | Prospective . | Retrospective . | P-value . |
|---|---|---|---|
| Number of patients | 82 | 85 | |
| Age (years) | 43.96 (24-72) | 43.97 (22-70) | 0.99 |
| BMI (kg/m2) | 28.2 (17.6-50.3) | 27.81 (19.08-39.29) | 0.577 |
| Weight resected (g) | 1355.9 (50-6900) | 1532 (154-13,154) | 0.401 |
| Diabetes | 1 | 5 | 0.22 |
| Smoking | 1 | 0 | — |
| History of massive weight lossa | 2 | 4 | 0.71 |
| History of bariatric Surgery | 12 | 12 | 0.887 |
| Healing imperfection | 8 | 10 | 0.86 |
| Wound complications | 2 | 11 | 0.025 |
| Major wound complication | 0 | 1 | — |
| Follow-up (days) | 218 (40-1069) | 267 (43-1782) |
| . | Prospective . | Retrospective . | P-value . |
|---|---|---|---|
| Number of patients | 82 | 85 | |
| Age (years) | 43.96 (24-72) | 43.97 (22-70) | 0.99 |
| BMI (kg/m2) | 28.2 (17.6-50.3) | 27.81 (19.08-39.29) | 0.577 |
| Weight resected (g) | 1355.9 (50-6900) | 1532 (154-13,154) | 0.401 |
| Diabetes | 1 | 5 | 0.22 |
| Smoking | 1 | 0 | — |
| History of massive weight lossa | 2 | 4 | 0.71 |
| History of bariatric Surgery | 12 | 12 | 0.887 |
| Healing imperfection | 8 | 10 | 0.86 |
| Wound complications | 2 | 11 | 0.025 |
| Major wound complication | 0 | 1 | — |
| Follow-up (days) | 218 (40-1069) | 267 (43-1782) |
Values are mean (range) or number.
aDefined as greater than 50-pound (22.7-kg) weight loss by nonsurgical means.
MGV was analyzed in relation to obesity (BMI) by linear regression (Figure 5). MGV is weakly inversely related to BMI. However, BMI cannot be used as a predictor of MGV or of the presence or absence of SFS due to wide patient-specific variability (Figure 6). A tabulation of MGV by procedure for the prospective group is shown in Table 4.
| . | Abdominoplasty . | Brachioplasty . | Thigh lifts . |
|---|---|---|---|
| Mean MGV | 0.13199607 | 0.13932039 | 0.1815325 |
| Minimum MGV | 0.03539026 | 0.09276608 | 0.14546674 |
| Maximum MGV | 0.29631758 | 0.18518523 | 0.2186263 |
| . | Abdominoplasty . | Brachioplasty . | Thigh lifts . |
|---|---|---|---|
| Mean MGV | 0.13199607 | 0.13932039 | 0.1815325 |
| Minimum MGV | 0.03539026 | 0.09276608 | 0.14546674 |
| Maximum MGV | 0.29631758 | 0.18518523 | 0.2186263 |
MGV, mean gray value.
| . | Abdominoplasty . | Brachioplasty . | Thigh lifts . |
|---|---|---|---|
| Mean MGV | 0.13199607 | 0.13932039 | 0.1815325 |
| Minimum MGV | 0.03539026 | 0.09276608 | 0.14546674 |
| Maximum MGV | 0.29631758 | 0.18518523 | 0.2186263 |
| . | Abdominoplasty . | Brachioplasty . | Thigh lifts . |
|---|---|---|---|
| Mean MGV | 0.13199607 | 0.13932039 | 0.1815325 |
| Minimum MGV | 0.03539026 | 0.09276608 | 0.14546674 |
| Maximum MGV | 0.29631758 | 0.18518523 | 0.2186263 |
MGV, mean gray value.
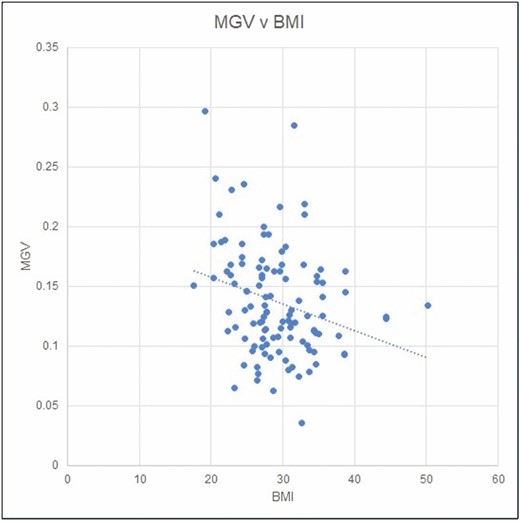
Linear regression analysis of mean gray value (MGV) in relation to obesity (BMI).
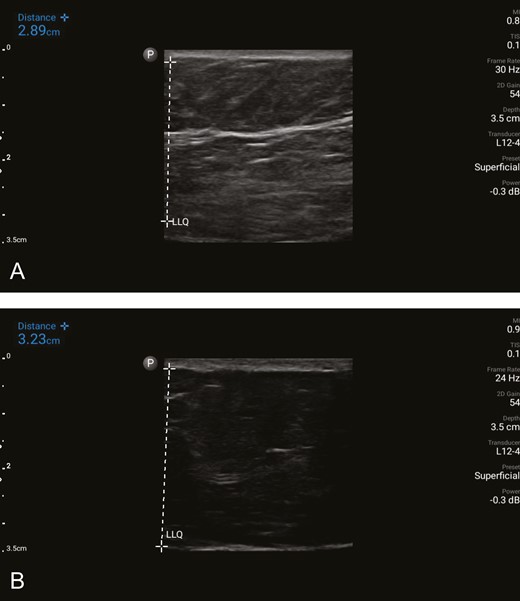
Variation of the SFS in the presence of obesity. (A) Ultrasound image of the subcutaneous tissue in the lower quadrant of the abdomen of a patient with a BMI of 33 kg/m2 undergoing abdominoplasty. A substantial, horizontally oriented, dominant band of SFS is identified in the subcutaneous tissue. The vertically oriented white dashed line marks the subcutaneous layer, measuring 2.89 cm. The corresponding mean gray value is 0.1800279, which indicates strong tissue quality and exceeds the threshold value of 0.127 in this study. (B) Ultrasound image of the subcutaneous tissue in the lower quadrant in a similar patient with a BMI of 33 kg/m2 also undergoing abdominoplasty. The SFS is not identified. The vertically oriented dashed line on the left marks the subcutaneous layer, measuring 3.23 cm. The corresponding mean gray value is 0.025769, indicating poor tissue quality. SFS, superficial fascial system.
There were no patients in the prospective group who were dissatisfied other than related to poor scarring secondary to wound-related complications. All revisions were by patient request in both cohorts, and all revisions were related to unsatisfactory scarring attributable to wound complications. All patients were fully informed of the goals and methods of this study, and all patients seemed to appreciate the additional ultrasound examination and physician attention and time. Calculation of MGV with the current process takes about 25 minutes (after the ultrasound examination). For that reason, MGVs were calculated after clinical hours and were not available at the time of the patient ultrasound examination. Patients were, however, able to see the presence or absence of the strand(s) of SFS in the subcutaneous fat during the ultrasound examination, and the senior author shared those findings with the patient. The senior author would advise patients with poor or absent SFS that they might be at higher risk for possible wound healing problems, and that a tension-reducing approach would be considered for safety. The final surgical plan was not made until the MGVs were known, however.
DISCUSSION
Ultrasound is used throughout both industry and medicine to investigate the underlying structural characteristics of materials and organs. For example, ultrasound elastography is used to quantify liver cirrhosis.19 Echocardiography has long been used to investigate cardiac disease, and the brightness of the sonographic image can be used to quantify myocardial scarring.20 To our knowledge, ultrasound has not been utilized in the interrogation of the integument. Ultrasound has, however, been used to document the variability of the SFS, and correlates well with both computed tomography and MRI analysis.15 Preoperative knowledge of this variability appears to have clinical relevance when planning body contouring procedures. This information can be obtained via low-cost, miniaturized, high-resolution, portable ultrasound technology and readily available image-processing programs. Although MGV was calculated for prospective patients in this study, visual inspection of the images can be useful, as it is easy to identify the presence or absence of 1 or more bands of the SFS, their thickness, and their distribution throughout the subcutaneous tissues. When bands of SFS predominate, MGV is predictably higher (Figure 7). An MGV of 0.127 was selected as the threshold value for performing tension-reducing procedures in this study because all wound complications in our previous study occurred in patients with this value or less.17
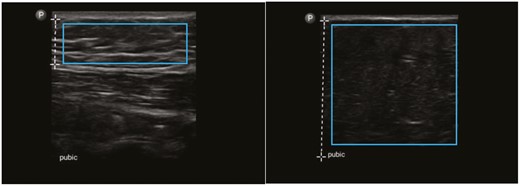
Ultrasound images marked by the vertical dashed lines indicating the differing depths of subcutaneous suprapubic fat in two patients undergoing abdominoplasty. The blue rectangles demonstrate the areas of subcutaneous tissue cropped by XnView and then analyzed by CellProfiler. Left: multiple horizontally-oriented streaks of white, reflective collagen indicate strong SFS in this area with a corresponding MGV of 0.16296. Right: subcutaneous tissue almost devoid of collagen indicates weak SFS with a corresponding MGV of 0.06206.
Ultrasound images can be correlated to intraoperative findings. The dominant band of SFS demonstrated on the preoperative ultrasound study can be identified intraoperatively (Figure 8). Although SFS often exists as a single layer of collagen that can be identified as a bright band on ultrasound, variability is the rule because the SFS layers can be highly variable: bi-, tri-, or multi-laminar, woven, or absent.
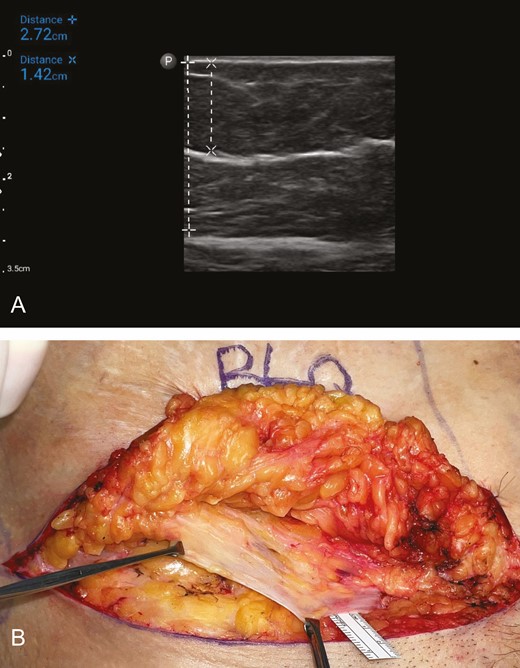
(A) Ultrasound image of the subcutaneous tissue in the right lower quadrant of the abdomen of a 66-year-old female patient with a BMI of 27 kg/m2 undergoing abdominoplasty. The thickness of the subcutaneous tissue is 2.72 cm (vertical dashed line, +---+), and the depth of the dominant band of SFS is 1.42 cm (vertical dashed line, ×---×). The mean gray value is 0.185487, which indicates a robust SFS. (B) Corresponding intraoperative photograph of the same area in the same patient undergoing abdominoplasty. The substantial layer of SFS (Scarpa’s fascia) has been dissected and is being held by the two Allis tissue forceps. SFS, superficial fascial system
The technology required to calculate MGV is far from perfect, however, as the wound complication rate was still 4.4% in the entire prospective group and 2.4% in the prospective subgroup undergoing abdominoplasty. These wounds were all managed in an office setting, and none required a return to the operating room. This study is unique in that we stratified our wound classification system to include healing imperfections, so that outcomes could be analyzed for all cases where healing was not perfect. Wounds classified as healing imperfections did not meet criteria as Dindo-Clavien Class I complications because no treatment was required other than topical antibiotic ointment, if any treatment was needed at all, and there was no delay in the patient’s recovery. According to that wound classification system, this study is remarkable in that it reports a wound complication rate that is clearly a magnitude less than in previous studies.
In a retrospective review of 317 postbariatric patients undergoing body contouring surgery, the major and minor dehiscence rate was 28.2%, the surgical infection rate was 13.2%, and the seroma rate was 8.2%.1 In a retrospective study of 1008 patients undergoing cosmetic traditional abdominoplasty or mini-abdominoplasty in an outpatient community setting, the major complication rate was 18.1% and the minor complication rate was 31.9%.21 In that study, patient demographics and surgical technique were very similar to ours. Seroma, stitch abscess, wound dehiscence, hematoma, abscess, and umbilical necrosis were counted as complications, as in this study. In a systematic review of outcomes of abdominoplasty (both with and without other procedures), the dehiscence rates ranged from 11% to 48%, skin or umbilical necrosis rates from 0% to 18%, seroma rates from 4.1% to 18.6%, and hematoma rates from 1% to 16%.22 In a retrospective study of 206 patients undergoing abdominoplasty either with or without other procedures in a university hospital setting, the overall complication rate was 37.4% and the major complication rate was 16%. This included a seroma rate of 17.4%, a dehiscence rate of 7.2%, a wound necrosis rate of 6.7%, and a hematoma rate of 5.8%.23 In a retrospective study of 80 patients undergoing abdominoplasty, complication rates were 76% in obese patients (which included wound breakdown and hematoma/seroma), 35% in overweight patients, and 33% in normal patients.24 In a retrospective review of 1128 patients undergoing abdominoplasty the hematoma rate was 5.7%, the infection rate was 4.5%, the cutaneous necrosis rate was 2.7%, the seroma rate was 2.7%, and the dehisence rate was 1.3%.25
It is always the goal of the plastic surgeon to achieve the best possible result in body contouring surgery. After optimization of physiologic parameters as much as possible, it always comes down to a subjective assessment between the balance of maximal contour improvement vs safe tissue resection and tension. Using a MGV of ≤0.127 preoperatively to direct more conservative contouring and reduced tension closures resulted in statistically significantly decreased wound complications, infections, and revisions.
It is also notable that complications related to seroma, hematoma, and infection, often reported as the most common complications of body contouring surgery, were also decreased compared with recent reports. The low revision rate (0.9%) in the prospective cohort reflects the lower incidence of wound complications in that group, as all revisions occurred in patients who experienced wound healing complications in both groups. There were statistically more patients with diabetes in the retrospective group, but upon further review of our data, patients with diabetes accounted for only 2 of the 20 wound complications in the retrospective group, and this did not skew the data or alter the statistical significance.
From the senior surgeon’s perspective, the significant reduction of these wound complications improved patient outcomes and experience, and also reduced office stress and expense: all interventions in the office setting involved patient discomfort, additional wound care burden, additional office visits, and often extension of the normal convalescent period. It is our opinion that the investment of time spent performing the ultrasound examination and the calculation of MGV was easily justified by improved patient experience and the reduced number of office interventions. Overall, these results are generalizable to plastic surgery practices with a similar patient demographic desiring body contouring surgery.
The limitations of this study are that it is Level 4 evidence, and these findings need to be further investigated by a randomized prospective controlled study. Further criticism is that it is a single-surgeon study, although that could also be interpreted as a strength because surgical techniques, suture materials, and protocols were consistent throughout the study period. Patient race was also not considered in our study, nor overall financial or educational background. Nonetheless, given patients were self-paying, it is assumed they had enough financial resources to pay for the surgeries. The authors received no funding for this study.
CONCLUSIONS
We introduce a noninvasively measured structural parameter of the subcutaneous tissue that may have clinical significance. MGV reflects the known variability of the SFS, and quantification of this variability preoperatively may have the potential to reduce wound morbidity by identifying body contouring patients who would benefit from tension-reducing strategies.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.



