-
PDF
- Split View
-
Views
-
Cite
Cite
Marshall E Kadin, Haiying Xu, Lisa M Hunsicker, Yingjie Guan, Nonmalignant CD30+ Cells in Contralateral Peri-Implant Capsule of Patient With BIA-ALCL: A Premalignant Step?, Aesthetic Surgery Journal, Volume 42, Issue 2, February 2022, Pages NP125–NP129, https://doi.org/10.1093/asj/sjab215
Close - Share Icon Share
Abstract
CD30 lymphocyte activation antigen and phosphorylated STAT3 (pSTAT3) are consistent markers of tumor cells in breast implant–associated anaplastic large cell lymphoma (BIA-ALCL). We present a case of BIA-ALCL in a breast implant capsule containing clustered tumor cells expressing CD30, pSTAT3, pSTAT6, interleukin 9, and granzyme B tumor cell biomarkers. Remarkably, the contralateral breast contained many scattered large, atypical CD30+ cells surrounded by inflammatory cells, raising a suspicion of bilateral BIA-ALCL, known to occur in some patients. To clarify the diagnosis, immunohistochemistry and multilabel immunofluorescence were performed. Unlike the tumor cells, the atypical CD30+ cells of the contralateral breast lacked pSTAT3, pSTAT6, interleukin 9, and granzyme B, eliminating a diagnosis of bilateral BIA-ALCL. This case highlights the importance of interpreting CD30 staining in the context of other tumor cell biomarkers and histopathology to avoid an incorrect diagnosis of BIA-ALCL. We believe the findings also suggest the possibility of CD30 expression as an early event in the multistep pathogenesis of BIA-ALCL.

CD30 is a lymphocyte activation antigen preferentially expressed by CD4+ T cells producing Th2-type cytokines.1 CD30 is also a consistent marker of malignant cells in anaplastic large cell lymphoma, including breast implant–associated anaplastic large cell lymphoma (BIA-ALCL).2 Hence, it is recommended that any seroma or capsule removed for complications of breast implant be investigated for CD30+ cells to diagnose or exclude BIA-ALCL.3,4 However, atypical CD30+ lymphocytes have been detected in clinically benign seromas, stressing the importance of correlating clinical and pathologic findings.5-7 Atypical CD30+ cells in capsules surrounding breast implants and their significance regarding malignancy have not previously been described, requiring further study as addressed herein.
At least 8 cases of bilateral BIA-ALCL have been reported to the PROFILE registry for BIA-ALCL as reported by Dr Colleen McCarthy in the 2nd World Consensus Conference on BIA-ALCL, November 6-7, 2020. In those cases, CD30+ malignant cells were found in capsules or fluids surrounding both breasts. However, it is unclear whether malignancy of CD30+ cells was established by coordinated expression of other tumor cell biomarkers in all cases. Further, clonality of tumor cells could not be established in the suspected contralateral BIA-ALCL of at least 1 reported case.8
Immunohistochemical detection of phosphorylated STAT3 (pSTAT3) has been reported in 100% of BIA-ALCL.9 pSTAT3 expression in BIA-ALCL is associated with mutations of STAT3 in approximately 20% of cases.9-11 These mutations are thought to drive proliferation of malignant cells when accompanied by appropriate cytokine signaling.12
Less well known is high expression of pSTAT6 in BIA-ALCL. We detected pSTAT6 by immunohistochemistry and immunoblotting in clinical samples and cell lines, respectively. STAT6 is downstream of IL-13 which is produced by malignant cells in most BIA-ALCL.13 Pharmacologic inhibition of pSTAT6 is cytotoxic to BIA-ALCL tumor cells.
Interleukin 9 (IL-9) is a cytokine produced by BIA-ALCL tumor cells and detected at significantly higher levels in malignant than in benign seromas.14,15 IL-9 was originally recognized as a growth factor for mast cells which are frequent in capsules infiltrated by BIA-ALCL. 13
Granzyme B (GrB) is a cytotoxic protein which produces cell death in target cells. GrB processes and activates various procaspases, inducing apoptosis in target cells. GrB is frequently expressed by malignant cells in BIA-ALCL.16 In tissue sections, GrB can be detected in the cytoplasm of tumor cells and is often associated with apoptosis. Soluble GrB is detected at higher levels in malignant than in benign seromas.16
Here we report a patient with BIA-ALCL in which CD30+ anaplastic cells produce GrB and IL-9 and express nuclear pSTAT3 and pSTAT6. In contrast CD30+ cells in the contralateral capsule are negative for these tumor biomarkers. The significance of these observations is discussed.
CASE REPORT
In June 2020, a 54-year-old Caucasian female presented for saline implant exchange to downsize and mastopexy for lift. She had no complaints except that her breasts were “too big and saggy.” She had undergone breast augmentation in 2004 with BIOCELL (Allergan, Irvine, CA) textured implants. Preoperative breast imaging was normal. Her implants ruptured in the clinic preoperatively and she decided not to replace them because her natural breast volume was adequate. Lift and implant removal was performed without capsulectomy because her implants appeared prepectoral. Implants were textured McGhan Style 468 495-cc saline implants. Intraoperatively, scant clear yellow fluid was seen in pockets post rupture but there was no significant fluid accumulation. Small heaped-up clumps of capsule were seen as rough spots in capsules bilaterally and these were biopsied and sent to pathology. Original pathology was reported as ALCL on the right and scattered CD30+ cells suspicious for ALCL on the left. Positron emission tomography-computed tomography showed metabolically active bilateral cervical and right axillary nodes thought to be postoperative changes. No metabolic evidence of disease was seen in the breast; only inflammatory uptake associated with bilateral postsurgical changes. The patient subsequently had complete open capsulectomies for surgical cure. She is doing well postoperatively with no further treatments needed after 10 months follow-up.
Pathology slides and blocks were sent for a second opinion. Hematoxylin and eosin stains showed clusters of large anaplastic cells surrounded by dense fibrosis at and near the surface in the right breast capsule. Scattered atypical large cells were seen away from the tumor cell clusters often surrounded by eosinophiles and clusters of plasma cells. The left breast capsule contained fat lobules, small vessels, and a mixed inflammatory infiltrate with lymphocytes, plasma cells, and eosinophiles. Scattered among the inflammatory cells were large, atypical cells (Figure 1). Immunoperoxidase stains showed that both the anaplastic tumor cells in the right breast and the large, atypical cells in the left breast capsule were CD30+ and of similar size and shape, raising a concern of BIA-ALCL.
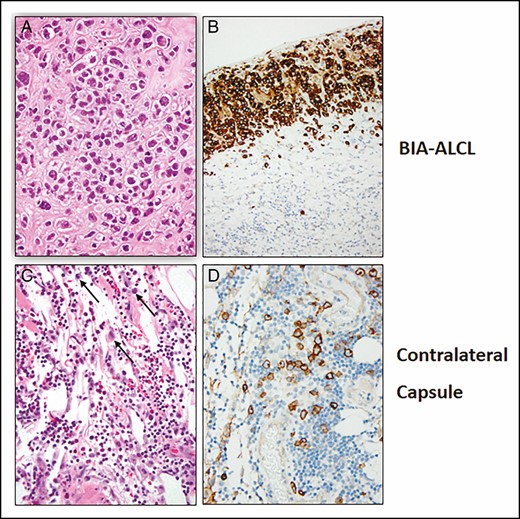
Hematoxylin and eosin–stained sections of tumor cells surrounded by dense fibrosis (A, B) and the contralateral capsule with inflammation and scattered large, atypical cells (black arrows) (C, D). Immunohistochemical demonstration of CD30 in tumor cells (B) and large, atypical cells in the contralateral capsule (D).
To clarify the diagnosis of the left breast, tumor and left breast tissue were dissected, fixed in 4% paraformaldehyde, and stained according to a standard protocol. The following primary antibodies were used: anti-pSTAT3 (Tyr705) (9145; Cell Signaling, Danvers, MA); anti-pSTAT6 (Tyr641) (SAB4300038; Sigma-Aldrich, St Louis, MO); anti-GrB antibody (ab134933; Abcam, Cambridge, UK); and anti-IL-9 antibody (ab181397; Abcam). Primary antibodies were detected with anti-rabbit or anti-mouse cell and tissue staining kits (CTS005 or CTS002; R&D Systems, Minneapolis, MN) or with fluorescent secondary antibodies including anti-mouse Alexa Fluor 488 or anti-rabbit Alexa Fluor 594 (Thermo Fisher Scientific, Waltham, MA). 4′,6-Diamidino-2-phenylindole (MBD0015; Sigma-Aldrich) was used for nuclear staining.
Tumor cells of the right breast stained for pSTAT3, pSTAT6, GrB, and IL-9 (Table 1; Figure 2). All of these stains were negative for the large, atypical cells of the left breast. To eliminate the possibility that these large, atypical cells were missed in consecutive sections in the left breast, double-label immunofluorescent stains were done with the same CD30 and pSTAT3 primary antibodies. Immunofluorescence staining results showed co-localized CD30 membrane staining and pSTAT3 nuclear staining of tumor cells in the right breast but showed only CD30 membrane staining of the large, atypical cells of the left breast (Figure 3). Small lymphocytes showed nuclear staining with 4′,6-diamidino-2-phenylindole but no CD30 staining. Together these results indicate that the large, atypical CD30+ cells of the left breast are not malignant and are surrounded by reactive lymphocytes as well as other inflammatory cells.
Staining Results Comparing BIA-ALCL Tumor Cells and Large Atypical Cells in the Contralateral Capsule
| Specimen . | CD30 . | pSTAT3 . | pSTAT6 . | IL-9 . | Granzyme B . |
|---|---|---|---|---|---|
| BIA-ALCL | + | + | + | + | + |
| Contralateral capsule | + | – | – | – | – |
| Specimen . | CD30 . | pSTAT3 . | pSTAT6 . | IL-9 . | Granzyme B . |
|---|---|---|---|---|---|
| BIA-ALCL | + | + | + | + | + |
| Contralateral capsule | + | – | – | – | – |
BIA-ALCL, breast implant–associated anaplastic large cell lymphoma; IL-9, interleukin 9; pSTAT3(6), phosphorylated STAT3(6).
Staining Results Comparing BIA-ALCL Tumor Cells and Large Atypical Cells in the Contralateral Capsule
| Specimen . | CD30 . | pSTAT3 . | pSTAT6 . | IL-9 . | Granzyme B . |
|---|---|---|---|---|---|
| BIA-ALCL | + | + | + | + | + |
| Contralateral capsule | + | – | – | – | – |
| Specimen . | CD30 . | pSTAT3 . | pSTAT6 . | IL-9 . | Granzyme B . |
|---|---|---|---|---|---|
| BIA-ALCL | + | + | + | + | + |
| Contralateral capsule | + | – | – | – | – |
BIA-ALCL, breast implant–associated anaplastic large cell lymphoma; IL-9, interleukin 9; pSTAT3(6), phosphorylated STAT3(6).
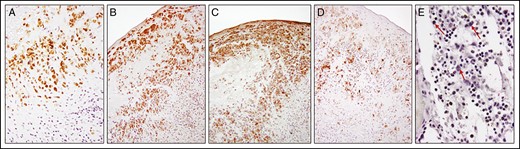
Immunohistochemistry analysis of pSTAT3 (A), pSTAT6 (B), interleukin 9 (C), and granzyme B (D) in tumor cells. Absence of staining of large, atypical cells (red arrows) for pSTAT3 (E) in the contralateral capsule. pSTAT3, phosphorylated STAT3.
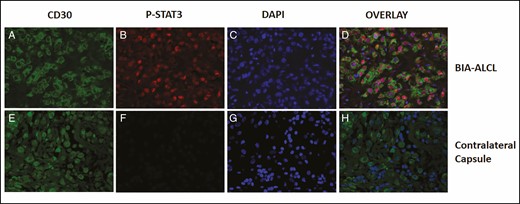
CD30 and pSTAT3 coexist in tumor cells but not in the large, atypical, CD30+ cells in the contralateral capsule. Immunofluorescence staining of CD30 and pSTAT3 in tumor cells (A-D) and large, atypical cells in the contralateral capsule (E-H). Breast implant–associated anaplastic large cell lymphoma immunofluorescence for CD30 (green; A and E), nuclear pSTAT3 (red; B and F), and co-stained for 4′,6-diamidino-2-phenylindole (blue; C and G). CD30+ tumor cells stain for both nuclear pSTAT3 and 4′,6-diamidino-2-phenylindole focally yielding purple color (D). Small lymphocytes stain for 4′,6-diamidino-2-phenylindole (blue) but not CD30 (H). pSTAT3, phosphorylated STAT3.
Discussion
CD30 is a lymphocyte activation antigen that also serves as a biomarker for BIA-ALCL.2,17 It is important to integrate CD30 expression into the evaluation of pathology specimens for possible diagnosis of BIA-ALCL.4 Importantly, identification of CD30+ cells should be evaluated in the context of routine histopathology and other characteristics of BIA-ALCL.18 This study demonstrates CD30+ cells lacking other tumor cell biomarkers, eg, pSTAT3, in a clinically and pathologically benign capsule around a breast implant. The opposite breast capsule showed unequivocal evidence of BIA-ALCL, containing aggregates of anaplastic cells expressing CD30, pSTAT3, pSTAT6, IL-9, and GrB. The CD30+ cells in the benign capsule were noncohesive and surrounded by small, reactive lymphocytes. Co-localization of pSTAT3 and CD30 in the tumor cells was not observed in these scattered CD30+ cells. Other BIA-ALCL biomarkers, including GrB, IL-9, and pSTAT6, were also absent in the benign contralateral capsule.14,16 Although the National Cancer Center Network guidelines do not yet include these biomarkers, we anticipate that with advancing knowledge, pSTAT3 and other recurrent biomarkers of BIA-ALCL will be recommended for evaluation of challenging cases such as the one we experienced. Optimal management of difficult cases may also support the establishment of reference centers with special expertise for diagnosis and treatment of BIA-ALCL.
These observations raise several important questions. First, does the absence of pSTAT3 and other tumor biomarkers exclude a diagnosis of BIA-ALCL? Second, are the CD30+ cells indicative of an early premalignant step in the evolution of BIA-ALCL?19 Is this the only case with CD30+ cells in the contralateral capsule of a patient with BIA-ALCL? It seems unlikely that this is the only one among hundreds of cases of BIA-ALCL in which “benign” CD30+ cells occur in the contralateral breast. Eight bilateral cases of BIA-ALCL have been accessioned in the PROFILE registry. It would be important to confirm none of these cases are similar to the one herein reported.
Conclusions
The use of a panel of biomarkers associated with BIA-ALCL can help to clarify the significance of suspicious CD30+ cells in capsules and seromas surrounding breast implants. It is suggested that future studies of BIA-ALCL should include thorough examination of the contralateral capsule for CD30+ cells and examine them for coexpression of other recurrent biomarkers of BIA-ALCL, eg, pSTAT3. All cases of presumed bilateral ALCL should be examined for tumor cell biomarkers and comparison of clonality when possible.
Acknowledgments
The authors appreciate the use of pathologic material provided by Dr Knight, Centura Laboratory Services, Littleton, CO.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
Dr Kadin's research was supported by the Aesthetic Surgery Education and Research Foundation (ASERF).
References
Author notes
Dr Hunsicker is a plastic surgeon in private practice, Littleton, CO, USA
- inflammatory cells
- immunohistochemistry
- antigens
- antigens, cd30
- biological markers
- fluorescent antibody technique
- interleukin-9
- lymphocyte activation
- ki-1+ anaplastic large cell lymphoma
- precancerous conditions
- breast
- breast implants
- diagnosis
- neoplasms
- tumor cells
- granzyme b
- stat3 protein
- histopathology tests
- implants
- breast implant–associated anaplastic large cell lymphoma



