-
PDF
- Split View
-
Views
-
Cite
Cite
Xuan-Jun Liu, Wen-Hui Liu, Shao-Wen Fang, Xin-Long Zhou, Jia-Xiang Xu, Guang-Shuai Li, Lasers and Intense Pulsed Light for the Treatment of Pathological Scars: A Network Meta-Analysis, Aesthetic Surgery Journal, Volume 42, Issue 11, November 2022, Pages NP675–NP687, https://doi.org/10.1093/asj/sjac175
Close - Share Icon Share
Abstract
Laser and intense pulsed light (IPL) therapies have shown promising effects on pathological scars, but the comparative effectiveness of laser and IPL therapies has not yet been studied.
The aim of this study was to compare and rank the efficacy of laser and IPL therapies to determine the most effective treatment method for pathological scars.
Relevant studies published up to February 2022 were identified by searching PubMed, Web of Science, Cochrane Library, CNKI, and Wanfang databases. We defined Vancouver Scar Scale score as the primary outcome. Both frequentist and Bayesian approaches were used to perform a network meta-analysis.
We included 25 trials with a total of 1688 participants. The rankings based on the surface under the cumulative ranking curve for the Vancouver Scar Scale score based on the Bayesian approach suggested IPL + CO2 (96.43%) > pulsed dye laser (PDL) + 1064-nm Nd:YAG (yttrium aluminum garnet) laser (86.21%) > PDL + CO2 (82.15%) > CO2 (58.97%) > 1064-nm Nd:YAG (57.03%) > PDL (52%) > 532-nm Nd:YAG (33.28%) > Er:YAG + IPL (28.38%) > Er:YAG (26.56%) > IPL (15.03%) > control (13.97%). The ranking results based on the frequentist approach were basically consistent with those based on the Bayesian approach.
The results of the network meta-analysis showed that the combination of IPL and CO2 laser has the highest probability of being the most effective intervention. However, our conclusions must be interpreted with caution due to the relatively few evaluation indicators included in our study. Future well-designed randomized controlled trials with large sample sizes are required to confirm our conclusions.

Pathologic scars are dermal fibrosis lesions with complicated etiology, characterized by abnormal hyperproliferation of fibroblasts and excessive deposition of collagen.1 Pathologic scars can be divided into hypertrophic scars and keloids. The former remain within the confines of the original wound and may regress over time, whereas the latter usually extend beyond the boundaries of the previous wound and do not show a tendency to regress spontaneously.2 Burn, deep trauma, and surgery are common causes of pathologic scars.3 The incidence of keloids varies by ethnicity, with Black people having a higher incidence than Caucasians.4 The incidence of keloids in Caucasians has been found to be less than 1% according to a study from the United Kingdom,5 whereas the incidence in Black and Hispanic populations varies from 4.5% to 16%.6 For this reason, there is a general consensus that darker-skinned populations are more likely to suffer from keloids than lighter-skinned populations.7 Oliver et al revealed, by means of self-administered questionnaires, a severe impairment of quality of life of patients with pathologic scars.8 Although these scars are not life-threatening, they can produce both functional and cosmetic concerns, resulting in low self-confidence and ultimately affecting the quality of life.9 Traditional treatments for hypertrophic scars and keloids include surgical excision, drug injection, radiotherapy, cryotherapy, etc.3,10,11 However, most of the therapeutic effects of these treatments are unsatisfactory.3,12,13
With the advancement of laser and light technology, intense pulsed light (IPL) and a variety of lasers have been used to treat pathologic scars, opening up new avenues for treatment.14 Laser and IPL therapies offer simple operation, fewer complications, and minimal trauma.15 Since Castro et al first described the use of neodymium:yttrium aluminum garnet (Nd:YAG) lasers to treat scars in 1983, laser and IPL therapies have made tremendous progress as a treatment modality for keloids and hypertrophic scars.16 Lasers commonly used to treat pathologic scars include, in addition to Nd:YAG lasers, erbium:yttrium aluminum garnet (Er:YAG) lasers, carbon dioxide (CO2) lasers, and pulsed dye lasers (PDLs).13 IPL delivers incoherent light: although not technically a laser, IPL similarly follows the principle of selective photothermolysis, making it promising for the treatment of pathologic scars.17
Cumulative evidence indicates that laser and IPL therapies have good curative effects for pathologic scars; 18 however, the optimal treatment strategy for such scars has not yet been determined. Therefore, our aim was to identify which treatment is the most effective for pathologic scars to help patients and clinicians select the optimal strategy.
METHODS
Protocol
This network meta-analysis (NMA) was registered in PROSPERO (International Prospective Register of Systematic Reviews, CRD42022311971).
Literature Search
PubMed (National Library of Medicine, Besthesda, MD), Web of Science (Clarivate Analytics PLC, Philadelphia, PA), the Cochrane Library (Wiley, Hoboken, NJ), CNKI (Tongfang Knowledge Network Technology Co., Ltd., Beijing, China), and Wanfang (Chinese Ministry of Science & Technology, Beijing, China) were searched for relevant studies investigating the effects of laser and IPL therapies on pathologic scars published from database inception until February 2022. The following keywords were used for the search: “laser*,” “light,” “hypertrophic scar,” “hypertrophic scars,” and “keloid*.” In addition, the reference lists of included studies and systematic reviews/meta-analyses were manually examined to identify additional eligible studies.
Study Selection
Two investigators (L.X.J. and F.S.W.) first screened the titles and abstracts of the retrieved literature. For each potentially eligible study, 2 investigators (L.X.J. and F.S.W.) then independently assessed the full text. In case of disagreement, consensus was reached by consultation with the corresponding author (L.G.S.).
Selection Criteria
Studies included in our analysis had to meet all the following criteria: (1) randomized clinical trials, retrospective studies, and observational studies (including cohort and case-control studies), irrespective of blinding or language; (2) patients with a diagnosis of hypertrophic or keloid scars (or both), regardless of age, sex, or ethnicity; (3) studies with an intervention group that received laser or IPL treatment; (4) studies with a comparison group that received a different type of laser treatment than the intervention group or received no treatment; (5) studies that include at least 1 of the following outcomes: Vancouver Scar Scale (VSS) score, thickness, pigmentation, vascularity, and pliability. The exclusion criteria were as follows: (1) meta-analyses, reviews, letters, conference abstracts, and case reports; (2) studies reporting insufficient data and additional data could not be obtained by contacting the corresponding author; (3) studies that only compared different parameters of the same type of laser; (4) studies reporting combinations with treatment other than laser and IPL therapies.
Data Extraction
Two investigators (L.X.J. and Z.X.L.) independently extracted data by means of a standardized form; all disagreements were settled through consultation with the corresponding author (L.G.S.). The following information was extracted from all included articles: first author’s name, publication year, country, participants’ demographic characteristics (age and sex), course of disease, etiology, sample size, control setting, intervention measures, number of sessions, duration of follow-up, and outcomes. VSS score was considered as the primary outcome, whereas thickness, pigmentation, vascularity, and pliability were considered as secondary outcomes. Because all the outcomes were continuous variables, we respectively extracted the mean, standard deviation (SD), and sample size for case and control groups. When the original trial only presented other forms of data instead of mean and SD, we converted these to mean and SD according to relevant formulas. When data were not reported for outcomes of interest, the senior author (L.G.S.) attempted to contact the corresponding author.
Risk of Bias
The risk of bias was independently assessed by 2 investigators (L.X.J. and Z.X.L.) based on the Cochrane Collaboration tool,19 which includes random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other potential sources of bias. Each item was assessed as presenting a low, high, or unclear risk of bias for each study. The overall risk of bias was considered low if all the items were deemed low risk. Studies were judged to be at high risk of bias if at least 1 item was considered as high risk. Otherwise, the overall risk of bias was considered unclear. Any disagreement was resolved by discussion or by consulting the corresponding author (L.G.S.).
Statistical Analysis
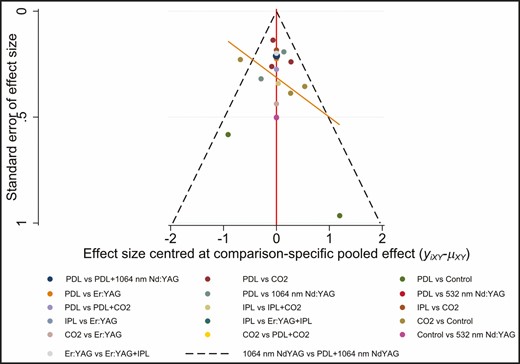
We constructed an NMA which combines direct and indirect treatment comparisons. This NMA was first confirmed by means of a frequentist approach in Stata 16.0 software (Stata Corporation, College Station, TX).20 A network plot was produced to show the comparisons of treatment regimens.21 Each node represents an intervention, and the size of the nodes represents the total sample size of interventions. The connecting lines represent the existence of direct comparisons between 2 interventions and the width of the lines corresponds to the number of trials. Because all the variables of interest were continuous, the standardized mean difference and 95% CI were used for data calculation. Global inconsistency was evaluated by the design-by-treatment interaction model.22 We summarized the standardized mean difference for all pairwise comparisons in a league table containing both direct and indirect comparisons. The intervention effects were ranked based on the surface under the cumulative ranking curve (SUCRA) probabilities. SUCRA is the ratio of the surface underneath the cumulative ranking curve to the entire plot area.23 The SUCRA value ranged from 0% to 100%, and higher SUCRA values indicated better efficacy.23 In addition, funnel plots were used to evaluate the publication bias.24
In addition, a Bayesian approach with a Markov chain Monte Carlo algorithm implemented in OpenBUGS 3.2.3 software (University of Helsinki, Helsinki, Finland) was applied to the VSS score to examine the robustness of the results based on the frequentist approach. We ran 3 Markov chains for 500,000 iterations and discarded the first 200,000 as the burn-in period to eliminate the influence of the initial value.25P < 0.05 was considered statistically significant.
RESULTS
Literature Search
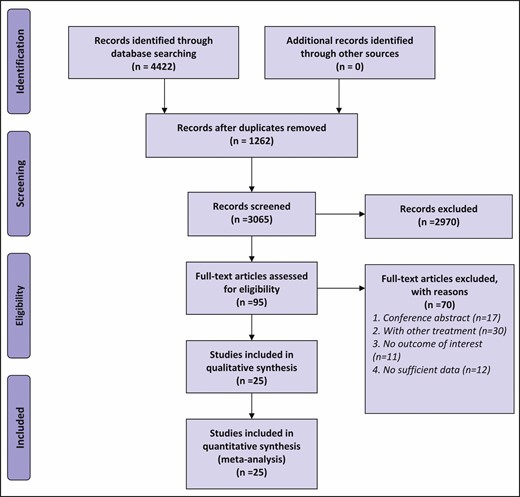
The procedure of study selection is shown in Figure 1. A total of 4422 articles were initially searched and 3160 articles remained after deduplication. Of these, 3065 articles were excluded based on the screening of titles and abstracts, leaving 95 articles for full-text review. Of these articles, 17 were excluded because they were conference abstracts, 30 were removed because they reported combinations with treatment other than laser and IPL therapies, 11 were removed because they did not report outcomes of interest, and 12 were removed because of lack of available data. Finally, 25 articles were selected for this NMA.26-50
Characteristics of Included Studies
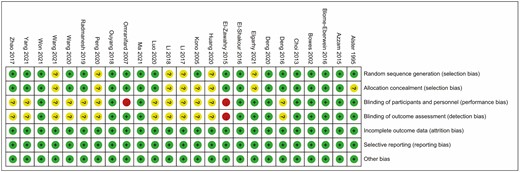
Overall, a total of 1688 patients were included in our analysis. All the articles included were published between 1995 and 2021. The sample size of included studies ranged between 6 and 221. Among these studies, 14 were conducted in China,31,32,36,38-41,43,44,46-50 4 in Egypt,27,33-35 3 in the United States,26,28,29 2 in Iran,42,45 1 in Japan,37 and 1 in Korea.30 IPL and 6 types of lasers were involved: 585/595-nm PDL, 10,600-nm CO2 laser, 2940-nm Er:YAG laser, 1064-nm Nd:YAG laser, and 532-nm Nd:YAG laser. In addition, 23 of the studies were 2-arm26-30,32-49 and 2 were 3-arm.31,50 The basic characteristics of the included studies are presented in Table 1. For overall risk of bias, 9 studies (36%) were considered to be at low risk of bias,27-30,32,34,41,43,48 14 studies (56%) were considered to be at unclear risk of bias,26,31,33,36-40,44-47,49,50 and 2 studies (8%) were considered to be at high risk of bias.35,42 Of the 2 studies rated as high risk, 1 was due to lack of blinding of participants and personnel,42 and the other was due to total nonblinding.35 Approximately half of the studies were not specifically conducted or reported as blinded, probably because blinding was difficult to achieve in practice. The summary of the risk of bias for the included studies is presented in Figure 2.
| Author (year) . | Country . | Control setting . | Participants . | Mean age or range (year) . | Male ratio . | Mean scar age or range . | Disease . | Etiology . | Skin type . | Number of sessions . | Follow-up . | Outcomes . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alster17 (1995) | USA | Self-control | A 16 D 16 | 49 | 62.5% | 17m | HS + K | S | I-III | 2 | 6m (since last laser) | Thickness, pliability |
| Azzam18 (2015) | Egypt | Self-control | C 19 D 19 | >15 | NR | NR | HS + K | NR | II-VI | 4 | 3m, 6m (since last laser) | VSS |
| Blome-Eberwein19 (2016) | USA | Standard control | C 48 D 32 | 39 for all | 37.5% for all | NR | HS | BU | NA | 3 | 4m-6m (since last laser) | VSS, thickness, pigmentation, pliability |
| Bowes20 (2002) | USA | Self-control | A 6 G 6 D 6 | 15-56 | NR | 3m-5y | HS | T, S | II-IV | 3-4 | 22w (since baseline) | VSS |
| Choi21 (2013) | Korea | Standard control | C 10 E 13 | 37.2 32.8 | 20.0% 30.8% | 7.5y 6.4y | HS | NR | III-V | 1-9 | 2m-10m (since baseline) | VSS |
| Deng22 (2016) | China | Standard control | A 52 F 57 A + F 53 | 26.5 27.2 26.8 | 30.8% 33.3% 28.3% | >6m | HS | T, S | NA | 4 | 1m (since last laser) | VSS |
| Deng23 (2020) | China | Standard control | A 22 D 23 | 35 42 | 60.87% 65.22% | 2m-6m for all | HS | BU, T | NA | 3 | 3m (since baseline) | Thickness, pigmentation, vascularity, pliability |
| Elgarhy24 (2021) | Egypt | Self-control | A 30 C 30 | 18 | 50% | 38.7m | HS | BU, T, S | NA | 3 | 1m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| El-Shakour25 (2016) | Egypt | Self-control | A 20 F 20 | 22.6 | 45% | 7m | HS + K | BU, T, S | NA | 6 | 1m (since last laser) | VSS |
| El-Zawahry26 (2015) | Egypt | Self-control | C 15 D 15 | 16-58 | 13.3% | >6m | HS + K | BU | II-IV | 3 | 3m (since last laser) | VSS |
| Huang27 (2020) | China | Standard control | E + F 28 E 28 | 36.85 37.14 | 35.7% 42.9% | NR | HS | AC, T, S | NA | 1 | NA | Thickness, pigmentation, vascularity, pliability |
| Kono28 (2005) | Japan | Self-control | A 15 D 15 | 13.7 | 53.3% | 12y | HS | BU, T, S | III-IV | 2 | 1m (since last laser) | VSS |
| Li29 (2017) | China | Standard control | C 73 F 64 | 28.53 for all | 55.5% for all | 4m-5y for all | HS | BU, T, S | III-IV | 3-6 | 6m (since last laser) | VSS |
| Li30 (2018) | China | Standard control | A 122 C 99 | 29 for all | 28.7% 46.5% | 2.1y 1.9y | HS | BU | III-IV | NA | NA | VSS |
| Luo31 (2020) | China | Standard control | A + C 48 C 48 | 34.95 35.42 | 56.3% 52.1% | 4.53y 4.86y | HS | BU | NA | 4 | 6m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| Ma32 (2021) | China | Self-control | C 15 D 15 | 2.14 | 66.7% | NR | HS | BU, T, S | NA | 3 | 6m (since last laser) | Thickness, pigmentation, vascularity, pliability |
| Omranifard33 (2007) | Iran | Standard control | A 40 E 40 | 27.2 for all | 22.5% 17.5% | 8.9m for all | HS | T, S | I-III | NA | 1m (since last laser) | VSS |
| Ouyang34 (2018) | China | Standard control | A + C 28 A 28 | 3-51 for all | 62.5% for all | <3m for all | HS | BU, T, S | NA | 2 | 2m (since baseline) 6m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
| Peng35 (2020) | China | Standard control | B + C30 B 30 | 29.28 28.75 | 56.7% 53.3% | 4.56m 4.43m | HS | BU, S | NA | 2 | 3m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| Radmanesh36 (2019) | Iran | Self-control | A 35 C 35 | 23 | 60% | 6m-72m | HS | BU, T, S | II-IV | 4 | 4m (since baseline) | VSS |
| Wang37 (2020) | China | Standard control | B + E 46 B 45 | 28.31 28.29 | 54.4% 60.0% | 8.31m 8.37m | HS | AC | NA | 3 | 2m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Wang38 (2021) | China | Standard control | A + C 48 C 45 | 32.9 33.5 | 44.1% for all | 2.48y 2.61y | K | NR | NA | 4 | 6m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Won39 (2021) | China | Self-control | C 20 D 20 | 2.35 | 65% | NR | HS | BU, T, S | III–IV | 3 | 6m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Yang40 (2021) | China | Standard control | B + C 81 B 81 | 35.69 35.72 | 48.1% 49.4% | 4.22m 4.02m | HS | BU, S | NA | 2 | 2m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
| Zhao41 (2017) | China | Standard control | B + E 45 E 45 B 45 | 21.80 21.90 21.85 | 48.9% 51.1% 53.3% | 11.05m 10.65m 10.58m | HS | AC | NA | 2 | 8m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
| Author (year) . | Country . | Control setting . | Participants . | Mean age or range (year) . | Male ratio . | Mean scar age or range . | Disease . | Etiology . | Skin type . | Number of sessions . | Follow-up . | Outcomes . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alster17 (1995) | USA | Self-control | A 16 D 16 | 49 | 62.5% | 17m | HS + K | S | I-III | 2 | 6m (since last laser) | Thickness, pliability |
| Azzam18 (2015) | Egypt | Self-control | C 19 D 19 | >15 | NR | NR | HS + K | NR | II-VI | 4 | 3m, 6m (since last laser) | VSS |
| Blome-Eberwein19 (2016) | USA | Standard control | C 48 D 32 | 39 for all | 37.5% for all | NR | HS | BU | NA | 3 | 4m-6m (since last laser) | VSS, thickness, pigmentation, pliability |
| Bowes20 (2002) | USA | Self-control | A 6 G 6 D 6 | 15-56 | NR | 3m-5y | HS | T, S | II-IV | 3-4 | 22w (since baseline) | VSS |
| Choi21 (2013) | Korea | Standard control | C 10 E 13 | 37.2 32.8 | 20.0% 30.8% | 7.5y 6.4y | HS | NR | III-V | 1-9 | 2m-10m (since baseline) | VSS |
| Deng22 (2016) | China | Standard control | A 52 F 57 A + F 53 | 26.5 27.2 26.8 | 30.8% 33.3% 28.3% | >6m | HS | T, S | NA | 4 | 1m (since last laser) | VSS |
| Deng23 (2020) | China | Standard control | A 22 D 23 | 35 42 | 60.87% 65.22% | 2m-6m for all | HS | BU, T | NA | 3 | 3m (since baseline) | Thickness, pigmentation, vascularity, pliability |
| Elgarhy24 (2021) | Egypt | Self-control | A 30 C 30 | 18 | 50% | 38.7m | HS | BU, T, S | NA | 3 | 1m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| El-Shakour25 (2016) | Egypt | Self-control | A 20 F 20 | 22.6 | 45% | 7m | HS + K | BU, T, S | NA | 6 | 1m (since last laser) | VSS |
| El-Zawahry26 (2015) | Egypt | Self-control | C 15 D 15 | 16-58 | 13.3% | >6m | HS + K | BU | II-IV | 3 | 3m (since last laser) | VSS |
| Huang27 (2020) | China | Standard control | E + F 28 E 28 | 36.85 37.14 | 35.7% 42.9% | NR | HS | AC, T, S | NA | 1 | NA | Thickness, pigmentation, vascularity, pliability |
| Kono28 (2005) | Japan | Self-control | A 15 D 15 | 13.7 | 53.3% | 12y | HS | BU, T, S | III-IV | 2 | 1m (since last laser) | VSS |
| Li29 (2017) | China | Standard control | C 73 F 64 | 28.53 for all | 55.5% for all | 4m-5y for all | HS | BU, T, S | III-IV | 3-6 | 6m (since last laser) | VSS |
| Li30 (2018) | China | Standard control | A 122 C 99 | 29 for all | 28.7% 46.5% | 2.1y 1.9y | HS | BU | III-IV | NA | NA | VSS |
| Luo31 (2020) | China | Standard control | A + C 48 C 48 | 34.95 35.42 | 56.3% 52.1% | 4.53y 4.86y | HS | BU | NA | 4 | 6m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| Ma32 (2021) | China | Self-control | C 15 D 15 | 2.14 | 66.7% | NR | HS | BU, T, S | NA | 3 | 6m (since last laser) | Thickness, pigmentation, vascularity, pliability |
| Omranifard33 (2007) | Iran | Standard control | A 40 E 40 | 27.2 for all | 22.5% 17.5% | 8.9m for all | HS | T, S | I-III | NA | 1m (since last laser) | VSS |
| Ouyang34 (2018) | China | Standard control | A + C 28 A 28 | 3-51 for all | 62.5% for all | <3m for all | HS | BU, T, S | NA | 2 | 2m (since baseline) 6m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
| Peng35 (2020) | China | Standard control | B + C30 B 30 | 29.28 28.75 | 56.7% 53.3% | 4.56m 4.43m | HS | BU, S | NA | 2 | 3m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| Radmanesh36 (2019) | Iran | Self-control | A 35 C 35 | 23 | 60% | 6m-72m | HS | BU, T, S | II-IV | 4 | 4m (since baseline) | VSS |
| Wang37 (2020) | China | Standard control | B + E 46 B 45 | 28.31 28.29 | 54.4% 60.0% | 8.31m 8.37m | HS | AC | NA | 3 | 2m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Wang38 (2021) | China | Standard control | A + C 48 C 45 | 32.9 33.5 | 44.1% for all | 2.48y 2.61y | K | NR | NA | 4 | 6m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Won39 (2021) | China | Self-control | C 20 D 20 | 2.35 | 65% | NR | HS | BU, T, S | III–IV | 3 | 6m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Yang40 (2021) | China | Standard control | B + C 81 B 81 | 35.69 35.72 | 48.1% 49.4% | 4.22m 4.02m | HS | BU, S | NA | 2 | 2m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
| Zhao41 (2017) | China | Standard control | B + E 45 E 45 B 45 | 21.80 21.90 21.85 | 48.9% 51.1% 53.3% | 11.05m 10.65m 10.58m | HS | AC | NA | 2 | 8m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
A, 585/595-nm PDL; B, intense pulsed light; C,10,600-nm CO2 laser; D, control; E, 2940-nm Er:YAG laser; F, 1064-nm Nd:YAG laser; G, 532-nm Nd:YAG laser; HS, hypertrophic scar; K, keloid; m, month; w, week; y, year; VSS, Vancouver Scar Scale; NR, not reported; NA, not available; AC, acne; BU, burn; T, trauma; S, surgery.
| Author (year) . | Country . | Control setting . | Participants . | Mean age or range (year) . | Male ratio . | Mean scar age or range . | Disease . | Etiology . | Skin type . | Number of sessions . | Follow-up . | Outcomes . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alster17 (1995) | USA | Self-control | A 16 D 16 | 49 | 62.5% | 17m | HS + K | S | I-III | 2 | 6m (since last laser) | Thickness, pliability |
| Azzam18 (2015) | Egypt | Self-control | C 19 D 19 | >15 | NR | NR | HS + K | NR | II-VI | 4 | 3m, 6m (since last laser) | VSS |
| Blome-Eberwein19 (2016) | USA | Standard control | C 48 D 32 | 39 for all | 37.5% for all | NR | HS | BU | NA | 3 | 4m-6m (since last laser) | VSS, thickness, pigmentation, pliability |
| Bowes20 (2002) | USA | Self-control | A 6 G 6 D 6 | 15-56 | NR | 3m-5y | HS | T, S | II-IV | 3-4 | 22w (since baseline) | VSS |
| Choi21 (2013) | Korea | Standard control | C 10 E 13 | 37.2 32.8 | 20.0% 30.8% | 7.5y 6.4y | HS | NR | III-V | 1-9 | 2m-10m (since baseline) | VSS |
| Deng22 (2016) | China | Standard control | A 52 F 57 A + F 53 | 26.5 27.2 26.8 | 30.8% 33.3% 28.3% | >6m | HS | T, S | NA | 4 | 1m (since last laser) | VSS |
| Deng23 (2020) | China | Standard control | A 22 D 23 | 35 42 | 60.87% 65.22% | 2m-6m for all | HS | BU, T | NA | 3 | 3m (since baseline) | Thickness, pigmentation, vascularity, pliability |
| Elgarhy24 (2021) | Egypt | Self-control | A 30 C 30 | 18 | 50% | 38.7m | HS | BU, T, S | NA | 3 | 1m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| El-Shakour25 (2016) | Egypt | Self-control | A 20 F 20 | 22.6 | 45% | 7m | HS + K | BU, T, S | NA | 6 | 1m (since last laser) | VSS |
| El-Zawahry26 (2015) | Egypt | Self-control | C 15 D 15 | 16-58 | 13.3% | >6m | HS + K | BU | II-IV | 3 | 3m (since last laser) | VSS |
| Huang27 (2020) | China | Standard control | E + F 28 E 28 | 36.85 37.14 | 35.7% 42.9% | NR | HS | AC, T, S | NA | 1 | NA | Thickness, pigmentation, vascularity, pliability |
| Kono28 (2005) | Japan | Self-control | A 15 D 15 | 13.7 | 53.3% | 12y | HS | BU, T, S | III-IV | 2 | 1m (since last laser) | VSS |
| Li29 (2017) | China | Standard control | C 73 F 64 | 28.53 for all | 55.5% for all | 4m-5y for all | HS | BU, T, S | III-IV | 3-6 | 6m (since last laser) | VSS |
| Li30 (2018) | China | Standard control | A 122 C 99 | 29 for all | 28.7% 46.5% | 2.1y 1.9y | HS | BU | III-IV | NA | NA | VSS |
| Luo31 (2020) | China | Standard control | A + C 48 C 48 | 34.95 35.42 | 56.3% 52.1% | 4.53y 4.86y | HS | BU | NA | 4 | 6m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| Ma32 (2021) | China | Self-control | C 15 D 15 | 2.14 | 66.7% | NR | HS | BU, T, S | NA | 3 | 6m (since last laser) | Thickness, pigmentation, vascularity, pliability |
| Omranifard33 (2007) | Iran | Standard control | A 40 E 40 | 27.2 for all | 22.5% 17.5% | 8.9m for all | HS | T, S | I-III | NA | 1m (since last laser) | VSS |
| Ouyang34 (2018) | China | Standard control | A + C 28 A 28 | 3-51 for all | 62.5% for all | <3m for all | HS | BU, T, S | NA | 2 | 2m (since baseline) 6m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
| Peng35 (2020) | China | Standard control | B + C30 B 30 | 29.28 28.75 | 56.7% 53.3% | 4.56m 4.43m | HS | BU, S | NA | 2 | 3m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| Radmanesh36 (2019) | Iran | Self-control | A 35 C 35 | 23 | 60% | 6m-72m | HS | BU, T, S | II-IV | 4 | 4m (since baseline) | VSS |
| Wang37 (2020) | China | Standard control | B + E 46 B 45 | 28.31 28.29 | 54.4% 60.0% | 8.31m 8.37m | HS | AC | NA | 3 | 2m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Wang38 (2021) | China | Standard control | A + C 48 C 45 | 32.9 33.5 | 44.1% for all | 2.48y 2.61y | K | NR | NA | 4 | 6m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Won39 (2021) | China | Self-control | C 20 D 20 | 2.35 | 65% | NR | HS | BU, T, S | III–IV | 3 | 6m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Yang40 (2021) | China | Standard control | B + C 81 B 81 | 35.69 35.72 | 48.1% 49.4% | 4.22m 4.02m | HS | BU, S | NA | 2 | 2m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
| Zhao41 (2017) | China | Standard control | B + E 45 E 45 B 45 | 21.80 21.90 21.85 | 48.9% 51.1% 53.3% | 11.05m 10.65m 10.58m | HS | AC | NA | 2 | 8m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
| Author (year) . | Country . | Control setting . | Participants . | Mean age or range (year) . | Male ratio . | Mean scar age or range . | Disease . | Etiology . | Skin type . | Number of sessions . | Follow-up . | Outcomes . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alster17 (1995) | USA | Self-control | A 16 D 16 | 49 | 62.5% | 17m | HS + K | S | I-III | 2 | 6m (since last laser) | Thickness, pliability |
| Azzam18 (2015) | Egypt | Self-control | C 19 D 19 | >15 | NR | NR | HS + K | NR | II-VI | 4 | 3m, 6m (since last laser) | VSS |
| Blome-Eberwein19 (2016) | USA | Standard control | C 48 D 32 | 39 for all | 37.5% for all | NR | HS | BU | NA | 3 | 4m-6m (since last laser) | VSS, thickness, pigmentation, pliability |
| Bowes20 (2002) | USA | Self-control | A 6 G 6 D 6 | 15-56 | NR | 3m-5y | HS | T, S | II-IV | 3-4 | 22w (since baseline) | VSS |
| Choi21 (2013) | Korea | Standard control | C 10 E 13 | 37.2 32.8 | 20.0% 30.8% | 7.5y 6.4y | HS | NR | III-V | 1-9 | 2m-10m (since baseline) | VSS |
| Deng22 (2016) | China | Standard control | A 52 F 57 A + F 53 | 26.5 27.2 26.8 | 30.8% 33.3% 28.3% | >6m | HS | T, S | NA | 4 | 1m (since last laser) | VSS |
| Deng23 (2020) | China | Standard control | A 22 D 23 | 35 42 | 60.87% 65.22% | 2m-6m for all | HS | BU, T | NA | 3 | 3m (since baseline) | Thickness, pigmentation, vascularity, pliability |
| Elgarhy24 (2021) | Egypt | Self-control | A 30 C 30 | 18 | 50% | 38.7m | HS | BU, T, S | NA | 3 | 1m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| El-Shakour25 (2016) | Egypt | Self-control | A 20 F 20 | 22.6 | 45% | 7m | HS + K | BU, T, S | NA | 6 | 1m (since last laser) | VSS |
| El-Zawahry26 (2015) | Egypt | Self-control | C 15 D 15 | 16-58 | 13.3% | >6m | HS + K | BU | II-IV | 3 | 3m (since last laser) | VSS |
| Huang27 (2020) | China | Standard control | E + F 28 E 28 | 36.85 37.14 | 35.7% 42.9% | NR | HS | AC, T, S | NA | 1 | NA | Thickness, pigmentation, vascularity, pliability |
| Kono28 (2005) | Japan | Self-control | A 15 D 15 | 13.7 | 53.3% | 12y | HS | BU, T, S | III-IV | 2 | 1m (since last laser) | VSS |
| Li29 (2017) | China | Standard control | C 73 F 64 | 28.53 for all | 55.5% for all | 4m-5y for all | HS | BU, T, S | III-IV | 3-6 | 6m (since last laser) | VSS |
| Li30 (2018) | China | Standard control | A 122 C 99 | 29 for all | 28.7% 46.5% | 2.1y 1.9y | HS | BU | III-IV | NA | NA | VSS |
| Luo31 (2020) | China | Standard control | A + C 48 C 48 | 34.95 35.42 | 56.3% 52.1% | 4.53y 4.86y | HS | BU | NA | 4 | 6m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| Ma32 (2021) | China | Self-control | C 15 D 15 | 2.14 | 66.7% | NR | HS | BU, T, S | NA | 3 | 6m (since last laser) | Thickness, pigmentation, vascularity, pliability |
| Omranifard33 (2007) | Iran | Standard control | A 40 E 40 | 27.2 for all | 22.5% 17.5% | 8.9m for all | HS | T, S | I-III | NA | 1m (since last laser) | VSS |
| Ouyang34 (2018) | China | Standard control | A + C 28 A 28 | 3-51 for all | 62.5% for all | <3m for all | HS | BU, T, S | NA | 2 | 2m (since baseline) 6m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
| Peng35 (2020) | China | Standard control | B + C30 B 30 | 29.28 28.75 | 56.7% 53.3% | 4.56m 4.43m | HS | BU, S | NA | 2 | 3m (since last laser) | VSS, thickness, pigmentation, vascularity, pliability |
| Radmanesh36 (2019) | Iran | Self-control | A 35 C 35 | 23 | 60% | 6m-72m | HS | BU, T, S | II-IV | 4 | 4m (since baseline) | VSS |
| Wang37 (2020) | China | Standard control | B + E 46 B 45 | 28.31 28.29 | 54.4% 60.0% | 8.31m 8.37m | HS | AC | NA | 3 | 2m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Wang38 (2021) | China | Standard control | A + C 48 C 45 | 32.9 33.5 | 44.1% for all | 2.48y 2.61y | K | NR | NA | 4 | 6m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Won39 (2021) | China | Self-control | C 20 D 20 | 2.35 | 65% | NR | HS | BU, T, S | III–IV | 3 | 6m (since last laser) | Thickness, pig entation, vascularity, pliability |
| Yang40 (2021) | China | Standard control | B + C 81 B 81 | 35.69 35.72 | 48.1% 49.4% | 4.22m 4.02m | HS | BU, S | NA | 2 | 2m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
| Zhao41 (2017) | China | Standard control | B + E 45 E 45 B 45 | 21.80 21.90 21.85 | 48.9% 51.1% 53.3% | 11.05m 10.65m 10.58m | HS | AC | NA | 2 | 8m (since baseline) | VSS, thickness, pigmentation, vascularity, pliability |
A, 585/595-nm PDL; B, intense pulsed light; C,10,600-nm CO2 laser; D, control; E, 2940-nm Er:YAG laser; F, 1064-nm Nd:YAG laser; G, 532-nm Nd:YAG laser; HS, hypertrophic scar; K, keloid; m, month; w, week; y, year; VSS, Vancouver Scar Scale; NR, not reported; NA, not available; AC, acne; BU, burn; T, trauma; S, surgery.
Primary Outcome
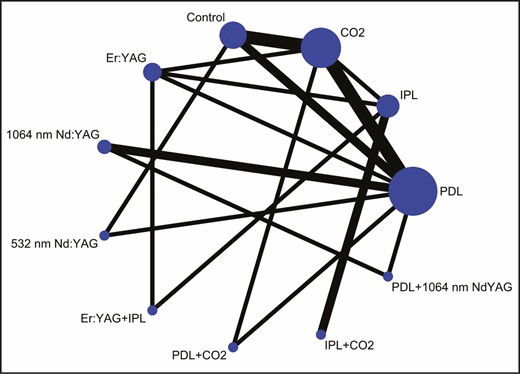
Figure 3 shows the network diagram for the VSS score. Eleven interventions were included in the NMA of the VSS score: PDL, IPL, CO2, Er:YAG, 1064-nm Nd:YAG, 532-nm Nd:YAG, Er:YAG + IPL, PDL + CO2, IPL + CO2, PDL + 1064-nm Nd:YAG, and control. PDL was reported in the largest number of studies and the largest number of studies compared the effectiveness of PDL and CO2 laser, followed by the comparison between CO2 laser and control group. This is probably because PDL is one of the first laser devices developed that can be used to treat pathologic scars. In addition, laser devices that are routinely used in clinical practice are more likely to have been studied. The inconsistency test revealed no significant global inconsistency between direct and indirect comparisons of VSS score (P = 0.36).
A league table for VSS score based on the frequentist approach is presented in Table 2. Compared with CO2, IPL + CO2 (–1.48; 95% CI, –2.23 to –0.72), PDL + 1064-nm Nd:YAG (–0.77; 95% CI, –1.50 to –0.05), and PDL + CO2 (–0.66; 95% CI, –1.19 to –0.12) resulted in a statistically significant reduction in VSS score. Compared with 1064-nm Nd:YAG, IPL + CO2 (–1.59; 95% CI, –2.54 to –0.63), PDL + 1064-nm Nd:YAG (–0.89; 95% CI, –1.53 to –0.24), and PDL + CO2 (–0.77; 95% CI, –1.53 to –0.01) resulted in a statistically significant reduction in VSS score. Compared with PDL, IPL + CO2 (–1.69; 95% CI, –2.48 to –0.89), PDL + 1064-nm Nd:YAG (–0.99; 95% CI, –1.63 to –0.34), and PDL + CO2 (–0.87; 95% CI, –1.42 to –0.32) resulted in a statistically significant reduction in VSS score. Compared with 532-nm Nd:YAG, IPL + CO2 (–1.97; 95% CI, –3.20 to –0.75), PDL + 1064-nm Nd:YAG (–1.27; 95% CI, –2.44 to –0.10), and PDL + CO2 (–1.15; 95% CI, –2.24 to –0.06) resulted in a statistically significant reduction in VSS score. Compared with Er:YAG + IPL, IPL + CO2 (–2.13; 95% CI, –2.98 to –1.28), PDL + 1064-nm Nd:YAG (–1.43; 95% CI, –2.43 to –0.42), and PDL + CO2 (–1.31; 95% CI, –2.21 to –0.41) resulted in a statistically significant reduction in VSS score. Compared with Er:YAG, IPL + CO2 (–2.14; 95% CI, –2.91 to –1.38), PDL + 1064-nm Nd:YAG (–1.44; 95% CI, –2.27 to –0.61), PDL + CO2 (–1.32; 95% CI, –2.04 to –0.61) and CO2 (–0.67; 95% Cl, –1.19 to –0.14) resulted in a statistically significant reduction in VSS score. Compared with the control group, IPL + CO2 (–2.16; 95% CI, –3.05 to –1.27), PDL + 1064-nm Nd:YAG (–1.46; 95% CI, –2.32 to –0.60), PDL + CO2 (–1.34; 95% CI, –2.04 to –0.64), and CO2 (–0.68; 95% Cl, –1.15 to –0.22) resulted in a statistically significant reduction in VSS score. Compared with IPL, IPL + CO2 (–2.36; 95% CI, –2.90 to –1.83), PDL + 1064-nm Nd:YAG (–1.66; 95% CI, –2.54 to –0.79), PDL + CO2 (–1.54; 95% CI, –2.29 to –0.80), and CO2 (–0.89; 95% Cl, –1.43 to –0.35) resulted in a statistically significant reduction in VSS score. Compared with IPL, IPL + CO2 (–2.36; 95% CI, –2.90 to –1.83), PDL + 1064-nm Nd:YAG (–1.66; 95% CI, –2.54 to –0.79), PDL + CO2 (–1.54; 95% CI, –2.29 to –0.80), CO2 (–0.89; 95% Cl, –1.43 to –0.35), and PDL (–0.68; 95% CI, –1.27 to –0.09) resulted in a statistically significant reduction in VSS score. Table 3 shows the results of this NMA based on the Bayesian approach. The results obtained from the Bayesian approach were basically consistent with the results obtained by the frequentist approach.
Results of Network Meta-analysis for Vancouver Scar Scale Score Based on the Frequentist Approach
| IPL + CO2 . | . | . | . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| –0.70 (–1.73, 0.33) | PDL + 1064-nm Nd:YAG | |||||||||
| –0.82 (–1.74, 0.10) | –0.12 (–0.97, 0.73) | PDL + CO2 | ||||||||
| –1.48 (–2.23, –0.72) | –0.77 (–1.50, –0.05) | –0.66 (–1.19, –0.12) | CO2 | |||||||
| –1.59 (–2.54, –0.63) | –0.89 (–1.53, –0.24) | –0.77 (–1.53, –0.01) | –0.11 (–0.74, 0.51) | 1064-nm Nd:YAG | ||||||
| –1.69 (–2.48, –0.89) | –0.99 (–1.63, –0.34) | –0, 87 (–1.42, –0.32) | –0.21 (–0.55, 0.13) | –0.10 (–0.62, 0.42) | PDL | |||||
| –1.97 (–3.20, –0.75) | –1.27 (–2.44, –0.10) | –1.15 (–2.24, –0.06) | –0.50 (–1.47, 0.48) | –0.39 (–1.49, 0.72) | –0.28 (–1.26, 0.69) | 532-nm Nd:YAG | ||||
| –2.13 (–2.98, –1.28) | –1.43 (–2.43, –0.42) | –1.31 (–2.21, –0.41) | –0.65 (–1.40, 0.10) | –0.54 (–1.47, 0.39) | –0.44 (–1.21, 0.33) | –0.16 (–1.37, 1.06) | Er:YAG + IPL | |||
| –2.14 (–2.91, –1.38) | –1.44 (–2.27, –0.61) | –1.32 (–2.04, –0.61) | –0.67 (–1.19, –0.14) | –0.56 (–1.30, 0.19) | –0.45 (–0.98, 0.07) | –0.17 (–1.25, 0.91) | –0.01 (–0.67, 0.64) | Er:YAG | ||
| –2.16 (–3.05, –1.27) | –1.46 (–2.32, –0.60) | –1.34 (–2.04, –0.64) | –0.68 (–1.15, –0.22) | –0.57 (–1.35, 0.20) | –0.47 (–1.03, 0.09) | –0.19 (–1.16, 0.79) | –0.03 (–0.91, 0.85) | –0.02 (–0.71, 0.68) | Control | |
| –2.36 (–2.90, –1.83) | –1.66 (–2.54, –0.79) | –1.54 (–2.29, –0.80) | –0.89 (–1.43, –0.35) | –0.78 (–1.57, 0.01) | –0.68 (–1.27, –0.09) | –0.39 (–1.50, 0.71) | –0.24 (–0.89, 0.42) | –0.22 (–0.77, 0.33) | –0.21 (–0.92, 0.50) | IPL |
| IPL + CO2 . | . | . | . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| –0.70 (–1.73, 0.33) | PDL + 1064-nm Nd:YAG | |||||||||
| –0.82 (–1.74, 0.10) | –0.12 (–0.97, 0.73) | PDL + CO2 | ||||||||
| –1.48 (–2.23, –0.72) | –0.77 (–1.50, –0.05) | –0.66 (–1.19, –0.12) | CO2 | |||||||
| –1.59 (–2.54, –0.63) | –0.89 (–1.53, –0.24) | –0.77 (–1.53, –0.01) | –0.11 (–0.74, 0.51) | 1064-nm Nd:YAG | ||||||
| –1.69 (–2.48, –0.89) | –0.99 (–1.63, –0.34) | –0, 87 (–1.42, –0.32) | –0.21 (–0.55, 0.13) | –0.10 (–0.62, 0.42) | PDL | |||||
| –1.97 (–3.20, –0.75) | –1.27 (–2.44, –0.10) | –1.15 (–2.24, –0.06) | –0.50 (–1.47, 0.48) | –0.39 (–1.49, 0.72) | –0.28 (–1.26, 0.69) | 532-nm Nd:YAG | ||||
| –2.13 (–2.98, –1.28) | –1.43 (–2.43, –0.42) | –1.31 (–2.21, –0.41) | –0.65 (–1.40, 0.10) | –0.54 (–1.47, 0.39) | –0.44 (–1.21, 0.33) | –0.16 (–1.37, 1.06) | Er:YAG + IPL | |||
| –2.14 (–2.91, –1.38) | –1.44 (–2.27, –0.61) | –1.32 (–2.04, –0.61) | –0.67 (–1.19, –0.14) | –0.56 (–1.30, 0.19) | –0.45 (–0.98, 0.07) | –0.17 (–1.25, 0.91) | –0.01 (–0.67, 0.64) | Er:YAG | ||
| –2.16 (–3.05, –1.27) | –1.46 (–2.32, –0.60) | –1.34 (–2.04, –0.64) | –0.68 (–1.15, –0.22) | –0.57 (–1.35, 0.20) | –0.47 (–1.03, 0.09) | –0.19 (–1.16, 0.79) | –0.03 (–0.91, 0.85) | –0.02 (–0.71, 0.68) | Control | |
| –2.36 (–2.90, –1.83) | –1.66 (–2.54, –0.79) | –1.54 (–2.29, –0.80) | –0.89 (–1.43, –0.35) | –0.78 (–1.57, 0.01) | –0.68 (–1.27, –0.09) | –0.39 (–1.50, 0.71) | –0.24 (–0.89, 0.42) | –0.22 (–0.77, 0.33) | –0.21 (–0.92, 0.50) | IPL |
IPL, intense pulsed light; PDL, pulsed dye laser; SMD, standard mean difference; YAG, yttrium aluminum garnet. The off-diagonal cells in the league table show the relative treatment effects (standard mean difference [SMD] and 95% confidence intervals) for pairwise comparisons estimated in the network meta-analysis. For any cell, a negative SMD favors the upper-left intervention; a positive SMD favors the lower-right intervention.
Results of Network Meta-analysis for Vancouver Scar Scale Score Based on the Frequentist Approach
| IPL + CO2 . | . | . | . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| –0.70 (–1.73, 0.33) | PDL + 1064-nm Nd:YAG | |||||||||
| –0.82 (–1.74, 0.10) | –0.12 (–0.97, 0.73) | PDL + CO2 | ||||||||
| –1.48 (–2.23, –0.72) | –0.77 (–1.50, –0.05) | –0.66 (–1.19, –0.12) | CO2 | |||||||
| –1.59 (–2.54, –0.63) | –0.89 (–1.53, –0.24) | –0.77 (–1.53, –0.01) | –0.11 (–0.74, 0.51) | 1064-nm Nd:YAG | ||||||
| –1.69 (–2.48, –0.89) | –0.99 (–1.63, –0.34) | –0, 87 (–1.42, –0.32) | –0.21 (–0.55, 0.13) | –0.10 (–0.62, 0.42) | PDL | |||||
| –1.97 (–3.20, –0.75) | –1.27 (–2.44, –0.10) | –1.15 (–2.24, –0.06) | –0.50 (–1.47, 0.48) | –0.39 (–1.49, 0.72) | –0.28 (–1.26, 0.69) | 532-nm Nd:YAG | ||||
| –2.13 (–2.98, –1.28) | –1.43 (–2.43, –0.42) | –1.31 (–2.21, –0.41) | –0.65 (–1.40, 0.10) | –0.54 (–1.47, 0.39) | –0.44 (–1.21, 0.33) | –0.16 (–1.37, 1.06) | Er:YAG + IPL | |||
| –2.14 (–2.91, –1.38) | –1.44 (–2.27, –0.61) | –1.32 (–2.04, –0.61) | –0.67 (–1.19, –0.14) | –0.56 (–1.30, 0.19) | –0.45 (–0.98, 0.07) | –0.17 (–1.25, 0.91) | –0.01 (–0.67, 0.64) | Er:YAG | ||
| –2.16 (–3.05, –1.27) | –1.46 (–2.32, –0.60) | –1.34 (–2.04, –0.64) | –0.68 (–1.15, –0.22) | –0.57 (–1.35, 0.20) | –0.47 (–1.03, 0.09) | –0.19 (–1.16, 0.79) | –0.03 (–0.91, 0.85) | –0.02 (–0.71, 0.68) | Control | |
| –2.36 (–2.90, –1.83) | –1.66 (–2.54, –0.79) | –1.54 (–2.29, –0.80) | –0.89 (–1.43, –0.35) | –0.78 (–1.57, 0.01) | –0.68 (–1.27, –0.09) | –0.39 (–1.50, 0.71) | –0.24 (–0.89, 0.42) | –0.22 (–0.77, 0.33) | –0.21 (–0.92, 0.50) | IPL |
| IPL + CO2 . | . | . | . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| –0.70 (–1.73, 0.33) | PDL + 1064-nm Nd:YAG | |||||||||
| –0.82 (–1.74, 0.10) | –0.12 (–0.97, 0.73) | PDL + CO2 | ||||||||
| –1.48 (–2.23, –0.72) | –0.77 (–1.50, –0.05) | –0.66 (–1.19, –0.12) | CO2 | |||||||
| –1.59 (–2.54, –0.63) | –0.89 (–1.53, –0.24) | –0.77 (–1.53, –0.01) | –0.11 (–0.74, 0.51) | 1064-nm Nd:YAG | ||||||
| –1.69 (–2.48, –0.89) | –0.99 (–1.63, –0.34) | –0, 87 (–1.42, –0.32) | –0.21 (–0.55, 0.13) | –0.10 (–0.62, 0.42) | PDL | |||||
| –1.97 (–3.20, –0.75) | –1.27 (–2.44, –0.10) | –1.15 (–2.24, –0.06) | –0.50 (–1.47, 0.48) | –0.39 (–1.49, 0.72) | –0.28 (–1.26, 0.69) | 532-nm Nd:YAG | ||||
| –2.13 (–2.98, –1.28) | –1.43 (–2.43, –0.42) | –1.31 (–2.21, –0.41) | –0.65 (–1.40, 0.10) | –0.54 (–1.47, 0.39) | –0.44 (–1.21, 0.33) | –0.16 (–1.37, 1.06) | Er:YAG + IPL | |||
| –2.14 (–2.91, –1.38) | –1.44 (–2.27, –0.61) | –1.32 (–2.04, –0.61) | –0.67 (–1.19, –0.14) | –0.56 (–1.30, 0.19) | –0.45 (–0.98, 0.07) | –0.17 (–1.25, 0.91) | –0.01 (–0.67, 0.64) | Er:YAG | ||
| –2.16 (–3.05, –1.27) | –1.46 (–2.32, –0.60) | –1.34 (–2.04, –0.64) | –0.68 (–1.15, –0.22) | –0.57 (–1.35, 0.20) | –0.47 (–1.03, 0.09) | –0.19 (–1.16, 0.79) | –0.03 (–0.91, 0.85) | –0.02 (–0.71, 0.68) | Control | |
| –2.36 (–2.90, –1.83) | –1.66 (–2.54, –0.79) | –1.54 (–2.29, –0.80) | –0.89 (–1.43, –0.35) | –0.78 (–1.57, 0.01) | –0.68 (–1.27, –0.09) | –0.39 (–1.50, 0.71) | –0.24 (–0.89, 0.42) | –0.22 (–0.77, 0.33) | –0.21 (–0.92, 0.50) | IPL |
IPL, intense pulsed light; PDL, pulsed dye laser; SMD, standard mean difference; YAG, yttrium aluminum garnet. The off-diagonal cells in the league table show the relative treatment effects (standard mean difference [SMD] and 95% confidence intervals) for pairwise comparisons estimated in the network meta-analysis. For any cell, a negative SMD favors the upper-left intervention; a positive SMD favors the lower-right intervention.
Results of Network Meta-analysis for Vancouver Scar Scale Score Based on the Bayesian Approach
| IPL + CO2 . | . | . | . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| –0.61 (–2.15, 0.98) | PDL + 1064-nm Nd:YAG | |||||||||
| –0.80 (–2.18, 0.59) | –0.19 (–1.52, 1.10) | PDL + CO2 | ||||||||
| –1.49 (–2.65, –0.36) | –0.89 (–2.05, 0.21) | –0.69 (–1.52, 0.11) | CO2 | |||||||
| –1.48 (–2.89, –0.01) | –0.87 (–1.88, 0.14) | –0.68 (–1.81, 0.49) | 0.01 (–0.90, 0.99) | 1064-nm Nd:YAG | ||||||
| –1.60 (–2.78, –0.38) | –0.99 (–2.01, 0.02) | –0.80 (–1.61, 0.03) | –0.11 (–0.58, 0.42) | –0.12 (–0.92, 0.67) | PDL | |||||
| –2.00 (–3.65, –0.39) | –1.39 (–2.96, 0.11) | –1.20 (–2.60, 0.20) | –0.50 (–1.71, 0.69) | –0.52 (–1.95, 0.86) | –0.40 (–1.58, 0.74) | 532-nm Nd:YAG | ||||
| –2.11 (–3.39, –0.83) | –1.50 (–3.08, 0.03) | –1.31 (–2.69, 0.07) | –0.62 (–1.77, 0.54) | –0.63 (–2.08, 0.78) | –0.51 (–1.71, 0.66) | –0.11 (–1.72, 1.54) | Er:YAG + IPL | |||
| –2.11 (–3.26, –0.97) | –1.50 (–2.79, –0.25) | –1.31 (–2.37, –0.25) | –0.62 (–1.36, 0.14) | –0.63 (–1.76, 0.45) | –0.51 (–1.29, 0.24) | –0.11 (–1.45, 1.27) | 3.036E–5 (–1.03, 1.03) | Er:YAG | ||
| –2.36 (–3.12, –1.59) | –1.75 (–3.15, –0.40) | –1.56 (–2.71, –0.41) | –0.86 (–1.70, –0.01) | –0.88 (–2.12, 0.31) | –0.76 (–1.69, 0.15) | –0.36 (–1.78, 1.10) | –0.24 (–1.27, 0.78) | –0.24 (–1.10, 0.60) | IPL | |
| –2.38 (–3.70, –1.18) | –1.78 (–3.07, –0.62) | –1.58 (–2.60, –0.65) | –0.89 (–1.51, –0.33) | –0.90 (–2.02, 0.09) | –0.78 (–1.52, –0.15) | –0.39 (–1.60, 0.80) | –0.27 (–1.59, 0.97) | –0.27 (–1.24, 0.61) | –0.03 (–1.08, 0.93) | Control |
| IPL + CO2 . | . | . | . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| –0.61 (–2.15, 0.98) | PDL + 1064-nm Nd:YAG | |||||||||
| –0.80 (–2.18, 0.59) | –0.19 (–1.52, 1.10) | PDL + CO2 | ||||||||
| –1.49 (–2.65, –0.36) | –0.89 (–2.05, 0.21) | –0.69 (–1.52, 0.11) | CO2 | |||||||
| –1.48 (–2.89, –0.01) | –0.87 (–1.88, 0.14) | –0.68 (–1.81, 0.49) | 0.01 (–0.90, 0.99) | 1064-nm Nd:YAG | ||||||
| –1.60 (–2.78, –0.38) | –0.99 (–2.01, 0.02) | –0.80 (–1.61, 0.03) | –0.11 (–0.58, 0.42) | –0.12 (–0.92, 0.67) | PDL | |||||
| –2.00 (–3.65, –0.39) | –1.39 (–2.96, 0.11) | –1.20 (–2.60, 0.20) | –0.50 (–1.71, 0.69) | –0.52 (–1.95, 0.86) | –0.40 (–1.58, 0.74) | 532-nm Nd:YAG | ||||
| –2.11 (–3.39, –0.83) | –1.50 (–3.08, 0.03) | –1.31 (–2.69, 0.07) | –0.62 (–1.77, 0.54) | –0.63 (–2.08, 0.78) | –0.51 (–1.71, 0.66) | –0.11 (–1.72, 1.54) | Er:YAG + IPL | |||
| –2.11 (–3.26, –0.97) | –1.50 (–2.79, –0.25) | –1.31 (–2.37, –0.25) | –0.62 (–1.36, 0.14) | –0.63 (–1.76, 0.45) | –0.51 (–1.29, 0.24) | –0.11 (–1.45, 1.27) | 3.036E–5 (–1.03, 1.03) | Er:YAG | ||
| –2.36 (–3.12, –1.59) | –1.75 (–3.15, –0.40) | –1.56 (–2.71, –0.41) | –0.86 (–1.70, –0.01) | –0.88 (–2.12, 0.31) | –0.76 (–1.69, 0.15) | –0.36 (–1.78, 1.10) | –0.24 (–1.27, 0.78) | –0.24 (–1.10, 0.60) | IPL | |
| –2.38 (–3.70, –1.18) | –1.78 (–3.07, –0.62) | –1.58 (–2.60, –0.65) | –0.89 (–1.51, –0.33) | –0.90 (–2.02, 0.09) | –0.78 (–1.52, –0.15) | –0.39 (–1.60, 0.80) | –0.27 (–1.59, 0.97) | –0.27 (–1.24, 0.61) | –0.03 (–1.08, 0.93) | Control |
IPL, intense pulsed light; PDL, pulsed dye laser; YAG, yttrium aluminum garnet. The off-diagonal cells in the league table show the relative treatment effects (standard mean difference [SMD] and 95% confidence intervals) for pairwise comparisons estimated in the network meta-analysis. For any cell, a negative SMD favors the upper-left intervention; a positive SMD favors the lower-right intervention.
Results of Network Meta-analysis for Vancouver Scar Scale Score Based on the Bayesian Approach
| IPL + CO2 . | . | . | . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| –0.61 (–2.15, 0.98) | PDL + 1064-nm Nd:YAG | |||||||||
| –0.80 (–2.18, 0.59) | –0.19 (–1.52, 1.10) | PDL + CO2 | ||||||||
| –1.49 (–2.65, –0.36) | –0.89 (–2.05, 0.21) | –0.69 (–1.52, 0.11) | CO2 | |||||||
| –1.48 (–2.89, –0.01) | –0.87 (–1.88, 0.14) | –0.68 (–1.81, 0.49) | 0.01 (–0.90, 0.99) | 1064-nm Nd:YAG | ||||||
| –1.60 (–2.78, –0.38) | –0.99 (–2.01, 0.02) | –0.80 (–1.61, 0.03) | –0.11 (–0.58, 0.42) | –0.12 (–0.92, 0.67) | PDL | |||||
| –2.00 (–3.65, –0.39) | –1.39 (–2.96, 0.11) | –1.20 (–2.60, 0.20) | –0.50 (–1.71, 0.69) | –0.52 (–1.95, 0.86) | –0.40 (–1.58, 0.74) | 532-nm Nd:YAG | ||||
| –2.11 (–3.39, –0.83) | –1.50 (–3.08, 0.03) | –1.31 (–2.69, 0.07) | –0.62 (–1.77, 0.54) | –0.63 (–2.08, 0.78) | –0.51 (–1.71, 0.66) | –0.11 (–1.72, 1.54) | Er:YAG + IPL | |||
| –2.11 (–3.26, –0.97) | –1.50 (–2.79, –0.25) | –1.31 (–2.37, –0.25) | –0.62 (–1.36, 0.14) | –0.63 (–1.76, 0.45) | –0.51 (–1.29, 0.24) | –0.11 (–1.45, 1.27) | 3.036E–5 (–1.03, 1.03) | Er:YAG | ||
| –2.36 (–3.12, –1.59) | –1.75 (–3.15, –0.40) | –1.56 (–2.71, –0.41) | –0.86 (–1.70, –0.01) | –0.88 (–2.12, 0.31) | –0.76 (–1.69, 0.15) | –0.36 (–1.78, 1.10) | –0.24 (–1.27, 0.78) | –0.24 (–1.10, 0.60) | IPL | |
| –2.38 (–3.70, –1.18) | –1.78 (–3.07, –0.62) | –1.58 (–2.60, –0.65) | –0.89 (–1.51, –0.33) | –0.90 (–2.02, 0.09) | –0.78 (–1.52, –0.15) | –0.39 (–1.60, 0.80) | –0.27 (–1.59, 0.97) | –0.27 (–1.24, 0.61) | –0.03 (–1.08, 0.93) | Control |
| IPL + CO2 . | . | . | . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|
| –0.61 (–2.15, 0.98) | PDL + 1064-nm Nd:YAG | |||||||||
| –0.80 (–2.18, 0.59) | –0.19 (–1.52, 1.10) | PDL + CO2 | ||||||||
| –1.49 (–2.65, –0.36) | –0.89 (–2.05, 0.21) | –0.69 (–1.52, 0.11) | CO2 | |||||||
| –1.48 (–2.89, –0.01) | –0.87 (–1.88, 0.14) | –0.68 (–1.81, 0.49) | 0.01 (–0.90, 0.99) | 1064-nm Nd:YAG | ||||||
| –1.60 (–2.78, –0.38) | –0.99 (–2.01, 0.02) | –0.80 (–1.61, 0.03) | –0.11 (–0.58, 0.42) | –0.12 (–0.92, 0.67) | PDL | |||||
| –2.00 (–3.65, –0.39) | –1.39 (–2.96, 0.11) | –1.20 (–2.60, 0.20) | –0.50 (–1.71, 0.69) | –0.52 (–1.95, 0.86) | –0.40 (–1.58, 0.74) | 532-nm Nd:YAG | ||||
| –2.11 (–3.39, –0.83) | –1.50 (–3.08, 0.03) | –1.31 (–2.69, 0.07) | –0.62 (–1.77, 0.54) | –0.63 (–2.08, 0.78) | –0.51 (–1.71, 0.66) | –0.11 (–1.72, 1.54) | Er:YAG + IPL | |||
| –2.11 (–3.26, –0.97) | –1.50 (–2.79, –0.25) | –1.31 (–2.37, –0.25) | –0.62 (–1.36, 0.14) | –0.63 (–1.76, 0.45) | –0.51 (–1.29, 0.24) | –0.11 (–1.45, 1.27) | 3.036E–5 (–1.03, 1.03) | Er:YAG | ||
| –2.36 (–3.12, –1.59) | –1.75 (–3.15, –0.40) | –1.56 (–2.71, –0.41) | –0.86 (–1.70, –0.01) | –0.88 (–2.12, 0.31) | –0.76 (–1.69, 0.15) | –0.36 (–1.78, 1.10) | –0.24 (–1.27, 0.78) | –0.24 (–1.10, 0.60) | IPL | |
| –2.38 (–3.70, –1.18) | –1.78 (–3.07, –0.62) | –1.58 (–2.60, –0.65) | –0.89 (–1.51, –0.33) | –0.90 (–2.02, 0.09) | –0.78 (–1.52, –0.15) | –0.39 (–1.60, 0.80) | –0.27 (–1.59, 0.97) | –0.27 (–1.24, 0.61) | –0.03 (–1.08, 0.93) | Control |
IPL, intense pulsed light; PDL, pulsed dye laser; YAG, yttrium aluminum garnet. The off-diagonal cells in the league table show the relative treatment effects (standard mean difference [SMD] and 95% confidence intervals) for pairwise comparisons estimated in the network meta-analysis. For any cell, a negative SMD favors the upper-left intervention; a positive SMD favors the lower-right intervention.
The SUCRA rankings based on the frequentist approach suggested IPL + CO2 (98.6%) as the best intervention followed by PDL + 1064-nm Nd:YAG (86.7%), PDL + CO2 (83.6%), CO2 (63.2%), 1064-nm Nd:YAG (55.1%), PDL (49.5%), 532-nm Nd:YAG (33.7%), Er:YAG + IPL (25.4%), Er:YAG (22.7%), control (21.5%), and IPL (10.1%) which ranked last (Figure 4). Treatment ranking based on SUCRA values, according to the Bayesian approach, was as follows: IPL + CO2 (96.43%) > PDL + 1064-nm Nd:YAG (86.21%) > PDL + CO2 (82.15%) > CO2 (58.97%) > 1064-nm Nd:YAG (57.03%) > PDL (52%) > 532-nm Nd:YAG (33.28%) > Er:YAG + IPL (28.38%) > Er:YAG (26.56%) > IPL (15.03%) > control (13.97%) (Figure 5). The funnel plot was basically symmetric, suggesting that there was no publication bias (Figure 6).
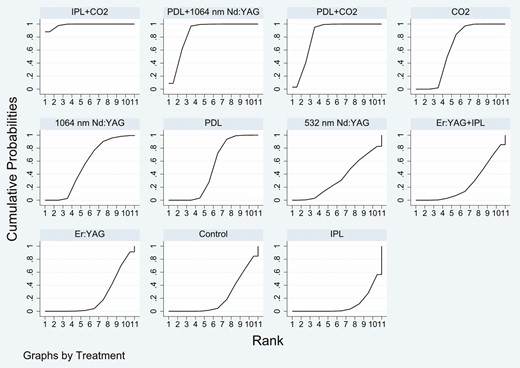
Plot of the surface under the cumulative ranking curve based on the frequentist approach for the Vancouver Scar Scale score.
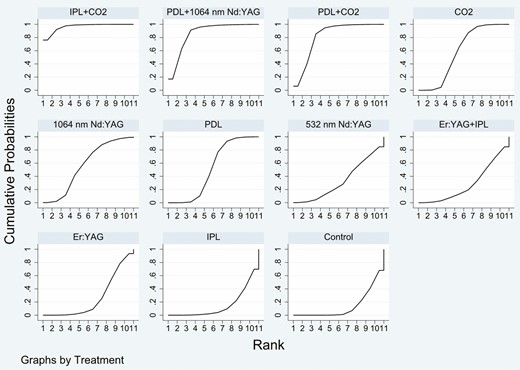
Plot of the surface under the cumulative ranking curve based on the Bayesian approach for the Vancouver Scar Scale score.
To further analyze the varying efficacy of lasers in reducing VSS score of hypertrophic scars, subgroup analyses were performed (Supplemental Table 1 and Supplemental Figure 1, available online at www.aestheticsurgeryjournal.com). The results showed that the most efficacious treatment was IPL + CO2, followed by PDL + 1064-nm Nd:YAG, PDL + CO2, and CO2. The results of subgroup analysis on hypertrophic scars were basically consistent with the overall group analysis.
Secondary Outcomes
Four secondary outcomes were reported in 5 studies, including 2 combination treatments and 3 monotherapies: IPL + CO2, Er:YAG + 1064-nm Nd:YAG, Er:YAG + IPL, Er:YAG, and IPL. The network diagram for all these 4 secondary outcomes is shown in Supplemental Figure 2, available online at www.aestheticsurgeryjournal.com.
The results of this NMA indicated that all types of combination treatments were superior to monotherapy treatment (Supplemental Tables 2-5, available online at www.aestheticsurgeryjournal.com). For thickness, Er:YAG + 1064-nm Nd:YAG (–1.08; 95% CI, –1.36 to –0.80) and Er:YAG + IPL (–2.57; 95% CI, –3.28 to –1.86) showed a significant improvement compared with IPL. IPL + CO2 (–1.04; 95% CI, –1.54 to –0.54) and Er:YAG + 1064 nm Nd:YAG (–0.78; 95% CI, –1.33 to –0.24) showed a significant improvement compared with Er:YAG. IPL + CO2 (–0.92; 95% CI, –1.42 to –0.42) showed a significant improvement compared with Er:YAG + IPL. For vascularity, Er:YAG + 1064-nm Nd:YAG (–1.24; 95% CI, –1.53 to –0.96) and Er:YAG + IPL (–2.25; 95% CI, –2.93 to –1.58) showed a significant improvement compared with IPL. IPL + CO2 (–1.22; 95% CI, –1.72 to –0.71) and Er:YAG + 1064-nm Nd:YAG (–0.77; 95% CI, –1.32 to –0.23) showed a significant improvement compared to Er:YAG. IPL + CO2 (–1.19; 95% CI, –1.69 to –0.68) and Er:YAG + 1064-nm Nd:YAG (–0.74; 95% CI, –1.43 to –0.06) showed a significant improvement compared with Er:YAG + IPL. For pigmentation, Er:YAG + 1064-nm Nd:YAG (–0.95; 95% CI, –1.23 to –0.67) and Er:YAG + IPL (–1.88; 95% CI, –2.54 to –1.23) showed a significant improvement compared with IPL. IPL + CO2 (–0.92; 95% CI, –1.42 to –0.42) and Er:YAG + 1064-nm Nd:YAG (–0.77; 95% CI, –1.31 to –0.22) showed a significant improvement compared with Er:YAG. IPL + CO2 (–0.81; 95% CI, –1.31 to –0.31) showed a significant improvement compared with Er:YAG + IPL. For pliability, Er:YAG + 1064-nm Nd:YAG (–0.85; 95% CI, –1.12 to –0.57) and Er:YAG + IPL (–2.98; 95% CI, –3.72 to –2.24) showed a significant improvement compared with IPL. IPL + CO2 (–0.82; 95% CI, –1.32 to –0.32) and Er:YAG + 1064-nm Nd:YAG (–0.64; 95% CI, –1.18 to –0.10) showed a significant improvement compared with Er:YAG. IPL + CO2 (–0.77; 95% CI, –1.26 to –0.27) showed a significant improvement compared with Er:YAG + IPL.
All of the results for these 4 secondary outcomes showed that the most efficacious treatment was IPL + CO2, followed by Er:YAG + 1064-nm Nd:YAG, Er:YAG + IPL, Er:YAG, and IPL (Supplemental Figures 3-6, available online at www.aestheticsurgeryjournal.com).
DISCUSSION
The main pathologic features of scars are excessive proliferation of fibroblasts, abnormal increase in capillaries, and excessive deposition of extracellular matrix.1 Therefore, there are 2 main strategies for scar treatment: inhibition of excessive fibroblast proliferation, and enhancement of angiogenesis. Although many treatments are currently used for pathologic scars, their therapeutic effects are not ideal.11
With the development of laser and light technology, IPL and a variety of lasers have been used to treat pathologic scars.15 Lasers used to treat pathologic scars can be roughly classified into 2 types: ablative and nonablative.51 The former destroys the epidermis in the process of reaching the chromophore, whereas the latter maintains the integrity of the epidermis during the treatment.18 Most of the proposed mechanisms for the use of lasers in the treatment of pathologic scars are based on selective photothermolysis, in which the light energy emitted by the laser can be absorbed by its intended target chromophore, leading to collagen denaturation, neocollagenesis, and finally tissue remodeling.13 Several clinical trials aimed at assessing the efficacy of laser and IPL therapies for pathologic scars have been conducted.26-50 Therefore, we conducted this NMA which summarized direct and indirect evidence to rank the efficacy of interventions.
Our results showed that combining different laser devices or utilizing devices with more than 1 target was more effective than utilizing single laser devices. This may be due to the fact that different wavelengths of the combined lasers target different chromophores expressed in the scar tissue, maximizing the laser-skin interaction and providing better efficacy than single-laser devices.52
According to our results, the combination of IPL and CO2 laser had the highest probability of being the most effective intervention. The results may be explained by the following mechanisms. Compared to other lasers, CO2 laser has a greater penetration depth and creates more thermal damage, allowing it to treat deeper dermal fibrotic components and leading to a more positive result.30 On the other hand, IPL can simultaneously target both erythema and dyspigmentation due to its wide wavelength spectrum.53 Furthermore, IPL has a large light spot size which allows it to treat larger areas of scars relatively quickly.54 A combination of IPL and CO2 laser produces an additive effect which leads to an optimal efficiency. However, probabilities indicating which intervention is the most effective should be interpreted with caution due to the limited data, and treatment effects should be given more attention.55
As for the comparison among different single-laser devices, PDL or a 532-nm Nd:YAG laser was less effective than a CO2 laser or a 1064-nm Nd:YAG laser. An explanation of this finding may be that although PDL and the 532-nm Nd:YAG laser are effective on oxygenated hemoglobin and melanin in the superficial skin layer, they cannot penetrate deeply into the deep layer of skin, to the dermis.34 This result was in contrast to Jin et al who reported that PDL and the 532-nm Nd:YAG laser yielded the best responses among all the laser systems they investigated.12 The reason for this difference may be the different evaluation indexes.
Several studies have demonstrated the safety and effectiveness of Er:YAG laser resurfacing of pathologic scars including improvement in scar thickness, vascularity, pigmentation, and pliability.56-58 The 2940-nm Er:YAG laser has a high affinity for water-containing tissue, resulting in high-precision skin ablation.57 In addition, the Er:YAG laser causes only minimal thermal necrosis with a lower incidence of severe side effects.59
It was also worth noting that treatment with IPL alone was the least effective. A possible explanation for this result is that the energy emitted from the IPL has a decreasing trend from the center of the light spectrum to the edge, whereas the light energy of the most useful segment of the spectrum of IPL is only a fraction of the total emitted energy.60 Therefore, the efficacy of IPL appears disappointing.
It appears that the clinical improvement of laser treatment may be reduced in dark-skinned individuals, which may be due to competitive absorption by melanin chromophore.34 In addition, evidence from previous studies has shown that hypertrophic scars tend to be more sensitive to laser treatment than keloids.3,34 Furthermore, keloids are more likely to recur after treatment.3 Koike et al have suggested that if the scars can become completely mature, no recurrence will be observed. Nevertheless, if there still remains only a little keloid redness and induration, these scars can have a higher rate of recurrence.61 At present, there does not appear to be a reliable report of a standard recurrence rate of laser treatment for pathologic scars. The recurrence rate of laser treatment for pathologic scars varies widely in different studies, ranging from 10.5% to 92%.62
Currently, laser therapy is usually used in combination with other therapies.63 The evidence available suggests that combined therapy can enhance the therapeutic efficacy, reduce the rate of recurrence, and lead to fewer intolerable side effects.51 In recent years, many have reported encouraging results, suggesting that a combination treatment consisting of laser treatment and drug injection is an effective method for the management of pathologic scars.64-66 Connell and Harland noted that PDL can reduce the pain caused by steroid injections. They also found that PDL promoted steroid injection by making the scar edematous and softer.66 Park et al have demonstrated the ability of fractional lasers to enhance the efficacy of topical medications by facilitating transdermal delivery.67 Wang et al reported on the long-term follow-up in a group of 41 patients with keloids.62 The results demonstrated that combination therapy with a CO2 laser and tropical triamcinolone was an effective method, producing a long-lasting effect and a low recurrence rate. The combination of laser and drug injection has a complimentary and summative effect which results in significant improvement and a low recurrence rate.64
To the best of our knowledge, this is the first NMA to compare the efficacy of laser and IPL therapies in patients with pathologic scars. Several strengths enhanced the validity of our NMA. We used relatively strict exclusion and inclusion criteria to reduce the risk of bias. Furthermore, we used both frequentist and Bayesian approaches to ensure the robustness of the results. In addition, the results were robust as demonstrated by the sensitivity analysis and funnel plots. Thus, we are confident that the results of our NMA are valid.
Although the results of our NMA are promising, we recognize several limitations. First, the number of sessions, the time intervals between treatments, and the follow-up time varied greatly across studies, which may be a potential source of heterogeneity. Second, the recurrence rate is an important index for evaluating the efficacy of the treatment for pathologic scars and few studies reported this. Future research should focus more on the recurrence rate after laser treatment. Finally, the quality of most of the included studies was not high.
CONCLUSIONS
This NMA is the first study that synthesizes the available evidence on laser and IPL therapies for pathologic scars. Our findings indicate that IPL combined with a CO2 laser has the potential to be the most effective treatment regimen for pathologic scars among all the regimens investigated. As for the secondary outcomes, an Er:YAG laser or an Er:YAG laser in combination with other laser systems appear favorable. However, due to the small number of studies, and the number of interventions involving secondary outcomes, this finding needs to be interpreted with caution. Further randomized controlled trials are needed to provide more direct evidence to verify our findings.
Disclosures
The author declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
Funded by the Key Scientific Research Projects of Colleges and Universities of Henan Province, China (grant 20A320033).



