-
PDF
- Split View
-
Views
-
Cite
Cite
Peter V Vester-Glowinski, Mikkel Herly, Mathias Ørholt, Bo S Rasmussen, Felix C Müller, Jens J Elberg, Carsten Thomsen, Krzysztof T Drzewiecki, Fat Grafting With Expanded Adipose-Derived Stromal Cells for Breast Augmentation: A Randomized Controlled Trial, Aesthetic Surgery Journal, Volume 42, Issue 11, November 2022, Pages 1279–1289, https://doi.org/10.1093/asj/sjac159
Close - Share Icon Share
Abstract
The main challenge with fat grafting is loss of some of the graft to postsurgery resorption. Previous studies suggest that adipose-derived stromal cells (ASCs) can improve the volume retention of fat grafts but there is a lack of randomized trials to support the use of ASCs in clinical practice.
This trial aimed to investigate whether ASCs improve fat graft volume retention in patients undergoing breast augmentation with lipofilling.
This was a double-blind, randomized controlled trial of breast augmentation with ASC-enriched fat grafting. Healthy women aged 30 to 45 years were enrolled. First, the participants underwent liposuction to obtain fat for culture expansion of ASCs. Then, the participants were randomly assigned to undergo a 300- to 350-mL breast augmentation with ASC-enriched fat grafting (10 × 106 ASCs/mL fat graft) to 1 of their breasts and placebo-enriched fat grafting of identical volume to the contralateral breast. The primary outcome was fat graft volume retention after a 1-year follow-up measured with MRI. The trial is registered at www.clinicaltrialsregister.eu (EudraCT-2014-000510-59).
Ten participants were included in the trial; all completed the treatment and follow-up. No serious adverse events occurred. Fat graft volume retention after 1 year was 54.0% (95% CI, 30.4%-77.6%) in the breasts treated with ASC-enriched fat grafting (n = 10) and 55.9% (95% CI, 28.9%-82.9%) in the contralateral breasts treated with placebo-enriched fat grafting (n = 10) (P = 0.566).
The findings of this trial do not support that ASC-enriched fat grafting is superior to standard fat grafting for breast augmentation.

See the Commentary on this article here.
Fat grafting is increasingly used for breast reconstruction1,2 and augmentation3-5 as an autologous and safe alternative to implants. However, fat grafting has a major drawback: between 20% and 80%6-12 of the graft is lost to postsurgery resorption.
It has been suggested that enrichment of fat grafts with adipose-derived stromal cells (ASCs)13 can increase volume retention and decrease the number of surgeries needed to achieve the desired result, which would markedly impact patient morbidity.14 Human studies of autologous high-dosage ASC-enriched fat grafting have previously indicated that the graft retention rates may be improved by cell enrichment14-16 but the effect has been disputed in some human studies.17,18 An international expert panel concluded that there is currently no definitive evidence that adding ASCs to a fat graft improves the outcome.19 Therefore, randomized controlled trials are needed to investigate the efficacy of ASC enrichment for clinically relevant outcomes. In this randomized controlled superiority trial, we investigate whether a breast augmentation with fat grafting enriched with expanded autologous ASCs leads to higher fat graft retention than placebo-enriched fat grafting.
METHODS
Trial Design
This was a randomized, double-blind, placebo-controlled superiority trial comparing ex vivo expanded ASC-enriched fat grafting with placebo-enriched fat grafting for breast augmentation.
The participants were randomly assigned to ASC-enriched fat grafting to 1 breast and placebo-enriched fat grafting to the contralateral breast. The trial was conducted in accordance with the CONSORT statement (Appendix, available online at www.aestheticsurgeryjournal.com) and the Declaration of Helsinki.20,21 The study design was approved by the Danish National Committee on Health Research Ethics and the Danish Medicines Agency (EudraCT 2014-000510-59), registered at the European Union Drug Regulating Authorities Clinical Trials Database (www.clinicaltrialsregister.eu) and monitored by the Good Clinical Practice (GCP) unit.
Participants
The trial was conducted at the Department of Plastic Surgery and Burns Treatment, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark. Healthy women aged 30 to 45 years with small, symmetric breasts were eligible for inclusion. Potential participants who had undergone previous breast surgery, were pregnant, or who had known chronic diseases were excluded. The participants provided written informed consent at least 7 days before treatment. The participants accepted that potential asymmetry from an increased volume retention on 1 of their breasts would necessitate 1 or more lipofilling procedures to achieve a symmetric final result.
Surgical Procedures
The lipoaspirate used to isolate stromal vascular fraction (SVF)22 for culture expansion of ASCs was obtained from the abdomen 17 days before the breast augmentation with a power-assisted liposuction device (Vibrasat, Möller Medical, Fulda, Germany) under local anesthesia. In a second procedure, each participant underwent breast augmentation under general anesthesia with fat grafting without ASC enrichment. The liposuction was performed with a water-jet-assisted device (Human Med AG, Schwerin, Germany) from the hips and thighs. The lipoaspirate was transferred to 50-mL syringes (BD, Albertslund, Denmark) and centrifuged at 100g for 3 minutes to remove the wetting solution. The fat grafts were mixed manually in an open sterile container with either ASCs or placebo and then transferred to 10-mL Luer Lock syringes (BD). The fat grafts were injected with blunt 14-G cannulas (Tulip Medical Products, San Diego, CA) by the structural grafting technique.23 One plastic surgeon performed all procedures.
Intervention
The included participants were randomized in 1 block to allocate which breast would receive ASC-enriched fat grafting. The contralateral breast was treated with placebo-enriched fat grafting. Thus, the allocation ratio between ASC enrichment and placebo enrichment was 1:1 within each participant. The randomization sequence was generated with www.randomization.com in a single block.
The fat obtained by liposuction was mixed with either the ASC solution or a placebo solution which consisted of normal centrifuged fat. The fat grafts were transferred to 10-mL syringes and placed on 2 surgical tables with a drape blocking the view to the surgical staff. Then, the syringes were brought to the surgical staff according to the randomized allocation. Figure 1 shows the study design and randomization process.
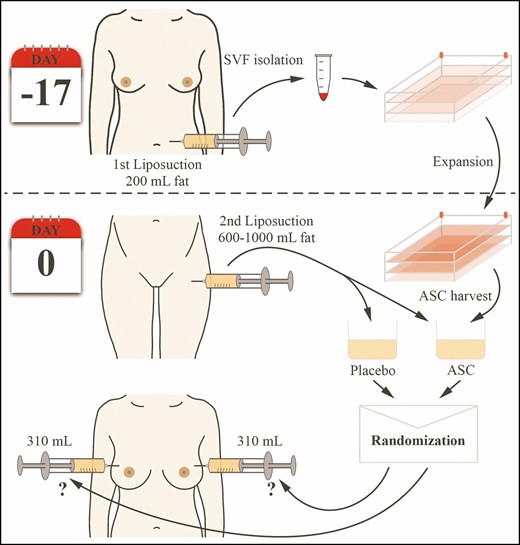
An illustration of the paired study design and randomization process.
ASC Expansion
The ASCs were expanded and harvested according to previously described principles15,24 using good manufacturing practice–grade reagents in a certified laboratory approved by the Danish National Board of Health for clinical stem cell expansion. The autologous ASCs for each participant were culture expanded in 8 pieces of CellStack-10 TC-treated (Corning Life Science, New York, NY) with a combined surface area of 5.1 m2 and harvested on the day of the breast augmentation surgery after 17 days of culture expansion. Figure 2 shows an overview of the expansion process.
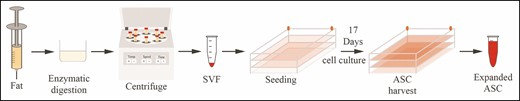
An illustration of the ASC expansion process from enzymatic digestion to ASC harvesting. ASC, adipose-derived stromal cell.
The cells were visually inspected to ensure normal spindle-shaped cell morphology before harvesting and the viability and ASC count was performed with a NucleoCounter NC-200 (ChemoMetec, Allerød, Denmark). The release criteria prior to treatment stipulated cell viability of >85% and that the culturing media should be free from microbiological contamination 1 week before the cell harvest and on the day of the breast augmentation surgery.
Phenotypic markers of the ASCs were measured from a sample taken from the ASC suspension on the day of surgery. An antibody mix against ASC-negative cell surface markers (anti-45 FITC and anti-CD31 FITC) and ASC-positive cell surface markers (anti-CD73 APC, anti-CD90 BV510, and anti-CD105 BV421) were added and incubated for 30 minutes. The stained samples were diluted with phosphate-buffered saline/ethylenediaminetetraacetic acid containing 0.1% hyaluronic acid and subsequently analyzed by flow cytometry (FACS Canto II flow cytometer, BD Biosciences, San Jose, CA).
Measurement of the Fat Graft Volume Retention
Fat graft volume retention was measured with a 3-T MRI unit (Siemens Magnetom Verio, Erlangen, Germany) equipped with a 4-channel breast coil. Imaging was obtained the day before breast augmentation surgery and at 4- and 12-month follow-up. The calculations of fat graft volume retention were done with Lipovol software based on a validated, automated method for measuring fat graft volume retention in the breast.25-27 Three blinded observers who were trained in the use of the software, and supervised by 2 radiologists, performed all fat graft volume retention calculations. An average of the 3 observers’ measurements was used in the analyses. An example of pre- and postoperative MRI is shown in Figure 3.
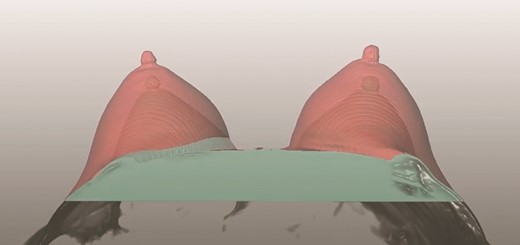
A screenshot of the Lipovol method where fat graft volume retention is measured.
Outcomes
The primary outcome was the percentage volume retention of the injected fat graft calculated as an average of the 3 observer’s measurements at the 12-month follow-up. The secondary outcomes were fat graft volume retention at the 4-month follow-up, radiologic changes in the breast evaluated with the Breast Imaging-Reporting Data System (BI-RADS),28 and cyst formation evaluated from MRI scans without contrast and mammography.
Statistical Analysis
A sample size calculation was performed before initiating the trial. A total of 10 participants were estimated to provide 90% power to detect a 30% difference in fat graft volume retention between the 2 groups (standard deviation, 20%) at a 2-tailed 5% significance level with a paired t test. The difference in fat graft volume retention between ASC enrichment and placebo enrichment after 4 and 12 months was assessed with a paired t test and P-values were evaluated at a 5% level. Data distributions were assessed with histograms, QQ-plots, and the Shapiro-Wilk test.29 All analyses and plots were performed in R, version 3.6.1 (www.r-project.org, R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Participants and Recruitment
Between January 2016 and May 2018, 306 women responded to the trial call, of whom 231 completed the assessment for eligibility. Ten female participants who met the inclusion criteria were recruited into the trial. A chart of the screening process can be seen in Figure 4. The median participant age was 33 years (interquartile range, 30-40 years). Baseline participant characteristics and data from the cell expansion and harvest are shown in Table 1. All the included participants completed the treatment and follow-up after 4 and 12 months according to protocol.
| Demography of the participants . | Median (IQR) . |
|---|---|
| Number of participants | 10 |
| Age (years) | 33 (30.3-40.0) |
| BMI (kg/m2) | 24.3 (22.9-25.7) |
| Isolation and seeding of SVF | |
| Lipoaspirate for SVF isolation (mL) | 200 (193-200) |
| SVF cells/mL lipoaspirate (live) | 1.39 × 106 (1.18-2.10 × 106) |
| Total SVF cells isolated (live) | 2.81 × 108 (2.32-4.13 × 108) |
| Viability of SVF cells (%) | 94.5 (93.3-95.7) |
| SVF cell size (µm) | 10.6 (10.5-10.7) |
| SVF seeding density (SVF/cm2) | 5.15 (4.46-6.00) |
| ASC harvest | |
| ASC/cm2 at harvest (live) | 8.66 × 104 (7.28 × 104 to 1.05 × 105) |
| Total ASCs at harvest (live) | 4.41 × 109 (3.70-5.32 × 109) |
| Viability (%) | 96.8 (96.3-97.4) |
| Culture time (days) | 17 (17-18) |
| ASC cell size (µm) | 17.2 (17.0-17.4) |
| Demography of the participants . | Median (IQR) . |
|---|---|
| Number of participants | 10 |
| Age (years) | 33 (30.3-40.0) |
| BMI (kg/m2) | 24.3 (22.9-25.7) |
| Isolation and seeding of SVF | |
| Lipoaspirate for SVF isolation (mL) | 200 (193-200) |
| SVF cells/mL lipoaspirate (live) | 1.39 × 106 (1.18-2.10 × 106) |
| Total SVF cells isolated (live) | 2.81 × 108 (2.32-4.13 × 108) |
| Viability of SVF cells (%) | 94.5 (93.3-95.7) |
| SVF cell size (µm) | 10.6 (10.5-10.7) |
| SVF seeding density (SVF/cm2) | 5.15 (4.46-6.00) |
| ASC harvest | |
| ASC/cm2 at harvest (live) | 8.66 × 104 (7.28 × 104 to 1.05 × 105) |
| Total ASCs at harvest (live) | 4.41 × 109 (3.70-5.32 × 109) |
| Viability (%) | 96.8 (96.3-97.4) |
| Culture time (days) | 17 (17-18) |
| ASC cell size (µm) | 17.2 (17.0-17.4) |
ASC, adipose-derived stromal cell; IQR, interquartile range; SVF, stromal vascular fraction.
| Demography of the participants . | Median (IQR) . |
|---|---|
| Number of participants | 10 |
| Age (years) | 33 (30.3-40.0) |
| BMI (kg/m2) | 24.3 (22.9-25.7) |
| Isolation and seeding of SVF | |
| Lipoaspirate for SVF isolation (mL) | 200 (193-200) |
| SVF cells/mL lipoaspirate (live) | 1.39 × 106 (1.18-2.10 × 106) |
| Total SVF cells isolated (live) | 2.81 × 108 (2.32-4.13 × 108) |
| Viability of SVF cells (%) | 94.5 (93.3-95.7) |
| SVF cell size (µm) | 10.6 (10.5-10.7) |
| SVF seeding density (SVF/cm2) | 5.15 (4.46-6.00) |
| ASC harvest | |
| ASC/cm2 at harvest (live) | 8.66 × 104 (7.28 × 104 to 1.05 × 105) |
| Total ASCs at harvest (live) | 4.41 × 109 (3.70-5.32 × 109) |
| Viability (%) | 96.8 (96.3-97.4) |
| Culture time (days) | 17 (17-18) |
| ASC cell size (µm) | 17.2 (17.0-17.4) |
| Demography of the participants . | Median (IQR) . |
|---|---|
| Number of participants | 10 |
| Age (years) | 33 (30.3-40.0) |
| BMI (kg/m2) | 24.3 (22.9-25.7) |
| Isolation and seeding of SVF | |
| Lipoaspirate for SVF isolation (mL) | 200 (193-200) |
| SVF cells/mL lipoaspirate (live) | 1.39 × 106 (1.18-2.10 × 106) |
| Total SVF cells isolated (live) | 2.81 × 108 (2.32-4.13 × 108) |
| Viability of SVF cells (%) | 94.5 (93.3-95.7) |
| SVF cell size (µm) | 10.6 (10.5-10.7) |
| SVF seeding density (SVF/cm2) | 5.15 (4.46-6.00) |
| ASC harvest | |
| ASC/cm2 at harvest (live) | 8.66 × 104 (7.28 × 104 to 1.05 × 105) |
| Total ASCs at harvest (live) | 4.41 × 109 (3.70-5.32 × 109) |
| Viability (%) | 96.8 (96.3-97.4) |
| Culture time (days) | 17 (17-18) |
| ASC cell size (µm) | 17.2 (17.0-17.4) |
ASC, adipose-derived stromal cell; IQR, interquartile range; SVF, stromal vascular fraction.
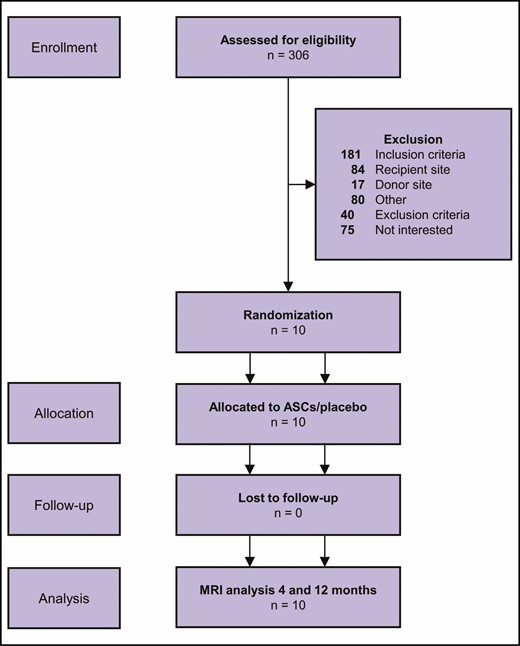
Flowchart of the screening process according to the CONSORT guidelines.
Primary Endpoint
Fat graft volume retention 12 months after surgery was 54.0% (95% CI, 30.4%-77.6%) in the breasts treated with ASC-enriched fat grafting (n = 10) and 55.9 % (95% CI, 28.9%-82.9%) in the contralateral breasts treated with placebo-enriched fat grafting (n = 10). The difference was not statistically significant (P = 0.566). Ladder plots connecting the fat graft retention rate in each participant’s breasts are shown in Figure 5 and the individual surgical data are presented in Table 2. None of the participants experienced serious adverse events or developed clinical asymmetry.
| Individual patient characteristics . | ASC-enriched side (R/L) . | Injected fat graft volume (mL) . | Fat graft retention at 12 months (%) . | |
|---|---|---|---|---|
| . | . | . | ASC-enriched . | Control . |
| Participant 1 | R | 300 | 64 | 70 |
| Participant 2 | R | 300 | 83 | 81 |
| Participant 3 | R | 300 | 4 | 6 |
| Participant 4 | L | 300 | 28 | 15 |
| Participant 5 | R | 350 | 70 | 70 |
| Participant 6 | R | 300 | 84 | 111 |
| Participant 7 | L | 330 | 83 | 81 |
| Participant 8 | L | 300 | 23 | 22 |
| Participant 9 | L | 320 | 14 | 14 |
| Participant 10 | L | 300 | 87 | 89 |
| Mean (95% CI) | – | 300 (300-315)a | 54.0 (30.4-77.6) | 55.9 (28.9-82.9) |
| Individual patient characteristics . | ASC-enriched side (R/L) . | Injected fat graft volume (mL) . | Fat graft retention at 12 months (%) . | |
|---|---|---|---|---|
| . | . | . | ASC-enriched . | Control . |
| Participant 1 | R | 300 | 64 | 70 |
| Participant 2 | R | 300 | 83 | 81 |
| Participant 3 | R | 300 | 4 | 6 |
| Participant 4 | L | 300 | 28 | 15 |
| Participant 5 | R | 350 | 70 | 70 |
| Participant 6 | R | 300 | 84 | 111 |
| Participant 7 | L | 330 | 83 | 81 |
| Participant 8 | L | 300 | 23 | 22 |
| Participant 9 | L | 320 | 14 | 14 |
| Participant 10 | L | 300 | 87 | 89 |
| Mean (95% CI) | – | 300 (300-315)a | 54.0 (30.4-77.6) | 55.9 (28.9-82.9) |
L, left; R, right.
aMedian (interquartile range).
| Individual patient characteristics . | ASC-enriched side (R/L) . | Injected fat graft volume (mL) . | Fat graft retention at 12 months (%) . | |
|---|---|---|---|---|
| . | . | . | ASC-enriched . | Control . |
| Participant 1 | R | 300 | 64 | 70 |
| Participant 2 | R | 300 | 83 | 81 |
| Participant 3 | R | 300 | 4 | 6 |
| Participant 4 | L | 300 | 28 | 15 |
| Participant 5 | R | 350 | 70 | 70 |
| Participant 6 | R | 300 | 84 | 111 |
| Participant 7 | L | 330 | 83 | 81 |
| Participant 8 | L | 300 | 23 | 22 |
| Participant 9 | L | 320 | 14 | 14 |
| Participant 10 | L | 300 | 87 | 89 |
| Mean (95% CI) | – | 300 (300-315)a | 54.0 (30.4-77.6) | 55.9 (28.9-82.9) |
| Individual patient characteristics . | ASC-enriched side (R/L) . | Injected fat graft volume (mL) . | Fat graft retention at 12 months (%) . | |
|---|---|---|---|---|
| . | . | . | ASC-enriched . | Control . |
| Participant 1 | R | 300 | 64 | 70 |
| Participant 2 | R | 300 | 83 | 81 |
| Participant 3 | R | 300 | 4 | 6 |
| Participant 4 | L | 300 | 28 | 15 |
| Participant 5 | R | 350 | 70 | 70 |
| Participant 6 | R | 300 | 84 | 111 |
| Participant 7 | L | 330 | 83 | 81 |
| Participant 8 | L | 300 | 23 | 22 |
| Participant 9 | L | 320 | 14 | 14 |
| Participant 10 | L | 300 | 87 | 89 |
| Mean (95% CI) | – | 300 (300-315)a | 54.0 (30.4-77.6) | 55.9 (28.9-82.9) |
L, left; R, right.
aMedian (interquartile range).
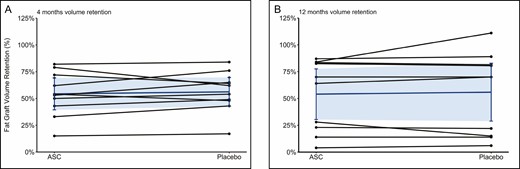
Ladder plots of the fat graft volume retention in both groups after 4 months (A) and 12 months (B). Each line represents a study participant where 1 breast was treated with adipose-derived stromal cells and the contralateral with placebo.
Secondary Endpoints
The fat graft volume retention 4 months after surgery was 1.9% (95% CI, –8.9% to –5.1%) lower in the ASC-enriched breasts (54.3%) than in the contralateral breasts treated with placebo-enriched fat grafting (56.2%) (P = 0.552). The interobserver variability between the 3 observers of the fat graft retention measurements was 6.9% (95% CI, 5.8%-8.1%) at the 12-month follow-up scans and 7.4% (95% CI, 6.1%-8.7%) at the 4-month follow-up scans.
Newly formed benign cysts were found in 9 out of 10 participants on the postoperative mammograms and/or ultrasound and were equally distributed between breasts treated with ASCs and placebo (Table 3). No malignancy was found. Preoperative and 12-month postoperative clinical photographs of 2 participants are shown in Figures 6 and 7.
Overview of the BI-RADS Classification, Complications, and Further Procedures After Fat Grafting
| . | BI-RADS . | Complications . | Further procedures . | ||
|---|---|---|---|---|---|
| . | Preoperative . | Postoperative . | ASC-enriched . | Fat (control) . | . |
| Summary (n) | I: 7 | II: 9 | Breasts with oily cysts: 9 | Breasts with oily cysts: 9 | FNAC and mammography + US |
| II: 3 | III: 1 | Breasts with fat necrosis: 2 | Breasts with fat necrosis: 1 | 2 ASC-enriched | |
| Palpable changes: 3 | Palpable changes: 2 | 1 control | |||
| . | BI-RADS . | Complications . | Further procedures . | ||
|---|---|---|---|---|---|
| . | Preoperative . | Postoperative . | ASC-enriched . | Fat (control) . | . |
| Summary (n) | I: 7 | II: 9 | Breasts with oily cysts: 9 | Breasts with oily cysts: 9 | FNAC and mammography + US |
| II: 3 | III: 1 | Breasts with fat necrosis: 2 | Breasts with fat necrosis: 1 | 2 ASC-enriched | |
| Palpable changes: 3 | Palpable changes: 2 | 1 control | |||
ASC, adipose-derived stromal cell; FNAC, fine needle aspiration cytology; US, ultrasound.
Overview of the BI-RADS Classification, Complications, and Further Procedures After Fat Grafting
| . | BI-RADS . | Complications . | Further procedures . | ||
|---|---|---|---|---|---|
| . | Preoperative . | Postoperative . | ASC-enriched . | Fat (control) . | . |
| Summary (n) | I: 7 | II: 9 | Breasts with oily cysts: 9 | Breasts with oily cysts: 9 | FNAC and mammography + US |
| II: 3 | III: 1 | Breasts with fat necrosis: 2 | Breasts with fat necrosis: 1 | 2 ASC-enriched | |
| Palpable changes: 3 | Palpable changes: 2 | 1 control | |||
| . | BI-RADS . | Complications . | Further procedures . | ||
|---|---|---|---|---|---|
| . | Preoperative . | Postoperative . | ASC-enriched . | Fat (control) . | . |
| Summary (n) | I: 7 | II: 9 | Breasts with oily cysts: 9 | Breasts with oily cysts: 9 | FNAC and mammography + US |
| II: 3 | III: 1 | Breasts with fat necrosis: 2 | Breasts with fat necrosis: 1 | 2 ASC-enriched | |
| Palpable changes: 3 | Palpable changes: 2 | 1 control | |||
ASC, adipose-derived stromal cell; FNAC, fine needle aspiration cytology; US, ultrasound.
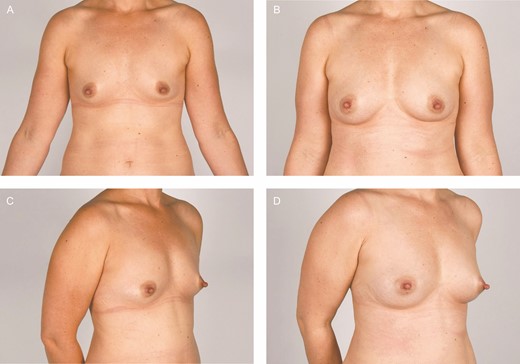
Clinical photographs of participant 7, a 40-year-old female. The fat graft volume retention was 81% in the breast treated with placebo (right breast) and 83% in the adipose-derived stromal cell–treated breast (left breast).
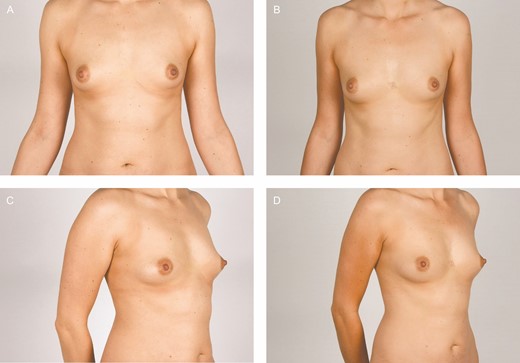
Clinical photographs of participant 9, a 30-year-old female. The fat graft volume retention was 14% in the breast treated with placebo (right breast) and 14% in the adipose-derived stromal cell–treated breast (left breast) after 12 months.
ASC Characteristics
The release criteria of the cells were fulfilled for all study participants. ASC viability at the end of harvest was 96.8% (95% CI, 96.3%-97.4%) The concentration of ASCs in the final cell suspension ranged from 167 to 200 × 106 ASCs/mL. The harvested ASCs from all participants were positive for CD73, CD90, and CD105, and negative for CD31, CD45, and HLA-DR, and thus fulfilled the release criteria. Examples of ASC phenotyping with flow cytometry are shown in Supplemental Figure 1, available online at www.aestheticsurgeryjournal.com. The capacity of the ASCs to differentiate into adipocytes, chondrocytes, and osteocytes has been proven previously with the same cell expansion protocol.24
The Effect of Weight Change on Fat Graft Volume Retention
Several participants underwent considerable body weight changes during the trial period. The median weight change was a weight gain of 1.1 kg (range, –12 to 4.9 kg) after 12 months and a weight gain of 1.0 kg (range, –10.5 to 5.20 kg) after 4 months. Fat graft retention and weight change was strongly correlated (P < 0.01) and is illustrated in Figure 8. However, the effect of the ASC enrichment on the fat graft volume retention remained nonsignificant across the patients who gained weight and the patients who lost weight during the follow-up (P = 0.396).
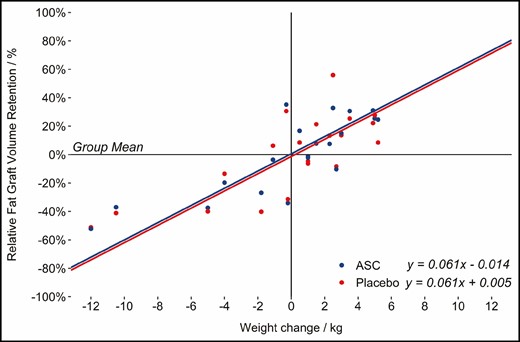
The effect of weight changes on fat graft volume retention adjusted for the treatment.
DISCUSSION
To our knowledge, this is the first blinded, randomized controlled trial to investigate ASC-enriched fat grafting to the breast. The participants received ASC-enriched fat grafting with 10 × 106 ASCs/mL fat graft into 1 of their breasts and placebo-enriched fat grafting into the contralateral breast. ASC enrichment was not superior to placebo enrichment in terms of fat graft volume retention after 4 months or 1 year of follow-up. The achieved fat graft retention rates for both groups were within the range reported in the literature30 and the patients experienced no complications besides an increase in their BI-RADS score. The general increase in BI-RADS score across patients is in line with the results of a systematic review of complications to breast augmentation with fat grafting in which 81.5% increased their BI-RADS score to I/II, which is in line with our results. The review found that 16.4% of patients needed additional imaging and 3.2 of patients had a biopsy to exclude malignancy5 which is comparable to 1 patient in our trial who had additional imaging and a biopsy. The increase in BI-RADS score did not differ between the ASC-enriched group and the placebo group and no malignant changes were found. The lack of effect from the ASC enrichment is contrary to previous studies on fat grafting enriched with expanded ASCs. In 2013, our group investigated ASC-enriched fat grafting using a methodology similar to the one used in the present trial.15 Contrary to our new results, Kølle et al found a 5-fold higher volume retention in the ASC-enriched grafts compared with the nonenriched control grafts (80.9% vs 16.3%) at 4-month follow-up.15 In both studies 10 trial participants were randomly assigned to receive ASC-enriched fat grafts and placebo with a paired study design. In the trial by Kølle et al, the participants received 30-mL bolus fat grafts to their upper arms, with 1 of the grafts being randomly assigned to ASC enrichment with 20 × 106 ASCs/mL.15 The main methodologic differences between the present trial and the previous one by Kølle et al are: the recipient site (breast vs upper arm), the injection technique (structural fat grafting31 vs bolus injection), the fat graft volume (310 mL vs 30 mL), the ASC concentration (10 × 106 ASC/mL vs 20 × 106 ASC/mL), and the measurement technique of the fat graft volume retention (3 blinded observers using a validated method compared with a single observer who calculated the graft retention manually). In our opinion, no single factor or difference in study design between this study and the previous one by Kølle et al fully explain the contrasting findings. However, we consider the present trial to reflect a more clinically relevant setting.
The lower dose in the present trial is of potential importance. We previously investigated the effect of ASC concentration in a porcine model32 using 30-mL bolus-injected fat grafts.33 The study showed no positive dose-response correlation between ASC concentration and fat graft retention. Grafts with 10 × 106 ASCs/mL were the only group to show a significantly higher retention rate (14.4%) than control grafts (9.2%) (P = 0.022), whereas the grafts enriched with 20 × 106 ASCs/mL had the lowest retention rate of the cell-enriched groups (11.8%).33 Therefore, we do not consider the lower ASC dosage a likely explanation for the lack of effect in the present trial.
In 2020, Kølle et al investigated ASC-enriched fat grafting for breast augmentation in an industry-sponsored, randomized controlled trial.34 The trial found significantly higher fat graft retention in the ASC-enriched group (80.2% vs 45.1%) with a concentration of 26 × 106 ASCs/mL. However, the most recent trial by Kølle et al has several limitations.34 According to clinicaltrials.gov, the trial exclusion criteria on weight changes was altered more than 2 years after treating the last patient (April 2017) and after the final follow-up.34 This is especially important when observing that the trial conclusion is highly reliant on an outlier in the treatment group with a retention rate of more than 200%. Furthermore, the trial was limited by a small sample size of 6 patients in each group who did not act as their own control; 34 it was not GCP monitored; the surgeons and patients were not blinded; and the fat graft retention rate was calculated with a nonvalidated technique by a single observer.
Limitations
Our study is limited by the small sample size of only 10 participants. Patient satisfaction was not formally assessed and was not considered relevant given that the patients received intervention treatment to 1 of their breasts and placebo to the contralateral breast. However, the strong study design of utilizing each participant as her own control enabled us to study the effect of the ASC enrichment without the influence of all the interindividual factors that affect the fat graft volume retention. The power calculation made before the trial estimated that a sample size of only 10 participants would provide 90% power to find a 30% effect on fat graft retention from the ASC enrichment. However, we observed a much lower standard deviation than the expected 20%, which points towards a higher power than what we expected. With the paired t test, we found that the breasts treated with ASC-enriched fat grafts had a 1.9% lower retention rate than the breasts treated with placebo-enriched fat grafting with a narrow confidence interval (95% CI, –9.1% to 5.3%). The interindividual variability of the fat graft volume retention rates was significantly associated with weight changes in a post hoc multivariate random effects linear model (P < 0.01). The effect of ASC enrichment remained insignificant when adjusting for weight changes (p = 0.396).
Strengths
The primary strength of this trial is the paired study design and the blinding of participants, surgeons, and the 3 observers who measured the fat graft volumes. By allocating 1 breast of each participant to ASC enrichment and the contralateral to placebo, we were able to isolate the effect of ASCs from all the interindividual factors that affect the fat graft volume retention.
CONCLUSIONS
Our trial did not find any effect of ASC enrichment on fat graft retention. Further studies are needed to confirm or refute the results of this trial. In our opinion, further research is also needed into the mode of action of ASCs on fat graft retention and whether certain cell characteristics of the expanded ASCs are crucial for a beneficial effect.
Presented in part at: Plastic Surgery The Meeting, San Diego, September 2019.
Acknowledgments
The authors wish to thank Dr. Niels Kroman for his contribution to the design of the study and Tim Kongsmark Weltz for his contribution to Figures 1 and 2. Drs Vester-Glowinski and Herly made an equal contribution to this work as co-first authors.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The study was funded by the Danish Cancer Society (Copenhagen, Denmark) (grant holder: Krzysztof T. Drzewiecki, R100-A6761). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.



