-
PDF
- Split View
-
Views
-
Cite
Cite
Biana Dubinsky-Pertzov, Francesco P Bernardini, Lior Or, Inbal Gazit, Morris E Hartstein, Late-Onset Upper Eyelid and Brow Edema as a Long-Term Complication of Hyaluronic Acid Filler Injection, Aesthetic Surgery Journal, Volume 41, Issue 6, June 2021, Pages NP464–NP471, https://doi.org/10.1093/asj/sjaa126
Close - Share Icon Share
Abstract
Late-onset upper eyelid edema is an uncommonly recognized complication of hyaluronic acid (HA)-based filler injection to the supraorbital area.
The authors sought to report their experience in diagnosing and managing late-onset upper eyelid edema.
This was a noncomparative, retrospective study of a series of 17 consecutive patients who presented with upper eyelid edema 6 to 24 months after uneventful HA filler injection in the supraorbital area.
The study group included 17 female patients. The average time of presentation was 13.9 months. Thirteen patients (76.4%) were satisfied after hyaluronidase and requested no further treatment (observation only); 4 patients (23.5%) elected to receive HA filler re-treatment, with satisfactory results. All patients were followed-up for at least 6 months after the re-treatment.
The incidence of late-onset upper eyelid edema is likely to increase as the number of patients undergoing HA filler injection to the supraorbital area increases. Our study emphasizes the importance of recognizing this condition and suggests a suitable noninvasive treatment with satisfying results for both the patient and the physician.

Hyaluronic acid (HA)-based fillers have grown in popularity in the last decade because they provide an efficient and safe nonsurgical solution for facial rejuvenation with good efficacy, immediate satisfaction, and short recovery time. HA fillers have been particularly popular in the periorbital region, specifically the lower lids and cheeks. More recently, there have been growing indications to restore volume, nonsurgically “elevate” the brows, treat the glabella, and correct the hollow upper sulcus.
However, HA-based fillers and the eyelids have a complicated relationship. As in the lower lid, filler use in the upper lid and brows can also lead to similar long-standing complications that need to be recognized and efficiently treated.
With aging comes a loss of volume and hollowing of the upper eyelid and thinning and sagging of the brows. This process can lead to exposure of the superior orbital rim, leading to an appearance of a sunken, hollowed eye with a deep superior sulcus. Also, age-related retro-orbicularis oculi fat (ROOF) atrophy causes loss of anterior 3-dimensional (3D) brow projection with loss of brow fat span show, contributing to lateral hooding and giving the entire upper eyelid/brow complex a tired and aged appearance. HA fillers offer a convenient and effective method to correct this process by restoring a convexity between the brow and lid crease and allowing for minimal tarsal platform show to restore the anterior projection and support to the brows, ultimately providing a “medical lift” to the brow and the eyelid.1
However, as in the lower eyelids, HA fillers in the upper eyelid and brow region can have immediate and delayed complications. Although extremely rare, the most severe vision-threatening immediate complication has been reported from embolization of filler material to the central retinal artery.2,3 Common complications reported after injections to the periocular area include bruising, redness, pain during the injection, contour irregularities, and filler visibility/bluish hue (Tyndall effect).4 Eyelid edema accompanied by the Tyndall effect has been reported as an early, not uncommon complication from HA filler injection and is explained by a poor injection technique or inappropriate injection volume5 and continues to be a predominant issue in the follow-up of periorbital filling. As mentioned, the exact etiology is poorly understood, and several explanations have been suggested. Edema can also develop as a result of immediate hypersensitivity reaction (type 1) to the injected filler or delayed hypersensitivity reaction (type 4) that may occur several weeks after injection. Reported risk factors for early onset eyelid edema are previously known allergies, rosacea, preexisting eyelid edema, and underlying tendency for fluid retention.6 Nevertheless, late-onset eyelid edema, noted more than 6 months after an uneventful injection, is an uncommon, rarely reported complication in the upper eyelids.7,8 We recently reported our experience in treating late-onset chronic edema of the lower eyelids.9 In this retrospective review, which represents the largest series of HA-related chronic upper eyelid edema to date, we report our experience in managing late-onset upper eyelid edema, sometimes presenting years after an uneventful filler treatment.
METHODS
The authors conducted a noncomparative, retrospective study of a series of patients who presented with upper eyelid edema 6 to 24 months after HA filler injection in the supraorbital area. The charts of 78 patients who received hyaluronidase (HYAL) treatment in the course of 3 consecutive years, from January 2016 to January 2019, to correct periocular edema after previous HA filler treatment elsewhere were collected from 2 busy oculoplastic practices. Of these, 61 patients were excluded as they presented with lower eyelid, leaving 17 patients, who formed our study group, with upper eyelid/brow edema that was managed by 2 authors.
This was a nonhomogeneous group consisting of patients treated by different physicians with different types of HA that was injected either with cannula or needle 1 or multiple times in the supraorbital area. All had in common the late occurrence of the upper eyelid swelling at least 6 months or more from an HA filler injection and a quick and complete response to HYAL. Because most of the patients were injected elsewhere, long before consultation, details regarding the original treatment and photos of the underlying periocular condition before the treatment were not available.
No institutional review board was necessary for this retrospective analysis, which was performed in the private practices of the 2 senior authors. Nevertheless, this study adhered to tenets of the current declaration of Helsinki and with the Health Insurance Portability and Accountability Act, and all the patients gave written informed consent.
Before administering the HYAL treatment, patients were informed of the potential of returning to their pre-filler state, with a superior sulcus hollow, and the potential need for a subsequent re-treatment following our own indications for the lower eyelids. Patients were scheduled for follow-up at least 2 weeks after HYAL injection. Photographic examples of patients before and after HYAL and the final result of each path were shown to all candidates before, and all patients accepted dissolution with HYAL.
Following our protocol for lower eyelid edema treatment, a starting dose of 3 IU of HYAL was the standard dose per point of edema, utilizing a needle or cannula; in other words, if the edema extended to the entire eyelid, we used 3 injection points of 3 IU per point, with a total of 9 IU. If the edema was only center-medially located, 2 points of 3 IU per point were used. Occasionally the amount per point could be doubled if the edema was considered severe. The HYAL was injected into the area of edema, usually the subcutaneous layer of the eyelid skin. See the video of the injection technique (available online as Supplemental Material) and Figure 1 for the treatment algorithm.
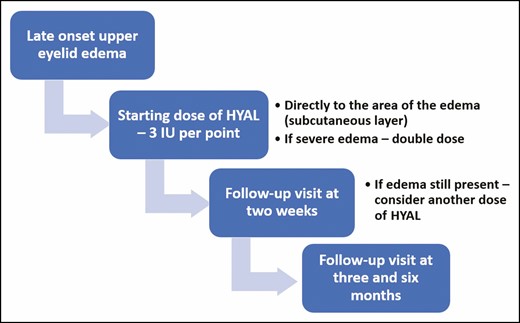
HYAL was reconstituted in 1 mL of saline, and 0.1 mL of this mixture was then diluted in 3 mL of saline. The HYAL was then injected into the edematous areas in 0.1-mL aliquots as needed. Immediate pressure was applied for several minutes after injection.
Re-treatment protocol for patients who were elected to receive refilling after HYAL included a filler with a lower G’ due to the thin eyelid skin, such as Restylane Refyne or Belotero, Teosyal Redensity II, or Stylage M. Either cannula or needle was utilized according to the surgeon’s preference. The injection was given very superficially, beginning at the superior orbital rim and slowly working inferiorly. At a certain point, the concavity becomes a convexity and further volume can be added as needed. In general, small volumes (0.2-0.3 mL) were sufficient.
RESULTS
The study group included 17 female patients whose age ranged from 26 to 80 years, with an average age of 54.9 years. The time to presentation ranged from 6 to 24 months after the filler treatment: 6 months in 8 patients, 1 year in 4 patients, 2 years in 3 patients, and 3 years after injection in 2 patients. The average time of presentation was 13.9 months. Edema resolved in all patients after HYAL treatment (Figures 2-6; Supplemental Figures 1 and 2). The average time of follow-up was 10.85 months (range, 6-36 months).
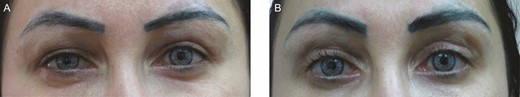
This 35-year-old female patient presented with upper eyelid edema in a centro-lateral brow edema pattern 6 months after hyaluronic acid injection to the upper eyelids with progressive swelling. (A) On presentation. (B) Three months after hyaluronidase treatment.
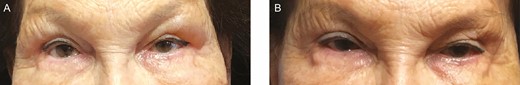
This 80-year-old female patient who underwent many hyaluronic acid treatments in the past (unknown hyaluronic acid filler was injected). (A) On presentation: centro-lateral brow edema pattern. (B) Three months after hyaluronidase treatment: 90 IU to the left upper eyelid and 90 IU to the right upper eyelid.
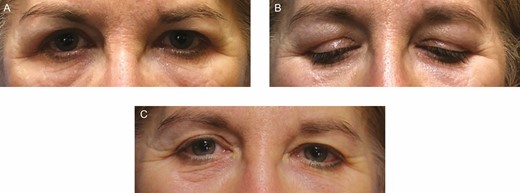
This 56-year-old female patient presenting with progressive swelling 1 year after filler injection to both upper eyelids. (A, B) On presentation. (C) Three months after injection of 90 IU of hyaluronidase to both upper eyelids.
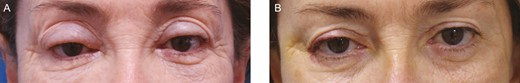
This 58-year-old female patient who underwent hyaluronic acid filler injection 1 year before the development of upper eyelid edema in a supero-medial pattern. (A) On presentation. (B) Three months after injection of 10 IU of hyaluronidase on each side.
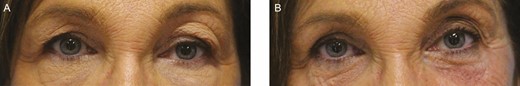
This 64-year-old female patient with history of previous hyaluronic acid injections in the past (unknown filler material). (A) On presentation – a centro-lateral brow edema pattern. (B) 3 months after 30 IU of hyaluronidase to each of 3 points in the left upper eyelid.
All the patients had a negative history of allergies, rosacea, chronic or fluctuating lower eyelid or malar edema of unknown origin, and of thyroid eye disease.
Thirteen patients (76.4%) were pleased with the aesthetic outcome after HYAL and requested no further treatment (observation only); 4 patients (23.5%) elected to undergo upper eyelid HA filler re-treatment, with satisfactory results. All patients were followed-up for at least 6 months after the re-treatment and at their follow-up visit reported satisfaction with their final result. No patients of the re-treatment group experienced a second episode of edema in the follow-up period.
Discussion
HA fillers provide a safe and well-tolerated rejuvenation method for the periocular region. As HA filler treatment becomes increasingly popular, reports of adverse events have increased, and there is a growing awareness among aesthetic practitioners of the vascular risks associated with filler injections that can lead to severe visual compromise. A recently published review of all cases of visual complications caused by filler injection between January 2015 and September 2018 demonstrated 2 cases of visual loss after HA injection to the brow and upper eyelid.10 Standard safety precautions must be followed as with injections elsewhere on the face.
On the other hand, chronic lower eyelid edema is a frequent HA-related complication and a significant cause of concern when patients are considering periocular HA treatment. It has been reported that edema develops in up to 11% of patients receiving HA for lower eyelid augmentation, typically presenting soon after injection.4
Late-onset chronic eyelid edema occurring after uneventful HA treatment is often misdiagnosed. The little awareness of this complication and the lag time between the procedure and the presentation of edema may prevent physicians from identifying the cause this edema. This can delay diagnosis and treatment. Further clouding the picture at presentation is the commonly held belief that hyaluronans are self-reabsorbing products that last no longer than approximately 9 to 12 months, as listed in the manufacturer packaging data. Once recognized, we believe the best method of managing eyelid edema is with HYAL. In a large series of patients with late-presenting chronic lower eyelid edema following HA injection, including the longest duration reported, our group has demonstrated that HA can still be utilized to treat lower eyelid hollows after HYAL.9
However, little is known about late-presenting upper eyelid edema after HA injection and the appropriate management of this complication. Until now, only a few scattered case reports have been described in the literature.7,8 This series is the largest to describe late-presenting chronic upper eyelid edema following HA injection, including the longest duration reported, with a range of 6 to 24 months after the patient’s last filler injection.
Unlike the dysmorphic lower eyelid, which is characterized by standard clinical presentations of edema, clinical features of upper eyelid edema are more variable based on the site of accumulation, which in turn may depend on the area of injection. In our series, we have recognized 3 different patterns of presentation: (1) supero-medial edema, with localized “clear bulging” at the level of the supero-medial bag; (2) centro-lateral brow edema; and (3) upper eyelid edema with eyelid ptosis. In all variants, the area of swelling alters the appearance of the eyelid skin similar to what happens to the lower eyelid; the affected area appears swollen and tense and displays some degree of Tyndall’s effect. The medical history is also quite similar to that of the lower eyelid, with progressive worsening of the swelling in the course of time; patients often do not relate the problem with the previous HA injection and often deny or forgot the treatment. Thus, it is entirely in the hands on the consulting physician to recognize the source of edema.
Late occurrence of edema appears to be an HA-related complication limited to the eyelids only. Recognized initially in the lowers, following the trend of expanding indications to filler treatment in the peri-ocular region, it starts to play a role in the uppers as well. The clinical manifestations of eyelid edema are strikingly similar between the upper and lower eyelids in many aspects. Compared with our recently published paper on late-onset lower eyelid edema following HA injections,9 the time of onset of edema is roughly the same, appearing on average 6 to 24 months after the reported treatment and slowly progressing. The appearance of edema is accompanied by the the Tyndall effect for the lower eyelid (Figure 3A).
Relative to the causes or the mechanism of early onset edema, technique and choice of material employed are usually the main risk factors. For late-onset edema presenting months and even years after an otherwise stable initial injection, we speculate that the unique anatomy and function of the eyelids play an important role. The thin upper eyelid skin, baring no subcutaneous fat, may allow less concentrated filler to diffuse with time, whereas more viscous fillers may cause visible lumpiness in this area. Specifically in the upper eyelid, the 3D structure of the hollow and deep superior sulcus makes it a challenging area to fill. This may require several filler injection sessions and precise and correct localization of the initial injection point to avoid overcorrection. Similar to what occurs in the lower eyelid, where injection at the level of the suborbicularis oculi fat of the upper cheek is likely to cause eyelid and malar edema, we believe that filler injection at the level of the ROOF is the main culprit of upper eyelid edema.
We further suspect that excessive treatment at the level of the glabella can also cause upper eyelid edema by slowly spilling/diffusing through the supra-trochlear neurovascular bundle/supraorbital notch route to the upper eyelid; this was considered the main mechanism in at least 2 cases of this series (Figures 2A and 3A). Where a significant edema was present at the level of the glabella and upper inner canthi, suggesting the original route of the edema extension. However, we did not use imaging modalities to assess this theoretical spread; hence, this speculation is yet to be proven and is suggested only by our clinical observations.
HA-related pretarsal upper eyelid edema causing eyelid ptosis has not been reported previously, and the concomitant presence of excessive volume at the level of the glabella with Tyndall effect at the upper inner canthi level is a possible explanation. Dissolution with HYAL at the level of the pre-tarsal eyelid and glabella completely reversed the swelling and the ptosis.
The third possible mechanism may well relate to injection at the level of the orbicularis muscle, which may cause impairment of the muscle fibers, reducing its lymphatic pump function with consequent fluid accumulation. Orbicularis muscle impairment was reported in 2 recent studies; De Pasquale et al showed evidence of degeneration of orbicularis oculi muscle fibers that accumulated water after HA injection to the adjacent extracellular matrix.11 Teo et al reported another case of lower eyelid edema progression after HA filler injection, showing histopathological evidence of striated muscle degeneration surrounding pools of abnormal organic extra-cellular matrix.12
Another possible explanation for this complication is repeated injections. A total 23.5% of the patients in our study group presenting with late-onset edema received repeated filler injections to the upper eyelid over the course of a year.
Choosing the appropriate filler, placing the filler with the appropriate injection technique, and being aware of the complex local anatomy may help mitigate the risk of this complication. However, it may not be able to be avoided completely. Thus, we need to learn how to recognize it and treat it effectively.
Management options include (1) observation only, (2) HYAL, and (3) HYAL and repeat injection or surgery. Observation alone is a valuable option for early-onset edema, enabling enough time for the HA to naturally reabsorb. However, this watchful waiting is not effective for late-onset edema, which only worsens with time. In addition, patients will likely not want to wait additional time until it possibly resolves. Surgery may be considered but may not be an appropriate treatment choice for upper eyelid edema. Because many of these patients underwent HA filling to correct a volume deficit, subtractive blepharoplasty will not necessarily be the right treatment option. The most frequent location of the edema resides in the ROOF and therefore falls out of the blepharoplasty excision, making this surgical approach poorly indicated.
Blepharoplasty can be considered once the HA is dissolved in selected cases. When centrally located, we recommend initial treatment with HYAL. We do not recommend surgery for HA-induced upper eyelid ptosis as HYAL is rapidly effective, making surgery grossly unnecessary. Four patients (23.5%) underwent secondary HA filler treatment performed by 1 of the 2 oculoplastic surgeons, similarly to what we previously reported in the lower lid, after HYAL. In each case, a different filler was chosen and a slow fill was conducted over several sessions. Another accepted option that can be considered is autologous fat transfer after HYAL.
Utilizing HYAL as a first-line treatment appears to be the most reasonable option. However, in the lower eyelid we have found that it is difficult to titrate to reliably dissolve just the “excess” fullness alone. As recently demonstrated by our group,9 dissolving the HA filler in the lower eyelid and the water that comes with it restored the patient to the baseline condition that initially led them to seek treatment. Subsequently, 80% percent of the patients with late-onset lower lid edema opted for secondary filler treatment and were satisfied with the final result.
In contrast, in our group of late-onset upper lid edema patients, most (76.4%) were satisfied with the results of HYAL only, highlighting the importance of regarding the anatomy and the specific effect of HYAL on the superior sulcus.
We speculate that because the volume deficit at the level of the brow/upper eyelid/glabella is less dramatic than that at the level of the infra-orbital region, the elimination of the filler does not cause such a dramatic posttreatment effect. Thus, many patients are satisfied after HYAL without requesting secondary treatment. Considering that almost 80% of patients are happy after HYAL is relevant because physicians can offer HYAL treatment to these patients with fewer precautions to consider. Secondary, side effects or complications with HYAL to the upper eyelid have never been reported before, so it appears to be the most effective and appropriate treatment for HA-related upper eyelid edema. From our experience, we advocate regular follow-ups of at least every 6 months on patients undergoing periocular injections.
Dermal filler use is expanding across many medical specialties, and HA filler is the most popular of the temporary fillers on the market due to its minimally immunogenic characteristics and reversibility (can be enzymatically degraded by HYAL). Therefore, it is reasonable to expect that periocular complications from HA fillers will increase proportionally. Practitioners will commonly face aesthetically relevant complications, with the need to be managed even in absence of complete information. First, the cosmetic physician should be suitably experienced to select and use HA fillers. HA is a simple linear polymer composed of repeating units of disaccharides, thus creating a highly hydrophilic molecule. This biochemical characteristic attracts water, drawing fluids into the HA matrix and creating turgor that enables the HA complex to withstand compressive forces. However, this hydrophilic nature might cause more water reabsorption, producing more tissue edema. As for research regarding chronic lower eyelid edema that we recently published in this journal, we could not speculate on any specific filler to be more prone than others to this complication. In our limited upper and lower eyelid series, encompassing 78 patients, all the commercially available fillers were included at least once, so we cannot say any one type is 100% safe. In terms of speculating if one brand is more likely to cause edema, we do not have data to reach that conclusion. Based on our experience, we advocate injectables of lower HA concentration for the periocular area and under-correction during first treatment. It is well known that as the HA slowly breaks down, each molecule binds to more water in a way that the same volume can be maintained progressively by more water and less HA in a metabolic process known as isovolumetric degradation.13 It is this progressive water accumulation by the naturally degrading hyaluronan over time that may also explain the delayed occurrence of the edema years after a successful HA treatment. Second, optimal complication management remains an unmet need in the field of aesthetic medicine. It is therefore recommended to treat this complication of late-onset upper eyelid edema rather than subject the patient to further and unnecessary medical testing. This only serves to further delay the treatment and causes additional patient aggravation. A noninvasive and aesthetically pleasing rehabilitation of this complication will result in a more favorable long-term experience, leading more patients and physicians to embrace this treatment.
This study has several limitations. First, information regarding the initial filler injection performed and the pretreatment condition is absent, including the number of treatments performed in the area, their specific locations, the technique, and the type and the quantity of filler used. Second, this study is a case series, it is retrospective, and has no comparative group. Third, the follow-up time after both “filler re-treated” patients and “HYAL-only” treated patients is limited; potentially, re-treated patients could experience recurrent eyelid edema during even longer follow-up. The number of patients with upper lid edema was much less than the number we reported for lower lid edema. This probably reflects the fact that lower lid filler is still much more popular than upper lid filler. The number of patients undergoing upper lid filler is likely to increase as well as the number of those presenting with upper lid edema. Therefore, any theories on the causes and the mechanism responsible for the development of this complication in our series are purely speculative. Establishing precisely why the eyelid edema occurred was beyond the goal of this study. However, it is clear from our experience that upper eyelid edema should be recognized and included as a known complication of HA fillers.
To the best of our knowledge, this is the first series of patients who underwent successful HYAL treatment to correct HA-related late-onset upper eyelid edema with a high rate of patient satisfaction, whereas only few of them were re-treated. Until future studies clarify the underlying mechanism of this complication, we feel that our experience will help increase its awareness among practitioners. This will offer a starting point for a better understanding of delayed HA-related complications and also provide useful guidelines for a more effective treatment.
Conclusions
As the number of patients receiving HA filler injection to the supraorbital area increases, the incidence of late-onset upper eyelid edema is likely to increase well. Our study emphasizes the importance of recognizing this condition and suggests a suitable noninvasive treatment with satisfying results for both the patient and the physician.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
References
Author notes
Dr Bernardini is an oculoplastic surgeon in private practice in Genova and Milano, Italy



