-
PDF
- Split View
-
Views
-
Cite
Cite
Yingjie Wu, Feng Hu, Xueping Li, Guoqian Yin, Autologous Fat Transplantation for Aesthetic Breast Augmentation: A Systematic Review and Meta-Analysis, Aesthetic Surgery Journal, Volume 41, Issue 6, June 2021, Pages NP402–NP429, https://doi.org/10.1093/asj/sjaa364
Close - Share Icon Share
Abstract
Autologous fat transplantation has already become a part of clinical practice for aesthetic breast augmentation even though evidence regarding its efficacy is still lacking.
The authors sought to determine the current worldwide status and efficacy, techniques, and oncologic safety on this subject.
PubMed, EMBASE, and Cochrane Library databases were searched to identify all relevant studies.
Eighty-four articles published between 1987 and April 2020, consisting of 6468 patients, were included, and 64 studies consisting of 5162 unique patients were included in the meta-analysis. Most studies had a low level of evidence (levels 2b-5); In this meta-analysis, there were 17 prospective cohort studies, 4 retrospective cohort studies, 6 case-control studies, and 38 case series. The publications were from 21 countries. Indications for autologous fat transplantation were aesthetic augmentation (93.2%) and congenital malformation (6.8%). Among the 5162 patients, 2 cases (0.04%) of cancer were reported. The meta-analysis revealed very high overall patient and surgeon satisfaction rates of 93% and 87%, respectively. Overall, only 1.56 sessions were needed to achieve the desired result. Long-term survival was calculated to be approximately 60% to 70% at 1-year follow-up. Only 8% of procedures resulted in clinical complications, and 5% of patients required biopsy because of abnormal clinical or radiological findings.
Autologous fat transplantation seems to be a major tool in aesthetic breast augmentation. Preoperative patient selection is essential but under-reported. Future research should focus on evaluating the technical and patient factors influencing the rate of fat survival and its oncological safety.

More than 100 years have passed since autologous fat transplantation (AFT) was utilized as a relatively simple and effective procedure for treating soft-tissue deficiencies. Neuber1 first reported the operation of filling soft tissue defects with several small, free fat blocks in 1893. In 1895, Czerny2 reconstructed the damaged breast with lipoma from the waist, which was the earliest record of breast augmentation with autogenous fat.
In 1987, Bircoll3 injected the breast with autologous fat grain for the first time. Since then, the application of AFT in breasts has been developing. Although AFT in breasts was forbidden by the American Society of Plastic Surgeons because of issues related to efficacy and safety, there is increasing interest in employing AFT for other indications. Thus, in 2009, AFT was not contraindicated for natural breasts but was regulated in the United States following the American Society of Plastic Surgeons Fat Graft Task Force report.4
AFT is utilized in congenital hypoplasia of the breast (unilateral or bilateral), such as micromastia, or congenital breast malformation (eg Poland syndrome, tuberous breast). It is also utilized for atrophic breasts after breast-feeding and residual local volume deficiencies after breast reconstruction following lumpectomy. Fat transplantation is an autologous reconstruction by a minimally invasive procedure compared with autogenous tissue, such as the myocutaneous flap.
Numerous studies5-25 about the widespread application of AFT in the breast have been published over the years. However, most of them were the individual findings of primarily case series and cohort studies that differed in indications, surgical techniques, and outcome measures that were highly fragmented and lacked reliable data from randomized controlled trials (RCTs). Thus, there is an urgent need for a thorough examination of the published literature on AFT in the form of a meta-analysis to facilitate a more straightforward interpretation by clinicians and help reach consensus regarding the efficacy of AFT in aesthetic augmentation of the breast.
New technologies and related research are updated continuously in the aspects of fat absorption, purification, and injection to avoid all kinds of complications and improve the survival rate of autogenous fat. For example, breast augmentation with the body-jet hydrodynamic liposuction system,5 Brava-assisted augmentation mammoplasty,6,7 cell-assisted augmentation,8,9 and augmentation mammoplasty with autologous fat combined with a prosthesis10,11 are recognized by an increasing number of patients and plastic surgeons.
This is the first review, to our knowledge, to determine the current worldwide status and efficacy, techniques, and oncologic safety of autologous fat transplantation for aesthetic breast augmentation.
METHODS
Search Strategy
This systematic review adhered to the standards of the Cochrane Handbook for Systematic Reviews of Interventions2 and was written in the format provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.3 A comprehensive, reproducible electronic search was conducted in the PubMed, EMBASE, and Cochrane Library databases to identify all published studies with human patients who underwent AFT for aesthetic breast augmentation. The search was performed on April 1, 2020, employing the following terms: “fat grafting,” “fat graft,” “fat transplantation,” “fat transfer,” “fat injection,” “fat implantation,” “fat filler,” “lipofilling,” “lipotransfer,” “lipomodeling,” “lipostructuring,” “lipofiller,” “lipoinjection,” “lipograft,” “adipocyte graft,” and “breast” (Details in Appendix).
Selection Criteria
We included all original articles that were published before April 1, 2020, concerning patients who underwent AFT for healthy native breasts, such as those with micromastia, Poland syndrome, tuberous breast deformity, and atrophic breast. The patients were required to have no personal or family history of breast cancer. Family was defined as first-degree relatives. The patients underwent only AFT or AFT combined with 1 of 3 auxiliary measures, including supplements (eg, cell-assisted lipotransfer, adipose-derived stem cells, platelet-rich plasma, and stromal vascular fraction [SVF]), preexpansion devices (eg, the Brava device), and implants. Exclusion criteria were unavailability of the full-text article, secondary sources (reviews and commentaries), and the utilization of AFT for breast reconstruction after cancer.
Data Collection
The full text of each article was read and reviewed by 2 independent reviewers (Y.J.W. and F.H.) based on predefined inclusion and exclusion criteria. Disagreements were resolved through discussion until a consensus was reached. First, we extracted general data from the literature with qualitative synthesis according to the predetermined table; the data information table included authors, publication date, study location, type of study, total number of patients, level of evidence, and average patient age. Second, basic data and quantitative data included treatment measures, body mass index (BMI), sessions of operations, follow-up duration, patient and surgeon satisfaction, complications, biopsies, mean fat volume injection, and volume retention. Third, perioperative information included the type of anesthesia, donor site, liposuction, fat treatment, fat injection, injection level, average operative time, and postoperative nursing. Fourth, postoperative follow-up included the type of imaging examination, radiological and oncological results, volume measurement method, time of breast volume change, and types and number of complications.
In addition, if studies from the same author or institution were conducted at the same time and reported the same outcomes, an overlap of more than 25% of the sample size was suspected. In case of the above situation, only the largest or most relevant study was included in our meta-analysis.
In some instances, authors were contacted for additional data. When necessary, units were converted to a standard format to ensure comparability and allow the pooling of data. Continuous variables that were reported as medians ± ranges were converted to means ± standard deviations employing the standard estimating equations utilized for meta-analyses. Similarly, categorical outcomes that were reported as Likert scale scores (eg, the degree of satisfaction) were dichotomized to allow analysis as proportions.
All references were stored in the Endnote Reference Management Tool (version X7.8, Thomson Reuters, Philadelphia, PA).
Outcome Effect Indexes
The combined effect indexes of a single-arm study included patient satisfaction, surgeon satisfaction, average sessions of operations, complications, and biopsy rate.
Statistical Analysis
Meta-analyses were performed with STATA/SE15.1 (TX77845, package meta; StataCorp, College Station, TX). To conduct the meta-analysis, the sample size of 1 arm was more than 4, and the performance of AFT had relevant outcome measures, including the rates of patient and surgeon satisfaction, volume retention, the procedure sessions, and associated clinical and radiological complications.
Heterogeneity tests were performed employing the I2 statistic, and the method of combining the effect index was inverse variance. All of the effect indexes were pooled in a standard random-effects model and presented as forest plots. All of the combined effects are expressed as rates and 95% confidence intervals.
The bias within a single study may be an indication bias, so 3 selection criteria were utilized to reduce the risk of indication bias: (1) exclude individuals with a personal history or first-degree relative family history of breast cancer and individuals who may already be at risk for cancer; (2) select individuals with only 1 combination of interventions; and (3) select individuals who underwent AFT of the breast for the first time.
At the same time, to reduce the heterogeneity among the studies, subgroup meta-analyses were performed for most outcomes by segregating studies based on known or presumed confounders (eg, interference measures). Depending on the type of interference and according to the technique and method of AFT, the studies were categorized into 4 subgroups: pure AFT (AFT group), combined supplement (AFT + SUPP group), combined Brava expansion technique (AFT + BRAVA group), and combined implants (AFT + IMP group).
To investigate the source of heterogeneity, a meta-regression model was established. There were multiple covariates: postoperative follow-up duration, manual or mechanical liposuction, fat transplantation subgroups, and fat survival rate. The meta-regression model trendline and respective 95% confidence intervals were utilized to estimate fat survival rate and volume retention over time.
RESULTS
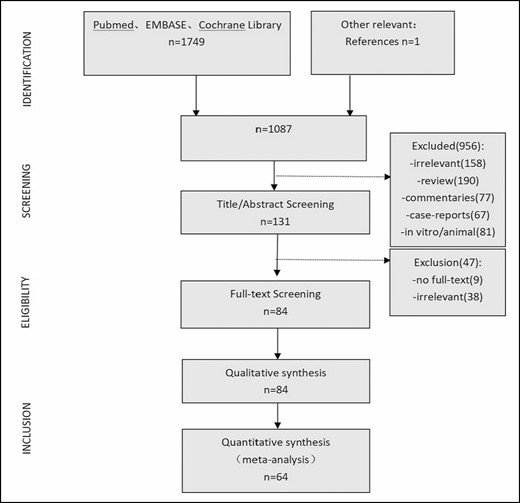
According to the systematic review selection criteria, the electronic search yielded 1749 articles, screening of related articles yielded 1 additional article, and 1087 articles were obtained after eliminating the duplicates. Screening of the title and abstract led to the inclusion of 131 records for further evaluation. Eighty-four studies were selected through further screening of the full-text articles. According to the meta-analysis selection criteria, 64 articles were finally included in the meta-analysis. The retrieval strategy flowchart is shown in Figure 1.
Study Characteristics
Eighty-four clinical studies, consisting of 6468 patients, were identified for inclusion (Table 1). Patient age ranged from 11 to 63 years, and the average age was 35.73 years. These studies were conducted between February 1987 and April 2020, mostly after 2008 (Figure 2). The studies were from 21 countries, with most being from North America, Europe, and Asia (Table 2). Most of these studies had a low level of evidence (levels 2b-5). Only 1 study was a randomized controlled cohort study, but the control groups were low-speed centrifugation group and sedimentation group according to different fat processing. Our control standard was according to the technique and method of AFT reinjection, and the remaining AFT arms were treated as case series. Sixty-four studies comprised 5162 unique patients who were included in the meta-analysis (Table 3). These studies consisted of 38 case series, 6 case-control studies, 17 prospective cohort studies, and 4 retrospective cohort studies. The average follow-up duration was 22 months (range, 3 months to 25 years). Patients’ average BMI was 17 to 30 kg/m2. The indications for AFT were aesthetic augmentation in 93.2% of patients (n = 4814) and congenital malformations (Poland syndrome and tuberous breasts) in 6.8% (n = 348).
| Study . | Location . | Design . | No. . | Levela . | Age (y) . |
|---|---|---|---|---|---|
| Abboud, 2015 | Belgium | Prospective cohort | 80 | 2b | 36 |
| Ahmad, 2017 | Pakistan | Case series | 2 | 5 | 27.5 |
| Atia, 2020 | Germany | Case series | 30 | 4 | 31.23 |
| Auclair, 2009 | France | Case series | 47 | 4 | — |
| Auclair, 2013 | France | Case series | 197 | 4 | — |
| Auclair, 2020 | France | Case series | 148 | 4 | 42 |
| Bircoll, 1987 | USA | Case report | 1 | 5 | — |
| Bircoll, 1987 | USA | Case report | 1 | 5 | 45 |
| Bircoll, 2010 | USA | Case series | 650 | 4 | — |
| Brault, 2017 | France | Case-control study | 15 | 3b | 21.1 |
| Bravo, 2014 | USA | Case-control study | 21 | 3b | — |
| Bresnick, 2016 | USA | Case series | 28 | 4 | — |
| Bulgin, 2013 | Croatia | Case report | 1 | 5 | 30 |
| Carvajal, 2008 | Colombia | Case series | 20 | 4 | 36.9 |
| Chiu, 2014 | Taiwan | Case series | 282 | 4 | 34.9 |
| Chiu, 2016 | Taiwan | Case series | 27 | 4 | 39.1 |
| Chiu, 2018 | Taiwan | Case-control study | 206 | 3b | 33 |
| Claudio, 2017 | Spain | Case series | 11 | 4 | 24 |
| Coleman, 2007 | USA | Retrospective cohort | 17 | 2b | 38.2 |
| Costantini, 2012 | Italy | Case series | 2 | 4 | 26,49 |
| Cotrufo, 2008 | Italy | Case series | 42 | 4 | 48 |
| Coudurie, 2015 | France | Case report | 1 | 5 | 12 |
| Del Vecchio, 2011 | USA | Prospective cohort | 25 | 2b | 21-60 |
| Del vecchio, 2012 | USA | Case report | 1 | 5 | 42 |
| Del Vecchio, 2014 | USA | Case series | 30 | 4 | — |
| Delay, 2009 | France | Case report | 1 | 5 | 11 |
| Delay, 2009 | France | Case series | 136 | 4 | — |
| Delay, 2013 | France | Case series | 31 | 4 | 23 |
| Derder, 2014 | France | Case series | 10 | 4 | 17.5 |
| Deschler, 2020 | France | Case series | 42 | 4 | 34 |
| Dos Anjos, 2015 | Spain | Case-control study | 47 | 3b | 37.8 |
| Fiaschetti, 2013 | Italy | Prospective cohort | 6 | 2b | 46.3 |
| Fisenko, 2017 | Russian | Case series | 2 | 5 | 42 |
| Gaston, 1994 | Switzerland | Case report | 1 | 5 | 20 |
| Graf, 2019 | Brazil | Case series | 26 | 4 | 59.1 |
| Guo, 2018 | China | Prospective cohort | 11 | 2b | 27 |
| Gutierrez-Ontalvilla, 2020 | Spain | Case series | 9 | 4 | 14.9 |
| Herly, 2019 | Denmark | Case series | 14 | 4 | 34.9 |
| Herold, 2010 | Germany | Prospective cohort | 10 | 2b | — |
| Ho Quoc, 2013 | France | Case series | 1000 | 4 | 39 |
| Ho Quoc, 2015 | France | Case report | 2 | 5 | 19,26 |
| Ho Quoc, 2015 | France | Case series | 10 | 4 | 21 |
| Illouz, 2009 | France | Case series | 439 | 4 | 45.6 |
| Jung, 2016 | South Korea | Prospective cohort | 5 | 2b | 34.4 |
| Kamakura, 2011 | Japan | Prospective cohort | 20 | 2b | 35.6 |
| Kang, 2018 | China | Randomized prospective controlled cohort | 100 | 2b | 43.6 |
| Kerfant, 2017 | France | Case series | 156 | 4 | 31.7 |
| Khouri, 2012 | USA | Prospective cohort | 81 | 2b | 17-63 |
| Khouri, 2014 | USA | Case series | 139 | 4 | 27-45.2 |
| Klit, 2015 | Denmark | Case series | 7 | 4 | 18 |
| Kwiatkowska, 2019 | Germany | Case series | 14 | 4 | — |
| La Marca, 2013 | France | Case series | 10 | 4 | 16 |
| Lancerotto, 2010 | USA | Case report | 1 | 5 | 16 |
| Li, 2014 | China | Case series | 105 | 4 | 31.3 |
| Maione, 2018 | Italy | Prospective cohort | 31 | 2b | 34.3 |
| Matsudo, 1988 | Switzerland | Case series | 21 | 4 | — |
| Muench, 2016 | Switzerland | Case series | 254 | 4 | 35.8 |
| Münch, 2013 | Switzerland | Case series | 84 | 4 | 36.7 |
| Ohashi, 2016 | Japan | Case series | 131 | 4 | 39.3 |
| Özalp, 2017 | Turkey | Case series | 34 | 4 | 31 |
| Peltoniemi, 2013 | Finland | Not-randomized prospective controlled cohort | 18 | 2b | 39-51 |
| Pinsolle, 2008 | France | Case series | 7 | 4 | 25 |
| Quoc, 2013 | France | Case series | 19 | 4 | 28 |
| Rubin, 2012 | Japan | Not-randomization controlled retrospective cohort | 27 | 2b | 35.9 |
| Salgarello, 2011 | Italy | Case series | 2 | 5 | — |
| Serra-Mestr, 2017 | Italy | Prospective cohort | 49 | 2b | 41 |
| Sforza, 2016 | England | Case series | 26 | 4 | 24 |
| Spear, 2014 | USA | Prospective cohort | 10 | 2b | 30 |
| Streit, 2017 | Czech Republic | Case report | 3 | 5 | 14,17,19 |
| Tassinari, 2016 | Italy | Case series | 242 | 5 | — |
| Ueberreiter, 2010 | Germany | Prospective cohort | 52 | 2b | — |
| Ueberreiter, 2013 | Germany | Prospective cohort | 56 | 2b | 22-58 |
| Veber, 2011 | France | Retrospective cohort | 31 | 2b | 38 |
| Visconti, 2019 | Italy | Case-control study | 29 | 3b | 26.5 |
| Walters, 2020 | USA | Case series | 140 | 4 | 39.7 |
| Wang, 2011 | China | Case series | 48 | 4 | 29.4 |
| Wang, 2012 | China | Prospective cohort | 18 | 2b | 32 |
| Wang, 2015 | China | Prospective cohort | 12 | 2b | 32 |
| Yoshimura, 2008 | Japan | Case series | 40 | 4 | 35.8 |
| Yoshimura, 2010 | Japan | Case series | 15 | 4 | 37.1 |
| Zheng, 2008 | China | Case series | 66 | 4 | 19-39 |
| Zheng, 2019 | China | Case-control study | 5 | 3b | 29.6 |
| Zocchi, 2008 | Italy | retrospective cohort | 181 | 2b | 33 |
| Zocchi, 2017 | Italy | Case series | 487 | 4 | 29.2 |
| Study . | Location . | Design . | No. . | Levela . | Age (y) . |
|---|---|---|---|---|---|
| Abboud, 2015 | Belgium | Prospective cohort | 80 | 2b | 36 |
| Ahmad, 2017 | Pakistan | Case series | 2 | 5 | 27.5 |
| Atia, 2020 | Germany | Case series | 30 | 4 | 31.23 |
| Auclair, 2009 | France | Case series | 47 | 4 | — |
| Auclair, 2013 | France | Case series | 197 | 4 | — |
| Auclair, 2020 | France | Case series | 148 | 4 | 42 |
| Bircoll, 1987 | USA | Case report | 1 | 5 | — |
| Bircoll, 1987 | USA | Case report | 1 | 5 | 45 |
| Bircoll, 2010 | USA | Case series | 650 | 4 | — |
| Brault, 2017 | France | Case-control study | 15 | 3b | 21.1 |
| Bravo, 2014 | USA | Case-control study | 21 | 3b | — |
| Bresnick, 2016 | USA | Case series | 28 | 4 | — |
| Bulgin, 2013 | Croatia | Case report | 1 | 5 | 30 |
| Carvajal, 2008 | Colombia | Case series | 20 | 4 | 36.9 |
| Chiu, 2014 | Taiwan | Case series | 282 | 4 | 34.9 |
| Chiu, 2016 | Taiwan | Case series | 27 | 4 | 39.1 |
| Chiu, 2018 | Taiwan | Case-control study | 206 | 3b | 33 |
| Claudio, 2017 | Spain | Case series | 11 | 4 | 24 |
| Coleman, 2007 | USA | Retrospective cohort | 17 | 2b | 38.2 |
| Costantini, 2012 | Italy | Case series | 2 | 4 | 26,49 |
| Cotrufo, 2008 | Italy | Case series | 42 | 4 | 48 |
| Coudurie, 2015 | France | Case report | 1 | 5 | 12 |
| Del Vecchio, 2011 | USA | Prospective cohort | 25 | 2b | 21-60 |
| Del vecchio, 2012 | USA | Case report | 1 | 5 | 42 |
| Del Vecchio, 2014 | USA | Case series | 30 | 4 | — |
| Delay, 2009 | France | Case report | 1 | 5 | 11 |
| Delay, 2009 | France | Case series | 136 | 4 | — |
| Delay, 2013 | France | Case series | 31 | 4 | 23 |
| Derder, 2014 | France | Case series | 10 | 4 | 17.5 |
| Deschler, 2020 | France | Case series | 42 | 4 | 34 |
| Dos Anjos, 2015 | Spain | Case-control study | 47 | 3b | 37.8 |
| Fiaschetti, 2013 | Italy | Prospective cohort | 6 | 2b | 46.3 |
| Fisenko, 2017 | Russian | Case series | 2 | 5 | 42 |
| Gaston, 1994 | Switzerland | Case report | 1 | 5 | 20 |
| Graf, 2019 | Brazil | Case series | 26 | 4 | 59.1 |
| Guo, 2018 | China | Prospective cohort | 11 | 2b | 27 |
| Gutierrez-Ontalvilla, 2020 | Spain | Case series | 9 | 4 | 14.9 |
| Herly, 2019 | Denmark | Case series | 14 | 4 | 34.9 |
| Herold, 2010 | Germany | Prospective cohort | 10 | 2b | — |
| Ho Quoc, 2013 | France | Case series | 1000 | 4 | 39 |
| Ho Quoc, 2015 | France | Case report | 2 | 5 | 19,26 |
| Ho Quoc, 2015 | France | Case series | 10 | 4 | 21 |
| Illouz, 2009 | France | Case series | 439 | 4 | 45.6 |
| Jung, 2016 | South Korea | Prospective cohort | 5 | 2b | 34.4 |
| Kamakura, 2011 | Japan | Prospective cohort | 20 | 2b | 35.6 |
| Kang, 2018 | China | Randomized prospective controlled cohort | 100 | 2b | 43.6 |
| Kerfant, 2017 | France | Case series | 156 | 4 | 31.7 |
| Khouri, 2012 | USA | Prospective cohort | 81 | 2b | 17-63 |
| Khouri, 2014 | USA | Case series | 139 | 4 | 27-45.2 |
| Klit, 2015 | Denmark | Case series | 7 | 4 | 18 |
| Kwiatkowska, 2019 | Germany | Case series | 14 | 4 | — |
| La Marca, 2013 | France | Case series | 10 | 4 | 16 |
| Lancerotto, 2010 | USA | Case report | 1 | 5 | 16 |
| Li, 2014 | China | Case series | 105 | 4 | 31.3 |
| Maione, 2018 | Italy | Prospective cohort | 31 | 2b | 34.3 |
| Matsudo, 1988 | Switzerland | Case series | 21 | 4 | — |
| Muench, 2016 | Switzerland | Case series | 254 | 4 | 35.8 |
| Münch, 2013 | Switzerland | Case series | 84 | 4 | 36.7 |
| Ohashi, 2016 | Japan | Case series | 131 | 4 | 39.3 |
| Özalp, 2017 | Turkey | Case series | 34 | 4 | 31 |
| Peltoniemi, 2013 | Finland | Not-randomized prospective controlled cohort | 18 | 2b | 39-51 |
| Pinsolle, 2008 | France | Case series | 7 | 4 | 25 |
| Quoc, 2013 | France | Case series | 19 | 4 | 28 |
| Rubin, 2012 | Japan | Not-randomization controlled retrospective cohort | 27 | 2b | 35.9 |
| Salgarello, 2011 | Italy | Case series | 2 | 5 | — |
| Serra-Mestr, 2017 | Italy | Prospective cohort | 49 | 2b | 41 |
| Sforza, 2016 | England | Case series | 26 | 4 | 24 |
| Spear, 2014 | USA | Prospective cohort | 10 | 2b | 30 |
| Streit, 2017 | Czech Republic | Case report | 3 | 5 | 14,17,19 |
| Tassinari, 2016 | Italy | Case series | 242 | 5 | — |
| Ueberreiter, 2010 | Germany | Prospective cohort | 52 | 2b | — |
| Ueberreiter, 2013 | Germany | Prospective cohort | 56 | 2b | 22-58 |
| Veber, 2011 | France | Retrospective cohort | 31 | 2b | 38 |
| Visconti, 2019 | Italy | Case-control study | 29 | 3b | 26.5 |
| Walters, 2020 | USA | Case series | 140 | 4 | 39.7 |
| Wang, 2011 | China | Case series | 48 | 4 | 29.4 |
| Wang, 2012 | China | Prospective cohort | 18 | 2b | 32 |
| Wang, 2015 | China | Prospective cohort | 12 | 2b | 32 |
| Yoshimura, 2008 | Japan | Case series | 40 | 4 | 35.8 |
| Yoshimura, 2010 | Japan | Case series | 15 | 4 | 37.1 |
| Zheng, 2008 | China | Case series | 66 | 4 | 19-39 |
| Zheng, 2019 | China | Case-control study | 5 | 3b | 29.6 |
| Zocchi, 2008 | Italy | retrospective cohort | 181 | 2b | 33 |
| Zocchi, 2017 | Italy | Case series | 487 | 4 | 29.2 |
—, not reported. aOxford Centre for Evidence-Based Medicine Levels of Evidence.
| Study . | Location . | Design . | No. . | Levela . | Age (y) . |
|---|---|---|---|---|---|
| Abboud, 2015 | Belgium | Prospective cohort | 80 | 2b | 36 |
| Ahmad, 2017 | Pakistan | Case series | 2 | 5 | 27.5 |
| Atia, 2020 | Germany | Case series | 30 | 4 | 31.23 |
| Auclair, 2009 | France | Case series | 47 | 4 | — |
| Auclair, 2013 | France | Case series | 197 | 4 | — |
| Auclair, 2020 | France | Case series | 148 | 4 | 42 |
| Bircoll, 1987 | USA | Case report | 1 | 5 | — |
| Bircoll, 1987 | USA | Case report | 1 | 5 | 45 |
| Bircoll, 2010 | USA | Case series | 650 | 4 | — |
| Brault, 2017 | France | Case-control study | 15 | 3b | 21.1 |
| Bravo, 2014 | USA | Case-control study | 21 | 3b | — |
| Bresnick, 2016 | USA | Case series | 28 | 4 | — |
| Bulgin, 2013 | Croatia | Case report | 1 | 5 | 30 |
| Carvajal, 2008 | Colombia | Case series | 20 | 4 | 36.9 |
| Chiu, 2014 | Taiwan | Case series | 282 | 4 | 34.9 |
| Chiu, 2016 | Taiwan | Case series | 27 | 4 | 39.1 |
| Chiu, 2018 | Taiwan | Case-control study | 206 | 3b | 33 |
| Claudio, 2017 | Spain | Case series | 11 | 4 | 24 |
| Coleman, 2007 | USA | Retrospective cohort | 17 | 2b | 38.2 |
| Costantini, 2012 | Italy | Case series | 2 | 4 | 26,49 |
| Cotrufo, 2008 | Italy | Case series | 42 | 4 | 48 |
| Coudurie, 2015 | France | Case report | 1 | 5 | 12 |
| Del Vecchio, 2011 | USA | Prospective cohort | 25 | 2b | 21-60 |
| Del vecchio, 2012 | USA | Case report | 1 | 5 | 42 |
| Del Vecchio, 2014 | USA | Case series | 30 | 4 | — |
| Delay, 2009 | France | Case report | 1 | 5 | 11 |
| Delay, 2009 | France | Case series | 136 | 4 | — |
| Delay, 2013 | France | Case series | 31 | 4 | 23 |
| Derder, 2014 | France | Case series | 10 | 4 | 17.5 |
| Deschler, 2020 | France | Case series | 42 | 4 | 34 |
| Dos Anjos, 2015 | Spain | Case-control study | 47 | 3b | 37.8 |
| Fiaschetti, 2013 | Italy | Prospective cohort | 6 | 2b | 46.3 |
| Fisenko, 2017 | Russian | Case series | 2 | 5 | 42 |
| Gaston, 1994 | Switzerland | Case report | 1 | 5 | 20 |
| Graf, 2019 | Brazil | Case series | 26 | 4 | 59.1 |
| Guo, 2018 | China | Prospective cohort | 11 | 2b | 27 |
| Gutierrez-Ontalvilla, 2020 | Spain | Case series | 9 | 4 | 14.9 |
| Herly, 2019 | Denmark | Case series | 14 | 4 | 34.9 |
| Herold, 2010 | Germany | Prospective cohort | 10 | 2b | — |
| Ho Quoc, 2013 | France | Case series | 1000 | 4 | 39 |
| Ho Quoc, 2015 | France | Case report | 2 | 5 | 19,26 |
| Ho Quoc, 2015 | France | Case series | 10 | 4 | 21 |
| Illouz, 2009 | France | Case series | 439 | 4 | 45.6 |
| Jung, 2016 | South Korea | Prospective cohort | 5 | 2b | 34.4 |
| Kamakura, 2011 | Japan | Prospective cohort | 20 | 2b | 35.6 |
| Kang, 2018 | China | Randomized prospective controlled cohort | 100 | 2b | 43.6 |
| Kerfant, 2017 | France | Case series | 156 | 4 | 31.7 |
| Khouri, 2012 | USA | Prospective cohort | 81 | 2b | 17-63 |
| Khouri, 2014 | USA | Case series | 139 | 4 | 27-45.2 |
| Klit, 2015 | Denmark | Case series | 7 | 4 | 18 |
| Kwiatkowska, 2019 | Germany | Case series | 14 | 4 | — |
| La Marca, 2013 | France | Case series | 10 | 4 | 16 |
| Lancerotto, 2010 | USA | Case report | 1 | 5 | 16 |
| Li, 2014 | China | Case series | 105 | 4 | 31.3 |
| Maione, 2018 | Italy | Prospective cohort | 31 | 2b | 34.3 |
| Matsudo, 1988 | Switzerland | Case series | 21 | 4 | — |
| Muench, 2016 | Switzerland | Case series | 254 | 4 | 35.8 |
| Münch, 2013 | Switzerland | Case series | 84 | 4 | 36.7 |
| Ohashi, 2016 | Japan | Case series | 131 | 4 | 39.3 |
| Özalp, 2017 | Turkey | Case series | 34 | 4 | 31 |
| Peltoniemi, 2013 | Finland | Not-randomized prospective controlled cohort | 18 | 2b | 39-51 |
| Pinsolle, 2008 | France | Case series | 7 | 4 | 25 |
| Quoc, 2013 | France | Case series | 19 | 4 | 28 |
| Rubin, 2012 | Japan | Not-randomization controlled retrospective cohort | 27 | 2b | 35.9 |
| Salgarello, 2011 | Italy | Case series | 2 | 5 | — |
| Serra-Mestr, 2017 | Italy | Prospective cohort | 49 | 2b | 41 |
| Sforza, 2016 | England | Case series | 26 | 4 | 24 |
| Spear, 2014 | USA | Prospective cohort | 10 | 2b | 30 |
| Streit, 2017 | Czech Republic | Case report | 3 | 5 | 14,17,19 |
| Tassinari, 2016 | Italy | Case series | 242 | 5 | — |
| Ueberreiter, 2010 | Germany | Prospective cohort | 52 | 2b | — |
| Ueberreiter, 2013 | Germany | Prospective cohort | 56 | 2b | 22-58 |
| Veber, 2011 | France | Retrospective cohort | 31 | 2b | 38 |
| Visconti, 2019 | Italy | Case-control study | 29 | 3b | 26.5 |
| Walters, 2020 | USA | Case series | 140 | 4 | 39.7 |
| Wang, 2011 | China | Case series | 48 | 4 | 29.4 |
| Wang, 2012 | China | Prospective cohort | 18 | 2b | 32 |
| Wang, 2015 | China | Prospective cohort | 12 | 2b | 32 |
| Yoshimura, 2008 | Japan | Case series | 40 | 4 | 35.8 |
| Yoshimura, 2010 | Japan | Case series | 15 | 4 | 37.1 |
| Zheng, 2008 | China | Case series | 66 | 4 | 19-39 |
| Zheng, 2019 | China | Case-control study | 5 | 3b | 29.6 |
| Zocchi, 2008 | Italy | retrospective cohort | 181 | 2b | 33 |
| Zocchi, 2017 | Italy | Case series | 487 | 4 | 29.2 |
| Study . | Location . | Design . | No. . | Levela . | Age (y) . |
|---|---|---|---|---|---|
| Abboud, 2015 | Belgium | Prospective cohort | 80 | 2b | 36 |
| Ahmad, 2017 | Pakistan | Case series | 2 | 5 | 27.5 |
| Atia, 2020 | Germany | Case series | 30 | 4 | 31.23 |
| Auclair, 2009 | France | Case series | 47 | 4 | — |
| Auclair, 2013 | France | Case series | 197 | 4 | — |
| Auclair, 2020 | France | Case series | 148 | 4 | 42 |
| Bircoll, 1987 | USA | Case report | 1 | 5 | — |
| Bircoll, 1987 | USA | Case report | 1 | 5 | 45 |
| Bircoll, 2010 | USA | Case series | 650 | 4 | — |
| Brault, 2017 | France | Case-control study | 15 | 3b | 21.1 |
| Bravo, 2014 | USA | Case-control study | 21 | 3b | — |
| Bresnick, 2016 | USA | Case series | 28 | 4 | — |
| Bulgin, 2013 | Croatia | Case report | 1 | 5 | 30 |
| Carvajal, 2008 | Colombia | Case series | 20 | 4 | 36.9 |
| Chiu, 2014 | Taiwan | Case series | 282 | 4 | 34.9 |
| Chiu, 2016 | Taiwan | Case series | 27 | 4 | 39.1 |
| Chiu, 2018 | Taiwan | Case-control study | 206 | 3b | 33 |
| Claudio, 2017 | Spain | Case series | 11 | 4 | 24 |
| Coleman, 2007 | USA | Retrospective cohort | 17 | 2b | 38.2 |
| Costantini, 2012 | Italy | Case series | 2 | 4 | 26,49 |
| Cotrufo, 2008 | Italy | Case series | 42 | 4 | 48 |
| Coudurie, 2015 | France | Case report | 1 | 5 | 12 |
| Del Vecchio, 2011 | USA | Prospective cohort | 25 | 2b | 21-60 |
| Del vecchio, 2012 | USA | Case report | 1 | 5 | 42 |
| Del Vecchio, 2014 | USA | Case series | 30 | 4 | — |
| Delay, 2009 | France | Case report | 1 | 5 | 11 |
| Delay, 2009 | France | Case series | 136 | 4 | — |
| Delay, 2013 | France | Case series | 31 | 4 | 23 |
| Derder, 2014 | France | Case series | 10 | 4 | 17.5 |
| Deschler, 2020 | France | Case series | 42 | 4 | 34 |
| Dos Anjos, 2015 | Spain | Case-control study | 47 | 3b | 37.8 |
| Fiaschetti, 2013 | Italy | Prospective cohort | 6 | 2b | 46.3 |
| Fisenko, 2017 | Russian | Case series | 2 | 5 | 42 |
| Gaston, 1994 | Switzerland | Case report | 1 | 5 | 20 |
| Graf, 2019 | Brazil | Case series | 26 | 4 | 59.1 |
| Guo, 2018 | China | Prospective cohort | 11 | 2b | 27 |
| Gutierrez-Ontalvilla, 2020 | Spain | Case series | 9 | 4 | 14.9 |
| Herly, 2019 | Denmark | Case series | 14 | 4 | 34.9 |
| Herold, 2010 | Germany | Prospective cohort | 10 | 2b | — |
| Ho Quoc, 2013 | France | Case series | 1000 | 4 | 39 |
| Ho Quoc, 2015 | France | Case report | 2 | 5 | 19,26 |
| Ho Quoc, 2015 | France | Case series | 10 | 4 | 21 |
| Illouz, 2009 | France | Case series | 439 | 4 | 45.6 |
| Jung, 2016 | South Korea | Prospective cohort | 5 | 2b | 34.4 |
| Kamakura, 2011 | Japan | Prospective cohort | 20 | 2b | 35.6 |
| Kang, 2018 | China | Randomized prospective controlled cohort | 100 | 2b | 43.6 |
| Kerfant, 2017 | France | Case series | 156 | 4 | 31.7 |
| Khouri, 2012 | USA | Prospective cohort | 81 | 2b | 17-63 |
| Khouri, 2014 | USA | Case series | 139 | 4 | 27-45.2 |
| Klit, 2015 | Denmark | Case series | 7 | 4 | 18 |
| Kwiatkowska, 2019 | Germany | Case series | 14 | 4 | — |
| La Marca, 2013 | France | Case series | 10 | 4 | 16 |
| Lancerotto, 2010 | USA | Case report | 1 | 5 | 16 |
| Li, 2014 | China | Case series | 105 | 4 | 31.3 |
| Maione, 2018 | Italy | Prospective cohort | 31 | 2b | 34.3 |
| Matsudo, 1988 | Switzerland | Case series | 21 | 4 | — |
| Muench, 2016 | Switzerland | Case series | 254 | 4 | 35.8 |
| Münch, 2013 | Switzerland | Case series | 84 | 4 | 36.7 |
| Ohashi, 2016 | Japan | Case series | 131 | 4 | 39.3 |
| Özalp, 2017 | Turkey | Case series | 34 | 4 | 31 |
| Peltoniemi, 2013 | Finland | Not-randomized prospective controlled cohort | 18 | 2b | 39-51 |
| Pinsolle, 2008 | France | Case series | 7 | 4 | 25 |
| Quoc, 2013 | France | Case series | 19 | 4 | 28 |
| Rubin, 2012 | Japan | Not-randomization controlled retrospective cohort | 27 | 2b | 35.9 |
| Salgarello, 2011 | Italy | Case series | 2 | 5 | — |
| Serra-Mestr, 2017 | Italy | Prospective cohort | 49 | 2b | 41 |
| Sforza, 2016 | England | Case series | 26 | 4 | 24 |
| Spear, 2014 | USA | Prospective cohort | 10 | 2b | 30 |
| Streit, 2017 | Czech Republic | Case report | 3 | 5 | 14,17,19 |
| Tassinari, 2016 | Italy | Case series | 242 | 5 | — |
| Ueberreiter, 2010 | Germany | Prospective cohort | 52 | 2b | — |
| Ueberreiter, 2013 | Germany | Prospective cohort | 56 | 2b | 22-58 |
| Veber, 2011 | France | Retrospective cohort | 31 | 2b | 38 |
| Visconti, 2019 | Italy | Case-control study | 29 | 3b | 26.5 |
| Walters, 2020 | USA | Case series | 140 | 4 | 39.7 |
| Wang, 2011 | China | Case series | 48 | 4 | 29.4 |
| Wang, 2012 | China | Prospective cohort | 18 | 2b | 32 |
| Wang, 2015 | China | Prospective cohort | 12 | 2b | 32 |
| Yoshimura, 2008 | Japan | Case series | 40 | 4 | 35.8 |
| Yoshimura, 2010 | Japan | Case series | 15 | 4 | 37.1 |
| Zheng, 2008 | China | Case series | 66 | 4 | 19-39 |
| Zheng, 2019 | China | Case-control study | 5 | 3b | 29.6 |
| Zocchi, 2008 | Italy | retrospective cohort | 181 | 2b | 33 |
| Zocchi, 2017 | Italy | Case series | 487 | 4 | 29.2 |
—, not reported. aOxford Centre for Evidence-Based Medicine Levels of Evidence.
| Country of origin . | No. of articles . | No. of patients . |
|---|---|---|
| France | 19 | 2302 |
| USA | 14 | 1145 |
| Italy | 10 | 1071 |
| China | 8 | 365 |
| Japan | 5 | 233 |
| Germany | 5 | 162 |
| Switzerland | 4 | 360 |
| Taiwan | 3 | 515 |
| Spain | 3 | 77 |
| Denmark | 2 | 21 |
| Belgium | 1 | 80 |
| Turkey | 1 | 34 |
| Brazil | 1 | 26 |
| England | 1 | 26 |
| Colombia | 1 | 20 |
| Finland | 1 | 18 |
| South Korea | 1 | 5 |
| Czech Republic | 1 | 3 |
| Pakistan | 1 | 2 |
| Russian | 1 | 2 |
| Croatia | 1 | 1 |
| Country of origin . | No. of articles . | No. of patients . |
|---|---|---|
| France | 19 | 2302 |
| USA | 14 | 1145 |
| Italy | 10 | 1071 |
| China | 8 | 365 |
| Japan | 5 | 233 |
| Germany | 5 | 162 |
| Switzerland | 4 | 360 |
| Taiwan | 3 | 515 |
| Spain | 3 | 77 |
| Denmark | 2 | 21 |
| Belgium | 1 | 80 |
| Turkey | 1 | 34 |
| Brazil | 1 | 26 |
| England | 1 | 26 |
| Colombia | 1 | 20 |
| Finland | 1 | 18 |
| South Korea | 1 | 5 |
| Czech Republic | 1 | 3 |
| Pakistan | 1 | 2 |
| Russian | 1 | 2 |
| Croatia | 1 | 1 |
| Country of origin . | No. of articles . | No. of patients . |
|---|---|---|
| France | 19 | 2302 |
| USA | 14 | 1145 |
| Italy | 10 | 1071 |
| China | 8 | 365 |
| Japan | 5 | 233 |
| Germany | 5 | 162 |
| Switzerland | 4 | 360 |
| Taiwan | 3 | 515 |
| Spain | 3 | 77 |
| Denmark | 2 | 21 |
| Belgium | 1 | 80 |
| Turkey | 1 | 34 |
| Brazil | 1 | 26 |
| England | 1 | 26 |
| Colombia | 1 | 20 |
| Finland | 1 | 18 |
| South Korea | 1 | 5 |
| Czech Republic | 1 | 3 |
| Pakistan | 1 | 2 |
| Russian | 1 | 2 |
| Croatia | 1 | 1 |
| Country of origin . | No. of articles . | No. of patients . |
|---|---|---|
| France | 19 | 2302 |
| USA | 14 | 1145 |
| Italy | 10 | 1071 |
| China | 8 | 365 |
| Japan | 5 | 233 |
| Germany | 5 | 162 |
| Switzerland | 4 | 360 |
| Taiwan | 3 | 515 |
| Spain | 3 | 77 |
| Denmark | 2 | 21 |
| Belgium | 1 | 80 |
| Turkey | 1 | 34 |
| Brazil | 1 | 26 |
| England | 1 | 26 |
| Colombia | 1 | 20 |
| Finland | 1 | 18 |
| South Korea | 1 | 5 |
| Czech Republic | 1 | 3 |
| Pakistan | 1 | 2 |
| Russian | 1 | 2 |
| Croatia | 1 | 1 |
| Study . | Design . | Level . | No. patients . | Treat group . | Age(y) . | BMI kg/m2 . | No. sessions . | Fellow-up (mo) . | Satisfaction No. Patients . | Satisfaction No. Surgeon . | No. Complications . | No. Biopsy . | Mean Volume injection (ml) . | Volume retention(%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | CH | 2b | 80 | AFT | 36 | 26 | 1 | 24 | 65 (n = 72) | — | 11 (n = 160) | 1 (n = 160) | 420 | 59.4% (12m) |
| Auclair, 2009 | CS | 4 | 1433 | AFT AFT+IMP | — | — | — | 16.7 | — | — | 0 | — | 260110 | — |
| Auclair et al, 201310 | CS | 4 | 197 | AFT+IMP | — | — | — | 5 | — | — | 9 | 2 | 369.9 (n = 20) | 57% (12m) |
| Atia, 2020 | CS | 4 | 30 | AFT | 31.23 | 26.9 | 1.2 | 12 | 27 | — | 12 | — | 259.83ml 252.17 ml | — |
| Auclair, 2020 | CS | 4 | 148 | AFT+IMP | 42 | 19.6 | — | 22 | — | — | 23 | — | 153ml | — |
| Bircoll, 2010 | CS | 4 | 650 | AFT | — | — | — | — | — | — | 8 | — | — | — |
| Brault et al, 201711 | CC | 3b | 1522 | AFT C:IMP | 21.1 | — | 2 | 17 | — | — | 2 | — | — | — |
| Bravo, 2014 | CC | 3b | 2138 | AFT+IMPC:IMP | — | — | — | 12 | — | — | 2 | — | 117 | — |
| Bresnick, 2016 | CS | 4 | 28 | AFT | — | — | 2.1 | — | 28 | — | 0 | - | 27 | — |
| Carvajal and Patino, 2008 | CS | 4 | 20 | AFT | 36.9 | — | — | 34.5 | — | — | 4 | 4 | 235 | — |
| Chiu, 2014 | CS | 4 | 205(HB) 77(LB) | AFT+SVF | 34.9 31.2 | 21.2 17.6 | — | 23.7 23.0 | 17665 | 17864 | 137 | — | 254241 | — |
| Chiu, 2016 | CS | 4 | 27 | AFT+SVF | 39.1 | 19.9 | — | 27.1 | — | — | 6 | — | 247 | — |
| Chiu, 201816 | CC | 3b | 105 101 | AFT AFT+SVF | 3337 | 18.8 20.3 | 11.2 | 15.8 13.4 | — | — | 46 | — | 310334 | 67.9% (12m) 68.7% (12m) |
| Claudio et al, 2017 | CS | 4 | 11 | AFT | 24 | 23.4 | 2 | 29.7 | — | — | 4 | 2 | 210 | — |
| Coleman and Saboeiro, 200713 | CH | 2b | 17 | AFT | 38.2 | — | 1.2 | 62.2 | — | — | 10 | 2 | 278.6 | — |
| Cotrufo et al, 2008 | CS | 4 | 42 | AFT | 48 | — | 1.3 | 7 | — | — | 1 | — | — | — |
| Del Vecchio and Bucky, 2011 | CH | 2b | 25 | AFT +BRAVA | 21–60 | — | — | 6 | — | — | 0 | — | 430 (n = 12) | 64% (6m) |
| Del Vecchio, 201419 | CS | 4 | 30 | AFT +BRAVA | — | — | — | 12 | — | — | — | — | 300 | 53% (12m) |
| Delay et al, 200918 | CS | 4 | 136 | AFT | — | — | 3–4 | 120 | — | — | 4 (n = 880) | — | — | 60~70% (3m) |
| Delay et al, 2013 | CS | 4 | 31 | AFT | 23 | 21.9 | 1.5 | 78 | 31 | 29 | 0 | — | 158 | — |
| 226 | ||||||||||||||
| Derder et al, 201420 | CS | 4 | 10 | AFT | 17.5 | — | 2 | 68 | 10 | — | — | — | 285 | — |
| Deschler et al, 202025 | CS | 4 | 42 | AFT | 34 | 22.9 | — | 25.6 | — | — | 3 | — | 312.2ml | — |
| Dos Anjos et al, 201517 | CC | 3b | 13 (LS) 44 (HS) | AFT+SVF | 37.8 39.4 | 21.6 21.6 | — | 18 | — | — | 3 | 3 | 229.1 270.7 | 50% (18m) 75% (18m) |
| Fiaschetti et al, 2013 | CH | 2b | 6 | AFT+PRP | 46.3 | — | 2 | — | — | — | — | — | 195.6 | 84.4% (6m) 72.1% (12m) |
| Guo et al, 201821 | CH | 2b | 11 | AFT | 27 | 20.2 | 1 | 3 | — | — | 0 | — | 207 | 56.6% (3m) |
| Gutierrez-Ontalvilla et al, 2020 | CS | 4 | 9 | AFT | 14.9 | — | 1.8 | 21.3 | — | — | 1 | — | 220ml | — |
| Herly et al, 2019 | CS | 4 | 14 | AFT | 34.9 | 24.2 | 1 | 4.5 | — | — | — | — | 304ml | 50.9% (4.5m) |
| Herold et al, 2010 | CH | 2b | 10 | AFT | — | — | — | — | — | — | — | — | 208 | 72.0% (6m) |
| Ho Quoc et al, 201312 | CS | 4 | 1000 | AFT | 39 | — | 1–3 | — | — | — | 40 | — | — | — |
| Ho Quoc et al, 2015 | CS | 4 | 10 | AFT | 21 | 21.5 | — | 72 | 10 | — | 0 | — | 380 | — |
| Illouz and Sterodimas, 2009 | CS | 4 | 439 | AFT | 45.6 | — | 3 | 12 | 399 | — | 53 | — | 145 | — |
| Jung et al, 2016 | CH | 2b | 5 | AFT+SVF | 34.4 | — | — | 12 | — | — | 5(n=10) | — | 221.2 | 65.1% (3m) 46.8% (12m) |
| Kamakura and Ito, 2011 | CH | 2b | 20 | AFT +ADRCS | 35.6 | — | — | 9 | 15 | 11(n=16) | 2 | 1 | 240 | — |
| Kang and Luan, 201814 | CH | 2b | 100 | AFT | 43.6 | 21.3 | 1.3 | 3 | — | — | 21 (n=167) | — | 176.1 | — |
| Kerfant et al, 2017 | CS | 4 | 156 | AFT+IMP | 31.7 | 18. 9 | 1.1 | 22.3 | — | — | 11 | — | 126 | — |
| Khouri et al, 2012 | CH | 2b | 81 | AFT +BRAVA | 17–63 | 19.8 | 1 | 44 | 81 | — | 1 | — | 282 (n=71) | 82.0% (12m) |
| Khouri et al, 2014 | CS | 4 | 4594 | AFT +BRAVAAFT | 27 45.2 | 21.6 | 1.2 1.4 | 9 | 4390 | — | —24 | — | 300354 | 79.0% (12m) 64.0% (12m) |
| Klit et al, 20155 | CS | 4 | 4 7 | AFT +BRAVAAFT | 18 | 20–25 | 1 | 13 | 9 | — | 0 | — | 245 147 | — |
| La Marca et al, 2013 | CS | 4 | 10 | AFT | 16 | — | 2.9 | 51 | 10 | — | 0 | — | 255 | — |
| Li et al, 2014 | CS | 4 | 105 | AFT | 31.3 | — | 1.3 | 18 | 105 | 88 | 5 | 3 | 205 | — |
| Maione et al, 2018 | CH | 2b | 31 | AFT+IMP | 34.3 | — | 1 | 3–12 | — | — | 0 | — | 134 | — |
| Matsudo and Toledo, 1988 | CS | 4 | 21 | AFT | — | — | — | 18 | — | — | — | 5–450ml | 20–50% | |
| Muench, 2016 | CS | 4 | 254 | AFT | 35.8 | 22.5 | 1.2 | 24.5 | 246 | — | 11 | — | 207 | — |
| Münch, 2013 | CS | 4 | 84 | AFT | 36.7 | 22.7 | 1.1 | 4.7 | 83 | — | 9 | — | 177 | — |
| Ohashi et al, 2016 | CS | 4 | 131 | AFT | 39.3 | 19.9 | — | 6 | 126 | — | 12 | — | 239.6 | — |
| Özalp and Aydinol, 2017 | CS | 4 | 34 | AFT+IMP | 31 | — | — | 22 | 30 | — | 6(n=68) | — | 114 | — |
| Peltoniemi et al, 201315 | CH | 2b | 108 | AFT+ ASCSAFT | 5139 | 23.423.4 | 1.81.8 | 6 | — | — | 21 | — | 178.5 204 | 74.2% (6m) 78.8% (6m) |
| Pinsolle et al, 2008 | CS | 4 | 71 | AFT1+IMP AFT | 25 | — | 2.1 | — | — | — | 1 | — | 96 | — |
| Quoc et al, 2013 | CS | 4 | 19 | AFT | 28 | 20.3 | 1.6 | — | 18 | 18 | 0 | — | 375 | — |
| Rubin et al, 2012 | CH | 2b | 27 23 | AFTC:reduction | 35.9 | — | — | 12 | — | — | — | — | 526.5 | — |
| Serra-Mestr et al, 2017 | CH | 2b | 49 | AFT | 41 | — | — | 12 | 48 | — | 3 | 1 | 42 | — |
| Sforza et al, 201622 | CS | 4 | 26 | AFT | 24 | — | — | — | 25 | 23 | 0 | — | 148 | 72.5% (12m) |
| Spear and Pittman, 201423 | CH | 2b | 10 | AFT | 30 | 23.3 | 1 | — | — | — | 0 | 1 | 243 | 39.8 % (12m) |
| Tassinari et al, 2016 | CS | 5 | 242 | AFT | — | — | — | 26.4 | — | — | 37 | — | 136 | — |
| Ueberreiter et al, 2010 | CH | 2b | 52 | AFT | — | — | 2.9 | 6–30 | — | — | 0 | — | 184 | 76% (6m) |
| Ueberreiter et al, 2013 | CH | 2b | 56 | AFT | 22–58 | 17–30 | 3 | 6–56 | 56 | — | — | — | 260 | — |
| Veber et al, 2011 | CH | 2b | 31 | AFT | 38 | — | 1.3 | 16.2 | - | — | — | — | 200.8 | — |
| Visconti and Salgarello, 2019 | CC | 3b | 29 | AFT +BRAVA | 26.5 | 26.6 | 1.3 | 12 | 40 in46 | — | 0 | — | 215ml | — |
| Wang et al, 2011 | CS | 4 | 48 | AFT | 29.4 | — | — | 18–72 | — | — | 8 | 8 | 50–170 | — |
| Wang et al, 2012 | CH | 2b | 18 | AFT+SVF | 32 | 22.1 | — | 6 | — | — | 1 | — | 256.5 (n=10) | 51.2% (3m) 54.2% (6m) |
| Wang et al, 2015 | CH | 2b | 12 | AFT+SVF | 32 | 22.1 | — | 6 | 11 | 11 | 0 | — | 256 | 60.7% (3m) 45.5% (6m) |
| Yoshimura et al, 20088 | CS | 4 | 40 | AFT+ CAL | 35.8 | 19.1 | 1 | 42 | 40 | — | 1 | — | 272.7 | 55.9% (6m) |
| Yoshimura et al, 20109 | CS | 4 | 15 | AFT+ CAL | 37.1 | 19.5 | 1 | 18 | 15 | — | 0 | — | 263.5 | 20~60% (n=6) |
| Zheng et al, 2008 | CS | 4 | 66 | AFT | 19–39 | — | 1.8 | 37 | 53 | 52 | 13 | 2 | 174 | — |
| Zheng et al, 2019 | CC | 3b | 5 | AFT | 29.6 | 21.1 | 1.2 | 4.4 | — | — | 0 | — | 175ml | 59.13%(4.4m) |
| Zocchi and Zuliani, 20086 | CH | 2b | 181 | AFT +BRAVA | 33 | — | — | 12 | 176 | 171 | 12 | — | 375 | 55% (12m) |
| Zocchi, 20177 | CS | 4 | 388 99 | AFT +BRAVAAFT+SVF | 29.2 26.8 | — | — | 12 | — | — | 25 | — | 287 380 | 74% (12m) 86% (12m) |
| Study . | Design . | Level . | No. patients . | Treat group . | Age(y) . | BMI kg/m2 . | No. sessions . | Fellow-up (mo) . | Satisfaction No. Patients . | Satisfaction No. Surgeon . | No. Complications . | No. Biopsy . | Mean Volume injection (ml) . | Volume retention(%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | CH | 2b | 80 | AFT | 36 | 26 | 1 | 24 | 65 (n = 72) | — | 11 (n = 160) | 1 (n = 160) | 420 | 59.4% (12m) |
| Auclair, 2009 | CS | 4 | 1433 | AFT AFT+IMP | — | — | — | 16.7 | — | — | 0 | — | 260110 | — |
| Auclair et al, 201310 | CS | 4 | 197 | AFT+IMP | — | — | — | 5 | — | — | 9 | 2 | 369.9 (n = 20) | 57% (12m) |
| Atia, 2020 | CS | 4 | 30 | AFT | 31.23 | 26.9 | 1.2 | 12 | 27 | — | 12 | — | 259.83ml 252.17 ml | — |
| Auclair, 2020 | CS | 4 | 148 | AFT+IMP | 42 | 19.6 | — | 22 | — | — | 23 | — | 153ml | — |
| Bircoll, 2010 | CS | 4 | 650 | AFT | — | — | — | — | — | — | 8 | — | — | — |
| Brault et al, 201711 | CC | 3b | 1522 | AFT C:IMP | 21.1 | — | 2 | 17 | — | — | 2 | — | — | — |
| Bravo, 2014 | CC | 3b | 2138 | AFT+IMPC:IMP | — | — | — | 12 | — | — | 2 | — | 117 | — |
| Bresnick, 2016 | CS | 4 | 28 | AFT | — | — | 2.1 | — | 28 | — | 0 | - | 27 | — |
| Carvajal and Patino, 2008 | CS | 4 | 20 | AFT | 36.9 | — | — | 34.5 | — | — | 4 | 4 | 235 | — |
| Chiu, 2014 | CS | 4 | 205(HB) 77(LB) | AFT+SVF | 34.9 31.2 | 21.2 17.6 | — | 23.7 23.0 | 17665 | 17864 | 137 | — | 254241 | — |
| Chiu, 2016 | CS | 4 | 27 | AFT+SVF | 39.1 | 19.9 | — | 27.1 | — | — | 6 | — | 247 | — |
| Chiu, 201816 | CC | 3b | 105 101 | AFT AFT+SVF | 3337 | 18.8 20.3 | 11.2 | 15.8 13.4 | — | — | 46 | — | 310334 | 67.9% (12m) 68.7% (12m) |
| Claudio et al, 2017 | CS | 4 | 11 | AFT | 24 | 23.4 | 2 | 29.7 | — | — | 4 | 2 | 210 | — |
| Coleman and Saboeiro, 200713 | CH | 2b | 17 | AFT | 38.2 | — | 1.2 | 62.2 | — | — | 10 | 2 | 278.6 | — |
| Cotrufo et al, 2008 | CS | 4 | 42 | AFT | 48 | — | 1.3 | 7 | — | — | 1 | — | — | — |
| Del Vecchio and Bucky, 2011 | CH | 2b | 25 | AFT +BRAVA | 21–60 | — | — | 6 | — | — | 0 | — | 430 (n = 12) | 64% (6m) |
| Del Vecchio, 201419 | CS | 4 | 30 | AFT +BRAVA | — | — | — | 12 | — | — | — | — | 300 | 53% (12m) |
| Delay et al, 200918 | CS | 4 | 136 | AFT | — | — | 3–4 | 120 | — | — | 4 (n = 880) | — | — | 60~70% (3m) |
| Delay et al, 2013 | CS | 4 | 31 | AFT | 23 | 21.9 | 1.5 | 78 | 31 | 29 | 0 | — | 158 | — |
| 226 | ||||||||||||||
| Derder et al, 201420 | CS | 4 | 10 | AFT | 17.5 | — | 2 | 68 | 10 | — | — | — | 285 | — |
| Deschler et al, 202025 | CS | 4 | 42 | AFT | 34 | 22.9 | — | 25.6 | — | — | 3 | — | 312.2ml | — |
| Dos Anjos et al, 201517 | CC | 3b | 13 (LS) 44 (HS) | AFT+SVF | 37.8 39.4 | 21.6 21.6 | — | 18 | — | — | 3 | 3 | 229.1 270.7 | 50% (18m) 75% (18m) |
| Fiaschetti et al, 2013 | CH | 2b | 6 | AFT+PRP | 46.3 | — | 2 | — | — | — | — | — | 195.6 | 84.4% (6m) 72.1% (12m) |
| Guo et al, 201821 | CH | 2b | 11 | AFT | 27 | 20.2 | 1 | 3 | — | — | 0 | — | 207 | 56.6% (3m) |
| Gutierrez-Ontalvilla et al, 2020 | CS | 4 | 9 | AFT | 14.9 | — | 1.8 | 21.3 | — | — | 1 | — | 220ml | — |
| Herly et al, 2019 | CS | 4 | 14 | AFT | 34.9 | 24.2 | 1 | 4.5 | — | — | — | — | 304ml | 50.9% (4.5m) |
| Herold et al, 2010 | CH | 2b | 10 | AFT | — | — | — | — | — | — | — | — | 208 | 72.0% (6m) |
| Ho Quoc et al, 201312 | CS | 4 | 1000 | AFT | 39 | — | 1–3 | — | — | — | 40 | — | — | — |
| Ho Quoc et al, 2015 | CS | 4 | 10 | AFT | 21 | 21.5 | — | 72 | 10 | — | 0 | — | 380 | — |
| Illouz and Sterodimas, 2009 | CS | 4 | 439 | AFT | 45.6 | — | 3 | 12 | 399 | — | 53 | — | 145 | — |
| Jung et al, 2016 | CH | 2b | 5 | AFT+SVF | 34.4 | — | — | 12 | — | — | 5(n=10) | — | 221.2 | 65.1% (3m) 46.8% (12m) |
| Kamakura and Ito, 2011 | CH | 2b | 20 | AFT +ADRCS | 35.6 | — | — | 9 | 15 | 11(n=16) | 2 | 1 | 240 | — |
| Kang and Luan, 201814 | CH | 2b | 100 | AFT | 43.6 | 21.3 | 1.3 | 3 | — | — | 21 (n=167) | — | 176.1 | — |
| Kerfant et al, 2017 | CS | 4 | 156 | AFT+IMP | 31.7 | 18. 9 | 1.1 | 22.3 | — | — | 11 | — | 126 | — |
| Khouri et al, 2012 | CH | 2b | 81 | AFT +BRAVA | 17–63 | 19.8 | 1 | 44 | 81 | — | 1 | — | 282 (n=71) | 82.0% (12m) |
| Khouri et al, 2014 | CS | 4 | 4594 | AFT +BRAVAAFT | 27 45.2 | 21.6 | 1.2 1.4 | 9 | 4390 | — | —24 | — | 300354 | 79.0% (12m) 64.0% (12m) |
| Klit et al, 20155 | CS | 4 | 4 7 | AFT +BRAVAAFT | 18 | 20–25 | 1 | 13 | 9 | — | 0 | — | 245 147 | — |
| La Marca et al, 2013 | CS | 4 | 10 | AFT | 16 | — | 2.9 | 51 | 10 | — | 0 | — | 255 | — |
| Li et al, 2014 | CS | 4 | 105 | AFT | 31.3 | — | 1.3 | 18 | 105 | 88 | 5 | 3 | 205 | — |
| Maione et al, 2018 | CH | 2b | 31 | AFT+IMP | 34.3 | — | 1 | 3–12 | — | — | 0 | — | 134 | — |
| Matsudo and Toledo, 1988 | CS | 4 | 21 | AFT | — | — | — | 18 | — | — | — | 5–450ml | 20–50% | |
| Muench, 2016 | CS | 4 | 254 | AFT | 35.8 | 22.5 | 1.2 | 24.5 | 246 | — | 11 | — | 207 | — |
| Münch, 2013 | CS | 4 | 84 | AFT | 36.7 | 22.7 | 1.1 | 4.7 | 83 | — | 9 | — | 177 | — |
| Ohashi et al, 2016 | CS | 4 | 131 | AFT | 39.3 | 19.9 | — | 6 | 126 | — | 12 | — | 239.6 | — |
| Özalp and Aydinol, 2017 | CS | 4 | 34 | AFT+IMP | 31 | — | — | 22 | 30 | — | 6(n=68) | — | 114 | — |
| Peltoniemi et al, 201315 | CH | 2b | 108 | AFT+ ASCSAFT | 5139 | 23.423.4 | 1.81.8 | 6 | — | — | 21 | — | 178.5 204 | 74.2% (6m) 78.8% (6m) |
| Pinsolle et al, 2008 | CS | 4 | 71 | AFT1+IMP AFT | 25 | — | 2.1 | — | — | — | 1 | — | 96 | — |
| Quoc et al, 2013 | CS | 4 | 19 | AFT | 28 | 20.3 | 1.6 | — | 18 | 18 | 0 | — | 375 | — |
| Rubin et al, 2012 | CH | 2b | 27 23 | AFTC:reduction | 35.9 | — | — | 12 | — | — | — | — | 526.5 | — |
| Serra-Mestr et al, 2017 | CH | 2b | 49 | AFT | 41 | — | — | 12 | 48 | — | 3 | 1 | 42 | — |
| Sforza et al, 201622 | CS | 4 | 26 | AFT | 24 | — | — | — | 25 | 23 | 0 | — | 148 | 72.5% (12m) |
| Spear and Pittman, 201423 | CH | 2b | 10 | AFT | 30 | 23.3 | 1 | — | — | — | 0 | 1 | 243 | 39.8 % (12m) |
| Tassinari et al, 2016 | CS | 5 | 242 | AFT | — | — | — | 26.4 | — | — | 37 | — | 136 | — |
| Ueberreiter et al, 2010 | CH | 2b | 52 | AFT | — | — | 2.9 | 6–30 | — | — | 0 | — | 184 | 76% (6m) |
| Ueberreiter et al, 2013 | CH | 2b | 56 | AFT | 22–58 | 17–30 | 3 | 6–56 | 56 | — | — | — | 260 | — |
| Veber et al, 2011 | CH | 2b | 31 | AFT | 38 | — | 1.3 | 16.2 | - | — | — | — | 200.8 | — |
| Visconti and Salgarello, 2019 | CC | 3b | 29 | AFT +BRAVA | 26.5 | 26.6 | 1.3 | 12 | 40 in46 | — | 0 | — | 215ml | — |
| Wang et al, 2011 | CS | 4 | 48 | AFT | 29.4 | — | — | 18–72 | — | — | 8 | 8 | 50–170 | — |
| Wang et al, 2012 | CH | 2b | 18 | AFT+SVF | 32 | 22.1 | — | 6 | — | — | 1 | — | 256.5 (n=10) | 51.2% (3m) 54.2% (6m) |
| Wang et al, 2015 | CH | 2b | 12 | AFT+SVF | 32 | 22.1 | — | 6 | 11 | 11 | 0 | — | 256 | 60.7% (3m) 45.5% (6m) |
| Yoshimura et al, 20088 | CS | 4 | 40 | AFT+ CAL | 35.8 | 19.1 | 1 | 42 | 40 | — | 1 | — | 272.7 | 55.9% (6m) |
| Yoshimura et al, 20109 | CS | 4 | 15 | AFT+ CAL | 37.1 | 19.5 | 1 | 18 | 15 | — | 0 | — | 263.5 | 20~60% (n=6) |
| Zheng et al, 2008 | CS | 4 | 66 | AFT | 19–39 | — | 1.8 | 37 | 53 | 52 | 13 | 2 | 174 | — |
| Zheng et al, 2019 | CC | 3b | 5 | AFT | 29.6 | 21.1 | 1.2 | 4.4 | — | — | 0 | — | 175ml | 59.13%(4.4m) |
| Zocchi and Zuliani, 20086 | CH | 2b | 181 | AFT +BRAVA | 33 | — | — | 12 | 176 | 171 | 12 | — | 375 | 55% (12m) |
| Zocchi, 20177 | CS | 4 | 388 99 | AFT +BRAVAAFT+SVF | 29.2 26.8 | — | — | 12 | — | — | 25 | — | 287 380 | 74% (12m) 86% (12m) |
—, not reported; ADRCS, autologous adipose-derived regenerative cells; AFT, autologous fat transplantation; ASCs, adipose-derived stem cells; C, control group; CAL, cell-assisted lipotransfer; CC, case-control study; CH, cohort study; CS, case series study; FU, fellow-up; HB, high BMI (BMI > 18.5); HS, high stromal vascular fraction; IMP, implant; LB; low BMI (BMI ≤ 18.5); LS, low stromal vascular fraction; m, month; PRP, platelet-rich plasma; SUPP, supplements as SVF, CAL, PRP, or ASCs; SVF, stromal vascular fraction; VOL, volume; n, number of related cases.
| Study . | Design . | Level . | No. patients . | Treat group . | Age(y) . | BMI kg/m2 . | No. sessions . | Fellow-up (mo) . | Satisfaction No. Patients . | Satisfaction No. Surgeon . | No. Complications . | No. Biopsy . | Mean Volume injection (ml) . | Volume retention(%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | CH | 2b | 80 | AFT | 36 | 26 | 1 | 24 | 65 (n = 72) | — | 11 (n = 160) | 1 (n = 160) | 420 | 59.4% (12m) |
| Auclair, 2009 | CS | 4 | 1433 | AFT AFT+IMP | — | — | — | 16.7 | — | — | 0 | — | 260110 | — |
| Auclair et al, 201310 | CS | 4 | 197 | AFT+IMP | — | — | — | 5 | — | — | 9 | 2 | 369.9 (n = 20) | 57% (12m) |
| Atia, 2020 | CS | 4 | 30 | AFT | 31.23 | 26.9 | 1.2 | 12 | 27 | — | 12 | — | 259.83ml 252.17 ml | — |
| Auclair, 2020 | CS | 4 | 148 | AFT+IMP | 42 | 19.6 | — | 22 | — | — | 23 | — | 153ml | — |
| Bircoll, 2010 | CS | 4 | 650 | AFT | — | — | — | — | — | — | 8 | — | — | — |
| Brault et al, 201711 | CC | 3b | 1522 | AFT C:IMP | 21.1 | — | 2 | 17 | — | — | 2 | — | — | — |
| Bravo, 2014 | CC | 3b | 2138 | AFT+IMPC:IMP | — | — | — | 12 | — | — | 2 | — | 117 | — |
| Bresnick, 2016 | CS | 4 | 28 | AFT | — | — | 2.1 | — | 28 | — | 0 | - | 27 | — |
| Carvajal and Patino, 2008 | CS | 4 | 20 | AFT | 36.9 | — | — | 34.5 | — | — | 4 | 4 | 235 | — |
| Chiu, 2014 | CS | 4 | 205(HB) 77(LB) | AFT+SVF | 34.9 31.2 | 21.2 17.6 | — | 23.7 23.0 | 17665 | 17864 | 137 | — | 254241 | — |
| Chiu, 2016 | CS | 4 | 27 | AFT+SVF | 39.1 | 19.9 | — | 27.1 | — | — | 6 | — | 247 | — |
| Chiu, 201816 | CC | 3b | 105 101 | AFT AFT+SVF | 3337 | 18.8 20.3 | 11.2 | 15.8 13.4 | — | — | 46 | — | 310334 | 67.9% (12m) 68.7% (12m) |
| Claudio et al, 2017 | CS | 4 | 11 | AFT | 24 | 23.4 | 2 | 29.7 | — | — | 4 | 2 | 210 | — |
| Coleman and Saboeiro, 200713 | CH | 2b | 17 | AFT | 38.2 | — | 1.2 | 62.2 | — | — | 10 | 2 | 278.6 | — |
| Cotrufo et al, 2008 | CS | 4 | 42 | AFT | 48 | — | 1.3 | 7 | — | — | 1 | — | — | — |
| Del Vecchio and Bucky, 2011 | CH | 2b | 25 | AFT +BRAVA | 21–60 | — | — | 6 | — | — | 0 | — | 430 (n = 12) | 64% (6m) |
| Del Vecchio, 201419 | CS | 4 | 30 | AFT +BRAVA | — | — | — | 12 | — | — | — | — | 300 | 53% (12m) |
| Delay et al, 200918 | CS | 4 | 136 | AFT | — | — | 3–4 | 120 | — | — | 4 (n = 880) | — | — | 60~70% (3m) |
| Delay et al, 2013 | CS | 4 | 31 | AFT | 23 | 21.9 | 1.5 | 78 | 31 | 29 | 0 | — | 158 | — |
| 226 | ||||||||||||||
| Derder et al, 201420 | CS | 4 | 10 | AFT | 17.5 | — | 2 | 68 | 10 | — | — | — | 285 | — |
| Deschler et al, 202025 | CS | 4 | 42 | AFT | 34 | 22.9 | — | 25.6 | — | — | 3 | — | 312.2ml | — |
| Dos Anjos et al, 201517 | CC | 3b | 13 (LS) 44 (HS) | AFT+SVF | 37.8 39.4 | 21.6 21.6 | — | 18 | — | — | 3 | 3 | 229.1 270.7 | 50% (18m) 75% (18m) |
| Fiaschetti et al, 2013 | CH | 2b | 6 | AFT+PRP | 46.3 | — | 2 | — | — | — | — | — | 195.6 | 84.4% (6m) 72.1% (12m) |
| Guo et al, 201821 | CH | 2b | 11 | AFT | 27 | 20.2 | 1 | 3 | — | — | 0 | — | 207 | 56.6% (3m) |
| Gutierrez-Ontalvilla et al, 2020 | CS | 4 | 9 | AFT | 14.9 | — | 1.8 | 21.3 | — | — | 1 | — | 220ml | — |
| Herly et al, 2019 | CS | 4 | 14 | AFT | 34.9 | 24.2 | 1 | 4.5 | — | — | — | — | 304ml | 50.9% (4.5m) |
| Herold et al, 2010 | CH | 2b | 10 | AFT | — | — | — | — | — | — | — | — | 208 | 72.0% (6m) |
| Ho Quoc et al, 201312 | CS | 4 | 1000 | AFT | 39 | — | 1–3 | — | — | — | 40 | — | — | — |
| Ho Quoc et al, 2015 | CS | 4 | 10 | AFT | 21 | 21.5 | — | 72 | 10 | — | 0 | — | 380 | — |
| Illouz and Sterodimas, 2009 | CS | 4 | 439 | AFT | 45.6 | — | 3 | 12 | 399 | — | 53 | — | 145 | — |
| Jung et al, 2016 | CH | 2b | 5 | AFT+SVF | 34.4 | — | — | 12 | — | — | 5(n=10) | — | 221.2 | 65.1% (3m) 46.8% (12m) |
| Kamakura and Ito, 2011 | CH | 2b | 20 | AFT +ADRCS | 35.6 | — | — | 9 | 15 | 11(n=16) | 2 | 1 | 240 | — |
| Kang and Luan, 201814 | CH | 2b | 100 | AFT | 43.6 | 21.3 | 1.3 | 3 | — | — | 21 (n=167) | — | 176.1 | — |
| Kerfant et al, 2017 | CS | 4 | 156 | AFT+IMP | 31.7 | 18. 9 | 1.1 | 22.3 | — | — | 11 | — | 126 | — |
| Khouri et al, 2012 | CH | 2b | 81 | AFT +BRAVA | 17–63 | 19.8 | 1 | 44 | 81 | — | 1 | — | 282 (n=71) | 82.0% (12m) |
| Khouri et al, 2014 | CS | 4 | 4594 | AFT +BRAVAAFT | 27 45.2 | 21.6 | 1.2 1.4 | 9 | 4390 | — | —24 | — | 300354 | 79.0% (12m) 64.0% (12m) |
| Klit et al, 20155 | CS | 4 | 4 7 | AFT +BRAVAAFT | 18 | 20–25 | 1 | 13 | 9 | — | 0 | — | 245 147 | — |
| La Marca et al, 2013 | CS | 4 | 10 | AFT | 16 | — | 2.9 | 51 | 10 | — | 0 | — | 255 | — |
| Li et al, 2014 | CS | 4 | 105 | AFT | 31.3 | — | 1.3 | 18 | 105 | 88 | 5 | 3 | 205 | — |
| Maione et al, 2018 | CH | 2b | 31 | AFT+IMP | 34.3 | — | 1 | 3–12 | — | — | 0 | — | 134 | — |
| Matsudo and Toledo, 1988 | CS | 4 | 21 | AFT | — | — | — | 18 | — | — | — | 5–450ml | 20–50% | |
| Muench, 2016 | CS | 4 | 254 | AFT | 35.8 | 22.5 | 1.2 | 24.5 | 246 | — | 11 | — | 207 | — |
| Münch, 2013 | CS | 4 | 84 | AFT | 36.7 | 22.7 | 1.1 | 4.7 | 83 | — | 9 | — | 177 | — |
| Ohashi et al, 2016 | CS | 4 | 131 | AFT | 39.3 | 19.9 | — | 6 | 126 | — | 12 | — | 239.6 | — |
| Özalp and Aydinol, 2017 | CS | 4 | 34 | AFT+IMP | 31 | — | — | 22 | 30 | — | 6(n=68) | — | 114 | — |
| Peltoniemi et al, 201315 | CH | 2b | 108 | AFT+ ASCSAFT | 5139 | 23.423.4 | 1.81.8 | 6 | — | — | 21 | — | 178.5 204 | 74.2% (6m) 78.8% (6m) |
| Pinsolle et al, 2008 | CS | 4 | 71 | AFT1+IMP AFT | 25 | — | 2.1 | — | — | — | 1 | — | 96 | — |
| Quoc et al, 2013 | CS | 4 | 19 | AFT | 28 | 20.3 | 1.6 | — | 18 | 18 | 0 | — | 375 | — |
| Rubin et al, 2012 | CH | 2b | 27 23 | AFTC:reduction | 35.9 | — | — | 12 | — | — | — | — | 526.5 | — |
| Serra-Mestr et al, 2017 | CH | 2b | 49 | AFT | 41 | — | — | 12 | 48 | — | 3 | 1 | 42 | — |
| Sforza et al, 201622 | CS | 4 | 26 | AFT | 24 | — | — | — | 25 | 23 | 0 | — | 148 | 72.5% (12m) |
| Spear and Pittman, 201423 | CH | 2b | 10 | AFT | 30 | 23.3 | 1 | — | — | — | 0 | 1 | 243 | 39.8 % (12m) |
| Tassinari et al, 2016 | CS | 5 | 242 | AFT | — | — | — | 26.4 | — | — | 37 | — | 136 | — |
| Ueberreiter et al, 2010 | CH | 2b | 52 | AFT | — | — | 2.9 | 6–30 | — | — | 0 | — | 184 | 76% (6m) |
| Ueberreiter et al, 2013 | CH | 2b | 56 | AFT | 22–58 | 17–30 | 3 | 6–56 | 56 | — | — | — | 260 | — |
| Veber et al, 2011 | CH | 2b | 31 | AFT | 38 | — | 1.3 | 16.2 | - | — | — | — | 200.8 | — |
| Visconti and Salgarello, 2019 | CC | 3b | 29 | AFT +BRAVA | 26.5 | 26.6 | 1.3 | 12 | 40 in46 | — | 0 | — | 215ml | — |
| Wang et al, 2011 | CS | 4 | 48 | AFT | 29.4 | — | — | 18–72 | — | — | 8 | 8 | 50–170 | — |
| Wang et al, 2012 | CH | 2b | 18 | AFT+SVF | 32 | 22.1 | — | 6 | — | — | 1 | — | 256.5 (n=10) | 51.2% (3m) 54.2% (6m) |
| Wang et al, 2015 | CH | 2b | 12 | AFT+SVF | 32 | 22.1 | — | 6 | 11 | 11 | 0 | — | 256 | 60.7% (3m) 45.5% (6m) |
| Yoshimura et al, 20088 | CS | 4 | 40 | AFT+ CAL | 35.8 | 19.1 | 1 | 42 | 40 | — | 1 | — | 272.7 | 55.9% (6m) |
| Yoshimura et al, 20109 | CS | 4 | 15 | AFT+ CAL | 37.1 | 19.5 | 1 | 18 | 15 | — | 0 | — | 263.5 | 20~60% (n=6) |
| Zheng et al, 2008 | CS | 4 | 66 | AFT | 19–39 | — | 1.8 | 37 | 53 | 52 | 13 | 2 | 174 | — |
| Zheng et al, 2019 | CC | 3b | 5 | AFT | 29.6 | 21.1 | 1.2 | 4.4 | — | — | 0 | — | 175ml | 59.13%(4.4m) |
| Zocchi and Zuliani, 20086 | CH | 2b | 181 | AFT +BRAVA | 33 | — | — | 12 | 176 | 171 | 12 | — | 375 | 55% (12m) |
| Zocchi, 20177 | CS | 4 | 388 99 | AFT +BRAVAAFT+SVF | 29.2 26.8 | — | — | 12 | — | — | 25 | — | 287 380 | 74% (12m) 86% (12m) |
| Study . | Design . | Level . | No. patients . | Treat group . | Age(y) . | BMI kg/m2 . | No. sessions . | Fellow-up (mo) . | Satisfaction No. Patients . | Satisfaction No. Surgeon . | No. Complications . | No. Biopsy . | Mean Volume injection (ml) . | Volume retention(%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | CH | 2b | 80 | AFT | 36 | 26 | 1 | 24 | 65 (n = 72) | — | 11 (n = 160) | 1 (n = 160) | 420 | 59.4% (12m) |
| Auclair, 2009 | CS | 4 | 1433 | AFT AFT+IMP | — | — | — | 16.7 | — | — | 0 | — | 260110 | — |
| Auclair et al, 201310 | CS | 4 | 197 | AFT+IMP | — | — | — | 5 | — | — | 9 | 2 | 369.9 (n = 20) | 57% (12m) |
| Atia, 2020 | CS | 4 | 30 | AFT | 31.23 | 26.9 | 1.2 | 12 | 27 | — | 12 | — | 259.83ml 252.17 ml | — |
| Auclair, 2020 | CS | 4 | 148 | AFT+IMP | 42 | 19.6 | — | 22 | — | — | 23 | — | 153ml | — |
| Bircoll, 2010 | CS | 4 | 650 | AFT | — | — | — | — | — | — | 8 | — | — | — |
| Brault et al, 201711 | CC | 3b | 1522 | AFT C:IMP | 21.1 | — | 2 | 17 | — | — | 2 | — | — | — |
| Bravo, 2014 | CC | 3b | 2138 | AFT+IMPC:IMP | — | — | — | 12 | — | — | 2 | — | 117 | — |
| Bresnick, 2016 | CS | 4 | 28 | AFT | — | — | 2.1 | — | 28 | — | 0 | - | 27 | — |
| Carvajal and Patino, 2008 | CS | 4 | 20 | AFT | 36.9 | — | — | 34.5 | — | — | 4 | 4 | 235 | — |
| Chiu, 2014 | CS | 4 | 205(HB) 77(LB) | AFT+SVF | 34.9 31.2 | 21.2 17.6 | — | 23.7 23.0 | 17665 | 17864 | 137 | — | 254241 | — |
| Chiu, 2016 | CS | 4 | 27 | AFT+SVF | 39.1 | 19.9 | — | 27.1 | — | — | 6 | — | 247 | — |
| Chiu, 201816 | CC | 3b | 105 101 | AFT AFT+SVF | 3337 | 18.8 20.3 | 11.2 | 15.8 13.4 | — | — | 46 | — | 310334 | 67.9% (12m) 68.7% (12m) |
| Claudio et al, 2017 | CS | 4 | 11 | AFT | 24 | 23.4 | 2 | 29.7 | — | — | 4 | 2 | 210 | — |
| Coleman and Saboeiro, 200713 | CH | 2b | 17 | AFT | 38.2 | — | 1.2 | 62.2 | — | — | 10 | 2 | 278.6 | — |
| Cotrufo et al, 2008 | CS | 4 | 42 | AFT | 48 | — | 1.3 | 7 | — | — | 1 | — | — | — |
| Del Vecchio and Bucky, 2011 | CH | 2b | 25 | AFT +BRAVA | 21–60 | — | — | 6 | — | — | 0 | — | 430 (n = 12) | 64% (6m) |
| Del Vecchio, 201419 | CS | 4 | 30 | AFT +BRAVA | — | — | — | 12 | — | — | — | — | 300 | 53% (12m) |
| Delay et al, 200918 | CS | 4 | 136 | AFT | — | — | 3–4 | 120 | — | — | 4 (n = 880) | — | — | 60~70% (3m) |
| Delay et al, 2013 | CS | 4 | 31 | AFT | 23 | 21.9 | 1.5 | 78 | 31 | 29 | 0 | — | 158 | — |
| 226 | ||||||||||||||
| Derder et al, 201420 | CS | 4 | 10 | AFT | 17.5 | — | 2 | 68 | 10 | — | — | — | 285 | — |
| Deschler et al, 202025 | CS | 4 | 42 | AFT | 34 | 22.9 | — | 25.6 | — | — | 3 | — | 312.2ml | — |
| Dos Anjos et al, 201517 | CC | 3b | 13 (LS) 44 (HS) | AFT+SVF | 37.8 39.4 | 21.6 21.6 | — | 18 | — | — | 3 | 3 | 229.1 270.7 | 50% (18m) 75% (18m) |
| Fiaschetti et al, 2013 | CH | 2b | 6 | AFT+PRP | 46.3 | — | 2 | — | — | — | — | — | 195.6 | 84.4% (6m) 72.1% (12m) |
| Guo et al, 201821 | CH | 2b | 11 | AFT | 27 | 20.2 | 1 | 3 | — | — | 0 | — | 207 | 56.6% (3m) |
| Gutierrez-Ontalvilla et al, 2020 | CS | 4 | 9 | AFT | 14.9 | — | 1.8 | 21.3 | — | — | 1 | — | 220ml | — |
| Herly et al, 2019 | CS | 4 | 14 | AFT | 34.9 | 24.2 | 1 | 4.5 | — | — | — | — | 304ml | 50.9% (4.5m) |
| Herold et al, 2010 | CH | 2b | 10 | AFT | — | — | — | — | — | — | — | — | 208 | 72.0% (6m) |
| Ho Quoc et al, 201312 | CS | 4 | 1000 | AFT | 39 | — | 1–3 | — | — | — | 40 | — | — | — |
| Ho Quoc et al, 2015 | CS | 4 | 10 | AFT | 21 | 21.5 | — | 72 | 10 | — | 0 | — | 380 | — |
| Illouz and Sterodimas, 2009 | CS | 4 | 439 | AFT | 45.6 | — | 3 | 12 | 399 | — | 53 | — | 145 | — |
| Jung et al, 2016 | CH | 2b | 5 | AFT+SVF | 34.4 | — | — | 12 | — | — | 5(n=10) | — | 221.2 | 65.1% (3m) 46.8% (12m) |
| Kamakura and Ito, 2011 | CH | 2b | 20 | AFT +ADRCS | 35.6 | — | — | 9 | 15 | 11(n=16) | 2 | 1 | 240 | — |
| Kang and Luan, 201814 | CH | 2b | 100 | AFT | 43.6 | 21.3 | 1.3 | 3 | — | — | 21 (n=167) | — | 176.1 | — |
| Kerfant et al, 2017 | CS | 4 | 156 | AFT+IMP | 31.7 | 18. 9 | 1.1 | 22.3 | — | — | 11 | — | 126 | — |
| Khouri et al, 2012 | CH | 2b | 81 | AFT +BRAVA | 17–63 | 19.8 | 1 | 44 | 81 | — | 1 | — | 282 (n=71) | 82.0% (12m) |
| Khouri et al, 2014 | CS | 4 | 4594 | AFT +BRAVAAFT | 27 45.2 | 21.6 | 1.2 1.4 | 9 | 4390 | — | —24 | — | 300354 | 79.0% (12m) 64.0% (12m) |
| Klit et al, 20155 | CS | 4 | 4 7 | AFT +BRAVAAFT | 18 | 20–25 | 1 | 13 | 9 | — | 0 | — | 245 147 | — |
| La Marca et al, 2013 | CS | 4 | 10 | AFT | 16 | — | 2.9 | 51 | 10 | — | 0 | — | 255 | — |
| Li et al, 2014 | CS | 4 | 105 | AFT | 31.3 | — | 1.3 | 18 | 105 | 88 | 5 | 3 | 205 | — |
| Maione et al, 2018 | CH | 2b | 31 | AFT+IMP | 34.3 | — | 1 | 3–12 | — | — | 0 | — | 134 | — |
| Matsudo and Toledo, 1988 | CS | 4 | 21 | AFT | — | — | — | 18 | — | — | — | 5–450ml | 20–50% | |
| Muench, 2016 | CS | 4 | 254 | AFT | 35.8 | 22.5 | 1.2 | 24.5 | 246 | — | 11 | — | 207 | — |
| Münch, 2013 | CS | 4 | 84 | AFT | 36.7 | 22.7 | 1.1 | 4.7 | 83 | — | 9 | — | 177 | — |
| Ohashi et al, 2016 | CS | 4 | 131 | AFT | 39.3 | 19.9 | — | 6 | 126 | — | 12 | — | 239.6 | — |
| Özalp and Aydinol, 2017 | CS | 4 | 34 | AFT+IMP | 31 | — | — | 22 | 30 | — | 6(n=68) | — | 114 | — |
| Peltoniemi et al, 201315 | CH | 2b | 108 | AFT+ ASCSAFT | 5139 | 23.423.4 | 1.81.8 | 6 | — | — | 21 | — | 178.5 204 | 74.2% (6m) 78.8% (6m) |
| Pinsolle et al, 2008 | CS | 4 | 71 | AFT1+IMP AFT | 25 | — | 2.1 | — | — | — | 1 | — | 96 | — |
| Quoc et al, 2013 | CS | 4 | 19 | AFT | 28 | 20.3 | 1.6 | — | 18 | 18 | 0 | — | 375 | — |
| Rubin et al, 2012 | CH | 2b | 27 23 | AFTC:reduction | 35.9 | — | — | 12 | — | — | — | — | 526.5 | — |
| Serra-Mestr et al, 2017 | CH | 2b | 49 | AFT | 41 | — | — | 12 | 48 | — | 3 | 1 | 42 | — |
| Sforza et al, 201622 | CS | 4 | 26 | AFT | 24 | — | — | — | 25 | 23 | 0 | — | 148 | 72.5% (12m) |
| Spear and Pittman, 201423 | CH | 2b | 10 | AFT | 30 | 23.3 | 1 | — | — | — | 0 | 1 | 243 | 39.8 % (12m) |
| Tassinari et al, 2016 | CS | 5 | 242 | AFT | — | — | — | 26.4 | — | — | 37 | — | 136 | — |
| Ueberreiter et al, 2010 | CH | 2b | 52 | AFT | — | — | 2.9 | 6–30 | — | — | 0 | — | 184 | 76% (6m) |
| Ueberreiter et al, 2013 | CH | 2b | 56 | AFT | 22–58 | 17–30 | 3 | 6–56 | 56 | — | — | — | 260 | — |
| Veber et al, 2011 | CH | 2b | 31 | AFT | 38 | — | 1.3 | 16.2 | - | — | — | — | 200.8 | — |
| Visconti and Salgarello, 2019 | CC | 3b | 29 | AFT +BRAVA | 26.5 | 26.6 | 1.3 | 12 | 40 in46 | — | 0 | — | 215ml | — |
| Wang et al, 2011 | CS | 4 | 48 | AFT | 29.4 | — | — | 18–72 | — | — | 8 | 8 | 50–170 | — |
| Wang et al, 2012 | CH | 2b | 18 | AFT+SVF | 32 | 22.1 | — | 6 | — | — | 1 | — | 256.5 (n=10) | 51.2% (3m) 54.2% (6m) |
| Wang et al, 2015 | CH | 2b | 12 | AFT+SVF | 32 | 22.1 | — | 6 | 11 | 11 | 0 | — | 256 | 60.7% (3m) 45.5% (6m) |
| Yoshimura et al, 20088 | CS | 4 | 40 | AFT+ CAL | 35.8 | 19.1 | 1 | 42 | 40 | — | 1 | — | 272.7 | 55.9% (6m) |
| Yoshimura et al, 20109 | CS | 4 | 15 | AFT+ CAL | 37.1 | 19.5 | 1 | 18 | 15 | — | 0 | — | 263.5 | 20~60% (n=6) |
| Zheng et al, 2008 | CS | 4 | 66 | AFT | 19–39 | — | 1.8 | 37 | 53 | 52 | 13 | 2 | 174 | — |
| Zheng et al, 2019 | CC | 3b | 5 | AFT | 29.6 | 21.1 | 1.2 | 4.4 | — | — | 0 | — | 175ml | 59.13%(4.4m) |
| Zocchi and Zuliani, 20086 | CH | 2b | 181 | AFT +BRAVA | 33 | — | — | 12 | 176 | 171 | 12 | — | 375 | 55% (12m) |
| Zocchi, 20177 | CS | 4 | 388 99 | AFT +BRAVAAFT+SVF | 29.2 26.8 | — | — | 12 | — | — | 25 | — | 287 380 | 74% (12m) 86% (12m) |
—, not reported; ADRCS, autologous adipose-derived regenerative cells; AFT, autologous fat transplantation; ASCs, adipose-derived stem cells; C, control group; CAL, cell-assisted lipotransfer; CC, case-control study; CH, cohort study; CS, case series study; FU, fellow-up; HB, high BMI (BMI > 18.5); HS, high stromal vascular fraction; IMP, implant; LB; low BMI (BMI ≤ 18.5); LS, low stromal vascular fraction; m, month; PRP, platelet-rich plasma; SUPP, supplements as SVF, CAL, PRP, or ASCs; SVF, stromal vascular fraction; VOL, volume; n, number of related cases.
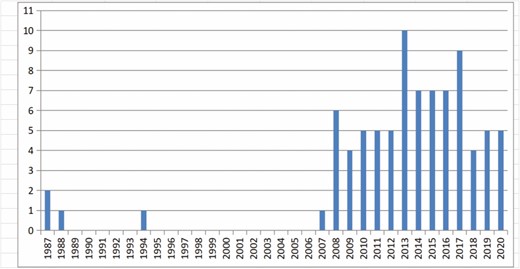
Publications concerning autologous fat transplantation in native healthy breasts by year.
Perioperative Management
Details of the studies are listed in Table 4.
| Study . | Anesthesia . | Donor site . | Fat harvesting . | Processing . | Injection . | Injection site . | Average time of the surgery (minutes) . | postoperative care . |
|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | general; tumescent | flanks; thighs; Lower; abdomen | mm multiple- hole cannula; lipomatic power-assisted machine | centrifugation 3000 rpm 0.7 atm | 3-mm customized v-shaped multi-hole cannula | Subcutaneous; parenchymal pericapsular muscular; submuscular spaces | 65 (45 ~ 90) | — |
| Auclair, 2009 | — | — | — | — | 15cm 1.5mm cannula | — | — | — |
| Auclair et al, 201310 | — | thighs | 3-mm cannula; 0.5 atm machine; “in-line” collection canister | 10-ml syringe centrifugation 3000rpm 2 min | 15cm 1.5mm cannula | subcutaneous | — | — |
| Atia, 2020 | general anesthesia; local anesthesia | abdomen; flanks; back; inner thigh; arms | 3-mm cannula 50-ml Luer lock syringe | Centrifugation 3000 rpm 3 min | 20G blunt cannula; 20-ml Luer lock syringe | multiple layers; multiple directions | — | pressure garment for 4 weeks; pain killers |
| Brault et al, 201711 | general; tumescent: saline solution, epinephrine (1 mg/L) | abdomen; trochanter-ic; inner thighs; inner knees | 3-mm cannula; vacuum pump 0.5 atm | centrifugation 1000rpm 1 min | 10-mL Luer-lock syringe; 2-mm transfer cannula | several layers | — | — |
| Bravo, 2014 | — | thighs; lower posterior trunk; abdomen | 10-ml Luer-Lock syringe; 3-mm blunt “bucket-handle” tip cannula | centrifugation 1200rpm 3 min | 1-ml syringes; 17-gauge sharp needle | subcutaneous; pectoralis fascia | — | — |
| Bresnick, 2016 | — | abdomen; outer thigh | — | drained; washed with saline | 1.5-mm blunt injection cannula; 20-gauge needle | subdermal; superficial breast tissue | — | — |
| Carvajal and Patino, 2008 | — | abdomen; back; thighs; arms | — | — | — | — | — | — |
| Chiu, 2014 | intravenous sedation; tumescent: 150~300mL (1000mL lactated Ringer’s solution, 80mL 2%lidocaine, 2 mL 1:1000 epinephrine) 10 min | abdomen; flanks; hips; thighs; calves | 3or4-mm cannula ; low-pressure suction machine –600 mm Hg | First: fat (100 mL) was mixed with 1% typel collagenase centrifuged at 800g for 5 min Second: centrifugation 800 g for 4 min | fanning pattern | subcutaneous; intramuscular; retromuscular; premuscular layers | — | — |
| Chiu, 2016 | tumescent: 150~300mL (1000mL lactated Ringer’s solution, 30mL 2% lidocaine, 1 mL 1:1000 epinephrine) 10~15 min | thighs; hips; flanks; abdomen; calves | 3or4-mm cannula; low pressure suction machine set at -400 to —-500 mm Hg | First: fat (100 mL) mixed with 1% typel collagenase centrifuged at 800g for 5min Second: centrifugation 800 g for 4 min | fanning pattern; 14-gauge 15-cm single-hole cannula | subcutaneous; subglandular; Supramuscular; intramuscular layers | — | — |
| Chiu, 201816 | tumescent: 150~300mL (1000mL lactated Ringer’s solution, 30 mL 2% lidocaine, 1 mL 1:1000 epinephrine ) 10~15 min | abdomen; flanks; hips; thighs; calves | 3or4-mm cannula; low-pressure suction machine -600 mm Hg | First: fat (100 mL) mixed with 0.075% type I collagenase centrifuged at 800g for 5min Second: centrifugation 800g for 4 min | Coleman Solid Injection Technique; 14g 15-cm single-hole cannula; 10-mlBD syringes | — | — | — |
| Claudio et al, 2017 | — | abdomen ; flanks | 3-mm cannula; 40kPa vacuum pump; 400-ml modified drainage bottle | centrifugation 2000rpm (400 G) 2 min | 10-ml syringes; 1.9-mm 9-cm blunt cannulas | — | — | — |
| Coleman and Saboeiro, 200713 | general; epidural plus sedation;local infiltration; intercostal nerve blocks | — | 10-ml syringe; 2-hole Coleman harvesting cannula | centrifugation and refinement | 3-ml syringes; 0.2ml each place | cutaneous muscle; retropectoral; prepectoral spaces | — | — |
| Cotrufo et al, 2008 | - | — | Coleman technique | — | — | — | — | — |
| Del Vecchio and Bucky, 2011 | 5L 30ml of 1% lidocaine with epinephrine, 1:100,000/L of normal saline | — | — | centrifugation low g forces (20 ~40 g) | — | — | 120 | — |
| Delay et al, 200918 | general tumescent | abdomen; inner thighs | 10-mL Luer-Lok syringe; 4-mm 15G blade cannula | Centrifugation 3200rpm 3min | 10-mL syringes; 17-gauge; small quantities; injection of 140% rule | — | — | infiltration of diluted ropivacaine a circular motion in local area; an abdominal support belt for six weeks 10 sessions |
| Delay et al, 2013 | general; tumescent: 1 mg adrenaline in 500mL physiological saline | — | 10-mL Luer-Lok syringe; 3.5-mm multihole cannula | centrifugation 500g (3000 rpm) for 3 min or 20sec | 2-mm single-hole cannula | deepest area of missing breast volume; edge of zone | — | — |
| Derder et al, 201420 | — | Abdomen; trochanter; inner thighs; inner knees; lumbar region | 3-mm cannulas; vacuum pump -0.5 atm | Centrifugation 3000 rpm for 3 min | 10-mL Luer-Lock syringes; 2-mm transfer cannulas | From deep to superficial plane | — | — |
| Deschler et al, 202025 | general anesthesia; tumescent: 1 mg epinephrine and 1 L Ringer’s lactate | supra- and infraumbilical abdomen; flanks/hips; medial and lateral thigh; medial knee | 3-to 6-mm diameter, 25 cm in length, blunt cannula;vacuum pressure of 500 mmHg | washed with Ringer’s lactate solution, Centrifugation 3000 rpm for 3 m`in | 3-mm diameter 18-cm long blunt cannula | subglandular; periglandular; subcutaneous | 113.2 | antithrombotic medication for a duration of 15 days, antibiotics,pain killers |
| Dos Anjos et al, 201517 | general; tumescent: Klein (include Ringer’s Lactate, no lidocaine) | infraumbilical area; flank | microaire cannula (PAL-404LS) ; vacuum pump 53.3 kPa (0.52 atm) | every 50 ml processed fat graft, 1-2ml resuspended SVF cells | 20ml Luer-Lock syringes ; 20-mm-long 2.1-mm-diameter Super Luer-Lock cannulas | — | — | — |
| Fiaschetti et al, 2013 | general; tumescent | — | specific canula Coleman Kit after infiltration | centrifugation 3000rpm 4min, combined with platelet-enriched plasma | microcanula ; Coleman Kit ; small pulses (0.2-1ml) | — | — | — |
| Guo et al, 201821 | tumescent: 400 mg lidocaine and 1 mg adrenaline per 1000 mL saline | abdomen; waist; thighs | 3.0mm sharp cannula; machine 500-600 mm Hg | Sedimentation 15 minutes | 1-mL syringes; 2-mm 150-250 mm in length blunt canula | subcutaneous; pectoralis muscle; retromammary | — | — |
| Gutierrez-Ontalvilla et al, 2020 | general anesthesia; Tumescent:1000 cc saline solution with 1 cc epinephrine | abdomen; trochanteric zones; inner thighs | 3-mm cannulas with a vacuum pressure of 300 mmHg | centrifuged at 3000 rpm for 3 min | 9-cm-long 1.6-mm-diameter Coleman-style 2 concave or straight blunt cannulas | pectoralis major muscle; subglandular; periglandular; subcutaneous | — | dressings consisting of paper tape were applied over and around the breast mound |
| Herold et al, 2010 | — | — | WAL:BEAULI | — | — | — | — | — |
| Ho Quoc et al, 201312 | tumescent: 1 mg adrenaline in 500 mL 0.9% saline | — | 10-mL Luer-Lok syringe with multiperforated cannula | centrifugation 3000 rpm 3 min between 2006 and 2009 and for 20 seconds after 2009 | disposable 2-mm monoperforated cannula | From deep zone toward surface; ribs; pectoralis major muscle; subcutaneous | — | — |
| Illouz and Sterodimas, 2009 | tumescent: normal saline 1:500,000 of adrenaline 15 min | abdomen; hips; flanks; knees | 60ml syringe; 4-mm canula | decantation | 10ml syringe; 2.5-mm canula | subcutaneous; intraglandular | — | — |
| Jung et al, 2016 | general; tumescent: 400mL(2% lidocaine50mL, 1:100,000 epinephrine 1ml in Ringer’s lactate solution) 10~15 min | posterior thighs ; flanks | Harvest-Jet ; 3-mm hole blunt cannula | sedimentation Svf: centrifugation and separation | 10-mL syringe ; screw-type injector (0.28mL each rotation) | subcutaneous; retromammary; pectoralis major | 240 | bandages and brassieres were not utilized |
| Kamakura and Ito, 2011 | — | abdomen; hip | 3-mm canula | fat tissue divided in to 2 equal parts:first used to isolate- stem cells(Celution800 System +proteolytic enzyme reagent),then added to second part and processed with Celution 800 System | seringue cellbrush and multilayer injection | Retro- and intra- muscular; subcutaneous; retroglandular | analgesics; limitation of activity for 3–7 days; cold compresses | |
| Kang and Luan, 201814 | tumescent: 1000 ml (normal saline 1 ml 1/200,000 epinephrine 600 mg lidocaine) | — | WAL; 3.8-mm cannula - 0.5 bar negative pressure sedimentation 15 min | sedimentation 15 min Or Centrifugation 800 r/min for 3 min | 2-mm fat injection needles; "3Ms" techniques, 1ml/each channel | subcutaneous; retromammar; pectoralis major; posterior of pectoralis major | — | shapewear for their recipient areas for 1 month |
| Kerfant et al, 2017 | tumescent: 400 ml saline, 40 ml lidocaine with 1%adrenaline 0.4 mg and 7.5 mg ropivacaine | buttocks; posterior thighs; abdomen; anteromedial thighs | 3-mm liposuction cannula ;low pressure,a sterile suction-assisted device | 10-ml syringes; centrifugation 3000 rpm for 1 min | 16- or18-gauge 15-cm-long cannula | subcutaneous in cleavage; submammary areas | 90 | — |
| Khouri et al, 2012 | — | — | 2.7-mm canula aspiration syringe; 300-mmHg closed system | centrifugation 15g 3min | 2.4-mm canula | Retro- and intra- muscular; subcutaneous; retroglandular | — | — |
| Khouri et al, 2014 | — | — | 2.7-mm canula aspiration syringe; 300-mmHg closed system | centrifugation 15g 3 min | 2.4-mm canula | — | — | — |
| Klit et al, 20155 | — | lower abdomen | Coleman 2-mm cannulas -0.5atm vacuum or BEAULI 3.8-mm cannula -0.5 bar WAL equipment (Body-jet) | centrifugation 3000 rpm for 3 min | 10-ml LuerLock syringes; 2-mm transfer cannulas | — | — | — |
| Kwiatkowska et al, 2019 | analog-sedation;local anesthesia; tumescent:1L 0.9%NaCl, 500mg lidocaine 1 mg epinephrine, 12.5ml sodium bicarbonate 8.4% solution | — | Berlin Autologous Lipotransfer, negative pressure of –0.5 bar Bodyjet level 1 | separation of fat from liquid with Lipo Col-lector | 10 cm length and simultaneous fat injection | — | — | — |
| La Marca et al, 2013 | — | abdomen; gluteal; thighs | 3mm canula syringe with minimal depression | Centrifugation 3000 rpm 4min | 17G Canule Coleman | Multilayer | — | — |
| Li et al, 2014 | general; tumescent: 20-mL 2% lidocaine with epinephrine, 1:100,000/L normal saline | abdomen; flanks ; trochanteric region; inner thigh; medial aspect of knees upper arms | 20-mL syringe; 3-mm 3-hole blunt cannula | Wash filtration | 14-gauge1-hole blunt cannula | superficial subcutaneous plane | — | — |
| Maione et al, 2018 | infiltrate only physiologic solution with epinephrine without local anesthetic | abdomen; hip | 2~3-mm 15~23- cm long blunt cannulas; 10-ml Luer-lock syringe | centrifugation at 3000 rpm for 5 min | 18-gauge angiographic needle with a snap-on wing | subcutaneous | 67 | — |
| Matsudo and Toledo, 1988 | — | inner of knee; dorsal regions; "jodhpur thigh" area; abdomen | — | — | — | — | — | — |
| Muench, 2016 | sedation with 7.5 mg Midazolam; gasmixture of 50% nitrous oxide and 50% oxygen; local anesthesia | abdomen; hip; groin region; thigh | 25-cm 3.8-mm double-lumen cannula | filtration: pore diameter 250 μm | 2-mm cannula; 12-gauge 150mm | fan-shaped manner;all layers “three-dimensional filling” | 113 | — |
| Münch, 2013 | sedation: 7.5 mg Midazolam; local anesthesia | abdomen; hip; groin region; thigh | WAL; 25 cm 3.8 mm double-lumen cannula | filtration: pore diameter 250 μm | 2-mm cannula; 12-gauge 150mm | fan-shaped manner;all layers “three-dimensional filling” | 135 | — |
| Ohashi et al, 2016 | tumescent: (1 ml epinephrine, 20 ml 8.4% sodium hydrogen carbonate, and 50 ml 1.0% lidocaine/1000 ml saline solution) | — | 3.0~4.6-mm 20-cm third- generation ultrasound device, VASER Lipo System low power(50 kPa) | centrifugation 1200g to 2000g for 3 min | 14-gauge Coleman cannulas (internal diameter 1.69mm) | — | — | — |
| Özalp and Aydinol, 2017 | Lactated Ringer’s solution with 1:200,000 epinephrine and 0.5% lidocaine | — | 3-mm blunt multihole aspiration cannula;20-ml Luer-Lok syringe | pure graft device washed with ringer lactate at 36°C | 5-ml and 10-ml syringes; closed system | multiplane superficial dermis; subcutaneous tissue;breast | 140 | — |
| Peltoniemi et al, 201315 | general; tumescent: 1 ml epinephrine 1:1000, 12.5 ml sodium bicarbonate 8ml and 500mg lidocaine each 1000ml 0.9% saline | — | WAL; 3.8-mm steel cannula ; 0.5 bar suction vacuum | Fat divided into 2 equal parts: first used to isolate vascular stromal fraction: Celution800/CRSsystem, second continuous rinse with tumescent solution at 37°C | 10-ml syringes; celbrush injector; 2-mm blunt cannula | pectoral muscles; retroglandular space; two-thirds subcutaneously | 120~180 | — |
| Pinsolle et al, 2008 | — | abdomen; trochanters | syringe | centrifugation | 1.5mm canula | — | — | — |
| Quoc et al, 2013 | — | — | 10 ml Luer-Lok syringes; 3.5-mm multiperforated cannulas | centrifugation at 3000 rpm for 3 min or 20 seconds | 14-gauge trocar 2-mm monoperforated cannula | from deep to superficial plane | — | — |
| Rubin et al, 2012 | — | — | 2.5-mm canula | Cell Assisted Lipotransfer ;Fat divided into 2 equal parts:first used to isolate vascular stromal fraction, second centrifugated ,then two parts mixed | reinjection in small aliquots | Intramuscular; subcutaneous | — | — |
| Serra-Mestr et al, 2017 | — | abdomen; inner thigh; knee | 2.4-mm microport harvester cannula with barbed; beveled 1-mm ports 10-ml Luer-Lok syringe | wash and decanted twice with lactated Ringer solution | 1-ml syringes; 1.5-mm blunt cannula | subcutaneous in crescent shape | — | — |
| Sforza et al, 201622 | general | abdomen | 60-mL Luer-Lock syringe; 2.4-mm cannula | the Pure graft system | 20-mL Luer-Lock syringe; 0.9-mm cannula | — | — | compression socks: a prophylactic pneumatic DVT system; soft dressings on the grafted areas |
| Spear and Pittman, 201423 | — | abdomen; hips | 3-mm canula; classic lipoaspiration 500 mmHg | Centrifugation 3000 rpm 3min | 10-ml syringe | Intramuscular; retroglandular; subcutaneous | 180 | — |
| Ueberreiter et al, 2010 | — | — | BEAULI:WAL | Separation of fat and water: Lipocollector | — | — | 90 | — |
| Ueberreiter et al, 2013 | tumescent: Klein 37 to 38°C (98–100°F) 10 min | abdomen; hip; thigh | WAL; 3.8mm cannula with effective suction openings of 0.9 mm–0.5 bar | Separation of fat and water: Lipocollector | 10 ml syringes | — | 70±15 | — |
| Veber et al, 2011 | — | — | Coleman | — | — | retro muscular; subcutaneous | — | — |
| Visconti and Salgarello, 2019 | normal saline and adrenaline 1:500,000 | flanks ; trochanter regions; inner thighs; medial aspect of knees | mercedes harvesting cannula (inner diameter, 1.8 mm) attached to vacuum canister | centrifuged at 1200g for 1 min | a Luer-Lok connector to 3-ml syringes | pectoralis major muscle; subglandular; Periglandular; subdermal | — | — |
| Wang et al, 2011 | tumescent | — | manual lipoaspiration 20-ml syringe | sedimentation | — | retroglandular | — | — |
| Wang et al, 2012 | — | abdomen; flanks; thigh | Lipokit machine | cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascular stromal fraction,second centrifugated,then two parts mixed. | 18-gauge trocar 1-mm monoperforated cannula | — | 180~240 | — |
| Wang et al, 2015 | — | thighs; flanks; lower abdomen | single combined machine Lipokit | isolate vascular stromal fraction centrifugation (800g for 5 min) 20-mL SVF-rich fat, centrifuged at 700g for 3 min | 16-gauge needle 20-mL | subcutaneous; under mammary glands; pectoralis muscles | — | — |
| Yoshimura et al, 20088 | general; tumescent: saline solution and diluted epinephrine (0.001%) | — | 2.5mm canula classic lipoaspiration | Cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascular stromal fraction, second centrifugated, then two parts mixed. | 17Gcanula 10-20ml syringe | periglandular; subcutaneous; intramuscular | injection time 35~60 | — |
| Yoshimura et al, 20109 | general tumescent: saline solution containing diluted epinephrine (0.0001%) | — | 2.5-mm canula conventional liposuction machine classic lipoaspiration | Cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascularstromal fraction, second centrifugated, then two parts mixed. | 16- or 18-gauge sharp needle (150-mm long), 10-20ml syringe | Plane formed between skin and periprosthetic capsula | — | — |
| Zheng et al, 2008 | — | abdomen; hips; trochanters | 2-holed blunt 3-mm canula; vacuum pump low negative pressure(0.5atm) | Saline solution cleaning; centrifugation 600rpm 2min | 5-ml syringe; 3-mm canula one-holed (3mm in diameter of the nozzle) blunt | subcutaneous; retroglandular | — | a surgical bra in the first 7 days postoperatively |
| Zheng et al, 2019 | general; anesthesia; Tumescent: 1 mg/L epinephrine in saline | thighs; abdomen | 4-mm suction cannula; constant negative pressure of 55 kPa | sedimented for 30 minutes | 2-mL syringe via 10-cm-long 2-mm-diameter cannula | subcutaneous; subglandular; intramuscular | — | — |
| Zocchi and Zuliani, 20086 | general; room-temperature saline using 2mg adrenaline per liter | trochanter; glutea | 2-mm canula 60- cc syringe | saline solution washing, stratification by vibration then decanting | 60-ccsyringe; 2-mm canula | subcutaneous; retroglandular | — | an elastic roll: maintain space between the breasts; a sports shaped-cups bra |
| Zocchi, 20177 | — | trochanteric; gluteal regions | FPU;2-mm single-hole; Tefloncoated special cannula; vacuum control | centrifugation 4500 rpm for 9 min | 2-mm cannulas; straight flexible 27-cm-long cannula; stiffer 25-cm-long curved one | — | — | — |
| Study . | Anesthesia . | Donor site . | Fat harvesting . | Processing . | Injection . | Injection site . | Average time of the surgery (minutes) . | postoperative care . |
|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | general; tumescent | flanks; thighs; Lower; abdomen | mm multiple- hole cannula; lipomatic power-assisted machine | centrifugation 3000 rpm 0.7 atm | 3-mm customized v-shaped multi-hole cannula | Subcutaneous; parenchymal pericapsular muscular; submuscular spaces | 65 (45 ~ 90) | — |
| Auclair, 2009 | — | — | — | — | 15cm 1.5mm cannula | — | — | — |
| Auclair et al, 201310 | — | thighs | 3-mm cannula; 0.5 atm machine; “in-line” collection canister | 10-ml syringe centrifugation 3000rpm 2 min | 15cm 1.5mm cannula | subcutaneous | — | — |
| Atia, 2020 | general anesthesia; local anesthesia | abdomen; flanks; back; inner thigh; arms | 3-mm cannula 50-ml Luer lock syringe | Centrifugation 3000 rpm 3 min | 20G blunt cannula; 20-ml Luer lock syringe | multiple layers; multiple directions | — | pressure garment for 4 weeks; pain killers |
| Brault et al, 201711 | general; tumescent: saline solution, epinephrine (1 mg/L) | abdomen; trochanter-ic; inner thighs; inner knees | 3-mm cannula; vacuum pump 0.5 atm | centrifugation 1000rpm 1 min | 10-mL Luer-lock syringe; 2-mm transfer cannula | several layers | — | — |
| Bravo, 2014 | — | thighs; lower posterior trunk; abdomen | 10-ml Luer-Lock syringe; 3-mm blunt “bucket-handle” tip cannula | centrifugation 1200rpm 3 min | 1-ml syringes; 17-gauge sharp needle | subcutaneous; pectoralis fascia | — | — |
| Bresnick, 2016 | — | abdomen; outer thigh | — | drained; washed with saline | 1.5-mm blunt injection cannula; 20-gauge needle | subdermal; superficial breast tissue | — | — |
| Carvajal and Patino, 2008 | — | abdomen; back; thighs; arms | — | — | — | — | — | — |
| Chiu, 2014 | intravenous sedation; tumescent: 150~300mL (1000mL lactated Ringer’s solution, 80mL 2%lidocaine, 2 mL 1:1000 epinephrine) 10 min | abdomen; flanks; hips; thighs; calves | 3or4-mm cannula ; low-pressure suction machine –600 mm Hg | First: fat (100 mL) was mixed with 1% typel collagenase centrifuged at 800g for 5 min Second: centrifugation 800 g for 4 min | fanning pattern | subcutaneous; intramuscular; retromuscular; premuscular layers | — | — |
| Chiu, 2016 | tumescent: 150~300mL (1000mL lactated Ringer’s solution, 30mL 2% lidocaine, 1 mL 1:1000 epinephrine) 10~15 min | thighs; hips; flanks; abdomen; calves | 3or4-mm cannula; low pressure suction machine set at -400 to —-500 mm Hg | First: fat (100 mL) mixed with 1% typel collagenase centrifuged at 800g for 5min Second: centrifugation 800 g for 4 min | fanning pattern; 14-gauge 15-cm single-hole cannula | subcutaneous; subglandular; Supramuscular; intramuscular layers | — | — |
| Chiu, 201816 | tumescent: 150~300mL (1000mL lactated Ringer’s solution, 30 mL 2% lidocaine, 1 mL 1:1000 epinephrine ) 10~15 min | abdomen; flanks; hips; thighs; calves | 3or4-mm cannula; low-pressure suction machine -600 mm Hg | First: fat (100 mL) mixed with 0.075% type I collagenase centrifuged at 800g for 5min Second: centrifugation 800g for 4 min | Coleman Solid Injection Technique; 14g 15-cm single-hole cannula; 10-mlBD syringes | — | — | — |
| Claudio et al, 2017 | — | abdomen ; flanks | 3-mm cannula; 40kPa vacuum pump; 400-ml modified drainage bottle | centrifugation 2000rpm (400 G) 2 min | 10-ml syringes; 1.9-mm 9-cm blunt cannulas | — | — | — |
| Coleman and Saboeiro, 200713 | general; epidural plus sedation;local infiltration; intercostal nerve blocks | — | 10-ml syringe; 2-hole Coleman harvesting cannula | centrifugation and refinement | 3-ml syringes; 0.2ml each place | cutaneous muscle; retropectoral; prepectoral spaces | — | — |
| Cotrufo et al, 2008 | - | — | Coleman technique | — | — | — | — | — |
| Del Vecchio and Bucky, 2011 | 5L 30ml of 1% lidocaine with epinephrine, 1:100,000/L of normal saline | — | — | centrifugation low g forces (20 ~40 g) | — | — | 120 | — |
| Delay et al, 200918 | general tumescent | abdomen; inner thighs | 10-mL Luer-Lok syringe; 4-mm 15G blade cannula | Centrifugation 3200rpm 3min | 10-mL syringes; 17-gauge; small quantities; injection of 140% rule | — | — | infiltration of diluted ropivacaine a circular motion in local area; an abdominal support belt for six weeks 10 sessions |
| Delay et al, 2013 | general; tumescent: 1 mg adrenaline in 500mL physiological saline | — | 10-mL Luer-Lok syringe; 3.5-mm multihole cannula | centrifugation 500g (3000 rpm) for 3 min or 20sec | 2-mm single-hole cannula | deepest area of missing breast volume; edge of zone | — | — |
| Derder et al, 201420 | — | Abdomen; trochanter; inner thighs; inner knees; lumbar region | 3-mm cannulas; vacuum pump -0.5 atm | Centrifugation 3000 rpm for 3 min | 10-mL Luer-Lock syringes; 2-mm transfer cannulas | From deep to superficial plane | — | — |
| Deschler et al, 202025 | general anesthesia; tumescent: 1 mg epinephrine and 1 L Ringer’s lactate | supra- and infraumbilical abdomen; flanks/hips; medial and lateral thigh; medial knee | 3-to 6-mm diameter, 25 cm in length, blunt cannula;vacuum pressure of 500 mmHg | washed with Ringer’s lactate solution, Centrifugation 3000 rpm for 3 m`in | 3-mm diameter 18-cm long blunt cannula | subglandular; periglandular; subcutaneous | 113.2 | antithrombotic medication for a duration of 15 days, antibiotics,pain killers |
| Dos Anjos et al, 201517 | general; tumescent: Klein (include Ringer’s Lactate, no lidocaine) | infraumbilical area; flank | microaire cannula (PAL-404LS) ; vacuum pump 53.3 kPa (0.52 atm) | every 50 ml processed fat graft, 1-2ml resuspended SVF cells | 20ml Luer-Lock syringes ; 20-mm-long 2.1-mm-diameter Super Luer-Lock cannulas | — | — | — |
| Fiaschetti et al, 2013 | general; tumescent | — | specific canula Coleman Kit after infiltration | centrifugation 3000rpm 4min, combined with platelet-enriched plasma | microcanula ; Coleman Kit ; small pulses (0.2-1ml) | — | — | — |
| Guo et al, 201821 | tumescent: 400 mg lidocaine and 1 mg adrenaline per 1000 mL saline | abdomen; waist; thighs | 3.0mm sharp cannula; machine 500-600 mm Hg | Sedimentation 15 minutes | 1-mL syringes; 2-mm 150-250 mm in length blunt canula | subcutaneous; pectoralis muscle; retromammary | — | — |
| Gutierrez-Ontalvilla et al, 2020 | general anesthesia; Tumescent:1000 cc saline solution with 1 cc epinephrine | abdomen; trochanteric zones; inner thighs | 3-mm cannulas with a vacuum pressure of 300 mmHg | centrifuged at 3000 rpm for 3 min | 9-cm-long 1.6-mm-diameter Coleman-style 2 concave or straight blunt cannulas | pectoralis major muscle; subglandular; periglandular; subcutaneous | — | dressings consisting of paper tape were applied over and around the breast mound |
| Herold et al, 2010 | — | — | WAL:BEAULI | — | — | — | — | — |
| Ho Quoc et al, 201312 | tumescent: 1 mg adrenaline in 500 mL 0.9% saline | — | 10-mL Luer-Lok syringe with multiperforated cannula | centrifugation 3000 rpm 3 min between 2006 and 2009 and for 20 seconds after 2009 | disposable 2-mm monoperforated cannula | From deep zone toward surface; ribs; pectoralis major muscle; subcutaneous | — | — |
| Illouz and Sterodimas, 2009 | tumescent: normal saline 1:500,000 of adrenaline 15 min | abdomen; hips; flanks; knees | 60ml syringe; 4-mm canula | decantation | 10ml syringe; 2.5-mm canula | subcutaneous; intraglandular | — | — |
| Jung et al, 2016 | general; tumescent: 400mL(2% lidocaine50mL, 1:100,000 epinephrine 1ml in Ringer’s lactate solution) 10~15 min | posterior thighs ; flanks | Harvest-Jet ; 3-mm hole blunt cannula | sedimentation Svf: centrifugation and separation | 10-mL syringe ; screw-type injector (0.28mL each rotation) | subcutaneous; retromammary; pectoralis major | 240 | bandages and brassieres were not utilized |
| Kamakura and Ito, 2011 | — | abdomen; hip | 3-mm canula | fat tissue divided in to 2 equal parts:first used to isolate- stem cells(Celution800 System +proteolytic enzyme reagent),then added to second part and processed with Celution 800 System | seringue cellbrush and multilayer injection | Retro- and intra- muscular; subcutaneous; retroglandular | analgesics; limitation of activity for 3–7 days; cold compresses | |
| Kang and Luan, 201814 | tumescent: 1000 ml (normal saline 1 ml 1/200,000 epinephrine 600 mg lidocaine) | — | WAL; 3.8-mm cannula - 0.5 bar negative pressure sedimentation 15 min | sedimentation 15 min Or Centrifugation 800 r/min for 3 min | 2-mm fat injection needles; "3Ms" techniques, 1ml/each channel | subcutaneous; retromammar; pectoralis major; posterior of pectoralis major | — | shapewear for their recipient areas for 1 month |
| Kerfant et al, 2017 | tumescent: 400 ml saline, 40 ml lidocaine with 1%adrenaline 0.4 mg and 7.5 mg ropivacaine | buttocks; posterior thighs; abdomen; anteromedial thighs | 3-mm liposuction cannula ;low pressure,a sterile suction-assisted device | 10-ml syringes; centrifugation 3000 rpm for 1 min | 16- or18-gauge 15-cm-long cannula | subcutaneous in cleavage; submammary areas | 90 | — |
| Khouri et al, 2012 | — | — | 2.7-mm canula aspiration syringe; 300-mmHg closed system | centrifugation 15g 3min | 2.4-mm canula | Retro- and intra- muscular; subcutaneous; retroglandular | — | — |
| Khouri et al, 2014 | — | — | 2.7-mm canula aspiration syringe; 300-mmHg closed system | centrifugation 15g 3 min | 2.4-mm canula | — | — | — |
| Klit et al, 20155 | — | lower abdomen | Coleman 2-mm cannulas -0.5atm vacuum or BEAULI 3.8-mm cannula -0.5 bar WAL equipment (Body-jet) | centrifugation 3000 rpm for 3 min | 10-ml LuerLock syringes; 2-mm transfer cannulas | — | — | — |
| Kwiatkowska et al, 2019 | analog-sedation;local anesthesia; tumescent:1L 0.9%NaCl, 500mg lidocaine 1 mg epinephrine, 12.5ml sodium bicarbonate 8.4% solution | — | Berlin Autologous Lipotransfer, negative pressure of –0.5 bar Bodyjet level 1 | separation of fat from liquid with Lipo Col-lector | 10 cm length and simultaneous fat injection | — | — | — |
| La Marca et al, 2013 | — | abdomen; gluteal; thighs | 3mm canula syringe with minimal depression | Centrifugation 3000 rpm 4min | 17G Canule Coleman | Multilayer | — | — |
| Li et al, 2014 | general; tumescent: 20-mL 2% lidocaine with epinephrine, 1:100,000/L normal saline | abdomen; flanks ; trochanteric region; inner thigh; medial aspect of knees upper arms | 20-mL syringe; 3-mm 3-hole blunt cannula | Wash filtration | 14-gauge1-hole blunt cannula | superficial subcutaneous plane | — | — |
| Maione et al, 2018 | infiltrate only physiologic solution with epinephrine without local anesthetic | abdomen; hip | 2~3-mm 15~23- cm long blunt cannulas; 10-ml Luer-lock syringe | centrifugation at 3000 rpm for 5 min | 18-gauge angiographic needle with a snap-on wing | subcutaneous | 67 | — |
| Matsudo and Toledo, 1988 | — | inner of knee; dorsal regions; "jodhpur thigh" area; abdomen | — | — | — | — | — | — |
| Muench, 2016 | sedation with 7.5 mg Midazolam; gasmixture of 50% nitrous oxide and 50% oxygen; local anesthesia | abdomen; hip; groin region; thigh | 25-cm 3.8-mm double-lumen cannula | filtration: pore diameter 250 μm | 2-mm cannula; 12-gauge 150mm | fan-shaped manner;all layers “three-dimensional filling” | 113 | — |
| Münch, 2013 | sedation: 7.5 mg Midazolam; local anesthesia | abdomen; hip; groin region; thigh | WAL; 25 cm 3.8 mm double-lumen cannula | filtration: pore diameter 250 μm | 2-mm cannula; 12-gauge 150mm | fan-shaped manner;all layers “three-dimensional filling” | 135 | — |
| Ohashi et al, 2016 | tumescent: (1 ml epinephrine, 20 ml 8.4% sodium hydrogen carbonate, and 50 ml 1.0% lidocaine/1000 ml saline solution) | — | 3.0~4.6-mm 20-cm third- generation ultrasound device, VASER Lipo System low power(50 kPa) | centrifugation 1200g to 2000g for 3 min | 14-gauge Coleman cannulas (internal diameter 1.69mm) | — | — | — |
| Özalp and Aydinol, 2017 | Lactated Ringer’s solution with 1:200,000 epinephrine and 0.5% lidocaine | — | 3-mm blunt multihole aspiration cannula;20-ml Luer-Lok syringe | pure graft device washed with ringer lactate at 36°C | 5-ml and 10-ml syringes; closed system | multiplane superficial dermis; subcutaneous tissue;breast | 140 | — |
| Peltoniemi et al, 201315 | general; tumescent: 1 ml epinephrine 1:1000, 12.5 ml sodium bicarbonate 8ml and 500mg lidocaine each 1000ml 0.9% saline | — | WAL; 3.8-mm steel cannula ; 0.5 bar suction vacuum | Fat divided into 2 equal parts: first used to isolate vascular stromal fraction: Celution800/CRSsystem, second continuous rinse with tumescent solution at 37°C | 10-ml syringes; celbrush injector; 2-mm blunt cannula | pectoral muscles; retroglandular space; two-thirds subcutaneously | 120~180 | — |
| Pinsolle et al, 2008 | — | abdomen; trochanters | syringe | centrifugation | 1.5mm canula | — | — | — |
| Quoc et al, 2013 | — | — | 10 ml Luer-Lok syringes; 3.5-mm multiperforated cannulas | centrifugation at 3000 rpm for 3 min or 20 seconds | 14-gauge trocar 2-mm monoperforated cannula | from deep to superficial plane | — | — |
| Rubin et al, 2012 | — | — | 2.5-mm canula | Cell Assisted Lipotransfer ;Fat divided into 2 equal parts:first used to isolate vascular stromal fraction, second centrifugated ,then two parts mixed | reinjection in small aliquots | Intramuscular; subcutaneous | — | — |
| Serra-Mestr et al, 2017 | — | abdomen; inner thigh; knee | 2.4-mm microport harvester cannula with barbed; beveled 1-mm ports 10-ml Luer-Lok syringe | wash and decanted twice with lactated Ringer solution | 1-ml syringes; 1.5-mm blunt cannula | subcutaneous in crescent shape | — | — |
| Sforza et al, 201622 | general | abdomen | 60-mL Luer-Lock syringe; 2.4-mm cannula | the Pure graft system | 20-mL Luer-Lock syringe; 0.9-mm cannula | — | — | compression socks: a prophylactic pneumatic DVT system; soft dressings on the grafted areas |
| Spear and Pittman, 201423 | — | abdomen; hips | 3-mm canula; classic lipoaspiration 500 mmHg | Centrifugation 3000 rpm 3min | 10-ml syringe | Intramuscular; retroglandular; subcutaneous | 180 | — |
| Ueberreiter et al, 2010 | — | — | BEAULI:WAL | Separation of fat and water: Lipocollector | — | — | 90 | — |
| Ueberreiter et al, 2013 | tumescent: Klein 37 to 38°C (98–100°F) 10 min | abdomen; hip; thigh | WAL; 3.8mm cannula with effective suction openings of 0.9 mm–0.5 bar | Separation of fat and water: Lipocollector | 10 ml syringes | — | 70±15 | — |
| Veber et al, 2011 | — | — | Coleman | — | — | retro muscular; subcutaneous | — | — |
| Visconti and Salgarello, 2019 | normal saline and adrenaline 1:500,000 | flanks ; trochanter regions; inner thighs; medial aspect of knees | mercedes harvesting cannula (inner diameter, 1.8 mm) attached to vacuum canister | centrifuged at 1200g for 1 min | a Luer-Lok connector to 3-ml syringes | pectoralis major muscle; subglandular; Periglandular; subdermal | — | — |
| Wang et al, 2011 | tumescent | — | manual lipoaspiration 20-ml syringe | sedimentation | — | retroglandular | — | — |
| Wang et al, 2012 | — | abdomen; flanks; thigh | Lipokit machine | cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascular stromal fraction,second centrifugated,then two parts mixed. | 18-gauge trocar 1-mm monoperforated cannula | — | 180~240 | — |
| Wang et al, 2015 | — | thighs; flanks; lower abdomen | single combined machine Lipokit | isolate vascular stromal fraction centrifugation (800g for 5 min) 20-mL SVF-rich fat, centrifuged at 700g for 3 min | 16-gauge needle 20-mL | subcutaneous; under mammary glands; pectoralis muscles | — | — |
| Yoshimura et al, 20088 | general; tumescent: saline solution and diluted epinephrine (0.001%) | — | 2.5mm canula classic lipoaspiration | Cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascular stromal fraction, second centrifugated, then two parts mixed. | 17Gcanula 10-20ml syringe | periglandular; subcutaneous; intramuscular | injection time 35~60 | — |
| Yoshimura et al, 20109 | general tumescent: saline solution containing diluted epinephrine (0.0001%) | — | 2.5-mm canula conventional liposuction machine classic lipoaspiration | Cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascularstromal fraction, second centrifugated, then two parts mixed. | 16- or 18-gauge sharp needle (150-mm long), 10-20ml syringe | Plane formed between skin and periprosthetic capsula | — | — |
| Zheng et al, 2008 | — | abdomen; hips; trochanters | 2-holed blunt 3-mm canula; vacuum pump low negative pressure(0.5atm) | Saline solution cleaning; centrifugation 600rpm 2min | 5-ml syringe; 3-mm canula one-holed (3mm in diameter of the nozzle) blunt | subcutaneous; retroglandular | — | a surgical bra in the first 7 days postoperatively |
| Zheng et al, 2019 | general; anesthesia; Tumescent: 1 mg/L epinephrine in saline | thighs; abdomen | 4-mm suction cannula; constant negative pressure of 55 kPa | sedimented for 30 minutes | 2-mL syringe via 10-cm-long 2-mm-diameter cannula | subcutaneous; subglandular; intramuscular | — | — |
| Zocchi and Zuliani, 20086 | general; room-temperature saline using 2mg adrenaline per liter | trochanter; glutea | 2-mm canula 60- cc syringe | saline solution washing, stratification by vibration then decanting | 60-ccsyringe; 2-mm canula | subcutaneous; retroglandular | — | an elastic roll: maintain space between the breasts; a sports shaped-cups bra |
| Zocchi, 20177 | — | trochanteric; gluteal regions | FPU;2-mm single-hole; Tefloncoated special cannula; vacuum control | centrifugation 4500 rpm for 9 min | 2-mm cannulas; straight flexible 27-cm-long cannula; stiffer 25-cm-long curved one | — | — | — |
—, not reported; DVT, deep vein thrombosis; FPU, fat processing unit; WAL, water-jet assisted liposuction.
| Study . | Anesthesia . | Donor site . | Fat harvesting . | Processing . | Injection . | Injection site . | Average time of the surgery (minutes) . | postoperative care . |
|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | general; tumescent | flanks; thighs; Lower; abdomen | mm multiple- hole cannula; lipomatic power-assisted machine | centrifugation 3000 rpm 0.7 atm | 3-mm customized v-shaped multi-hole cannula | Subcutaneous; parenchymal pericapsular muscular; submuscular spaces | 65 (45 ~ 90) | — |
| Auclair, 2009 | — | — | — | — | 15cm 1.5mm cannula | — | — | — |
| Auclair et al, 201310 | — | thighs | 3-mm cannula; 0.5 atm machine; “in-line” collection canister | 10-ml syringe centrifugation 3000rpm 2 min | 15cm 1.5mm cannula | subcutaneous | — | — |
| Atia, 2020 | general anesthesia; local anesthesia | abdomen; flanks; back; inner thigh; arms | 3-mm cannula 50-ml Luer lock syringe | Centrifugation 3000 rpm 3 min | 20G blunt cannula; 20-ml Luer lock syringe | multiple layers; multiple directions | — | pressure garment for 4 weeks; pain killers |
| Brault et al, 201711 | general; tumescent: saline solution, epinephrine (1 mg/L) | abdomen; trochanter-ic; inner thighs; inner knees | 3-mm cannula; vacuum pump 0.5 atm | centrifugation 1000rpm 1 min | 10-mL Luer-lock syringe; 2-mm transfer cannula | several layers | — | — |
| Bravo, 2014 | — | thighs; lower posterior trunk; abdomen | 10-ml Luer-Lock syringe; 3-mm blunt “bucket-handle” tip cannula | centrifugation 1200rpm 3 min | 1-ml syringes; 17-gauge sharp needle | subcutaneous; pectoralis fascia | — | — |
| Bresnick, 2016 | — | abdomen; outer thigh | — | drained; washed with saline | 1.5-mm blunt injection cannula; 20-gauge needle | subdermal; superficial breast tissue | — | — |
| Carvajal and Patino, 2008 | — | abdomen; back; thighs; arms | — | — | — | — | — | — |
| Chiu, 2014 | intravenous sedation; tumescent: 150~300mL (1000mL lactated Ringer’s solution, 80mL 2%lidocaine, 2 mL 1:1000 epinephrine) 10 min | abdomen; flanks; hips; thighs; calves | 3or4-mm cannula ; low-pressure suction machine –600 mm Hg | First: fat (100 mL) was mixed with 1% typel collagenase centrifuged at 800g for 5 min Second: centrifugation 800 g for 4 min | fanning pattern | subcutaneous; intramuscular; retromuscular; premuscular layers | — | — |
| Chiu, 2016 | tumescent: 150~300mL (1000mL lactated Ringer’s solution, 30mL 2% lidocaine, 1 mL 1:1000 epinephrine) 10~15 min | thighs; hips; flanks; abdomen; calves | 3or4-mm cannula; low pressure suction machine set at -400 to —-500 mm Hg | First: fat (100 mL) mixed with 1% typel collagenase centrifuged at 800g for 5min Second: centrifugation 800 g for 4 min | fanning pattern; 14-gauge 15-cm single-hole cannula | subcutaneous; subglandular; Supramuscular; intramuscular layers | — | — |
| Chiu, 201816 | tumescent: 150~300mL (1000mL lactated Ringer’s solution, 30 mL 2% lidocaine, 1 mL 1:1000 epinephrine ) 10~15 min | abdomen; flanks; hips; thighs; calves | 3or4-mm cannula; low-pressure suction machine -600 mm Hg | First: fat (100 mL) mixed with 0.075% type I collagenase centrifuged at 800g for 5min Second: centrifugation 800g for 4 min | Coleman Solid Injection Technique; 14g 15-cm single-hole cannula; 10-mlBD syringes | — | — | — |
| Claudio et al, 2017 | — | abdomen ; flanks | 3-mm cannula; 40kPa vacuum pump; 400-ml modified drainage bottle | centrifugation 2000rpm (400 G) 2 min | 10-ml syringes; 1.9-mm 9-cm blunt cannulas | — | — | — |
| Coleman and Saboeiro, 200713 | general; epidural plus sedation;local infiltration; intercostal nerve blocks | — | 10-ml syringe; 2-hole Coleman harvesting cannula | centrifugation and refinement | 3-ml syringes; 0.2ml each place | cutaneous muscle; retropectoral; prepectoral spaces | — | — |
| Cotrufo et al, 2008 | - | — | Coleman technique | — | — | — | — | — |
| Del Vecchio and Bucky, 2011 | 5L 30ml of 1% lidocaine with epinephrine, 1:100,000/L of normal saline | — | — | centrifugation low g forces (20 ~40 g) | — | — | 120 | — |
| Delay et al, 200918 | general tumescent | abdomen; inner thighs | 10-mL Luer-Lok syringe; 4-mm 15G blade cannula | Centrifugation 3200rpm 3min | 10-mL syringes; 17-gauge; small quantities; injection of 140% rule | — | — | infiltration of diluted ropivacaine a circular motion in local area; an abdominal support belt for six weeks 10 sessions |
| Delay et al, 2013 | general; tumescent: 1 mg adrenaline in 500mL physiological saline | — | 10-mL Luer-Lok syringe; 3.5-mm multihole cannula | centrifugation 500g (3000 rpm) for 3 min or 20sec | 2-mm single-hole cannula | deepest area of missing breast volume; edge of zone | — | — |
| Derder et al, 201420 | — | Abdomen; trochanter; inner thighs; inner knees; lumbar region | 3-mm cannulas; vacuum pump -0.5 atm | Centrifugation 3000 rpm for 3 min | 10-mL Luer-Lock syringes; 2-mm transfer cannulas | From deep to superficial plane | — | — |
| Deschler et al, 202025 | general anesthesia; tumescent: 1 mg epinephrine and 1 L Ringer’s lactate | supra- and infraumbilical abdomen; flanks/hips; medial and lateral thigh; medial knee | 3-to 6-mm diameter, 25 cm in length, blunt cannula;vacuum pressure of 500 mmHg | washed with Ringer’s lactate solution, Centrifugation 3000 rpm for 3 m`in | 3-mm diameter 18-cm long blunt cannula | subglandular; periglandular; subcutaneous | 113.2 | antithrombotic medication for a duration of 15 days, antibiotics,pain killers |
| Dos Anjos et al, 201517 | general; tumescent: Klein (include Ringer’s Lactate, no lidocaine) | infraumbilical area; flank | microaire cannula (PAL-404LS) ; vacuum pump 53.3 kPa (0.52 atm) | every 50 ml processed fat graft, 1-2ml resuspended SVF cells | 20ml Luer-Lock syringes ; 20-mm-long 2.1-mm-diameter Super Luer-Lock cannulas | — | — | — |
| Fiaschetti et al, 2013 | general; tumescent | — | specific canula Coleman Kit after infiltration | centrifugation 3000rpm 4min, combined with platelet-enriched plasma | microcanula ; Coleman Kit ; small pulses (0.2-1ml) | — | — | — |
| Guo et al, 201821 | tumescent: 400 mg lidocaine and 1 mg adrenaline per 1000 mL saline | abdomen; waist; thighs | 3.0mm sharp cannula; machine 500-600 mm Hg | Sedimentation 15 minutes | 1-mL syringes; 2-mm 150-250 mm in length blunt canula | subcutaneous; pectoralis muscle; retromammary | — | — |
| Gutierrez-Ontalvilla et al, 2020 | general anesthesia; Tumescent:1000 cc saline solution with 1 cc epinephrine | abdomen; trochanteric zones; inner thighs | 3-mm cannulas with a vacuum pressure of 300 mmHg | centrifuged at 3000 rpm for 3 min | 9-cm-long 1.6-mm-diameter Coleman-style 2 concave or straight blunt cannulas | pectoralis major muscle; subglandular; periglandular; subcutaneous | — | dressings consisting of paper tape were applied over and around the breast mound |
| Herold et al, 2010 | — | — | WAL:BEAULI | — | — | — | — | — |
| Ho Quoc et al, 201312 | tumescent: 1 mg adrenaline in 500 mL 0.9% saline | — | 10-mL Luer-Lok syringe with multiperforated cannula | centrifugation 3000 rpm 3 min between 2006 and 2009 and for 20 seconds after 2009 | disposable 2-mm monoperforated cannula | From deep zone toward surface; ribs; pectoralis major muscle; subcutaneous | — | — |
| Illouz and Sterodimas, 2009 | tumescent: normal saline 1:500,000 of adrenaline 15 min | abdomen; hips; flanks; knees | 60ml syringe; 4-mm canula | decantation | 10ml syringe; 2.5-mm canula | subcutaneous; intraglandular | — | — |
| Jung et al, 2016 | general; tumescent: 400mL(2% lidocaine50mL, 1:100,000 epinephrine 1ml in Ringer’s lactate solution) 10~15 min | posterior thighs ; flanks | Harvest-Jet ; 3-mm hole blunt cannula | sedimentation Svf: centrifugation and separation | 10-mL syringe ; screw-type injector (0.28mL each rotation) | subcutaneous; retromammary; pectoralis major | 240 | bandages and brassieres were not utilized |
| Kamakura and Ito, 2011 | — | abdomen; hip | 3-mm canula | fat tissue divided in to 2 equal parts:first used to isolate- stem cells(Celution800 System +proteolytic enzyme reagent),then added to second part and processed with Celution 800 System | seringue cellbrush and multilayer injection | Retro- and intra- muscular; subcutaneous; retroglandular | analgesics; limitation of activity for 3–7 days; cold compresses | |
| Kang and Luan, 201814 | tumescent: 1000 ml (normal saline 1 ml 1/200,000 epinephrine 600 mg lidocaine) | — | WAL; 3.8-mm cannula - 0.5 bar negative pressure sedimentation 15 min | sedimentation 15 min Or Centrifugation 800 r/min for 3 min | 2-mm fat injection needles; "3Ms" techniques, 1ml/each channel | subcutaneous; retromammar; pectoralis major; posterior of pectoralis major | — | shapewear for their recipient areas for 1 month |
| Kerfant et al, 2017 | tumescent: 400 ml saline, 40 ml lidocaine with 1%adrenaline 0.4 mg and 7.5 mg ropivacaine | buttocks; posterior thighs; abdomen; anteromedial thighs | 3-mm liposuction cannula ;low pressure,a sterile suction-assisted device | 10-ml syringes; centrifugation 3000 rpm for 1 min | 16- or18-gauge 15-cm-long cannula | subcutaneous in cleavage; submammary areas | 90 | — |
| Khouri et al, 2012 | — | — | 2.7-mm canula aspiration syringe; 300-mmHg closed system | centrifugation 15g 3min | 2.4-mm canula | Retro- and intra- muscular; subcutaneous; retroglandular | — | — |
| Khouri et al, 2014 | — | — | 2.7-mm canula aspiration syringe; 300-mmHg closed system | centrifugation 15g 3 min | 2.4-mm canula | — | — | — |
| Klit et al, 20155 | — | lower abdomen | Coleman 2-mm cannulas -0.5atm vacuum or BEAULI 3.8-mm cannula -0.5 bar WAL equipment (Body-jet) | centrifugation 3000 rpm for 3 min | 10-ml LuerLock syringes; 2-mm transfer cannulas | — | — | — |
| Kwiatkowska et al, 2019 | analog-sedation;local anesthesia; tumescent:1L 0.9%NaCl, 500mg lidocaine 1 mg epinephrine, 12.5ml sodium bicarbonate 8.4% solution | — | Berlin Autologous Lipotransfer, negative pressure of –0.5 bar Bodyjet level 1 | separation of fat from liquid with Lipo Col-lector | 10 cm length and simultaneous fat injection | — | — | — |
| La Marca et al, 2013 | — | abdomen; gluteal; thighs | 3mm canula syringe with minimal depression | Centrifugation 3000 rpm 4min | 17G Canule Coleman | Multilayer | — | — |
| Li et al, 2014 | general; tumescent: 20-mL 2% lidocaine with epinephrine, 1:100,000/L normal saline | abdomen; flanks ; trochanteric region; inner thigh; medial aspect of knees upper arms | 20-mL syringe; 3-mm 3-hole blunt cannula | Wash filtration | 14-gauge1-hole blunt cannula | superficial subcutaneous plane | — | — |
| Maione et al, 2018 | infiltrate only physiologic solution with epinephrine without local anesthetic | abdomen; hip | 2~3-mm 15~23- cm long blunt cannulas; 10-ml Luer-lock syringe | centrifugation at 3000 rpm for 5 min | 18-gauge angiographic needle with a snap-on wing | subcutaneous | 67 | — |
| Matsudo and Toledo, 1988 | — | inner of knee; dorsal regions; "jodhpur thigh" area; abdomen | — | — | — | — | — | — |
| Muench, 2016 | sedation with 7.5 mg Midazolam; gasmixture of 50% nitrous oxide and 50% oxygen; local anesthesia | abdomen; hip; groin region; thigh | 25-cm 3.8-mm double-lumen cannula | filtration: pore diameter 250 μm | 2-mm cannula; 12-gauge 150mm | fan-shaped manner;all layers “three-dimensional filling” | 113 | — |
| Münch, 2013 | sedation: 7.5 mg Midazolam; local anesthesia | abdomen; hip; groin region; thigh | WAL; 25 cm 3.8 mm double-lumen cannula | filtration: pore diameter 250 μm | 2-mm cannula; 12-gauge 150mm | fan-shaped manner;all layers “three-dimensional filling” | 135 | — |
| Ohashi et al, 2016 | tumescent: (1 ml epinephrine, 20 ml 8.4% sodium hydrogen carbonate, and 50 ml 1.0% lidocaine/1000 ml saline solution) | — | 3.0~4.6-mm 20-cm third- generation ultrasound device, VASER Lipo System low power(50 kPa) | centrifugation 1200g to 2000g for 3 min | 14-gauge Coleman cannulas (internal diameter 1.69mm) | — | — | — |
| Özalp and Aydinol, 2017 | Lactated Ringer’s solution with 1:200,000 epinephrine and 0.5% lidocaine | — | 3-mm blunt multihole aspiration cannula;20-ml Luer-Lok syringe | pure graft device washed with ringer lactate at 36°C | 5-ml and 10-ml syringes; closed system | multiplane superficial dermis; subcutaneous tissue;breast | 140 | — |
| Peltoniemi et al, 201315 | general; tumescent: 1 ml epinephrine 1:1000, 12.5 ml sodium bicarbonate 8ml and 500mg lidocaine each 1000ml 0.9% saline | — | WAL; 3.8-mm steel cannula ; 0.5 bar suction vacuum | Fat divided into 2 equal parts: first used to isolate vascular stromal fraction: Celution800/CRSsystem, second continuous rinse with tumescent solution at 37°C | 10-ml syringes; celbrush injector; 2-mm blunt cannula | pectoral muscles; retroglandular space; two-thirds subcutaneously | 120~180 | — |
| Pinsolle et al, 2008 | — | abdomen; trochanters | syringe | centrifugation | 1.5mm canula | — | — | — |
| Quoc et al, 2013 | — | — | 10 ml Luer-Lok syringes; 3.5-mm multiperforated cannulas | centrifugation at 3000 rpm for 3 min or 20 seconds | 14-gauge trocar 2-mm monoperforated cannula | from deep to superficial plane | — | — |
| Rubin et al, 2012 | — | — | 2.5-mm canula | Cell Assisted Lipotransfer ;Fat divided into 2 equal parts:first used to isolate vascular stromal fraction, second centrifugated ,then two parts mixed | reinjection in small aliquots | Intramuscular; subcutaneous | — | — |
| Serra-Mestr et al, 2017 | — | abdomen; inner thigh; knee | 2.4-mm microport harvester cannula with barbed; beveled 1-mm ports 10-ml Luer-Lok syringe | wash and decanted twice with lactated Ringer solution | 1-ml syringes; 1.5-mm blunt cannula | subcutaneous in crescent shape | — | — |
| Sforza et al, 201622 | general | abdomen | 60-mL Luer-Lock syringe; 2.4-mm cannula | the Pure graft system | 20-mL Luer-Lock syringe; 0.9-mm cannula | — | — | compression socks: a prophylactic pneumatic DVT system; soft dressings on the grafted areas |
| Spear and Pittman, 201423 | — | abdomen; hips | 3-mm canula; classic lipoaspiration 500 mmHg | Centrifugation 3000 rpm 3min | 10-ml syringe | Intramuscular; retroglandular; subcutaneous | 180 | — |
| Ueberreiter et al, 2010 | — | — | BEAULI:WAL | Separation of fat and water: Lipocollector | — | — | 90 | — |
| Ueberreiter et al, 2013 | tumescent: Klein 37 to 38°C (98–100°F) 10 min | abdomen; hip; thigh | WAL; 3.8mm cannula with effective suction openings of 0.9 mm–0.5 bar | Separation of fat and water: Lipocollector | 10 ml syringes | — | 70±15 | — |
| Veber et al, 2011 | — | — | Coleman | — | — | retro muscular; subcutaneous | — | — |
| Visconti and Salgarello, 2019 | normal saline and adrenaline 1:500,000 | flanks ; trochanter regions; inner thighs; medial aspect of knees | mercedes harvesting cannula (inner diameter, 1.8 mm) attached to vacuum canister | centrifuged at 1200g for 1 min | a Luer-Lok connector to 3-ml syringes | pectoralis major muscle; subglandular; Periglandular; subdermal | — | — |
| Wang et al, 2011 | tumescent | — | manual lipoaspiration 20-ml syringe | sedimentation | — | retroglandular | — | — |
| Wang et al, 2012 | — | abdomen; flanks; thigh | Lipokit machine | cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascular stromal fraction,second centrifugated,then two parts mixed. | 18-gauge trocar 1-mm monoperforated cannula | — | 180~240 | — |
| Wang et al, 2015 | — | thighs; flanks; lower abdomen | single combined machine Lipokit | isolate vascular stromal fraction centrifugation (800g for 5 min) 20-mL SVF-rich fat, centrifuged at 700g for 3 min | 16-gauge needle 20-mL | subcutaneous; under mammary glands; pectoralis muscles | — | — |
| Yoshimura et al, 20088 | general; tumescent: saline solution and diluted epinephrine (0.001%) | — | 2.5mm canula classic lipoaspiration | Cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascular stromal fraction, second centrifugated, then two parts mixed. | 17Gcanula 10-20ml syringe | periglandular; subcutaneous; intramuscular | injection time 35~60 | — |
| Yoshimura et al, 20109 | general tumescent: saline solution containing diluted epinephrine (0.0001%) | — | 2.5-mm canula conventional liposuction machine classic lipoaspiration | Cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascularstromal fraction, second centrifugated, then two parts mixed. | 16- or 18-gauge sharp needle (150-mm long), 10-20ml syringe | Plane formed between skin and periprosthetic capsula | — | — |
| Zheng et al, 2008 | — | abdomen; hips; trochanters | 2-holed blunt 3-mm canula; vacuum pump low negative pressure(0.5atm) | Saline solution cleaning; centrifugation 600rpm 2min | 5-ml syringe; 3-mm canula one-holed (3mm in diameter of the nozzle) blunt | subcutaneous; retroglandular | — | a surgical bra in the first 7 days postoperatively |
| Zheng et al, 2019 | general; anesthesia; Tumescent: 1 mg/L epinephrine in saline | thighs; abdomen | 4-mm suction cannula; constant negative pressure of 55 kPa | sedimented for 30 minutes | 2-mL syringe via 10-cm-long 2-mm-diameter cannula | subcutaneous; subglandular; intramuscular | — | — |
| Zocchi and Zuliani, 20086 | general; room-temperature saline using 2mg adrenaline per liter | trochanter; glutea | 2-mm canula 60- cc syringe | saline solution washing, stratification by vibration then decanting | 60-ccsyringe; 2-mm canula | subcutaneous; retroglandular | — | an elastic roll: maintain space between the breasts; a sports shaped-cups bra |
| Zocchi, 20177 | — | trochanteric; gluteal regions | FPU;2-mm single-hole; Tefloncoated special cannula; vacuum control | centrifugation 4500 rpm for 9 min | 2-mm cannulas; straight flexible 27-cm-long cannula; stiffer 25-cm-long curved one | — | — | — |
| Study . | Anesthesia . | Donor site . | Fat harvesting . | Processing . | Injection . | Injection site . | Average time of the surgery (minutes) . | postoperative care . |
|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | general; tumescent | flanks; thighs; Lower; abdomen | mm multiple- hole cannula; lipomatic power-assisted machine | centrifugation 3000 rpm 0.7 atm | 3-mm customized v-shaped multi-hole cannula | Subcutaneous; parenchymal pericapsular muscular; submuscular spaces | 65 (45 ~ 90) | — |
| Auclair, 2009 | — | — | — | — | 15cm 1.5mm cannula | — | — | — |
| Auclair et al, 201310 | — | thighs | 3-mm cannula; 0.5 atm machine; “in-line” collection canister | 10-ml syringe centrifugation 3000rpm 2 min | 15cm 1.5mm cannula | subcutaneous | — | — |
| Atia, 2020 | general anesthesia; local anesthesia | abdomen; flanks; back; inner thigh; arms | 3-mm cannula 50-ml Luer lock syringe | Centrifugation 3000 rpm 3 min | 20G blunt cannula; 20-ml Luer lock syringe | multiple layers; multiple directions | — | pressure garment for 4 weeks; pain killers |
| Brault et al, 201711 | general; tumescent: saline solution, epinephrine (1 mg/L) | abdomen; trochanter-ic; inner thighs; inner knees | 3-mm cannula; vacuum pump 0.5 atm | centrifugation 1000rpm 1 min | 10-mL Luer-lock syringe; 2-mm transfer cannula | several layers | — | — |
| Bravo, 2014 | — | thighs; lower posterior trunk; abdomen | 10-ml Luer-Lock syringe; 3-mm blunt “bucket-handle” tip cannula | centrifugation 1200rpm 3 min | 1-ml syringes; 17-gauge sharp needle | subcutaneous; pectoralis fascia | — | — |
| Bresnick, 2016 | — | abdomen; outer thigh | — | drained; washed with saline | 1.5-mm blunt injection cannula; 20-gauge needle | subdermal; superficial breast tissue | — | — |
| Carvajal and Patino, 2008 | — | abdomen; back; thighs; arms | — | — | — | — | — | — |
| Chiu, 2014 | intravenous sedation; tumescent: 150~300mL (1000mL lactated Ringer’s solution, 80mL 2%lidocaine, 2 mL 1:1000 epinephrine) 10 min | abdomen; flanks; hips; thighs; calves | 3or4-mm cannula ; low-pressure suction machine –600 mm Hg | First: fat (100 mL) was mixed with 1% typel collagenase centrifuged at 800g for 5 min Second: centrifugation 800 g for 4 min | fanning pattern | subcutaneous; intramuscular; retromuscular; premuscular layers | — | — |
| Chiu, 2016 | tumescent: 150~300mL (1000mL lactated Ringer’s solution, 30mL 2% lidocaine, 1 mL 1:1000 epinephrine) 10~15 min | thighs; hips; flanks; abdomen; calves | 3or4-mm cannula; low pressure suction machine set at -400 to —-500 mm Hg | First: fat (100 mL) mixed with 1% typel collagenase centrifuged at 800g for 5min Second: centrifugation 800 g for 4 min | fanning pattern; 14-gauge 15-cm single-hole cannula | subcutaneous; subglandular; Supramuscular; intramuscular layers | — | — |
| Chiu, 201816 | tumescent: 150~300mL (1000mL lactated Ringer’s solution, 30 mL 2% lidocaine, 1 mL 1:1000 epinephrine ) 10~15 min | abdomen; flanks; hips; thighs; calves | 3or4-mm cannula; low-pressure suction machine -600 mm Hg | First: fat (100 mL) mixed with 0.075% type I collagenase centrifuged at 800g for 5min Second: centrifugation 800g for 4 min | Coleman Solid Injection Technique; 14g 15-cm single-hole cannula; 10-mlBD syringes | — | — | — |
| Claudio et al, 2017 | — | abdomen ; flanks | 3-mm cannula; 40kPa vacuum pump; 400-ml modified drainage bottle | centrifugation 2000rpm (400 G) 2 min | 10-ml syringes; 1.9-mm 9-cm blunt cannulas | — | — | — |
| Coleman and Saboeiro, 200713 | general; epidural plus sedation;local infiltration; intercostal nerve blocks | — | 10-ml syringe; 2-hole Coleman harvesting cannula | centrifugation and refinement | 3-ml syringes; 0.2ml each place | cutaneous muscle; retropectoral; prepectoral spaces | — | — |
| Cotrufo et al, 2008 | - | — | Coleman technique | — | — | — | — | — |
| Del Vecchio and Bucky, 2011 | 5L 30ml of 1% lidocaine with epinephrine, 1:100,000/L of normal saline | — | — | centrifugation low g forces (20 ~40 g) | — | — | 120 | — |
| Delay et al, 200918 | general tumescent | abdomen; inner thighs | 10-mL Luer-Lok syringe; 4-mm 15G blade cannula | Centrifugation 3200rpm 3min | 10-mL syringes; 17-gauge; small quantities; injection of 140% rule | — | — | infiltration of diluted ropivacaine a circular motion in local area; an abdominal support belt for six weeks 10 sessions |
| Delay et al, 2013 | general; tumescent: 1 mg adrenaline in 500mL physiological saline | — | 10-mL Luer-Lok syringe; 3.5-mm multihole cannula | centrifugation 500g (3000 rpm) for 3 min or 20sec | 2-mm single-hole cannula | deepest area of missing breast volume; edge of zone | — | — |
| Derder et al, 201420 | — | Abdomen; trochanter; inner thighs; inner knees; lumbar region | 3-mm cannulas; vacuum pump -0.5 atm | Centrifugation 3000 rpm for 3 min | 10-mL Luer-Lock syringes; 2-mm transfer cannulas | From deep to superficial plane | — | — |
| Deschler et al, 202025 | general anesthesia; tumescent: 1 mg epinephrine and 1 L Ringer’s lactate | supra- and infraumbilical abdomen; flanks/hips; medial and lateral thigh; medial knee | 3-to 6-mm diameter, 25 cm in length, blunt cannula;vacuum pressure of 500 mmHg | washed with Ringer’s lactate solution, Centrifugation 3000 rpm for 3 m`in | 3-mm diameter 18-cm long blunt cannula | subglandular; periglandular; subcutaneous | 113.2 | antithrombotic medication for a duration of 15 days, antibiotics,pain killers |
| Dos Anjos et al, 201517 | general; tumescent: Klein (include Ringer’s Lactate, no lidocaine) | infraumbilical area; flank | microaire cannula (PAL-404LS) ; vacuum pump 53.3 kPa (0.52 atm) | every 50 ml processed fat graft, 1-2ml resuspended SVF cells | 20ml Luer-Lock syringes ; 20-mm-long 2.1-mm-diameter Super Luer-Lock cannulas | — | — | — |
| Fiaschetti et al, 2013 | general; tumescent | — | specific canula Coleman Kit after infiltration | centrifugation 3000rpm 4min, combined with platelet-enriched plasma | microcanula ; Coleman Kit ; small pulses (0.2-1ml) | — | — | — |
| Guo et al, 201821 | tumescent: 400 mg lidocaine and 1 mg adrenaline per 1000 mL saline | abdomen; waist; thighs | 3.0mm sharp cannula; machine 500-600 mm Hg | Sedimentation 15 minutes | 1-mL syringes; 2-mm 150-250 mm in length blunt canula | subcutaneous; pectoralis muscle; retromammary | — | — |
| Gutierrez-Ontalvilla et al, 2020 | general anesthesia; Tumescent:1000 cc saline solution with 1 cc epinephrine | abdomen; trochanteric zones; inner thighs | 3-mm cannulas with a vacuum pressure of 300 mmHg | centrifuged at 3000 rpm for 3 min | 9-cm-long 1.6-mm-diameter Coleman-style 2 concave or straight blunt cannulas | pectoralis major muscle; subglandular; periglandular; subcutaneous | — | dressings consisting of paper tape were applied over and around the breast mound |
| Herold et al, 2010 | — | — | WAL:BEAULI | — | — | — | — | — |
| Ho Quoc et al, 201312 | tumescent: 1 mg adrenaline in 500 mL 0.9% saline | — | 10-mL Luer-Lok syringe with multiperforated cannula | centrifugation 3000 rpm 3 min between 2006 and 2009 and for 20 seconds after 2009 | disposable 2-mm monoperforated cannula | From deep zone toward surface; ribs; pectoralis major muscle; subcutaneous | — | — |
| Illouz and Sterodimas, 2009 | tumescent: normal saline 1:500,000 of adrenaline 15 min | abdomen; hips; flanks; knees | 60ml syringe; 4-mm canula | decantation | 10ml syringe; 2.5-mm canula | subcutaneous; intraglandular | — | — |
| Jung et al, 2016 | general; tumescent: 400mL(2% lidocaine50mL, 1:100,000 epinephrine 1ml in Ringer’s lactate solution) 10~15 min | posterior thighs ; flanks | Harvest-Jet ; 3-mm hole blunt cannula | sedimentation Svf: centrifugation and separation | 10-mL syringe ; screw-type injector (0.28mL each rotation) | subcutaneous; retromammary; pectoralis major | 240 | bandages and brassieres were not utilized |
| Kamakura and Ito, 2011 | — | abdomen; hip | 3-mm canula | fat tissue divided in to 2 equal parts:first used to isolate- stem cells(Celution800 System +proteolytic enzyme reagent),then added to second part and processed with Celution 800 System | seringue cellbrush and multilayer injection | Retro- and intra- muscular; subcutaneous; retroglandular | analgesics; limitation of activity for 3–7 days; cold compresses | |
| Kang and Luan, 201814 | tumescent: 1000 ml (normal saline 1 ml 1/200,000 epinephrine 600 mg lidocaine) | — | WAL; 3.8-mm cannula - 0.5 bar negative pressure sedimentation 15 min | sedimentation 15 min Or Centrifugation 800 r/min for 3 min | 2-mm fat injection needles; "3Ms" techniques, 1ml/each channel | subcutaneous; retromammar; pectoralis major; posterior of pectoralis major | — | shapewear for their recipient areas for 1 month |
| Kerfant et al, 2017 | tumescent: 400 ml saline, 40 ml lidocaine with 1%adrenaline 0.4 mg and 7.5 mg ropivacaine | buttocks; posterior thighs; abdomen; anteromedial thighs | 3-mm liposuction cannula ;low pressure,a sterile suction-assisted device | 10-ml syringes; centrifugation 3000 rpm for 1 min | 16- or18-gauge 15-cm-long cannula | subcutaneous in cleavage; submammary areas | 90 | — |
| Khouri et al, 2012 | — | — | 2.7-mm canula aspiration syringe; 300-mmHg closed system | centrifugation 15g 3min | 2.4-mm canula | Retro- and intra- muscular; subcutaneous; retroglandular | — | — |
| Khouri et al, 2014 | — | — | 2.7-mm canula aspiration syringe; 300-mmHg closed system | centrifugation 15g 3 min | 2.4-mm canula | — | — | — |
| Klit et al, 20155 | — | lower abdomen | Coleman 2-mm cannulas -0.5atm vacuum or BEAULI 3.8-mm cannula -0.5 bar WAL equipment (Body-jet) | centrifugation 3000 rpm for 3 min | 10-ml LuerLock syringes; 2-mm transfer cannulas | — | — | — |
| Kwiatkowska et al, 2019 | analog-sedation;local anesthesia; tumescent:1L 0.9%NaCl, 500mg lidocaine 1 mg epinephrine, 12.5ml sodium bicarbonate 8.4% solution | — | Berlin Autologous Lipotransfer, negative pressure of –0.5 bar Bodyjet level 1 | separation of fat from liquid with Lipo Col-lector | 10 cm length and simultaneous fat injection | — | — | — |
| La Marca et al, 2013 | — | abdomen; gluteal; thighs | 3mm canula syringe with minimal depression | Centrifugation 3000 rpm 4min | 17G Canule Coleman | Multilayer | — | — |
| Li et al, 2014 | general; tumescent: 20-mL 2% lidocaine with epinephrine, 1:100,000/L normal saline | abdomen; flanks ; trochanteric region; inner thigh; medial aspect of knees upper arms | 20-mL syringe; 3-mm 3-hole blunt cannula | Wash filtration | 14-gauge1-hole blunt cannula | superficial subcutaneous plane | — | — |
| Maione et al, 2018 | infiltrate only physiologic solution with epinephrine without local anesthetic | abdomen; hip | 2~3-mm 15~23- cm long blunt cannulas; 10-ml Luer-lock syringe | centrifugation at 3000 rpm for 5 min | 18-gauge angiographic needle with a snap-on wing | subcutaneous | 67 | — |
| Matsudo and Toledo, 1988 | — | inner of knee; dorsal regions; "jodhpur thigh" area; abdomen | — | — | — | — | — | — |
| Muench, 2016 | sedation with 7.5 mg Midazolam; gasmixture of 50% nitrous oxide and 50% oxygen; local anesthesia | abdomen; hip; groin region; thigh | 25-cm 3.8-mm double-lumen cannula | filtration: pore diameter 250 μm | 2-mm cannula; 12-gauge 150mm | fan-shaped manner;all layers “three-dimensional filling” | 113 | — |
| Münch, 2013 | sedation: 7.5 mg Midazolam; local anesthesia | abdomen; hip; groin region; thigh | WAL; 25 cm 3.8 mm double-lumen cannula | filtration: pore diameter 250 μm | 2-mm cannula; 12-gauge 150mm | fan-shaped manner;all layers “three-dimensional filling” | 135 | — |
| Ohashi et al, 2016 | tumescent: (1 ml epinephrine, 20 ml 8.4% sodium hydrogen carbonate, and 50 ml 1.0% lidocaine/1000 ml saline solution) | — | 3.0~4.6-mm 20-cm third- generation ultrasound device, VASER Lipo System low power(50 kPa) | centrifugation 1200g to 2000g for 3 min | 14-gauge Coleman cannulas (internal diameter 1.69mm) | — | — | — |
| Özalp and Aydinol, 2017 | Lactated Ringer’s solution with 1:200,000 epinephrine and 0.5% lidocaine | — | 3-mm blunt multihole aspiration cannula;20-ml Luer-Lok syringe | pure graft device washed with ringer lactate at 36°C | 5-ml and 10-ml syringes; closed system | multiplane superficial dermis; subcutaneous tissue;breast | 140 | — |
| Peltoniemi et al, 201315 | general; tumescent: 1 ml epinephrine 1:1000, 12.5 ml sodium bicarbonate 8ml and 500mg lidocaine each 1000ml 0.9% saline | — | WAL; 3.8-mm steel cannula ; 0.5 bar suction vacuum | Fat divided into 2 equal parts: first used to isolate vascular stromal fraction: Celution800/CRSsystem, second continuous rinse with tumescent solution at 37°C | 10-ml syringes; celbrush injector; 2-mm blunt cannula | pectoral muscles; retroglandular space; two-thirds subcutaneously | 120~180 | — |
| Pinsolle et al, 2008 | — | abdomen; trochanters | syringe | centrifugation | 1.5mm canula | — | — | — |
| Quoc et al, 2013 | — | — | 10 ml Luer-Lok syringes; 3.5-mm multiperforated cannulas | centrifugation at 3000 rpm for 3 min or 20 seconds | 14-gauge trocar 2-mm monoperforated cannula | from deep to superficial plane | — | — |
| Rubin et al, 2012 | — | — | 2.5-mm canula | Cell Assisted Lipotransfer ;Fat divided into 2 equal parts:first used to isolate vascular stromal fraction, second centrifugated ,then two parts mixed | reinjection in small aliquots | Intramuscular; subcutaneous | — | — |
| Serra-Mestr et al, 2017 | — | abdomen; inner thigh; knee | 2.4-mm microport harvester cannula with barbed; beveled 1-mm ports 10-ml Luer-Lok syringe | wash and decanted twice with lactated Ringer solution | 1-ml syringes; 1.5-mm blunt cannula | subcutaneous in crescent shape | — | — |
| Sforza et al, 201622 | general | abdomen | 60-mL Luer-Lock syringe; 2.4-mm cannula | the Pure graft system | 20-mL Luer-Lock syringe; 0.9-mm cannula | — | — | compression socks: a prophylactic pneumatic DVT system; soft dressings on the grafted areas |
| Spear and Pittman, 201423 | — | abdomen; hips | 3-mm canula; classic lipoaspiration 500 mmHg | Centrifugation 3000 rpm 3min | 10-ml syringe | Intramuscular; retroglandular; subcutaneous | 180 | — |
| Ueberreiter et al, 2010 | — | — | BEAULI:WAL | Separation of fat and water: Lipocollector | — | — | 90 | — |
| Ueberreiter et al, 2013 | tumescent: Klein 37 to 38°C (98–100°F) 10 min | abdomen; hip; thigh | WAL; 3.8mm cannula with effective suction openings of 0.9 mm–0.5 bar | Separation of fat and water: Lipocollector | 10 ml syringes | — | 70±15 | — |
| Veber et al, 2011 | — | — | Coleman | — | — | retro muscular; subcutaneous | — | — |
| Visconti and Salgarello, 2019 | normal saline and adrenaline 1:500,000 | flanks ; trochanter regions; inner thighs; medial aspect of knees | mercedes harvesting cannula (inner diameter, 1.8 mm) attached to vacuum canister | centrifuged at 1200g for 1 min | a Luer-Lok connector to 3-ml syringes | pectoralis major muscle; subglandular; Periglandular; subdermal | — | — |
| Wang et al, 2011 | tumescent | — | manual lipoaspiration 20-ml syringe | sedimentation | — | retroglandular | — | — |
| Wang et al, 2012 | — | abdomen; flanks; thigh | Lipokit machine | cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascular stromal fraction,second centrifugated,then two parts mixed. | 18-gauge trocar 1-mm monoperforated cannula | — | 180~240 | — |
| Wang et al, 2015 | — | thighs; flanks; lower abdomen | single combined machine Lipokit | isolate vascular stromal fraction centrifugation (800g for 5 min) 20-mL SVF-rich fat, centrifuged at 700g for 3 min | 16-gauge needle 20-mL | subcutaneous; under mammary glands; pectoralis muscles | — | — |
| Yoshimura et al, 20088 | general; tumescent: saline solution and diluted epinephrine (0.001%) | — | 2.5mm canula classic lipoaspiration | Cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascular stromal fraction, second centrifugated, then two parts mixed. | 17Gcanula 10-20ml syringe | periglandular; subcutaneous; intramuscular | injection time 35~60 | — |
| Yoshimura et al, 20109 | general tumescent: saline solution containing diluted epinephrine (0.0001%) | — | 2.5-mm canula conventional liposuction machine classic lipoaspiration | Cell Assisted Lipotransfer. Fat divided into 2 equal parts: first used to isolate vascularstromal fraction, second centrifugated, then two parts mixed. | 16- or 18-gauge sharp needle (150-mm long), 10-20ml syringe | Plane formed between skin and periprosthetic capsula | — | — |
| Zheng et al, 2008 | — | abdomen; hips; trochanters | 2-holed blunt 3-mm canula; vacuum pump low negative pressure(0.5atm) | Saline solution cleaning; centrifugation 600rpm 2min | 5-ml syringe; 3-mm canula one-holed (3mm in diameter of the nozzle) blunt | subcutaneous; retroglandular | — | a surgical bra in the first 7 days postoperatively |
| Zheng et al, 2019 | general; anesthesia; Tumescent: 1 mg/L epinephrine in saline | thighs; abdomen | 4-mm suction cannula; constant negative pressure of 55 kPa | sedimented for 30 minutes | 2-mL syringe via 10-cm-long 2-mm-diameter cannula | subcutaneous; subglandular; intramuscular | — | — |
| Zocchi and Zuliani, 20086 | general; room-temperature saline using 2mg adrenaline per liter | trochanter; glutea | 2-mm canula 60- cc syringe | saline solution washing, stratification by vibration then decanting | 60-ccsyringe; 2-mm canula | subcutaneous; retroglandular | — | an elastic roll: maintain space between the breasts; a sports shaped-cups bra |
| Zocchi, 20177 | — | trochanteric; gluteal regions | FPU;2-mm single-hole; Tefloncoated special cannula; vacuum control | centrifugation 4500 rpm for 9 min | 2-mm cannulas; straight flexible 27-cm-long cannula; stiffer 25-cm-long curved one | — | — | — |
—, not reported; DVT, deep vein thrombosis; FPU, fat processing unit; WAL, water-jet assisted liposuction.
Anesthesia
In most studies, surgery was performed with the patients under general anesthesia combined with local infiltration anesthesia. In some cases, only local infiltration anesthesia, namely tumescent technology, was employed. Ingredients were 30 to 50 mL of 1% lidocaine and 1 mL of 1:100,000 adrenaline in each liter of normal saline or sodium lactate format solution. The infiltration lasted for 10 to 15 minutes. However, lidocaine was not present in the tumescent fluid in 9 studies (833 patients). Five studies (373 patients) utilized antibiotics after the operation, and only 2 studies detailed the method of antibiotics.
Brava Device
Pre-expansion with the Brava device was employed in 7 studies (779 patients). The main objective was to improve the surfaces that interfere with AFT through neovascularization by pre-expanding the receiving tissues. The method involved wearing the Brava device for 3 to 4 weeks before the operation for 10 to 12 hours per day and 2 to 4 weeks after the operation.
Preparation of the Breast
Only 2 studies mentioned the preoperative preparation of the breast area. Ho Quoc et al12 utilized a 14-gauge trocar to perform fasciotomies of the breast area so the fat could survive more easily under less pressure. Zocchi and colleagues7 pressurized inflation with carbon dioxide in the breast area.
Donor Areas
The typical fat donor areas employed were the abdomen and thighs, but this varied depending on both the individual patient fat-tissue distribution and where the surgeon could most easily access the donor sites.
Fat Harvesting
The principle of liposuction is based on the Coleman technology,13 2- to 4-mm-diameter casing, and negative 0.4 to 0.6 standard atmospheric pressure. However, there were some small differences among the studies, such as the negative pressure source, including a manual syringe and machine. Twenty-two studies performed liposuction with the manual syringe, 37 studies with a machine, and 7 studies (in 37 studies) utilized a hydrodynamic liposuction machine.
Fat Processing
Fat tissues were usually processed by centrifugation in 37 studies. The optimal rotation speed and time were not provided, but most of them reported values of 3000 rpm and about 2 to 4 minutes, respectively. Sedimentation was employed in 12 studies, filtration in 3, and the cleaning method in 4. Kang and Luan14 reported the effect of low-speed centrifugation and sedimentation on postoperative fat necrosis. This study suggested that low-speed centrifugation was more likely to cause refractory nodules, especially for patients who have had breast surgery or need multiple fat transplantations. Twelve studies utilized SVF or adipose stem cells derived from autogenous fat, one of which reported a case of auto-concentration of monocytes derived from bone marrow obtained from the ilium. Peltoniemi et al15 and Chiu16 reported no significant difference in the fat survival rate between the AFT group and AFT combined with SVF at 6 and 12 months postoperatively. Dos Anjos et al17 categorized SVF into a low-concentration group and a high-concentration group for comparison, and the fat survival rate at 18 months was significantly different between the 2 groups (50% vs 75%).
Fat Reinjection
Multi-tunnel, multi-layer, multi-point, fan-shaped, and small have been commonly employed to describe the fat injection. Syringes range from 1 mL to 60 mL in capacity. A spiral-propelling syringe was also utilized, and each spiral rotation injected 0.28 mL of fat. Most studies injected fat in the hypodermic, hypoglandular, upper, and lower spaces of the pectoralis major muscle. However, it is controversial if the injection level in the pectoralis major muscle causes intramuscular nodules and pain during activity. Different researchers have different opinions on the amount of injection to employ. The amount of a single injection in the AFT group was 50 to 420 mL, that in the AFT + SUPP group was 195 to 380 mL, that in the AFT + BRAVA group was 245 to 430 mL, and that in the AFT + IMP group was 27 to 134 mL. Five studies18-22 proposed that because fat tissue must be absorbed after the operation, the amount of transplanted fat should be 30% to 50% more than the expected amount. Thus, the amount of relative stable fat survival at the final 12 months minimum was appropriate and satisfactory. Longer term satisfaction still needs to be observed. In addition, 2 studies proposed that the interval between 2 operations was 3 months or 6 months, which was consistent with our results in the meta-regression model established with postoperative follow-up duration as the covariate and fat survival rate as the dependent variable. The sessions of operations were usually approximately 1 to 5 times for achieving better results.
Postoperative Follow-up
Details of the postoperative follow-up for all studies are listed in Table 5.
Table 5. Objective Assessment of Postoperative Follow-Up
| Study . | Radiologic examination . | Fellow-up (mo) . | Examination results . | Chest Measurement(cm) . | Volume Measurement Method . | Timing of Measuremet(mo) . | complications . | number . |
|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | ultrasound mammographyMRI | 0,12 | negative for pathologic findings | — | MRIbra size | 0,120,6 | cyst formation infection | 9,2 |
| Auclair, 2009 | mammography | — | — | — | — | — | — | 0 |
| Auclair et al, 201310 | photographsmammography | 0,12 | negative for pathologic findings | — | quantitativethree-dimensional breast imaging | 0,12 | cystic masscapsular contractureinsufficient soft-tissue coveragedonor-site deformity | 2,1,5,1 |
| Atia, 2020 | mammography ultrasound scans | 0,6,12 | solid nodules cystic lesions microcalcifications | — | — | — | pain fat necrosis irregularities fluid collection asymmetry lipo-necrotic cyst | 4,1,3,2,1,1 |
| Auclair, 2020 | mammogram | 0,1,6,12 | — | — | — | — | seromainsufficient soft-tissue coveragecapsular contracture | 2,13,8 |
| Bircoll, 2010 | — | — | — | — | — | — | microcalcifications | 8 |
| Brault et al, 201711 | — | — | — | — | digital imaging software | 0,12 | oil cysts | 2 |
| Bravo, 2014 | — | — | — | mean length between parasternal vertical aesthetic lines preop 1.43 ± 0.61 cm postop 0.60 ± 0.32 cm | photographs | 12 | unilateral Baker grade II capsular contracture | 2 |
| Bresnick, 2016 | MRI | — | — | — | — | — | — | 0 |
| Carvajal and Patino, 2008 | mammogram | 0,6–84 | BI–RADS 2 (85%) and 3 (15%) microcalcifications oil cystslipid cysts | — | — | — | oil cysts | 4 |
| Chiu, 2014 | ultrasound MRI | 0,2–11 0,1–8 | — | mean postoperative change in BCD3.5 cm | physical examination | 6.8 | recipient site infection fat necrosis | 13,7 |
| Chiu, 2016 | ultrasound | 0,3,6,12 | — | breast thickness change was 13.1 mm | ultrasonography | 12 | recipient site infection fat necrosis small induration | 6 |
| Chiu, 201816 | ultrasound MRI | 0,3,6,12 | induration necrosis cysts | — | 3D laser scanning | 0,3,6,12 | indurationnecrosis cysts | 4,6 |
| Claudio et al, 2017 | ultrasound | — | oil cyst | — | — | — | oil cyst | 4 |
| Coleman and Saboeiro, 200713 | mammograms | 0,12 | breast cancer | — | — | — | local infection small nodule calcificationsbreast cancer | 102 |
| Cotrufo et al, 2008 | — | — | — | — | — | — | necrosis | 1 |
| Del Vecchio and Bucky, 2011 | MRI | 0,6 | — | — | MRI | 0,6 | — | 0 |
| Del Vecchio, 201419 | — | — | — | — | quantitative volumetric breast imaging | 0,12 | — | — |
| Delay et al, 200918 | mammography ultrasound MRI | 0.5, 3,12 | oily cysts 15% | — | photographs MRI | 0.5 3 12 | infection | 4 |
| Delay et al, 2013 | mammography ultrasound MRI | 0,12 | oily cysts 20% | — | photographs MRI | 0,12 | — | 0 |
| Derder et al, 201420 | ultrasound mammography MRI | 0,0.5, 3,6,12 | ACR 1(92.3%) ACR 2(7.7%) oil cysts | — | photographs mammography ultrasoundMRI | 0,0.5,3,6,12 | — | — |
| Deschler et al, 202025 | MRI | 0,12 | cytosteatonecrosis | — | — | — | oil cysts hypertrophic scars | 2, 1 |
| Dos Anjos et al, 201517 | ultrasound | 6 | oil cysts | — | 3D | 18 | fat necrosis | 3 |
| Fiaschetti et al, 2013 | mammography ultrasound MRI | 0,3,6 | — | — | mammography ultrasound MRI | 0,3,6,12 | — | — |
| Guo et al, 201821 | MRIultrasound | 0,3 | BI–RADS 1– 2 | — | MRI | 0,3 | — | 0 |
| Gutierrez-Ontalvilla et al, 2020 | ultrasound | 0,1,3,6,12 | oil cysts | — | — | — | painful nodule | 1 |
| Herly et al, 2019 | MRI | — | — | — | MRI | 4.5 | — | — |
| Herold et al, 2010 | MRI | 0,6 | - | MRI | 0,6 | — | ||
| Ho Quoc et al, 201312 | — | — | — | — | — | — | infectionswound healing fat necrosis | 40 |
| Ho Quoc et al, 2015 | MRI | 0,12 | — | — | MRI | 0,12 | — | 0 |
| Illouz and Sterodimas, 2009 | mammography ultrasound | 0,6,12 | BI–RADS 1or 2 | — | MRI | 0,12 | striae hematomas infections | 53 |
| Jung et al, 2016 | mammography ultrasoundMRI | 0,3,12 | BI–RADS 1or 2 oil cysts 3 | — | MRI | 0,3,12 | oil cystssole cyst multiple cysts | 3,1,1 |
| Kamakura and Ito, 2011 | mammograms | 9 | liponecrotic cyst 2 | — | clinicalmammograms | 9 | liponecrotic cyst | 2 |
| Kang and Luan, 201814 | ultrasounds | 3 | — | — | breast palpation ultrasound | 3 | palpable nodules | 21 |
| Kerfant et al, 2017 | mammograms | 0 | ACR 1– 2 | — | MRI | — | hematoma infectionsbaker grade II/III contractures malrotation | 11 |
| Khouri et al, 2012 | mammography ultrasound | 0,3,6,12 | fat necrosis calcifications | — | MRI | 0,3,6,12 | atypicalmycobacterial infection | 1 |
| Khouri et al, 2014 | mammography ultrasound | 12 | cyst | — | 3Dphotographic imagingMRI | 0,6,12 | cyst infectionpneumothorax1 | 24 |
| Klit et al, 20155 | — | — | — | — | — | — | — | 0 |
| La Marca et al, 2013 | — | — | — | — | — | — | — | 0 |
| Li et al, 2014 | mammography MRI | 0 | — | — | — | — | calcificationssmall nodules | 5 |
| Maione et al, 2018 | ultrasound mammograph | 0,0.5,3, 12 | — | — | — | — | — | 0 |
| Muench, 2016 | mammography ultrasound | 0,6 | — | — | — | — | contour irregu- laritiesasymmetries | 6,5 |
| Münch, 2013 | — | — | — | — | — | — | hematomaasymmetries | 9 |
| Ohashi et al, 2016 | ultrasound | 3,6 | partial fat necrosis | overall bust size decreased slightly 2.0 ± 3.0 cm | top bust and under bust measurements | 6 | implant removal seromanoduleshematomafat necrosis | 4,4,1, 3 |
| Özalp and Aydinol, 2017 | ultrasound | 18 | — | Mean increase above inframammary fold 1.3 cm | ultrasonograph | 18 | cyst formationcapsular contracture | 2,4 |
| Peltoniemi et al, 2013 15 | mammogram ultrasound | 0,18,24 | small oil cysts | — | MRI | 6 | cysts | 3 |
| Pinsolle et al, 2008 | — | — | — | — | — | — | necrosis | 1 |
| Quoc et al, 2013 | ultrasoundmammography MRI | 0,12 | oil cysts | — | — | — | — | 0 |
| Rubin et al, 2012 | mammograms | 0,12 | oil cysts calcificationsfat necrosis | — | mammogram | 0,6,12 | — | — |
| Serra-Mestr et al, 2017 | — | — | — | mean inter mammary distance from 3 ± 0.6 cm to 1.7 ± 0.4 cm | intermammary distances | 0,12 | oil cystdissymmetry | 1,2 |
| Sforza et al, 201622 | — | — | — | — | 3D imaging | 0,12 | — | — |
| Spear and Pittman, 201423 | mammograms MRI | 0,12 | BI–RADS 4 mammograms | — | MRI2D3D | 0,12 | — | — |
| Tassinari et al, 2016 | — | — | — | — | — | — | cyst formation | 37 |
| Ueberreiter et al, 2010 | — | — | — | — | MRI | 0,6 | — | — |
| Ueberreiter et al, 2013 | MRI | 0,6 | — | — | MRI | 6 | — | — |
| Veber et al, 2011 | mammograms | 0,16 | microcalcifications macrocalcifications cystic lesions | — | — | — | — | — |
| Visconti and Salgarello, 2019 | mammographyultrasound scans | 0,12 | — | — | — | — | — | 0 |
| Wang et al, 2011 | mammograms | 18 | microcalcifications | — | — | — | microcalcifications | 8 |
| Wang et al, 2012 | MRI | 0,3,6 | — | — | MRI | 0,3,6 | small nodules | 1 |
| Wang et al, 2015 | mammography | 0,3,6 | BI–RADS 2–3 | — | MRI | 0,3,6 | — | 0 |
| Yoshimura et al, 20088 | mammography MRI | 0,6 | cyst microcalcifications | chest circumferenceincreased 4-8cm | clinical MRI | 6 | palpable nodules | 1 |
| Yoshimura et al, 20109 | mammography MRI | 0,12 | — | — | MRI3D measurements | 0,12 | — | 0 |
| Zheng et al, 2008 | ultrasound mammographyMRI | 0,1,3,6,12 | cystsliponecrotic cysts calcification | — | — | — | liponecrotic cysts palpable nodules | 11, 2 |
| Zheng et al, 2019 | MRI | 0,3,6 | — | — | 3D scanner | 0,3 | — | — |
| Zocchi and Zuliani, 20086 | ultrasoundmammography MRI | 0,6,12 | — | — | mammograms ultrasonograms | 12 | liponecrosis microcyst microcalcifications | 5,7 |
| Zocchi, 20177 | — | — | — | — | mammograms ultrasonograms | 12 | infectionslocalized liponecrosisoil cystmacro calcificationslocalized liponecrosis oil cystmacro calcifications | 25,5 |
| Study . | Radiologic examination . | Fellow-up (mo) . | Examination results . | Chest Measurement(cm) . | Volume Measurement Method . | Timing of Measuremet(mo) . | complications . | number . |
|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | ultrasound mammographyMRI | 0,12 | negative for pathologic findings | — | MRIbra size | 0,120,6 | cyst formation infection | 9,2 |
| Auclair, 2009 | mammography | — | — | — | — | — | — | 0 |
| Auclair et al, 201310 | photographsmammography | 0,12 | negative for pathologic findings | — | quantitativethree-dimensional breast imaging | 0,12 | cystic masscapsular contractureinsufficient soft-tissue coveragedonor-site deformity | 2,1,5,1 |
| Atia, 2020 | mammography ultrasound scans | 0,6,12 | solid nodules cystic lesions microcalcifications | — | — | — | pain fat necrosis irregularities fluid collection asymmetry lipo-necrotic cyst | 4,1,3,2,1,1 |
| Auclair, 2020 | mammogram | 0,1,6,12 | — | — | — | — | seromainsufficient soft-tissue coveragecapsular contracture | 2,13,8 |
| Bircoll, 2010 | — | — | — | — | — | — | microcalcifications | 8 |
| Brault et al, 201711 | — | — | — | — | digital imaging software | 0,12 | oil cysts | 2 |
| Bravo, 2014 | — | — | — | mean length between parasternal vertical aesthetic lines preop 1.43 ± 0.61 cm postop 0.60 ± 0.32 cm | photographs | 12 | unilateral Baker grade II capsular contracture | 2 |
| Bresnick, 2016 | MRI | — | — | — | — | — | — | 0 |
| Carvajal and Patino, 2008 | mammogram | 0,6–84 | BI–RADS 2 (85%) and 3 (15%) microcalcifications oil cystslipid cysts | — | — | — | oil cysts | 4 |
| Chiu, 2014 | ultrasound MRI | 0,2–11 0,1–8 | — | mean postoperative change in BCD3.5 cm | physical examination | 6.8 | recipient site infection fat necrosis | 13,7 |
| Chiu, 2016 | ultrasound | 0,3,6,12 | — | breast thickness change was 13.1 mm | ultrasonography | 12 | recipient site infection fat necrosis small induration | 6 |
| Chiu, 201816 | ultrasound MRI | 0,3,6,12 | induration necrosis cysts | — | 3D laser scanning | 0,3,6,12 | indurationnecrosis cysts | 4,6 |
| Claudio et al, 2017 | ultrasound | — | oil cyst | — | — | — | oil cyst | 4 |
| Coleman and Saboeiro, 200713 | mammograms | 0,12 | breast cancer | — | — | — | local infection small nodule calcificationsbreast cancer | 102 |
| Cotrufo et al, 2008 | — | — | — | — | — | — | necrosis | 1 |
| Del Vecchio and Bucky, 2011 | MRI | 0,6 | — | — | MRI | 0,6 | — | 0 |
| Del Vecchio, 201419 | — | — | — | — | quantitative volumetric breast imaging | 0,12 | — | — |
| Delay et al, 200918 | mammography ultrasound MRI | 0.5, 3,12 | oily cysts 15% | — | photographs MRI | 0.5 3 12 | infection | 4 |
| Delay et al, 2013 | mammography ultrasound MRI | 0,12 | oily cysts 20% | — | photographs MRI | 0,12 | — | 0 |
| Derder et al, 201420 | ultrasound mammography MRI | 0,0.5, 3,6,12 | ACR 1(92.3%) ACR 2(7.7%) oil cysts | — | photographs mammography ultrasoundMRI | 0,0.5,3,6,12 | — | — |
| Deschler et al, 202025 | MRI | 0,12 | cytosteatonecrosis | — | — | — | oil cysts hypertrophic scars | 2, 1 |
| Dos Anjos et al, 201517 | ultrasound | 6 | oil cysts | — | 3D | 18 | fat necrosis | 3 |
| Fiaschetti et al, 2013 | mammography ultrasound MRI | 0,3,6 | — | — | mammography ultrasound MRI | 0,3,6,12 | — | — |
| Guo et al, 201821 | MRIultrasound | 0,3 | BI–RADS 1– 2 | — | MRI | 0,3 | — | 0 |
| Gutierrez-Ontalvilla et al, 2020 | ultrasound | 0,1,3,6,12 | oil cysts | — | — | — | painful nodule | 1 |
| Herly et al, 2019 | MRI | — | — | — | MRI | 4.5 | — | — |
| Herold et al, 2010 | MRI | 0,6 | - | MRI | 0,6 | — | ||
| Ho Quoc et al, 201312 | — | — | — | — | — | — | infectionswound healing fat necrosis | 40 |
| Ho Quoc et al, 2015 | MRI | 0,12 | — | — | MRI | 0,12 | — | 0 |
| Illouz and Sterodimas, 2009 | mammography ultrasound | 0,6,12 | BI–RADS 1or 2 | — | MRI | 0,12 | striae hematomas infections | 53 |
| Jung et al, 2016 | mammography ultrasoundMRI | 0,3,12 | BI–RADS 1or 2 oil cysts 3 | — | MRI | 0,3,12 | oil cystssole cyst multiple cysts | 3,1,1 |
| Kamakura and Ito, 2011 | mammograms | 9 | liponecrotic cyst 2 | — | clinicalmammograms | 9 | liponecrotic cyst | 2 |
| Kang and Luan, 201814 | ultrasounds | 3 | — | — | breast palpation ultrasound | 3 | palpable nodules | 21 |
| Kerfant et al, 2017 | mammograms | 0 | ACR 1– 2 | — | MRI | — | hematoma infectionsbaker grade II/III contractures malrotation | 11 |
| Khouri et al, 2012 | mammography ultrasound | 0,3,6,12 | fat necrosis calcifications | — | MRI | 0,3,6,12 | atypicalmycobacterial infection | 1 |
| Khouri et al, 2014 | mammography ultrasound | 12 | cyst | — | 3Dphotographic imagingMRI | 0,6,12 | cyst infectionpneumothorax1 | 24 |
| Klit et al, 20155 | — | — | — | — | — | — | — | 0 |
| La Marca et al, 2013 | — | — | — | — | — | — | — | 0 |
| Li et al, 2014 | mammography MRI | 0 | — | — | — | — | calcificationssmall nodules | 5 |
| Maione et al, 2018 | ultrasound mammograph | 0,0.5,3, 12 | — | — | — | — | — | 0 |
| Muench, 2016 | mammography ultrasound | 0,6 | — | — | — | — | contour irregu- laritiesasymmetries | 6,5 |
| Münch, 2013 | — | — | — | — | — | — | hematomaasymmetries | 9 |
| Ohashi et al, 2016 | ultrasound | 3,6 | partial fat necrosis | overall bust size decreased slightly 2.0 ± 3.0 cm | top bust and under bust measurements | 6 | implant removal seromanoduleshematomafat necrosis | 4,4,1, 3 |
| Özalp and Aydinol, 2017 | ultrasound | 18 | — | Mean increase above inframammary fold 1.3 cm | ultrasonograph | 18 | cyst formationcapsular contracture | 2,4 |
| Peltoniemi et al, 2013 15 | mammogram ultrasound | 0,18,24 | small oil cysts | — | MRI | 6 | cysts | 3 |
| Pinsolle et al, 2008 | — | — | — | — | — | — | necrosis | 1 |
| Quoc et al, 2013 | ultrasoundmammography MRI | 0,12 | oil cysts | — | — | — | — | 0 |
| Rubin et al, 2012 | mammograms | 0,12 | oil cysts calcificationsfat necrosis | — | mammogram | 0,6,12 | — | — |
| Serra-Mestr et al, 2017 | — | — | — | mean inter mammary distance from 3 ± 0.6 cm to 1.7 ± 0.4 cm | intermammary distances | 0,12 | oil cystdissymmetry | 1,2 |
| Sforza et al, 201622 | — | — | — | — | 3D imaging | 0,12 | — | — |
| Spear and Pittman, 201423 | mammograms MRI | 0,12 | BI–RADS 4 mammograms | — | MRI2D3D | 0,12 | — | — |
| Tassinari et al, 2016 | — | — | — | — | — | — | cyst formation | 37 |
| Ueberreiter et al, 2010 | — | — | — | — | MRI | 0,6 | — | — |
| Ueberreiter et al, 2013 | MRI | 0,6 | — | — | MRI | 6 | — | — |
| Veber et al, 2011 | mammograms | 0,16 | microcalcifications macrocalcifications cystic lesions | — | — | — | — | — |
| Visconti and Salgarello, 2019 | mammographyultrasound scans | 0,12 | — | — | — | — | — | 0 |
| Wang et al, 2011 | mammograms | 18 | microcalcifications | — | — | — | microcalcifications | 8 |
| Wang et al, 2012 | MRI | 0,3,6 | — | — | MRI | 0,3,6 | small nodules | 1 |
| Wang et al, 2015 | mammography | 0,3,6 | BI–RADS 2–3 | — | MRI | 0,3,6 | — | 0 |
| Yoshimura et al, 20088 | mammography MRI | 0,6 | cyst microcalcifications | chest circumferenceincreased 4-8cm | clinical MRI | 6 | palpable nodules | 1 |
| Yoshimura et al, 20109 | mammography MRI | 0,12 | — | — | MRI3D measurements | 0,12 | — | 0 |
| Zheng et al, 2008 | ultrasound mammographyMRI | 0,1,3,6,12 | cystsliponecrotic cysts calcification | — | — | — | liponecrotic cysts palpable nodules | 11, 2 |
| Zheng et al, 2019 | MRI | 0,3,6 | — | — | 3D scanner | 0,3 | — | — |
| Zocchi and Zuliani, 20086 | ultrasoundmammography MRI | 0,6,12 | — | — | mammograms ultrasonograms | 12 | liponecrosis microcyst microcalcifications | 5,7 |
| Zocchi, 20177 | — | — | — | — | mammograms ultrasonograms | 12 | infectionslocalized liponecrosisoil cystmacro calcificationslocalized liponecrosis oil cystmacro calcifications | 25,5 |
—, not reported; ACR, American College of Radiology; BCD, breast circumference; BI–RADS, Breast Imaging Reporting and Data System; 2D, 2-dimensional; 3D, 3-dimensional; MRI, magnetic resonance imaging.
Table 5. Objective Assessment of Postoperative Follow-Up
| Study . | Radiologic examination . | Fellow-up (mo) . | Examination results . | Chest Measurement(cm) . | Volume Measurement Method . | Timing of Measuremet(mo) . | complications . | number . |
|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | ultrasound mammographyMRI | 0,12 | negative for pathologic findings | — | MRIbra size | 0,120,6 | cyst formation infection | 9,2 |
| Auclair, 2009 | mammography | — | — | — | — | — | — | 0 |
| Auclair et al, 201310 | photographsmammography | 0,12 | negative for pathologic findings | — | quantitativethree-dimensional breast imaging | 0,12 | cystic masscapsular contractureinsufficient soft-tissue coveragedonor-site deformity | 2,1,5,1 |
| Atia, 2020 | mammography ultrasound scans | 0,6,12 | solid nodules cystic lesions microcalcifications | — | — | — | pain fat necrosis irregularities fluid collection asymmetry lipo-necrotic cyst | 4,1,3,2,1,1 |
| Auclair, 2020 | mammogram | 0,1,6,12 | — | — | — | — | seromainsufficient soft-tissue coveragecapsular contracture | 2,13,8 |
| Bircoll, 2010 | — | — | — | — | — | — | microcalcifications | 8 |
| Brault et al, 201711 | — | — | — | — | digital imaging software | 0,12 | oil cysts | 2 |
| Bravo, 2014 | — | — | — | mean length between parasternal vertical aesthetic lines preop 1.43 ± 0.61 cm postop 0.60 ± 0.32 cm | photographs | 12 | unilateral Baker grade II capsular contracture | 2 |
| Bresnick, 2016 | MRI | — | — | — | — | — | — | 0 |
| Carvajal and Patino, 2008 | mammogram | 0,6–84 | BI–RADS 2 (85%) and 3 (15%) microcalcifications oil cystslipid cysts | — | — | — | oil cysts | 4 |
| Chiu, 2014 | ultrasound MRI | 0,2–11 0,1–8 | — | mean postoperative change in BCD3.5 cm | physical examination | 6.8 | recipient site infection fat necrosis | 13,7 |
| Chiu, 2016 | ultrasound | 0,3,6,12 | — | breast thickness change was 13.1 mm | ultrasonography | 12 | recipient site infection fat necrosis small induration | 6 |
| Chiu, 201816 | ultrasound MRI | 0,3,6,12 | induration necrosis cysts | — | 3D laser scanning | 0,3,6,12 | indurationnecrosis cysts | 4,6 |
| Claudio et al, 2017 | ultrasound | — | oil cyst | — | — | — | oil cyst | 4 |
| Coleman and Saboeiro, 200713 | mammograms | 0,12 | breast cancer | — | — | — | local infection small nodule calcificationsbreast cancer | 102 |
| Cotrufo et al, 2008 | — | — | — | — | — | — | necrosis | 1 |
| Del Vecchio and Bucky, 2011 | MRI | 0,6 | — | — | MRI | 0,6 | — | 0 |
| Del Vecchio, 201419 | — | — | — | — | quantitative volumetric breast imaging | 0,12 | — | — |
| Delay et al, 200918 | mammography ultrasound MRI | 0.5, 3,12 | oily cysts 15% | — | photographs MRI | 0.5 3 12 | infection | 4 |
| Delay et al, 2013 | mammography ultrasound MRI | 0,12 | oily cysts 20% | — | photographs MRI | 0,12 | — | 0 |
| Derder et al, 201420 | ultrasound mammography MRI | 0,0.5, 3,6,12 | ACR 1(92.3%) ACR 2(7.7%) oil cysts | — | photographs mammography ultrasoundMRI | 0,0.5,3,6,12 | — | — |
| Deschler et al, 202025 | MRI | 0,12 | cytosteatonecrosis | — | — | — | oil cysts hypertrophic scars | 2, 1 |
| Dos Anjos et al, 201517 | ultrasound | 6 | oil cysts | — | 3D | 18 | fat necrosis | 3 |
| Fiaschetti et al, 2013 | mammography ultrasound MRI | 0,3,6 | — | — | mammography ultrasound MRI | 0,3,6,12 | — | — |
| Guo et al, 201821 | MRIultrasound | 0,3 | BI–RADS 1– 2 | — | MRI | 0,3 | — | 0 |
| Gutierrez-Ontalvilla et al, 2020 | ultrasound | 0,1,3,6,12 | oil cysts | — | — | — | painful nodule | 1 |
| Herly et al, 2019 | MRI | — | — | — | MRI | 4.5 | — | — |
| Herold et al, 2010 | MRI | 0,6 | - | MRI | 0,6 | — | ||
| Ho Quoc et al, 201312 | — | — | — | — | — | — | infectionswound healing fat necrosis | 40 |
| Ho Quoc et al, 2015 | MRI | 0,12 | — | — | MRI | 0,12 | — | 0 |
| Illouz and Sterodimas, 2009 | mammography ultrasound | 0,6,12 | BI–RADS 1or 2 | — | MRI | 0,12 | striae hematomas infections | 53 |
| Jung et al, 2016 | mammography ultrasoundMRI | 0,3,12 | BI–RADS 1or 2 oil cysts 3 | — | MRI | 0,3,12 | oil cystssole cyst multiple cysts | 3,1,1 |
| Kamakura and Ito, 2011 | mammograms | 9 | liponecrotic cyst 2 | — | clinicalmammograms | 9 | liponecrotic cyst | 2 |
| Kang and Luan, 201814 | ultrasounds | 3 | — | — | breast palpation ultrasound | 3 | palpable nodules | 21 |
| Kerfant et al, 2017 | mammograms | 0 | ACR 1– 2 | — | MRI | — | hematoma infectionsbaker grade II/III contractures malrotation | 11 |
| Khouri et al, 2012 | mammography ultrasound | 0,3,6,12 | fat necrosis calcifications | — | MRI | 0,3,6,12 | atypicalmycobacterial infection | 1 |
| Khouri et al, 2014 | mammography ultrasound | 12 | cyst | — | 3Dphotographic imagingMRI | 0,6,12 | cyst infectionpneumothorax1 | 24 |
| Klit et al, 20155 | — | — | — | — | — | — | — | 0 |
| La Marca et al, 2013 | — | — | — | — | — | — | — | 0 |
| Li et al, 2014 | mammography MRI | 0 | — | — | — | — | calcificationssmall nodules | 5 |
| Maione et al, 2018 | ultrasound mammograph | 0,0.5,3, 12 | — | — | — | — | — | 0 |
| Muench, 2016 | mammography ultrasound | 0,6 | — | — | — | — | contour irregu- laritiesasymmetries | 6,5 |
| Münch, 2013 | — | — | — | — | — | — | hematomaasymmetries | 9 |
| Ohashi et al, 2016 | ultrasound | 3,6 | partial fat necrosis | overall bust size decreased slightly 2.0 ± 3.0 cm | top bust and under bust measurements | 6 | implant removal seromanoduleshematomafat necrosis | 4,4,1, 3 |
| Özalp and Aydinol, 2017 | ultrasound | 18 | — | Mean increase above inframammary fold 1.3 cm | ultrasonograph | 18 | cyst formationcapsular contracture | 2,4 |
| Peltoniemi et al, 2013 15 | mammogram ultrasound | 0,18,24 | small oil cysts | — | MRI | 6 | cysts | 3 |
| Pinsolle et al, 2008 | — | — | — | — | — | — | necrosis | 1 |
| Quoc et al, 2013 | ultrasoundmammography MRI | 0,12 | oil cysts | — | — | — | — | 0 |
| Rubin et al, 2012 | mammograms | 0,12 | oil cysts calcificationsfat necrosis | — | mammogram | 0,6,12 | — | — |
| Serra-Mestr et al, 2017 | — | — | — | mean inter mammary distance from 3 ± 0.6 cm to 1.7 ± 0.4 cm | intermammary distances | 0,12 | oil cystdissymmetry | 1,2 |
| Sforza et al, 201622 | — | — | — | — | 3D imaging | 0,12 | — | — |
| Spear and Pittman, 201423 | mammograms MRI | 0,12 | BI–RADS 4 mammograms | — | MRI2D3D | 0,12 | — | — |
| Tassinari et al, 2016 | — | — | — | — | — | — | cyst formation | 37 |
| Ueberreiter et al, 2010 | — | — | — | — | MRI | 0,6 | — | — |
| Ueberreiter et al, 2013 | MRI | 0,6 | — | — | MRI | 6 | — | — |
| Veber et al, 2011 | mammograms | 0,16 | microcalcifications macrocalcifications cystic lesions | — | — | — | — | — |
| Visconti and Salgarello, 2019 | mammographyultrasound scans | 0,12 | — | — | — | — | — | 0 |
| Wang et al, 2011 | mammograms | 18 | microcalcifications | — | — | — | microcalcifications | 8 |
| Wang et al, 2012 | MRI | 0,3,6 | — | — | MRI | 0,3,6 | small nodules | 1 |
| Wang et al, 2015 | mammography | 0,3,6 | BI–RADS 2–3 | — | MRI | 0,3,6 | — | 0 |
| Yoshimura et al, 20088 | mammography MRI | 0,6 | cyst microcalcifications | chest circumferenceincreased 4-8cm | clinical MRI | 6 | palpable nodules | 1 |
| Yoshimura et al, 20109 | mammography MRI | 0,12 | — | — | MRI3D measurements | 0,12 | — | 0 |
| Zheng et al, 2008 | ultrasound mammographyMRI | 0,1,3,6,12 | cystsliponecrotic cysts calcification | — | — | — | liponecrotic cysts palpable nodules | 11, 2 |
| Zheng et al, 2019 | MRI | 0,3,6 | — | — | 3D scanner | 0,3 | — | — |
| Zocchi and Zuliani, 20086 | ultrasoundmammography MRI | 0,6,12 | — | — | mammograms ultrasonograms | 12 | liponecrosis microcyst microcalcifications | 5,7 |
| Zocchi, 20177 | — | — | — | — | mammograms ultrasonograms | 12 | infectionslocalized liponecrosisoil cystmacro calcificationslocalized liponecrosis oil cystmacro calcifications | 25,5 |
| Study . | Radiologic examination . | Fellow-up (mo) . | Examination results . | Chest Measurement(cm) . | Volume Measurement Method . | Timing of Measuremet(mo) . | complications . | number . |
|---|---|---|---|---|---|---|---|---|
| Abboud and Dibo, 201524 | ultrasound mammographyMRI | 0,12 | negative for pathologic findings | — | MRIbra size | 0,120,6 | cyst formation infection | 9,2 |
| Auclair, 2009 | mammography | — | — | — | — | — | — | 0 |
| Auclair et al, 201310 | photographsmammography | 0,12 | negative for pathologic findings | — | quantitativethree-dimensional breast imaging | 0,12 | cystic masscapsular contractureinsufficient soft-tissue coveragedonor-site deformity | 2,1,5,1 |
| Atia, 2020 | mammography ultrasound scans | 0,6,12 | solid nodules cystic lesions microcalcifications | — | — | — | pain fat necrosis irregularities fluid collection asymmetry lipo-necrotic cyst | 4,1,3,2,1,1 |
| Auclair, 2020 | mammogram | 0,1,6,12 | — | — | — | — | seromainsufficient soft-tissue coveragecapsular contracture | 2,13,8 |
| Bircoll, 2010 | — | — | — | — | — | — | microcalcifications | 8 |
| Brault et al, 201711 | — | — | — | — | digital imaging software | 0,12 | oil cysts | 2 |
| Bravo, 2014 | — | — | — | mean length between parasternal vertical aesthetic lines preop 1.43 ± 0.61 cm postop 0.60 ± 0.32 cm | photographs | 12 | unilateral Baker grade II capsular contracture | 2 |
| Bresnick, 2016 | MRI | — | — | — | — | — | — | 0 |
| Carvajal and Patino, 2008 | mammogram | 0,6–84 | BI–RADS 2 (85%) and 3 (15%) microcalcifications oil cystslipid cysts | — | — | — | oil cysts | 4 |
| Chiu, 2014 | ultrasound MRI | 0,2–11 0,1–8 | — | mean postoperative change in BCD3.5 cm | physical examination | 6.8 | recipient site infection fat necrosis | 13,7 |
| Chiu, 2016 | ultrasound | 0,3,6,12 | — | breast thickness change was 13.1 mm | ultrasonography | 12 | recipient site infection fat necrosis small induration | 6 |
| Chiu, 201816 | ultrasound MRI | 0,3,6,12 | induration necrosis cysts | — | 3D laser scanning | 0,3,6,12 | indurationnecrosis cysts | 4,6 |
| Claudio et al, 2017 | ultrasound | — | oil cyst | — | — | — | oil cyst | 4 |
| Coleman and Saboeiro, 200713 | mammograms | 0,12 | breast cancer | — | — | — | local infection small nodule calcificationsbreast cancer | 102 |
| Cotrufo et al, 2008 | — | — | — | — | — | — | necrosis | 1 |
| Del Vecchio and Bucky, 2011 | MRI | 0,6 | — | — | MRI | 0,6 | — | 0 |
| Del Vecchio, 201419 | — | — | — | — | quantitative volumetric breast imaging | 0,12 | — | — |
| Delay et al, 200918 | mammography ultrasound MRI | 0.5, 3,12 | oily cysts 15% | — | photographs MRI | 0.5 3 12 | infection | 4 |
| Delay et al, 2013 | mammography ultrasound MRI | 0,12 | oily cysts 20% | — | photographs MRI | 0,12 | — | 0 |
| Derder et al, 201420 | ultrasound mammography MRI | 0,0.5, 3,6,12 | ACR 1(92.3%) ACR 2(7.7%) oil cysts | — | photographs mammography ultrasoundMRI | 0,0.5,3,6,12 | — | — |
| Deschler et al, 202025 | MRI | 0,12 | cytosteatonecrosis | — | — | — | oil cysts hypertrophic scars | 2, 1 |
| Dos Anjos et al, 201517 | ultrasound | 6 | oil cysts | — | 3D | 18 | fat necrosis | 3 |
| Fiaschetti et al, 2013 | mammography ultrasound MRI | 0,3,6 | — | — | mammography ultrasound MRI | 0,3,6,12 | — | — |
| Guo et al, 201821 | MRIultrasound | 0,3 | BI–RADS 1– 2 | — | MRI | 0,3 | — | 0 |
| Gutierrez-Ontalvilla et al, 2020 | ultrasound | 0,1,3,6,12 | oil cysts | — | — | — | painful nodule | 1 |
| Herly et al, 2019 | MRI | — | — | — | MRI | 4.5 | — | — |
| Herold et al, 2010 | MRI | 0,6 | - | MRI | 0,6 | — | ||
| Ho Quoc et al, 201312 | — | — | — | — | — | — | infectionswound healing fat necrosis | 40 |
| Ho Quoc et al, 2015 | MRI | 0,12 | — | — | MRI | 0,12 | — | 0 |
| Illouz and Sterodimas, 2009 | mammography ultrasound | 0,6,12 | BI–RADS 1or 2 | — | MRI | 0,12 | striae hematomas infections | 53 |
| Jung et al, 2016 | mammography ultrasoundMRI | 0,3,12 | BI–RADS 1or 2 oil cysts 3 | — | MRI | 0,3,12 | oil cystssole cyst multiple cysts | 3,1,1 |
| Kamakura and Ito, 2011 | mammograms | 9 | liponecrotic cyst 2 | — | clinicalmammograms | 9 | liponecrotic cyst | 2 |
| Kang and Luan, 201814 | ultrasounds | 3 | — | — | breast palpation ultrasound | 3 | palpable nodules | 21 |
| Kerfant et al, 2017 | mammograms | 0 | ACR 1– 2 | — | MRI | — | hematoma infectionsbaker grade II/III contractures malrotation | 11 |
| Khouri et al, 2012 | mammography ultrasound | 0,3,6,12 | fat necrosis calcifications | — | MRI | 0,3,6,12 | atypicalmycobacterial infection | 1 |
| Khouri et al, 2014 | mammography ultrasound | 12 | cyst | — | 3Dphotographic imagingMRI | 0,6,12 | cyst infectionpneumothorax1 | 24 |
| Klit et al, 20155 | — | — | — | — | — | — | — | 0 |
| La Marca et al, 2013 | — | — | — | — | — | — | — | 0 |
| Li et al, 2014 | mammography MRI | 0 | — | — | — | — | calcificationssmall nodules | 5 |
| Maione et al, 2018 | ultrasound mammograph | 0,0.5,3, 12 | — | — | — | — | — | 0 |
| Muench, 2016 | mammography ultrasound | 0,6 | — | — | — | — | contour irregu- laritiesasymmetries | 6,5 |
| Münch, 2013 | — | — | — | — | — | — | hematomaasymmetries | 9 |
| Ohashi et al, 2016 | ultrasound | 3,6 | partial fat necrosis | overall bust size decreased slightly 2.0 ± 3.0 cm | top bust and under bust measurements | 6 | implant removal seromanoduleshematomafat necrosis | 4,4,1, 3 |
| Özalp and Aydinol, 2017 | ultrasound | 18 | — | Mean increase above inframammary fold 1.3 cm | ultrasonograph | 18 | cyst formationcapsular contracture | 2,4 |
| Peltoniemi et al, 2013 15 | mammogram ultrasound | 0,18,24 | small oil cysts | — | MRI | 6 | cysts | 3 |
| Pinsolle et al, 2008 | — | — | — | — | — | — | necrosis | 1 |
| Quoc et al, 2013 | ultrasoundmammography MRI | 0,12 | oil cysts | — | — | — | — | 0 |
| Rubin et al, 2012 | mammograms | 0,12 | oil cysts calcificationsfat necrosis | — | mammogram | 0,6,12 | — | — |
| Serra-Mestr et al, 2017 | — | — | — | mean inter mammary distance from 3 ± 0.6 cm to 1.7 ± 0.4 cm | intermammary distances | 0,12 | oil cystdissymmetry | 1,2 |
| Sforza et al, 201622 | — | — | — | — | 3D imaging | 0,12 | — | — |
| Spear and Pittman, 201423 | mammograms MRI | 0,12 | BI–RADS 4 mammograms | — | MRI2D3D | 0,12 | — | — |
| Tassinari et al, 2016 | — | — | — | — | — | — | cyst formation | 37 |
| Ueberreiter et al, 2010 | — | — | — | — | MRI | 0,6 | — | — |
| Ueberreiter et al, 2013 | MRI | 0,6 | — | — | MRI | 6 | — | — |
| Veber et al, 2011 | mammograms | 0,16 | microcalcifications macrocalcifications cystic lesions | — | — | — | — | — |
| Visconti and Salgarello, 2019 | mammographyultrasound scans | 0,12 | — | — | — | — | — | 0 |
| Wang et al, 2011 | mammograms | 18 | microcalcifications | — | — | — | microcalcifications | 8 |
| Wang et al, 2012 | MRI | 0,3,6 | — | — | MRI | 0,3,6 | small nodules | 1 |
| Wang et al, 2015 | mammography | 0,3,6 | BI–RADS 2–3 | — | MRI | 0,3,6 | — | 0 |
| Yoshimura et al, 20088 | mammography MRI | 0,6 | cyst microcalcifications | chest circumferenceincreased 4-8cm | clinical MRI | 6 | palpable nodules | 1 |
| Yoshimura et al, 20109 | mammography MRI | 0,12 | — | — | MRI3D measurements | 0,12 | — | 0 |
| Zheng et al, 2008 | ultrasound mammographyMRI | 0,1,3,6,12 | cystsliponecrotic cysts calcification | — | — | — | liponecrotic cysts palpable nodules | 11, 2 |
| Zheng et al, 2019 | MRI | 0,3,6 | — | — | 3D scanner | 0,3 | — | — |
| Zocchi and Zuliani, 20086 | ultrasoundmammography MRI | 0,6,12 | — | — | mammograms ultrasonograms | 12 | liponecrosis microcyst microcalcifications | 5,7 |
| Zocchi, 20177 | — | — | — | — | mammograms ultrasonograms | 12 | infectionslocalized liponecrosisoil cystmacro calcificationslocalized liponecrosis oil cystmacro calcifications | 25,5 |
—, not reported; ACR, American College of Radiology; BCD, breast circumference; BI–RADS, Breast Imaging Reporting and Data System; 2D, 2-dimensional; 3D, 3-dimensional; MRI, magnetic resonance imaging.
Imaging Examination
Almost all of the studies reported 1 or more imaging examinations utilizing ultrasonography, mammography, and magnetic resonance imaging (MRI). Patients underwent preoperative and/or postoperative radiographic imaging. Mammography was the most common, with 24 studies reporting abnormalities, most of which were cysts and calcifications. Eight studies were conducted in accordance with the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) standard mammography evaluation, most of which were BI-RADS 1 or 2 before the operation or BI-RADS 2 or 3 after the operation. In the control examination after 6 months, most of the mammograms classified as BI-RADS 3 were reclassified as BI-RADS 1 or 2. Spear and Pittman23 reported a case of BI-RADS 4 that showed a negative pathological finding after biopsy.
Regarding the assessment of volume gain, most of the studies mainly reported MRI (24 studies) and 3-dimensional (3D) laser scanning (7 studies), which were performed at 3, 6, and 12 months postoperatively. In addition, some other methods were utilized: 5 studies measured the chest circumference, 2 studies measured the distance from the sternum to the inner edge of the breast, 1 study measured the thickness of the breast, and 2 studies employed digital software imaging.
Complications
In the 53 studies retrieved, complications were identified in approximately 8% of patients (388/5162). The main complication was oil cysts. Other common complications were calcification, fat necrosis, small nodule, infection in the receiving area, asymmetry, insufficient transplantation, depression in the donor area, and hematoma. Pneumothorax was rare and occurred in only 1 case. Thirty patients required further surgical treatment.
Oncological Risk
Of 5162 patients followed-up for an average of 22 months (range, 5-72 months), 2 were diagnosed with breast cancer (0.04%). The 2 cases were reported by Coleman and Saboeiro13 in a case series of 15 patients published in 2007, with cancer in the non-transplant area in 1 case and cancer possibly in the transplant area in another case.
Meta-analysis
Patient and Surgeon Satisfaction
The data of 4413 patients in 29 studies were converted by double anti-sine and combined with the random effect model employing the D-L method. After a mean follow-up of 22 months (range, 6 months to 10 years), the meta-analysis revealed an overall patient satisfaction of 93% (95% CI = 89%-97%) (Figure 3). According to the heterogeneity test, there was no significant difference between the subgroups (P > 0.01). Similarly, the satisfaction of surgeons was 87% (95% CI = 80%-94%) in 742 patients from 11 studies (Figure 4).
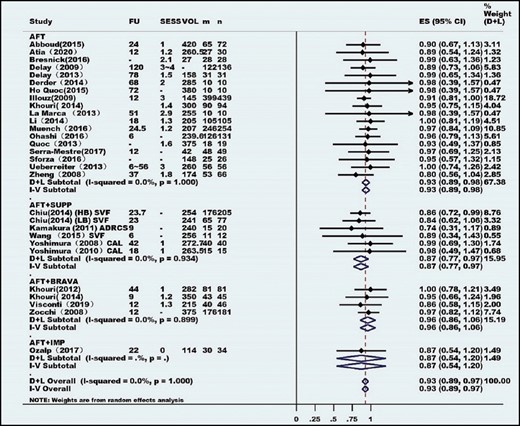
Meta-analysis of patient satisfaction. ADRCS, autologous adipose-derived regenerative cells; AFT, autologous fat transplantation; ASCs, adipose-derived stem cells; CAL, cell-assisted lipotransfer; CI, confidence interval; ES, satisfaction rates; FU, follow-up; IMP, implant; m, number of patients satisfaction; n, total number of patients; PRP, platelet-rich plasma; SESS, number of autologous fat transplantation sessions; SUPP, supplements as SVF, CAL, PRP, or ASCs; SVF, stromal vascular fraction; VOL, mean injected volume.
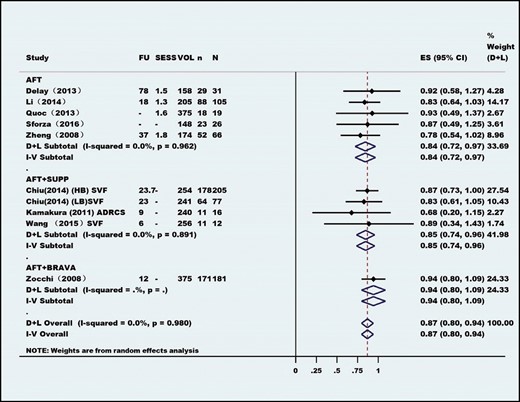
Meta-analysis of surgeon satisfaction. ADRCS, autologous adipose-derived regenerative cells; AFT, autologous fat transplantation; CI, confidence interval; ES, satisfaction rates; FU, follow-up; n, number of patients of surgeon satisfaction; N, total number of patients; SESS, number of autologous fat transplantation sessions; SUPP, supplements as SVF, CAL, PRP, or ASCs; SVF, stromal vascular fraction; VOL, mean injected volume.
Complications
The incidence of AFT-related clinical complications based on our meta-analysis was 8% (95% CI = 5%-11%) in 53 studies (Figure 5). There was no significant heterogeneity between the subgroups (P > 0.01).
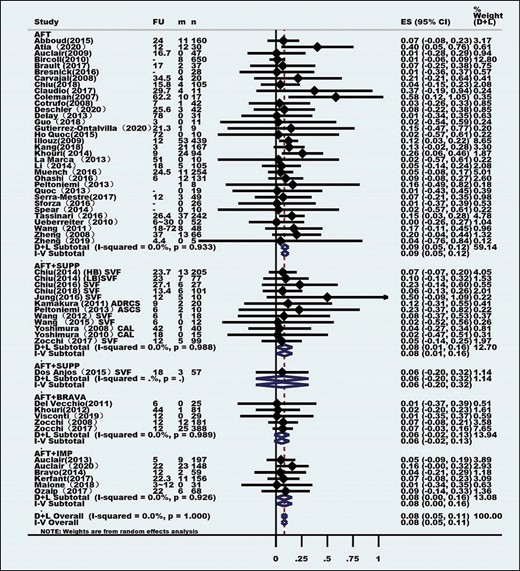
Meta-analysis of the autologous fat transplantation-related complication rates. ADRCS, autologous adipose-derived regenerative cells; AFT, autologous fat transplantation; ASCs, adipose-derived stem cells; CAL, cell-assisted lipotransfer; CI, confidence interval; ES, complication rates; FU, follow-up; IMP, implant; m, number of patients with complication; n, total number of patients; SESS, number of autologous fat transplantation sessions; SUPP, supplements as SVF, CAL, PRP, or ASCs; SVF, stromal vascular fraction.
Number of AFT Sessions
The meta-analysis of data from 2180 patients from 36 studies provided an overall mean number of AFT sessions of 1.56 (95% CI = 1.26-1.85) (Figure 6). The test result of heterogeneity between the subgroups was I2 = 98.8% (P < 0.01). There was significant heterogeneity between the groups, which may be related to the implementation of surgery by different surgeons, patients’ conditions, and perioperative conditions.
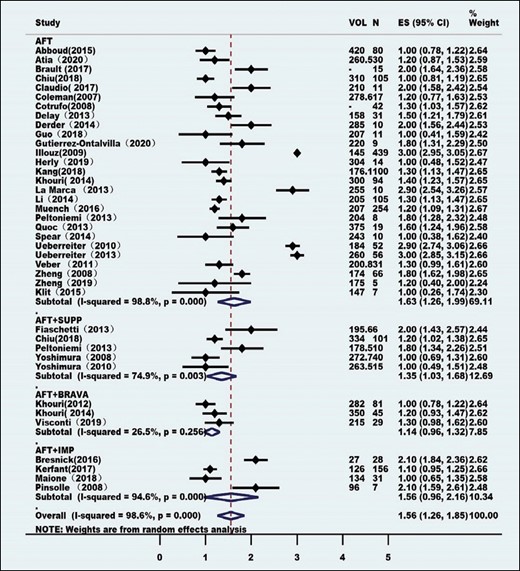
Meta-analysis of the mean number of autologous fat transplantation sessions. AFT, autologous fat transplantation; CI, confidence interval; ES, the mean number of autologous fat transplantation sessions; FU, follow-up; IMP, implant; N, total number of autologous fat transplantation sessions; SUPP, supplements as SVF, CAL, PRP, or ASCs.
Autologous Fat Transplantation-Related Biopsy Rate
The final meta-analysis showed that 5% (95% CI = 2%-12%) of breasts treated with AFT in 12 studies (670 patients) had to eventually undergo additional histopathological examination because of the presence of abnormal radiological images or a clinically detected palpable mass (Figure 7). Among the 30 cases of biopsy, 28 were found to be poor on pathological examination, and 2 were considered breast cancer cases.
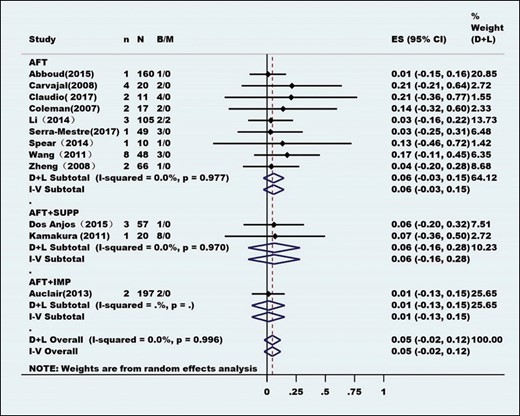
Meta-analysis of the autologous fat transplantation-related biopsy rates. AFT, autologous fat transplantation; B, number of benign lesions (fat necrosis); CI, confidence interval; ES, biopsy rates; IMP, implant; M, number of malignant lesions (cancer relapse); n, total number of biopsies; N, total number of breasts; SUPP, supplements as SVF, CAL, PRP, or ASCs.
Meta-regression Model
Among the results of 21 studies (1483 patients), 3 factors, including the postoperative follow-up time, manual or mechanical liposuction method, and 4 subgroups of fat transplantation methods, were employed as covariates, and the fat survival rate was utilized as the dependent variable to establish the regression model (P < 0.01). The model suggested that there was heterogeneity among the studies in postoperative follow-up duration, especially at 12 months postoperatively (Table 6). There was no heterogeneity (P > 0.01) between the studies that employed the liposuction and fat transplantation methods (Table 7).
Heterogeneity Test Result of Meta-Regression Analysis When Concomitant Variable Was Time. The Fat Survival Rate Was Heterogeneity Among the Studies in Postoperative Follow-Up Duration, Especially at 6 and 12 Months Postoperatively (P < 0.01)
| logy | . | t . | P>|t| . | [95% CI] . | . |
|---|---|---|---|---|
| x2 | | −1.74 | 0.087 | 0.3159328 | 1.084268 |
| x3 | | −3.18 | 0.003 | 0.5750615 | 0.8827491 |
| x4 | | −8.90 | 0.000 | 0.6829244 | 0.7858744 |
| x5 | | −1.88 | 0.067 | 0.5184975 | 1.022669 |
| logy | . | t . | P>|t| . | [95% CI] . | . |
|---|---|---|---|---|
| x2 | | −1.74 | 0.087 | 0.3159328 | 1.084268 |
| x3 | | −3.18 | 0.003 | 0.5750615 | 0.8827491 |
| x4 | | −8.90 | 0.000 | 0.6829244 | 0.7858744 |
| x5 | | −1.88 | 0.067 | 0.5184975 | 1.022669 |
log, logarithm; X, time; x2, 3 months; x3, 6 months; x4, 12 months; x5, 18 months; CI, confidence interval.
Heterogeneity Test Result of Meta-Regression Analysis When Concomitant Variable Was Time. The Fat Survival Rate Was Heterogeneity Among the Studies in Postoperative Follow-Up Duration, Especially at 6 and 12 Months Postoperatively (P < 0.01)
| logy | . | t . | P>|t| . | [95% CI] . | . |
|---|---|---|---|---|
| x2 | | −1.74 | 0.087 | 0.3159328 | 1.084268 |
| x3 | | −3.18 | 0.003 | 0.5750615 | 0.8827491 |
| x4 | | −8.90 | 0.000 | 0.6829244 | 0.7858744 |
| x5 | | −1.88 | 0.067 | 0.5184975 | 1.022669 |
| logy | . | t . | P>|t| . | [95% CI] . | . |
|---|---|---|---|---|
| x2 | | −1.74 | 0.087 | 0.3159328 | 1.084268 |
| x3 | | −3.18 | 0.003 | 0.5750615 | 0.8827491 |
| x4 | | −8.90 | 0.000 | 0.6829244 | 0.7858744 |
| x5 | | −1.88 | 0.067 | 0.5184975 | 1.022669 |
log, logarithm; X, time; x2, 3 months; x3, 6 months; x4, 12 months; x5, 18 months; CI, confidence interval.
Heterogeneity Test Result of Meta-Regression Analysis. The Fat Survival Rate Was No Heterogeneity (P > 0.01) Between the Studies that Used the Liposuction and Fat Transplantation Methods and Was Heterogeneity Among the Studies in Postoperative Follow-Up Time (P < 0.01)
| logy | . | t . | P>|t| . | [95% CI] . | . |
|---|---|---|---|---|
| group | | 0.02 | 0.988 | 0.9948549 | 1.005253 |
| treatgroup | | 0.03 | 0.977 | 0.9973304 | 1.002757 |
| x | | −9.46 | 0.000 | 0.968913 | 0.979682 |
| logy | . | t . | P>|t| . | [95% CI] . | . |
|---|---|---|---|---|
| group | | 0.02 | 0.988 | 0.9948549 | 1.005253 |
| treatgroup | | 0.03 | 0.977 | 0.9973304 | 1.002757 |
| x | | −9.46 | 0.000 | 0.968913 | 0.979682 |
Group, 2 groups: manual liposuction and machine liposuction; treatgroup, 4 treatgroups: AFT (pure autologous fat transplantation), AFT + SUPP (autologous fat transplantation combined supplement), AFT + BRAVA (autologous fat transplantation combined Brava expansion technique), AFT + IMP (autologous fat transplantation combined implants); x, time; CI, confidence interval.
Heterogeneity Test Result of Meta-Regression Analysis. The Fat Survival Rate Was No Heterogeneity (P > 0.01) Between the Studies that Used the Liposuction and Fat Transplantation Methods and Was Heterogeneity Among the Studies in Postoperative Follow-Up Time (P < 0.01)
| logy | . | t . | P>|t| . | [95% CI] . | . |
|---|---|---|---|---|
| group | | 0.02 | 0.988 | 0.9948549 | 1.005253 |
| treatgroup | | 0.03 | 0.977 | 0.9973304 | 1.002757 |
| x | | −9.46 | 0.000 | 0.968913 | 0.979682 |
| logy | . | t . | P>|t| . | [95% CI] . | . |
|---|---|---|---|---|
| group | | 0.02 | 0.988 | 0.9948549 | 1.005253 |
| treatgroup | | 0.03 | 0.977 | 0.9973304 | 1.002757 |
| x | | −9.46 | 0.000 | 0.968913 | 0.979682 |
Group, 2 groups: manual liposuction and machine liposuction; treatgroup, 4 treatgroups: AFT (pure autologous fat transplantation), AFT + SUPP (autologous fat transplantation combined supplement), AFT + BRAVA (autologous fat transplantation combined Brava expansion technique), AFT + IMP (autologous fat transplantation combined implants); x, time; CI, confidence interval.
Twenty-one studies provided data on volume retention, which was plotted against time in a meta-regression model (Figure 8). The meta-regression model trend line was 60% to 70% at 1-year follow-up and reached a plateau at approximately 65% when extrapolated on the long term. Only 1 study (57 patients) provided follow-up data at 18 months, and this had a great influence on the trend-line.
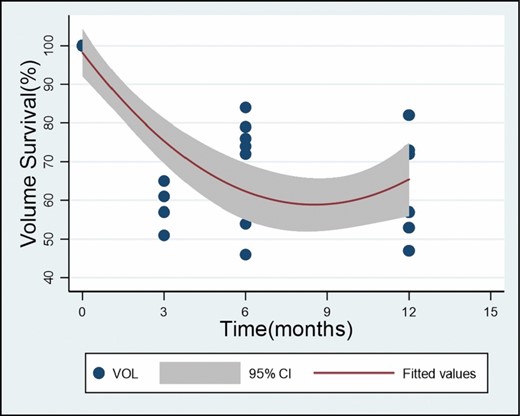
Meta-analysis of graft survival over time. The meta-regression model trendline and respective 95% confidence interval estimate the volume retention over time. CI, confidence interval; VOL, mean injected volume.
DISCUSSION
Synthesis of Results
Over the past decade, there has been renewed interest in AFT for breast augmentation. Instead of focusing on the application of AFT for breast reconstruction, research has gradually shifted to aesthetic breast augmentation, and many studies have been performed. This systematic review and meta-analysis of 6468 cases from 84 studies of aesthetic breast augmentation by AFT in the past 33 years provides a more sophisticated and balanced interpretation of study evidence on AFT to allow healthcare providers to make evidence-based recommendations on the efficacy of the technique.
Sixty-four studies reporting 1 or more relevant outcomes reflecting the efficacy of AFT were selected for this meta-analysis. Patient satisfaction was the most commonly reported outcome, and it was 93% in 4413 unique patients from 29 studies. Similarly, 87% of plastic surgeons were pleased with the result based on preoperative and postoperative photographs. Other evaluation methods of breast augmentation were the satisfaction rating, Likert scale, visual analog scale, and Breast-Q. Only 5 studies11,21,23-25 employed the Breast-Q to investigate patient satisfaction (158 patients), which involves the evaluation of breast shape, size, and texture as well as the quality of life. Volume retention was evaluated in 1483 patients from 21 studies by performing serial measurements utilizing 3D imaging, MRI, or computed tomography. In the past, only MRI and computed tomography could achieve such measurements, but their utilization was limited because of their high cost and high radiation exposure. The recent development of 3D imaging equipment made these measurements more practical, and it is the most suitable and reliable method for correcting insufficient volume in AFT. Volume retention results varied greatly between studies, but the meta-regression model against time showed 50% volume retention at 1 year. Unfortunately, the data were severely limited, because measurements beyond 6 and 12 months were lacking. Moreover, the possible effects of other important confounders such as fat harvesting, fat processing, and fat reinjection could not be demonstrated until now. Interestingly, the overall number of sessions required was relatively low (1.56). Although it can be argued that this number would become higher if excessive fat resorption would result in patients demanding extra AFT sessions, the mean follow-up duration of 22 months would be sufficient to accommodate for this. Unfortunately, the rate of clinical complications per procedure was 8%, and these complications were not minor. Finally, the risk that a breast treated with AFT would result in clinical or radiological abnormalities and require histopathological examination (biopsy) was 5%. Two cases of cancer diagnosis were reported among the 5162 patients (0.04%). At present, there is no special study on tumor oncological safety of AFT to our knowledge, but these cases were not representative of the whole population. Further study with long-term follow-up is needed.
Limitations
This meta-analysis has a couple of limitations. First, there was a low level of evidence due to the lack of RCTs. AFT has become a part of clinical practice, and it may be considered unethical to establish an RCT with a standard blind method and control group. In addition, because of the lack of appropriate and safe alternative methods, few studies have successfully included a control group. Therefore, data collected over the past 30 years were sufficient for a single-arm meta-analysis. By determining the size, direction, and CI of each result, the comprehensive effect evaluation of a large number of observational studies is mainly provided. Second, standard and validated measurement results tools (eg, Breast-Q) were rarely employed to improve the effectiveness and comparability of different studies. Over the years, researchers have relied on various unverified, customized scales, and questionnaires or utilized preoperative and postoperative photographs. Third, the follow-up duration was too short. If examining cancer and outcomes of AFT, 2 years would be the minimum follow-up. In addition, the results of effect indicators are often regarded as continuous data (mean ± standard deviations) or classified variables (frequency). The main problem with continuous data is that there is no direct reference value or comparison between the control group and the combined effect results. Even if these data are available, it is difficult to assess whether the overall data have the same distribution. However, classification methods (eg, dichotomies) tend to oversimplify results by assigning patients to specific categories (dissatisfaction and satisfaction). Because these data do not depend on the reference value, they can more accurately reflect the absolute effect of autogenous fat transplantation, but they lack the accuracy of continuous data. As with all meta-analyses, heterogeneity between studies can have an important effect on the results. Therefore, all of the analyses in this study adopted the random effect model and analyzed different interference measures in the subgroups to consider the heterogeneity between the studies.
Future Recommendations
This meta-analysis provides strong evidence for the overall efficacy of AFT in breast augmentation and establishes a foundation for further research in this field. A specific subgroup analysis will improve the quality of future research methods. Such future studies should be conducted employing prospective study designs, including appropriate control groups and the utilization of validated outcome measurement instruments or standards. According to the current data, in the next 5 years, the global plastic surgery community should solve the following research problems. First, clarify the long-term oncological safety of AFT. Second, determine the best AFT technology that can predict the long-term survival rate. Third, determine the factors that affect the survival rate, such as height, chest circumference, and BMI, and the relationship between them as well as the maximum amount of AFT.
CONCLUSIONS
After an average of 1.56 operations (range, 1-5), patient satisfaction (93%) and surgeon satisfaction (87%) reached high levels, indicating that AFT has a significant effect on breast volume and contour improvement in breast augmentation. The volume retention after 1 year was 60% to 70%; however, further research is needed to confirm this finding. Mild complications occurred in 8% of patients, and biopsies were performed in 5% of patients. AFT can be employed as one of the methods of breast augmentation. However, preoperative imaging examinations, such as breast ultrasonography, mammography, or MRI, should be performed. The indications of AFT should be clear, the operation should be performed carefully and in a standardized manner, complications should be avoided as much as possible, and long-term follow-up should be conducted after the operation to ensure the oncological safety of AFT.
Acknowledgments
We thank Editage (www.editage.cn) for English language editing.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Availability of Data and Materials
The datasets generated in this study are available from the corresponding author on reasonable request.
REFERENCES
Author notes
Dr Li is a plastic surgeon in private practice in Nanning City, Guangxi, China



