-
PDF
- Split View
-
Views
-
Cite
Cite
Graeme Ewan Glass, Photobiomodulation: The Clinical Applications of Low-Level Light Therapy, Aesthetic Surgery Journal, Volume 41, Issue 6, June 2021, Pages 723–738, https://doi.org/10.1093/asj/sjab025
Close - Share Icon Share
Abstract
Low-level light therapy (LLLT) is a recent addition to the pantheon of light-based therapeutic interventions. The absorption of red/near-infrared light energy, a process termed “photobiomodulation,” enhances mitochondrial ATP production, cell signaling, and growth factor synthesis, and attenuates oxidative stress. Photobiomodulation is now highly commercialized with devices marketed directly to the consumer. In the gray area between the commercial and therapeutic sectors, harnessing the clinical potential in reproducible and scientifically measurable ways remains challenging.
The aim of this article was to summarize the clinical evidence for photobiomodulation and discuss the regulatory framework for this therapy
A review of the clinical literature pertaining to the use of LLLT for skin rejuvenation (facial rhytids and dyschromias), acne vulgaris, wound healing, body contouring, and androgenic alopecia was performed.
A reasonable body of clinical trial evidence exists to support the role of low-energy red/near-infrared light as a safe and effective method of skin rejuvenation, treatment of acne vulgaris and alopecia, and, especially, body contouring. Methodologic flaws, small patient cohorts, and industry funding mean there is ample scope to improve the quality of evidence. It remains unclear if light-emitting diode sources induce physiologic effects of compararable nature and magnitude to those of the laser-based systems used in most of the higher-quality studies.
LLLT is here to stay. However, its ubiquity and commercial success have outpaced empirical approaches on which solid clinical evidence is established. Thus, the challenge is to prove its therapeutic utility in retrospect. Well-designed, adequately powered, independent clinical trials will help us answer some of the unresolved questions and enable the potential of this therapy to be realized.
Light and Skin Aging
As we age, so, inevitably, does our skin. Intrinsic skin aging occurs as a result of the relentless passage of time, whereas extrinsic aging arises as the cumulative result of our environmental exposures.1 With age, progressive loss of telomere length leads to cellular senescence and a failure of cell-mediated tissue regeneration, the histopathologic manifestations of which include thinning of both the epidermis and dermis, flattening of the rete ridges, and decline in synthesis of type 1 collagen.2 Changes in soft tissue volume and distribution and in the structure of the skeletal framework lead to age-associated facial aging.3,4 Concurrently, extrinsic changes manifest as loss of tone and elasticity caused by fragmentation of collagen, elastin, and anchoring fibrils induced by alterations in the ratio of matrix metalloproteinase to metalloproteinase-inhibitor expression; loss of extracellular matrix glycosaminoglycans; and pigmentary variations (ephilides/freckles and lentigines) due to localized changes in melanocyte and melanosome activity.5 The basis for extrinsic aging is free radical damage to nuclear and mictochondrial DNA, cellular proteins, and cellular and mitochondrial membrane lipoproteins causing apoptosis and necrosis. Modulating these manifestations of aging is both a societal preoccupation and a growth industry projected to be worth $10 billion annually within the next few years.6
The use of light as a therapeutic intervention is an ancient concept. In its modern incarnation, light therapy probably began with the selective use of ultraviolet radiation to treat lupus vulgaris, an innovation for which the Nobel Prize for medicine was awarded in 1903.7 Recently, low-level light therapy (LLLT) has been added to the pantheon of light-based therapies. This type of therapy is based on photobiomodulation,8 a cascade of clinically and aesthetically beneficial cellular responses to nonablative red and near-infrared light.9 Before we evaluate the clinical evidence for the use of LLLT, we will first examine this option in the context of other light-based therapies utilized by aesthetic surgeons and practitioners.
Lasers
Over the last 30 years, lasers have become a well-established method of energy delivery to the skin for the purpose of inducing the tissue repair cascade (photorejuvenation). Their therapeutic utility lies with the specificity of the monochromic light for specific photoacceptor molecules (chromophores). Additionally, the light is monophasic, collimated, polarized, and coherent, and these features may confer additional photobiomodulatory advantages. Whereas the chromophore absorbs the light energy, adjacent molecules do not. When the chromophore is water (the major constituent of cell cytoplasm and extracellular matrix), cell lysis and protein denaturation induces an immune response and hence the wound-healing cascade. Ablative laser rejuvenation (eg, CO2 laser treatment) results in loss of the epidermal barrier, whereas nonablative laser rejuvenation (eg, Nd:YAG laser treatment) penetrates to the dermis without disturbing the overlying epidermis. Pigment (ephilides and lentigines) and hemoglobin (telangiectasias) may also be targeted specifically. The efficacy of laser photorejuvenation is well supported but traditional lasers are expensive and subject to stringent regulation, making them suitable for use only in a clinical setting. Moreover, a narrow beam width limits their use for the rejuvenation of large surface areas unless directed by an experienced practitioner.
Intense Pulsed Light
The traditional, nonablative alternative to laser resurfacing is intense (intermittent) pulsed light (IPL) therapy, which uses pulses of high-energy, polychromatic light to heat target tissue. Filters are sometimes used to achieve a degree of selectivity for target chromophores. Again, the theoretical principle is based on photothermolysis but IPL, delivering light at wavelengths of 500 to 1200 nm, exhibits less chromophore selectivity than monochromatic lasers, even when filters are used, and relies on the fact that chomophores may effectively absorb light energy within a range of wavelengths either side of their absorption peak.10 IPL, filtered to selectively remove light at shorter wavelengths and delivered at a fluence of 30 to 60 J/cm2 has been shown to be efficacious in the management of superficial rhytids and vascular lesions.11-13 The perceived advantages of IPL over lasers include versatility of clinical use owing to the use of different filters and their large footprint enabling swift coverage of large surface areas. The disadvantages include the lack of chromophore specificity.14 IPL has also found favor when used in conjunction with topically applied or systemic photosensitizers which accumulate in target tissue and are activated by changes in molecular structure following absorption of light energy. This technique is the basis of photodynamic therapy (PDT).
Low-Level Light Therapy
Around 50 years ago, experiments were performed to establish the oncologic safety of low-energy defocused red laser light by irradiating shaved murine skin. The investigators found no evidence of neoplastic changes but did observe an unexpected acceleration in subsequent hair regrowth.15 The same group later observed enhanced wound healing in various wound models following irradiation with defocused red laser light.16 Subsequent in vitro experiments reported enhanced cell proliferation following irradiation with red and near-infrared light.17 In the 1970s, 1980s, and early 1990s Karu and colleagues undertook much of the experimental work that was crucial in unraveling the physiologic mechanisms responsible for these findings. They established that activation of cytochrome c oxidase boosts mitochondrial ATP production, which, in turn, enhances the metabolic activity of the cell. Simultaneously, regulation of the reduction/oxidation (redox) state of the intracellular microenvironment favors the expression of genes associated with tissue regeneration and repair. Immune modulation ensures a coordinated regenerative effort. Crucially, these processes take place in the absence of inciting tissue injury, photothermal effects, or photoacoustic effects.18-20
Simultaneously, the National Aeronautics and Space Administration was at the forefront of the development of light-emitting diodes (LEDs) with near-monochromatic light at the red (670, 720 nm) and near-infrared (880 nm) end of the visible spectrum. Initially designed for plant growth experiments in space, these LEDs were also found to enhance cellular proliferation in vitro and to improve wound healing in a number of experimental and clinical studies.21 These findings led to speculation that LEDs might be used not only to minimize tissue atrophy among astronauts at zero gravity22 but might also be employed as an alternative source of light for photorejuvenation.23
Today LLLT (being the method by which photobiomodulation is induced) is in widespread use. As with IPL before it, it has taken many years for the therapeutic potential of photobiomodulation to become generally accepted, and controversies remain.24 Because LEDs operate at power levels below that which is considered by the FDA to constitute a medical hazard25 they have not been subject to therapeutic device regulation and this has paved the way for the commercial exploitation. The number of controllable variables, including wavelength, spatial coherence, polarity (the geometric orientation of the light wave with respect to the direction of travel), pulse structure, fluence (total energy), irradiance (energy density), and exposure frequency have rendered LLLT challenging to study through clinical trials or systematic reviews.26 A great deal of preclinical research has now been done to optimize these parameters. To summarize, the physiologic potential of photobiomodulation cannot be harnessed by light at one wavelength alone. Red and near-infrared light is associated with the proliferation of a number of cell types across different species.27-31 The optimal fluence is probably no greater than 4 J/cm2.32,33 When it contacts tissue, coherent (laser) light produces an event known as speckle and the resultant polarization phenomenon allows light to be absorbed by deep tissues at intensity thresholds sufficient to initiate desirable biochemical cascades.34,35 Speckle and polarization do not arise with LED illumination and this has led some to postulate that LLLT delivered by laser light is more effective when the target lies deeper than a few millimeters. Studies examining the polarity of light have yielded some interesting data but polarity and coherence remain intimately connected and there is not yet enough experimental evidence to inform clinical practice.36 A diagrammatic summary of the physiologic effects of LLLT is shown in Figure 1.
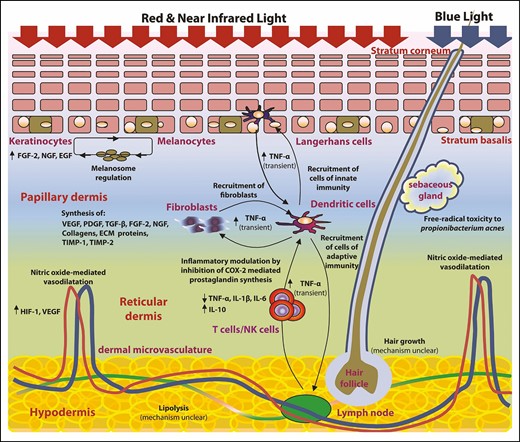
Diagrammatic summary of the effects of red/near-infrared and blue light on epidermis, dermis, sebaceous glands, hair follicles, and subcutaneous fat. ECM, extracellular matrix; EGF, epidermal growth factor; FGF-2, fibroblast growth factor 2; HIF-1, hypoxia-inducible factor 1; IL, interleukin; NGF, nerve growth factor; PDGF, platelet-derived growth factor; TIMP, tissue inhibitor of matrix metalloproteinase; TGF-β, transforming growth factor β; TNF-α: tissue necrosis factor α; VEGF, vascular endothelial growth factor.
The Commercialization of LLLT
There are many commercially available LED devices for skin rejuvenation. Some of these systems are designed for clinic use although the majority have been marketed directly to the consumer for domestic use. Most have proprietary elements or innovations to differentiate them from their competitors. An exhaustive list is beyond the scope of this paper (see Supplemental Table). The efficacy of LLLT has been investigated by way of preclinical proof-of-concept studies and clinical trials for a number of indications of interest to plastic and/or aesthetic surgeons. A review of the literature was conducted as described below.
METHODS
A list of therapeutic indications for LLLT was established by literature review (performed by G.G. and repeated by A.H. and A.S. as per the Acknowledgments). Thus, 3 independent searches were performed. Discrepancies were handled by the sole author (G.G.) who made the final decision on source inclusion. The search was conducted with Google Scholar (no date limit; Google, Mountain View, CA), PubMed (no date limit; United States National Library of Medicine [NLM], Bethesda, MD), Ovid MEDLINE (January 1980 to June 2020; Wolters Kluwer, Alphen aan den Rijn, the Netherlands), the Cochrane Database of Systematic Reviews, and the Cochrane Controlled Trials Register (searched June 6, 2020; Wiley, Hoboken, NJ). For each indication of relevance to plastic and/or aesthetic surgeons, the literature was examined to establish the existence (or otherwise) of proof of efficacy in principle and peer-review clinical evidence of efficacy and/or safety. In each case the parameters of light therapy were noted, including the source of light, wavelength, and fluence (total energy per unit area), as well as the characteristics of the study, including participants, therapeutic protocol, objective outcome measures, and findings. Synthesis of the evidence took the form of a narrative review as the source data were too heterogeneous to be able to draw meaningful conclusions from systematic review methodology. For the same reason, it was not possible to conduct a meta-analysis of the data for any indication.
Exclusion Criteria
Only LLLT was considered here. Focused laser therapy, IPL, and PDT were excluded. Established or experimental uses for LLLT that fall outside the remit of the plastic and/or aesthetic surgeon, such as psoriasis, joint pain, neonatal jaundice, and seasonal affective disorder, were also excluded. Low-quality clinical trials that relied exclusively on subjective outcome measures were not considered.
RESULTS
LLLT for Skin Rejuvenation
Skin rejuvenation is the focus of much of the experimental and clinical evidence for LLLT. The experimental evidence for LLLT and skin rejuvenation can be subdivided into evidence for collagen and extracellular matrix regeneration, regulation of melanogenesis, regulation of sebum production, perifollicular inflammation, and microbial activity. The experimental findings translate to studies examining the influence of LLLT on facial rhytids, dyschromias, and acne vulgaris.
Facial Rhytids
A proof-of-concept (phase 2) study evaluating the morphologic histology of human skin samples harvested from 6 volunteers after exposure to LED phototherapy at 633 nm (Omnilux Revive, GlobalMed Technologies, Napa, CA; 8 sessions over 8 weeks at 94 J/cm2) reported increased numbers of dermal fibroblasts and increased numbers of mitochondria and vimectin filaments (hence metabolic activity) within the fibroblasts after treatment.37 In a randomized, double-blinded, placebo-controlled, split-face clinical trial of LED phototherapy for skin rejuvenation, Lee et al observed enhanced fibroblast activity, collagen and elastin synthesis, and expression of the proinflammatory cytokines interleukin 1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α) and tissue inhibitors of matrix metalloproteinase (TIMP-1 and TIMP-2) in response to LED light at 830 nm, 633 nm or both (alternately) for 20 minutes twice per week; the observed effects persisted for up to 12 weeks after cessation of therapy.38 Clinically, this manifested as reduced wrinkles and improved skin elasticity, measured objectively by profilometric evaluation of silicon imprints and with use of a device to measure skin elasticity (Cutometer, Courage & Khazaka Electronic GmbH, Köln, Germany), respectively. These data suggest that red and near-infrared light promoted skin rejuvenation and did so through mechanisms akin to tissue healing following trauma, as has been observed in other wound-healing studies.39 Similarly, Weiss et al reported improvements in skin appearance, profilometric smoothness, and dermal collagen deposition with a pulsed LED device at 590 nm twice weekly for 4 weeks delivering 0.1 J/cm2 per treatment. Their study was nonrandomized, noncontrolled, and partially blinded.40 A number of additional studies have also been published,41-44 yielding variable results as summarized in Table 1. This summary table excludes self-reported and subjective outcomes that almost all studies included as part of their results. Examples of the authors’ experience with photorejuvenation using LLLT are shown in Figure 2.
Clinical Trials of LLLT Alone for Facial Rhytids (Quantifiable Objective Measures)
| Study . | Light source . | Λ (nm)/ fluence (J/cm2) . | Study participants/design . | Protocol . | Measurement . | Findings . | Notes . |
|---|---|---|---|---|---|---|---|
| Bhat et al41 | OmniLux Revive LED, Globalmed Technologies, Napa, CA | 630/96 | 22 participants (of 23); blinded photographic assessment | Hemiface, 9 sessions over 3 weeks; results evaluated at weeks 3, 8, and 12 | Cutometer | No difference in elasticity and hydration. Some evidence of improvement on photographic assessment | Poor correlation between perceived treatment response and side of treatment |
| Lee et al38 | Omnilux Revive and Omnilux Plus, Globalmed Technologies, Napa, CA | 633 and 830/126 and 66 | 76 participants (of 112); 4 study groups: 633 nm only; 830 nm only; 633 and 830 nm sham treatment | Hemiface, 8 sessions over 4 weeks; results evaluated at 3 weeks during treatment, and at 2, 4, 8, and 12 weeks posttreatment; compared with baseline | Profilometry, Mexameter (melanin), Cutometer, RT-PCR, histology, electron microscopy | Significant improvements in wrinkle reduction, skin elasticity; increase in number and metabolic activity of fibroblasts; increase in collagen and elastin fibers; increase in IL-1β, TNF-α and reduction in IL-6; benefits persisted to the end of the study period | Power calculation used to support study design; placebo controlled; attempted double blinding; high dropout rate (almost half in sham group) |
| Nam et al44 | SkinLabs red LED and white LED, BS & Co, Seoul, Korea | 640-680 nm (red) or 411-777 nm (polychromatic)/5.2 | 50 participants (of 52) | Full face once-daily session for 12 weeks; results evaluated at weeks 0, 6, and 12 | Profilometry; blinded photographic assessment | Slight improvement over 12 weeks with both red and white LEDs; no difference between light sources | Claim of efficacy for red light LED on basis of slight difference in improvement grade; statistically dubious |
| Russell et al43 | Omnilux Revive and Omnilux Plus, Globalmed Technologies, Napa, CA | 633 and 830/126 and 66 | 31 participants (of 38) | Full face, 9 sessions over 5 weeks; results evaluated at weeks 6, 9, and 12 | Profilometry | Significant increase in smoothness on profilometry at week 12 | Comparison made with baseline (pretreatment) |
| Weiss et al40 | Gentlewaves LED, Virginia Beach, VA | 590/0.1 | 90 participants (of 90); partially blinded photographic assessment | Full face, 8 sessions over 4 weeks; results evaluated at weeks 4, 8, 12, 26, and 52 | Profilometry; histology | Improvements in (blinded) appearance on photographic review; smoothness in profilometry and collagen deposition | Comparison made with baseline (pretreatment); investigators not told which photos were pre- and posttreatment |
| Wunsch and Matuschka42 | Fluorescent lamps | 611–650 (red) or 570–850 (polychromatic)/9 | 128 participants (of 144); 4 study groups (+ 23 controls) | Full face, 30 sessions over 15 weeks; results evaluated 6 months after completion | Profilometry; ultrasound-measured collagen density | Significant improvements in collagen density on ultrasound and smoothness on profilometry | No difference between red and polychromatic light |
| Study . | Light source . | Λ (nm)/ fluence (J/cm2) . | Study participants/design . | Protocol . | Measurement . | Findings . | Notes . |
|---|---|---|---|---|---|---|---|
| Bhat et al41 | OmniLux Revive LED, Globalmed Technologies, Napa, CA | 630/96 | 22 participants (of 23); blinded photographic assessment | Hemiface, 9 sessions over 3 weeks; results evaluated at weeks 3, 8, and 12 | Cutometer | No difference in elasticity and hydration. Some evidence of improvement on photographic assessment | Poor correlation between perceived treatment response and side of treatment |
| Lee et al38 | Omnilux Revive and Omnilux Plus, Globalmed Technologies, Napa, CA | 633 and 830/126 and 66 | 76 participants (of 112); 4 study groups: 633 nm only; 830 nm only; 633 and 830 nm sham treatment | Hemiface, 8 sessions over 4 weeks; results evaluated at 3 weeks during treatment, and at 2, 4, 8, and 12 weeks posttreatment; compared with baseline | Profilometry, Mexameter (melanin), Cutometer, RT-PCR, histology, electron microscopy | Significant improvements in wrinkle reduction, skin elasticity; increase in number and metabolic activity of fibroblasts; increase in collagen and elastin fibers; increase in IL-1β, TNF-α and reduction in IL-6; benefits persisted to the end of the study period | Power calculation used to support study design; placebo controlled; attempted double blinding; high dropout rate (almost half in sham group) |
| Nam et al44 | SkinLabs red LED and white LED, BS & Co, Seoul, Korea | 640-680 nm (red) or 411-777 nm (polychromatic)/5.2 | 50 participants (of 52) | Full face once-daily session for 12 weeks; results evaluated at weeks 0, 6, and 12 | Profilometry; blinded photographic assessment | Slight improvement over 12 weeks with both red and white LEDs; no difference between light sources | Claim of efficacy for red light LED on basis of slight difference in improvement grade; statistically dubious |
| Russell et al43 | Omnilux Revive and Omnilux Plus, Globalmed Technologies, Napa, CA | 633 and 830/126 and 66 | 31 participants (of 38) | Full face, 9 sessions over 5 weeks; results evaluated at weeks 6, 9, and 12 | Profilometry | Significant increase in smoothness on profilometry at week 12 | Comparison made with baseline (pretreatment) |
| Weiss et al40 | Gentlewaves LED, Virginia Beach, VA | 590/0.1 | 90 participants (of 90); partially blinded photographic assessment | Full face, 8 sessions over 4 weeks; results evaluated at weeks 4, 8, 12, 26, and 52 | Profilometry; histology | Improvements in (blinded) appearance on photographic review; smoothness in profilometry and collagen deposition | Comparison made with baseline (pretreatment); investigators not told which photos were pre- and posttreatment |
| Wunsch and Matuschka42 | Fluorescent lamps | 611–650 (red) or 570–850 (polychromatic)/9 | 128 participants (of 144); 4 study groups (+ 23 controls) | Full face, 30 sessions over 15 weeks; results evaluated 6 months after completion | Profilometry; ultrasound-measured collagen density | Significant improvements in collagen density on ultrasound and smoothness on profilometry | No difference between red and polychromatic light |
Λ, wavelength; IL, interleukin; LED, light-emitting diode; LLLT, low-level light therapy; RT-PCR, reverse transcriptase-polymerase chain reaction; TNF, tissue necrosis factor.
Clinical Trials of LLLT Alone for Facial Rhytids (Quantifiable Objective Measures)
| Study . | Light source . | Λ (nm)/ fluence (J/cm2) . | Study participants/design . | Protocol . | Measurement . | Findings . | Notes . |
|---|---|---|---|---|---|---|---|
| Bhat et al41 | OmniLux Revive LED, Globalmed Technologies, Napa, CA | 630/96 | 22 participants (of 23); blinded photographic assessment | Hemiface, 9 sessions over 3 weeks; results evaluated at weeks 3, 8, and 12 | Cutometer | No difference in elasticity and hydration. Some evidence of improvement on photographic assessment | Poor correlation between perceived treatment response and side of treatment |
| Lee et al38 | Omnilux Revive and Omnilux Plus, Globalmed Technologies, Napa, CA | 633 and 830/126 and 66 | 76 participants (of 112); 4 study groups: 633 nm only; 830 nm only; 633 and 830 nm sham treatment | Hemiface, 8 sessions over 4 weeks; results evaluated at 3 weeks during treatment, and at 2, 4, 8, and 12 weeks posttreatment; compared with baseline | Profilometry, Mexameter (melanin), Cutometer, RT-PCR, histology, electron microscopy | Significant improvements in wrinkle reduction, skin elasticity; increase in number and metabolic activity of fibroblasts; increase in collagen and elastin fibers; increase in IL-1β, TNF-α and reduction in IL-6; benefits persisted to the end of the study period | Power calculation used to support study design; placebo controlled; attempted double blinding; high dropout rate (almost half in sham group) |
| Nam et al44 | SkinLabs red LED and white LED, BS & Co, Seoul, Korea | 640-680 nm (red) or 411-777 nm (polychromatic)/5.2 | 50 participants (of 52) | Full face once-daily session for 12 weeks; results evaluated at weeks 0, 6, and 12 | Profilometry; blinded photographic assessment | Slight improvement over 12 weeks with both red and white LEDs; no difference between light sources | Claim of efficacy for red light LED on basis of slight difference in improvement grade; statistically dubious |
| Russell et al43 | Omnilux Revive and Omnilux Plus, Globalmed Technologies, Napa, CA | 633 and 830/126 and 66 | 31 participants (of 38) | Full face, 9 sessions over 5 weeks; results evaluated at weeks 6, 9, and 12 | Profilometry | Significant increase in smoothness on profilometry at week 12 | Comparison made with baseline (pretreatment) |
| Weiss et al40 | Gentlewaves LED, Virginia Beach, VA | 590/0.1 | 90 participants (of 90); partially blinded photographic assessment | Full face, 8 sessions over 4 weeks; results evaluated at weeks 4, 8, 12, 26, and 52 | Profilometry; histology | Improvements in (blinded) appearance on photographic review; smoothness in profilometry and collagen deposition | Comparison made with baseline (pretreatment); investigators not told which photos were pre- and posttreatment |
| Wunsch and Matuschka42 | Fluorescent lamps | 611–650 (red) or 570–850 (polychromatic)/9 | 128 participants (of 144); 4 study groups (+ 23 controls) | Full face, 30 sessions over 15 weeks; results evaluated 6 months after completion | Profilometry; ultrasound-measured collagen density | Significant improvements in collagen density on ultrasound and smoothness on profilometry | No difference between red and polychromatic light |
| Study . | Light source . | Λ (nm)/ fluence (J/cm2) . | Study participants/design . | Protocol . | Measurement . | Findings . | Notes . |
|---|---|---|---|---|---|---|---|
| Bhat et al41 | OmniLux Revive LED, Globalmed Technologies, Napa, CA | 630/96 | 22 participants (of 23); blinded photographic assessment | Hemiface, 9 sessions over 3 weeks; results evaluated at weeks 3, 8, and 12 | Cutometer | No difference in elasticity and hydration. Some evidence of improvement on photographic assessment | Poor correlation between perceived treatment response and side of treatment |
| Lee et al38 | Omnilux Revive and Omnilux Plus, Globalmed Technologies, Napa, CA | 633 and 830/126 and 66 | 76 participants (of 112); 4 study groups: 633 nm only; 830 nm only; 633 and 830 nm sham treatment | Hemiface, 8 sessions over 4 weeks; results evaluated at 3 weeks during treatment, and at 2, 4, 8, and 12 weeks posttreatment; compared with baseline | Profilometry, Mexameter (melanin), Cutometer, RT-PCR, histology, electron microscopy | Significant improvements in wrinkle reduction, skin elasticity; increase in number and metabolic activity of fibroblasts; increase in collagen and elastin fibers; increase in IL-1β, TNF-α and reduction in IL-6; benefits persisted to the end of the study period | Power calculation used to support study design; placebo controlled; attempted double blinding; high dropout rate (almost half in sham group) |
| Nam et al44 | SkinLabs red LED and white LED, BS & Co, Seoul, Korea | 640-680 nm (red) or 411-777 nm (polychromatic)/5.2 | 50 participants (of 52) | Full face once-daily session for 12 weeks; results evaluated at weeks 0, 6, and 12 | Profilometry; blinded photographic assessment | Slight improvement over 12 weeks with both red and white LEDs; no difference between light sources | Claim of efficacy for red light LED on basis of slight difference in improvement grade; statistically dubious |
| Russell et al43 | Omnilux Revive and Omnilux Plus, Globalmed Technologies, Napa, CA | 633 and 830/126 and 66 | 31 participants (of 38) | Full face, 9 sessions over 5 weeks; results evaluated at weeks 6, 9, and 12 | Profilometry | Significant increase in smoothness on profilometry at week 12 | Comparison made with baseline (pretreatment) |
| Weiss et al40 | Gentlewaves LED, Virginia Beach, VA | 590/0.1 | 90 participants (of 90); partially blinded photographic assessment | Full face, 8 sessions over 4 weeks; results evaluated at weeks 4, 8, 12, 26, and 52 | Profilometry; histology | Improvements in (blinded) appearance on photographic review; smoothness in profilometry and collagen deposition | Comparison made with baseline (pretreatment); investigators not told which photos were pre- and posttreatment |
| Wunsch and Matuschka42 | Fluorescent lamps | 611–650 (red) or 570–850 (polychromatic)/9 | 128 participants (of 144); 4 study groups (+ 23 controls) | Full face, 30 sessions over 15 weeks; results evaluated 6 months after completion | Profilometry; ultrasound-measured collagen density | Significant improvements in collagen density on ultrasound and smoothness on profilometry | No difference between red and polychromatic light |
Λ, wavelength; IL, interleukin; LED, light-emitting diode; LLLT, low-level light therapy; RT-PCR, reverse transcriptase-polymerase chain reaction; TNF, tissue necrosis factor.
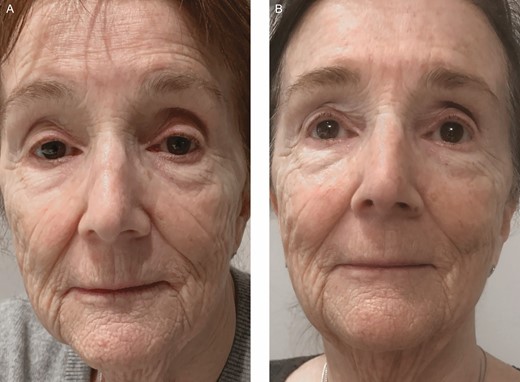
Clinical example of skin rejuvenation (rhytids and dyschromia) with LLLT. A 73-year-old female treated with a homeuse dual-wavelength LLLT device at 470 and 808 nm for 20 minutes/day for 12 weeks. (A) Pretreatment and (B) 1 week after discontinuation of treatment. LLLT, low-level light therapy.
Dyschromias
Lan et al irradiated melanoblast and melanocyte cells lines with a low-energy He-Ne laser at 632.8 nm and demonstrated that LLLT induced responses that were dependent on the maturation stage of the cell. Melanoblasts exhibited enhanced migration whereas melanocytes exhibited enhanced melanogenesis.45 They further demonstrated melanocyte proliferation by the He-Ne laser and provided evidence in support of a mitochondrial focus for this stimulus, in keeping with our understanding of the role of mitochondrial ATP production in the physiology of photobiomodulation.46
In a review of therapeutic interventions for vitiligo, Mandel et al mentioned hitherto unpublished data in which they observed marked repigmentation in two-thirds of patients treated with a prolonged regimen (5 days per week for 6-8 months) of low-energy laser therapy at 623 nm.47 Yu et al demonstrated variable repigmentation in 30 patients with vitiligo treated with a He-Ne laser at 3 J/cm2. After a mean of 16 treatments (once to twice weekly), 60% of patients exhibited at least 50% repigmentation per surface area of the lesion. The study was not blinded and a maintenance therapy protocol was advocated after the result stabilized in order to maintain the repigmentation.48 A later trial by the same group, this time comprising 40 patients with segmental vitiligo of the head and neck region, used a He-Ne laser at 632.8 nm and 3 J/cm2. After a mean of 17 sessions, greater than 50% repigmentation was observed in 60% of the treatment cohort. Moreover, the study identified dysfunctional cutaneous blood flow in the vitiligo lesions, a finding that was normalized by treatment with the laser.49 By contrast, a single study of a combination of blue and red LED-based light therapy for acne vulgaris in 24 patients with Fitzpatrick type 4 skin observed an incidental skin-lightening effect which, on further analysis, was attributed to the red light.50 Despite anecdotal reports this has not been reconfirmed in the clinical literature.
LLLT for Acne Vulgaris
The elimination of facial blemishes, comedones, papules, and pustules by LLLT has also attracted interest. Acne vulgaris has been defined as a “chronic inflammatory disease of the pilosebaceous unit resulting from androgen-induced increased sebum production, altered keratinization, inflammation, and bacterial colonization of hair follicles … by Propionibacterium acnes.” 51 Red/infrared light is believed to have a beneficial effect on acne vulgaris by increasing keratinocyte turnover and inducing an anti-inflammatory microenvironment. The other experimental area of interest is bacterial colonization, for which the physiologic response to LLLT is quite different. The influence of light irradiation on bacterial species varies with the species concerned and the wavelength and irradiance of the light source.52Propionibacterium synthesize and store porphyrins, which are photosensitive molecules.53 The absorption of blue light by porphyrins (with an absorption peak of between 380 and 440 nm) causes a photochemical reaction with the formation of free radicals that, in turn, kill the host bacteria.54,55 This phenomenon has also been observed for other bacterial species (reviewed)56 and can be exploited to manage acne vulgaris with light treatment.
A double-blinded randomized controlled trial of a single treatment of pulsed-dye laser at 585 nm for the management of mild to moderate inflammatory facial acne revealed a rapid reduction in lesion burden by 4 weeks which persisted until 12 weeks at the conclusion of the study.57 Conversely, another randomized controlled trial conducted around the same time did not find any significant improvements using a pulsed-dye laser.58 Interestingly, this study was designed as a split-face study with the contralateral untreated side serving as the control. The authors observed improvements in both sides, which might have accounted for the lack of statistical proof of efficacy and perhaps hinted at generalized effects, including the effect of nonablative pulsed-dye laser on the local and (perhaps) regional or even systemic expression of growth factors including transforming growth factor β1 (TGF-β1).59 Some studies have also reported successful outcomes with a 532-nm KTP laser60 and a 1450-nm diode laser, alone61 or in combination with the pulsed-dye laser.62 However, other studies have yielded equivocal or difficult-to-interpret results.63
A small, single-blinded randomized controlled trial of 26 patients and 15 controls with mild to moderate acne vulgaris reported that exposure to blue light at 414 nm every second day for 8 weeks resulted in a significant reduction in inflammatory lesions.64 These findings have been supported by a number of additional studies of variable methodologic quality65-68 and the use of home (blue light) LED devices for the treatment of acne is now commercially supported.69 A randomized controlled trial of 107 patients with mild to moderate acne vulgaris revealed that a combination of blue (415 nm) and red (660 nm) light, delivered by fluorescent lamps, produced a significant improvement which was greater than with blue light alone and, for inflammatory lesions, performed better than benzoyl peroxide, the positive control.70 Similarly, a randomized controlled trial of combination (blue and red light) LED therapy involving 35 patients reported significant reductions in both inflammatory and noninflammatory lesions, sebum output, and sebaceous gland size.71 Two smaller studies of 24 and 22 patients with mild to severe acne vulgaris treated with a combination of blue (415 nm) and red (633 nm) LED light also revealed improvements in lesion count from the baseline over the 8- and 12-week duration of follow-up, respectively.50,72 In 1 study, the patients also underwent microdermabrasion72 but these studies were neither randomized into different treatment groups nor were the researchers blinded to the treatment method. A study of 22 adolescents randomized to receive weekly salicylic acid peels or combination light therapy using a 470-nm LED and a 660-nm red laser concluded that phototherapy was at least as effective as the positive control.73 A pilot study found the combination of blue (415 nm) and near-infrared (830 nm) LED therapy to be less effective.74 As Hamilton et al concluded in their review of the subject, the existing trial data are limited by short follow-up times, the exclusion of more severe acne in the study protocols, and a lack of direct comparisons with conventional acne treatments.75
LLLT for Androgenic Alopecia
Androgenic alopecia remains a phenomenon for which many theories have been proposed but which remains incompletely understood.76 Notwithstanding the fact the very first observation of a beneficial physiologic effect of low-level light was hair regrowth,15 phototrichogenesis also remains incompletely understood.
The evidence for the use of LLLT in the treatment of androgenic alopecia has been reviewed several times, most recently in 2019.77-79 Liu et al also performed a meta-analysis of the study data which included 11 randomized controlled trials (from 8 publications80-87) and a total of 667 test subjects, of whom approximately 40% were female.79 The reviews all reported that LLLT was efficacious in improving mean hair density and hair thickness with minimal side effects, and the meta-analysis supported the claim of efficacy for mean hair density, with no discernible gender difference. Interestingly, subgroup meta-analysis suggested that a low frequency of treatment (<60 minutes/week) was more efficacious. Although the results are encouraging, there was heterogeneity between studies. Although most studies used light at wavelengths of 630 to 660 nm, some used dual-wavelength devices. Total fluence also varied between studies. Moreover, 9 of the 11 randomized controlled trials were manufacturer-funded, and in 7 of the 11 trials, 1 or more of the authors had a direct commercial interest in the device tested.80,82,83,87 In fact, the only study that declared no conflicts of interest was also the only study to report that subjective self-assessment data yielded no significant difference in perception of change.81 It is interesting to observe that LLLT has been shown to inhibit both androgen-mediated sebum production and androgen-mediated hair loss, but, as yet, the true influence of LLLT on androgenic receptors of the pilosebaceous unit remains a matter of speculation.
LLLT for Wound Healing
The influence of LLLT on fibroblast and myofibroblast growth and differentiation is well established.17,88 In addition, preclinical studies based on a number of wound models have provided proof in principle of enhanced wound healing following low-energy laser irradiation. Prabdu and colleagues examined the biostimulatory effect of a He-Ne laser at 632.8 nm in a murine study and revealed enhanced wound healing in an environment of reduced inflammatory stigmata, which was confirmed by both histology and laser-induced fluorescence.89 Similarly, Yasukawa et al demonstrated enhanced wound healing by way of greater scar strength and reduced inflammation, with the optimal result following an irradiation protocol of around 4 J/cm2 every other day.90 A human experimental wound (abrasion) model treated with a diode laser (820 nm, 8 J/cm2) exhibited enhanced wound healing compared with sham controls. Interestingly, wounds were produced in duplicate in the experimental model and the adjacent, untreated wounds also exhibited enhanced wound healing relative to sham controls or their adjacent untreated wounds.91 Crucially, this suggests a wider physiologic effect than simply the area irradiated.
The clinical outcomes of laser-based LLLT in the management of diabetic wounds have been examined elsewhere and will not be reiterated here. A number of surgical wounds have been treated with LLLT. Two split-mouth studies examining re-epithelialization after gingivectomy with/without gingivoplasty reported enhanced healing on the LLLT-treated side.92,93 LLLT has also been shown to enhance the healing of intraoral bony94 and palatal mucosal defects.95 Additional reports provide evidence for the role of LLLT in enhancing healing of burns96 and in the appearance of surgical scars97 but high-quality randomized controlled trials are lacking. Some anecdotal accounts of wound healing with LED-based LLLT have been published but it is difficult to define the role of LED-based LLLT based on these data.98
LLLT for Body Contouring
Noninvasive body contouring and/or localized subcutaneous fat reduction remains a hugely popular alternative to surgical fat loss strategies. The main options available include cryolipolysis, high-intensity focused ultrasound, radiofrequency ablation, and LLLT.99 The physiologic mechanism responsible for LLLT-induced subcutaneous adipolysis is incompletely understood. Hypotheses include light-induced transitory pore formation within lipid cell membranes with the loss of lipid contents into the interstitial space,100 alterations in adipocyte lipid metabolism without liquefaction,101 and a generalized alteration in adipocyte behavior secondary to reduced oxidative stress with resultant increase in adiponectin secretion and reduction in insulin resistance.102 Whereas the first 2 hypotheses consider the actions of LLLT to be local, the third proposes a systemic effect. Neira et al supplemented their microscopic study with a radiologic study. On performing MRI to observe the radiologic effects of tumescent infiltration on subcutaneous fat, they observed a change in the radiologic signal after LLLT treatment, with almost complete homogeneity of the fat signal, which they attribute to the release of fat into the interstitial space.103 An animal model designed to investigate the transcutaneous effect of irradiating subcutaneous fat with light energy utilized the dorsal fat pad of rats and gallium-aluminum-arsenide laser-induced light at 670 nm and variable fluences. Although changes in brown fat, including coalescence and fusion, were observed, no changes akin to lipolysis were observed in the yellow fat component. Because the differences observed in the clinical and preclinical studies might be due to study design, the mechanism remains incompletely elucidated.
In general, the first clinical studies evaluated the use of LLLT as an adjunct to other measures. Neira and Ortiz-Neira published a personal case series of 700 patients who had undergone LLLT-assisted liposculpture. The technique utilized a 635-nm diode laser at 14 mW applied to the infiltrated area for 6 to 12 minutes prior to liposuction. They reported subjective improvements in the ease of performing liposuction as well as aesthetic contour and skin retraction with the use of the laser.104 Jackson et al performed a multicenter, double-blinded, placebo-controlled, randomized clinical trial of LLLT-assisted liposculpture. Seventy patients were included. They utilized the Zerona laser (Erchonia Medical Inc., McKinney, TX), emitting light at 635 nm and 14 mW. When applied for 12 minutes preoperatively after tumescent infiltration, LLLT enhanced the ease of subsequent fat removal, reduced operating times, and improved recovery.105 It should be noted that this is a fundamentally different technique from laser-assisted liposuction which uses a high powered Nd:YAG laser, introduced through a cannula via a fiber optic cable, to directly vaporize fat.106
LLLT has also been used as an independent, noninvasive modality in body contouring. A summary of the clinical data is presented in Table 2. A number of proprietary laser-based LLLT devices, emitting light at the red/near-infrared end of the spectrum, have been investigated for their effect on the subcutaneous fat deposits of the abdomen, buttocks, thighs, and arms by way of clinical trials and case series. Four randomized controlled trials,107-110 2 large retrospective cases series111,112 and 1 small prospective cohort study113 concluded that laser-based LLLT was effective in reducing subcutaneous fat deposits, whereas 1 randomized controlled trial101 and 2 small prospective split-abdomen cohorts102,114 did not find any significant differences. Although industry funding and/or methodologic flaws were common features of several of these studies, the evidence was overwhelmingly favorable. Interestingly, 1 study used LED-based light as the sham control.108 There remains a lack of evidence to support a role for LED-based LLLT in body contouring.
Clinical Trials of LLLT Alone for Body Contouring (Quantifiable Objective Measures)
| Study . | Light source . | Λ (nm)/fluence (J/cm2) . | Study participants/design . | Protocol . | Measurement . | Findings . | Notes . |
|---|---|---|---|---|---|---|---|
| Caruso-Davies et al101 | LAPEX-2000 lipolaser, Meridian Medical, Kr. | 635-680 | 34 females, 6 males; randomized 1:1 to treatment or sham groups, BMI < 30 kg/m2 | Device applied to waist, 2 × 30 minutes/week for 4 weeks | Waist circumference pretreatment and at 1, 3, and 8 weeks posttreatment | No significant differences observed by intention-to-treat analysis. | Significant improvement in treatment group in blinded photographic assessment |
| Elm et al114 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 5 subjects; one half of body treated; BMI ≤ 29 kg/m2 | Device applied to waist and thighs; 3×/week for 2 weeks | Waist and thigh circumference pretreatment and at 1 and 4 weeks posttreatment | No significant differences observed | Curva nutraceutical supplement also taken by 3 patients |
| Gold et al109 | SmoothShapes, Cynosure Inc., MA | 650 (LED) and 915(laser) | 83 females; thighs (R + L) randomized to LLLT + massage or no treatment (control) | Device applied to thigh for 2 × 30 minutes/week for 4 weeks | Thigh circumference in 3 places pretreatment and 4, 8 days and 1 month posttreatment | Significant reduction in thigh circumference observed in the treatment group at 1 month | Magnitude of change small despite statistical significance; industry funded |
| Jackson et al108 | Zerona laser, Erchonia Corp., McKinney, TX | 635nm/6.6 | 67 subjects: 35 randomized to treatment; 32 randomized to sham (LED-based light); BMI 25-30 kg/m2 | Device applied to waist, hips, and thighs; 3 × 40 minutes/week for 2 weeks | Waist, hip, and thigh circumference pretreatment, midtreatment, end treatment, and 2 weeks posttreatment | Significant and progressive reduction in combined circumference observed. | Fat reduction attributed to laser only, not LED light; industry funded |
| Jackson et al111 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 636 females and 53 males treated; retrospective multicenter study | Device applied to waist, hips, and thighs; 3 × 40 minute/week for 2 weeks | Waist, hips, thighs, arms, knees, chest, and neck circumference | Significant reductions in all measured circumferential areas posttreatment | Nutraceutical supplements also taken; retrospective, no controls |
| Jankowski et al102 | Lipo Laser, Mimari, Poland | 650/9.14 mW/cm2 | 17 subjects; split-abdomen study; BMI < 30 kg/m2 | Device applied to 1 side of abdomen; 6 treatments over 2 weeks | Ultrasonic measurement of abdominal adipose thickness pretreatment and at 2 weeks posttreatment | No significant differences observed | High dropout rate (24 initially recruited); side effects included skin ulceration in 2 patients |
| Lach et al107 | SmoothShapes, Cynosure Inc., MA. | 650 (LED) and 915 (laser) | 102 females; thighs (R + L) randomized to LLLT + massage or massage alone | Device applied to 1 thigh for 40-120 minutes/week for 4–6 weeks | Thigh circumference and subcutaneous fat thickness measured by MRI pretreatment and posttreatment | Significant reduction in fat thickness of thigh treated with LLLT + massage vs massage alone | High dropout rate (28 of 102); industry funded |
| McRae and Boris112 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 86 subjects treated; retrospective, single-center study | Device applied to waist, hips, and thighs; 3 × 40 minutes/week for 2 weeks | Waist, hip, and thigh circumference pretreatment and 1 week posttreatment | Significant reduction in each individual and combined circumference; low correlation with weight change | Nutraceutical supplements also taken; retrospective, no controls |
| Nestor et al110 | Zerona laser, Erchonia Corp., McKinney, TX | 635/3.94 | 30 females, 1 male; randomized 1:1 to treatment or sham groups; BMI 20-35 kg/m2 | Device applied to upper arm; 3× per week for 2 weeks | Upper arm circumference measured at 3 and 6 treatments and 2 weeks posttreatment | Significant and progressive reduction in arm circumference with LLLT not related to changes in BMI | Good study design and statistical analyses |
| Savoia et al113 | VibroLight, PromoItalia Group., Naples, Italy | 635 | 24 females, 9 males; prospective cohort study of treatment for localized adiposity or fibrous cellulite | Device applied where necessary: 1 session (23 minutes) per week for 6 weeks (localized adiposity); 2 sessions (56 minutes) per week for 4 weeks (fibrous cellulite) | Waist, hip (buttocks), thigh circumference (as per treatment area) pretreatment, during treatment, and 2 and 4 weeks posttreatment | Abdomen treated in 18, thighs in 12, buttocks in 3; significant reduction in combined circumference observed | Device uses vibration therapy in addition to LLLT; heterogeneous sample; no controls; industry funded |
| Study . | Light source . | Λ (nm)/fluence (J/cm2) . | Study participants/design . | Protocol . | Measurement . | Findings . | Notes . |
|---|---|---|---|---|---|---|---|
| Caruso-Davies et al101 | LAPEX-2000 lipolaser, Meridian Medical, Kr. | 635-680 | 34 females, 6 males; randomized 1:1 to treatment or sham groups, BMI < 30 kg/m2 | Device applied to waist, 2 × 30 minutes/week for 4 weeks | Waist circumference pretreatment and at 1, 3, and 8 weeks posttreatment | No significant differences observed by intention-to-treat analysis. | Significant improvement in treatment group in blinded photographic assessment |
| Elm et al114 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 5 subjects; one half of body treated; BMI ≤ 29 kg/m2 | Device applied to waist and thighs; 3×/week for 2 weeks | Waist and thigh circumference pretreatment and at 1 and 4 weeks posttreatment | No significant differences observed | Curva nutraceutical supplement also taken by 3 patients |
| Gold et al109 | SmoothShapes, Cynosure Inc., MA | 650 (LED) and 915(laser) | 83 females; thighs (R + L) randomized to LLLT + massage or no treatment (control) | Device applied to thigh for 2 × 30 minutes/week for 4 weeks | Thigh circumference in 3 places pretreatment and 4, 8 days and 1 month posttreatment | Significant reduction in thigh circumference observed in the treatment group at 1 month | Magnitude of change small despite statistical significance; industry funded |
| Jackson et al108 | Zerona laser, Erchonia Corp., McKinney, TX | 635nm/6.6 | 67 subjects: 35 randomized to treatment; 32 randomized to sham (LED-based light); BMI 25-30 kg/m2 | Device applied to waist, hips, and thighs; 3 × 40 minutes/week for 2 weeks | Waist, hip, and thigh circumference pretreatment, midtreatment, end treatment, and 2 weeks posttreatment | Significant and progressive reduction in combined circumference observed. | Fat reduction attributed to laser only, not LED light; industry funded |
| Jackson et al111 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 636 females and 53 males treated; retrospective multicenter study | Device applied to waist, hips, and thighs; 3 × 40 minute/week for 2 weeks | Waist, hips, thighs, arms, knees, chest, and neck circumference | Significant reductions in all measured circumferential areas posttreatment | Nutraceutical supplements also taken; retrospective, no controls |
| Jankowski et al102 | Lipo Laser, Mimari, Poland | 650/9.14 mW/cm2 | 17 subjects; split-abdomen study; BMI < 30 kg/m2 | Device applied to 1 side of abdomen; 6 treatments over 2 weeks | Ultrasonic measurement of abdominal adipose thickness pretreatment and at 2 weeks posttreatment | No significant differences observed | High dropout rate (24 initially recruited); side effects included skin ulceration in 2 patients |
| Lach et al107 | SmoothShapes, Cynosure Inc., MA. | 650 (LED) and 915 (laser) | 102 females; thighs (R + L) randomized to LLLT + massage or massage alone | Device applied to 1 thigh for 40-120 minutes/week for 4–6 weeks | Thigh circumference and subcutaneous fat thickness measured by MRI pretreatment and posttreatment | Significant reduction in fat thickness of thigh treated with LLLT + massage vs massage alone | High dropout rate (28 of 102); industry funded |
| McRae and Boris112 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 86 subjects treated; retrospective, single-center study | Device applied to waist, hips, and thighs; 3 × 40 minutes/week for 2 weeks | Waist, hip, and thigh circumference pretreatment and 1 week posttreatment | Significant reduction in each individual and combined circumference; low correlation with weight change | Nutraceutical supplements also taken; retrospective, no controls |
| Nestor et al110 | Zerona laser, Erchonia Corp., McKinney, TX | 635/3.94 | 30 females, 1 male; randomized 1:1 to treatment or sham groups; BMI 20-35 kg/m2 | Device applied to upper arm; 3× per week for 2 weeks | Upper arm circumference measured at 3 and 6 treatments and 2 weeks posttreatment | Significant and progressive reduction in arm circumference with LLLT not related to changes in BMI | Good study design and statistical analyses |
| Savoia et al113 | VibroLight, PromoItalia Group., Naples, Italy | 635 | 24 females, 9 males; prospective cohort study of treatment for localized adiposity or fibrous cellulite | Device applied where necessary: 1 session (23 minutes) per week for 6 weeks (localized adiposity); 2 sessions (56 minutes) per week for 4 weeks (fibrous cellulite) | Waist, hip (buttocks), thigh circumference (as per treatment area) pretreatment, during treatment, and 2 and 4 weeks posttreatment | Abdomen treated in 18, thighs in 12, buttocks in 3; significant reduction in combined circumference observed | Device uses vibration therapy in addition to LLLT; heterogeneous sample; no controls; industry funded |
LED, light-emitting diode; LLLT, low-level light therapy.
Clinical Trials of LLLT Alone for Body Contouring (Quantifiable Objective Measures)
| Study . | Light source . | Λ (nm)/fluence (J/cm2) . | Study participants/design . | Protocol . | Measurement . | Findings . | Notes . |
|---|---|---|---|---|---|---|---|
| Caruso-Davies et al101 | LAPEX-2000 lipolaser, Meridian Medical, Kr. | 635-680 | 34 females, 6 males; randomized 1:1 to treatment or sham groups, BMI < 30 kg/m2 | Device applied to waist, 2 × 30 minutes/week for 4 weeks | Waist circumference pretreatment and at 1, 3, and 8 weeks posttreatment | No significant differences observed by intention-to-treat analysis. | Significant improvement in treatment group in blinded photographic assessment |
| Elm et al114 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 5 subjects; one half of body treated; BMI ≤ 29 kg/m2 | Device applied to waist and thighs; 3×/week for 2 weeks | Waist and thigh circumference pretreatment and at 1 and 4 weeks posttreatment | No significant differences observed | Curva nutraceutical supplement also taken by 3 patients |
| Gold et al109 | SmoothShapes, Cynosure Inc., MA | 650 (LED) and 915(laser) | 83 females; thighs (R + L) randomized to LLLT + massage or no treatment (control) | Device applied to thigh for 2 × 30 minutes/week for 4 weeks | Thigh circumference in 3 places pretreatment and 4, 8 days and 1 month posttreatment | Significant reduction in thigh circumference observed in the treatment group at 1 month | Magnitude of change small despite statistical significance; industry funded |
| Jackson et al108 | Zerona laser, Erchonia Corp., McKinney, TX | 635nm/6.6 | 67 subjects: 35 randomized to treatment; 32 randomized to sham (LED-based light); BMI 25-30 kg/m2 | Device applied to waist, hips, and thighs; 3 × 40 minutes/week for 2 weeks | Waist, hip, and thigh circumference pretreatment, midtreatment, end treatment, and 2 weeks posttreatment | Significant and progressive reduction in combined circumference observed. | Fat reduction attributed to laser only, not LED light; industry funded |
| Jackson et al111 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 636 females and 53 males treated; retrospective multicenter study | Device applied to waist, hips, and thighs; 3 × 40 minute/week for 2 weeks | Waist, hips, thighs, arms, knees, chest, and neck circumference | Significant reductions in all measured circumferential areas posttreatment | Nutraceutical supplements also taken; retrospective, no controls |
| Jankowski et al102 | Lipo Laser, Mimari, Poland | 650/9.14 mW/cm2 | 17 subjects; split-abdomen study; BMI < 30 kg/m2 | Device applied to 1 side of abdomen; 6 treatments over 2 weeks | Ultrasonic measurement of abdominal adipose thickness pretreatment and at 2 weeks posttreatment | No significant differences observed | High dropout rate (24 initially recruited); side effects included skin ulceration in 2 patients |
| Lach et al107 | SmoothShapes, Cynosure Inc., MA. | 650 (LED) and 915 (laser) | 102 females; thighs (R + L) randomized to LLLT + massage or massage alone | Device applied to 1 thigh for 40-120 minutes/week for 4–6 weeks | Thigh circumference and subcutaneous fat thickness measured by MRI pretreatment and posttreatment | Significant reduction in fat thickness of thigh treated with LLLT + massage vs massage alone | High dropout rate (28 of 102); industry funded |
| McRae and Boris112 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 86 subjects treated; retrospective, single-center study | Device applied to waist, hips, and thighs; 3 × 40 minutes/week for 2 weeks | Waist, hip, and thigh circumference pretreatment and 1 week posttreatment | Significant reduction in each individual and combined circumference; low correlation with weight change | Nutraceutical supplements also taken; retrospective, no controls |
| Nestor et al110 | Zerona laser, Erchonia Corp., McKinney, TX | 635/3.94 | 30 females, 1 male; randomized 1:1 to treatment or sham groups; BMI 20-35 kg/m2 | Device applied to upper arm; 3× per week for 2 weeks | Upper arm circumference measured at 3 and 6 treatments and 2 weeks posttreatment | Significant and progressive reduction in arm circumference with LLLT not related to changes in BMI | Good study design and statistical analyses |
| Savoia et al113 | VibroLight, PromoItalia Group., Naples, Italy | 635 | 24 females, 9 males; prospective cohort study of treatment for localized adiposity or fibrous cellulite | Device applied where necessary: 1 session (23 minutes) per week for 6 weeks (localized adiposity); 2 sessions (56 minutes) per week for 4 weeks (fibrous cellulite) | Waist, hip (buttocks), thigh circumference (as per treatment area) pretreatment, during treatment, and 2 and 4 weeks posttreatment | Abdomen treated in 18, thighs in 12, buttocks in 3; significant reduction in combined circumference observed | Device uses vibration therapy in addition to LLLT; heterogeneous sample; no controls; industry funded |
| Study . | Light source . | Λ (nm)/fluence (J/cm2) . | Study participants/design . | Protocol . | Measurement . | Findings . | Notes . |
|---|---|---|---|---|---|---|---|
| Caruso-Davies et al101 | LAPEX-2000 lipolaser, Meridian Medical, Kr. | 635-680 | 34 females, 6 males; randomized 1:1 to treatment or sham groups, BMI < 30 kg/m2 | Device applied to waist, 2 × 30 minutes/week for 4 weeks | Waist circumference pretreatment and at 1, 3, and 8 weeks posttreatment | No significant differences observed by intention-to-treat analysis. | Significant improvement in treatment group in blinded photographic assessment |
| Elm et al114 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 5 subjects; one half of body treated; BMI ≤ 29 kg/m2 | Device applied to waist and thighs; 3×/week for 2 weeks | Waist and thigh circumference pretreatment and at 1 and 4 weeks posttreatment | No significant differences observed | Curva nutraceutical supplement also taken by 3 patients |
| Gold et al109 | SmoothShapes, Cynosure Inc., MA | 650 (LED) and 915(laser) | 83 females; thighs (R + L) randomized to LLLT + massage or no treatment (control) | Device applied to thigh for 2 × 30 minutes/week for 4 weeks | Thigh circumference in 3 places pretreatment and 4, 8 days and 1 month posttreatment | Significant reduction in thigh circumference observed in the treatment group at 1 month | Magnitude of change small despite statistical significance; industry funded |
| Jackson et al108 | Zerona laser, Erchonia Corp., McKinney, TX | 635nm/6.6 | 67 subjects: 35 randomized to treatment; 32 randomized to sham (LED-based light); BMI 25-30 kg/m2 | Device applied to waist, hips, and thighs; 3 × 40 minutes/week for 2 weeks | Waist, hip, and thigh circumference pretreatment, midtreatment, end treatment, and 2 weeks posttreatment | Significant and progressive reduction in combined circumference observed. | Fat reduction attributed to laser only, not LED light; industry funded |
| Jackson et al111 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 636 females and 53 males treated; retrospective multicenter study | Device applied to waist, hips, and thighs; 3 × 40 minute/week for 2 weeks | Waist, hips, thighs, arms, knees, chest, and neck circumference | Significant reductions in all measured circumferential areas posttreatment | Nutraceutical supplements also taken; retrospective, no controls |
| Jankowski et al102 | Lipo Laser, Mimari, Poland | 650/9.14 mW/cm2 | 17 subjects; split-abdomen study; BMI < 30 kg/m2 | Device applied to 1 side of abdomen; 6 treatments over 2 weeks | Ultrasonic measurement of abdominal adipose thickness pretreatment and at 2 weeks posttreatment | No significant differences observed | High dropout rate (24 initially recruited); side effects included skin ulceration in 2 patients |
| Lach et al107 | SmoothShapes, Cynosure Inc., MA. | 650 (LED) and 915 (laser) | 102 females; thighs (R + L) randomized to LLLT + massage or massage alone | Device applied to 1 thigh for 40-120 minutes/week for 4–6 weeks | Thigh circumference and subcutaneous fat thickness measured by MRI pretreatment and posttreatment | Significant reduction in fat thickness of thigh treated with LLLT + massage vs massage alone | High dropout rate (28 of 102); industry funded |
| McRae and Boris112 | Zerona laser, Erchonia Corp., McKinney, TX | 635 | 86 subjects treated; retrospective, single-center study | Device applied to waist, hips, and thighs; 3 × 40 minutes/week for 2 weeks | Waist, hip, and thigh circumference pretreatment and 1 week posttreatment | Significant reduction in each individual and combined circumference; low correlation with weight change | Nutraceutical supplements also taken; retrospective, no controls |
| Nestor et al110 | Zerona laser, Erchonia Corp., McKinney, TX | 635/3.94 | 30 females, 1 male; randomized 1:1 to treatment or sham groups; BMI 20-35 kg/m2 | Device applied to upper arm; 3× per week for 2 weeks | Upper arm circumference measured at 3 and 6 treatments and 2 weeks posttreatment | Significant and progressive reduction in arm circumference with LLLT not related to changes in BMI | Good study design and statistical analyses |
| Savoia et al113 | VibroLight, PromoItalia Group., Naples, Italy | 635 | 24 females, 9 males; prospective cohort study of treatment for localized adiposity or fibrous cellulite | Device applied where necessary: 1 session (23 minutes) per week for 6 weeks (localized adiposity); 2 sessions (56 minutes) per week for 4 weeks (fibrous cellulite) | Waist, hip (buttocks), thigh circumference (as per treatment area) pretreatment, during treatment, and 2 and 4 weeks posttreatment | Abdomen treated in 18, thighs in 12, buttocks in 3; significant reduction in combined circumference observed | Device uses vibration therapy in addition to LLLT; heterogeneous sample; no controls; industry funded |
LED, light-emitting diode; LLLT, low-level light therapy.
The physiologic effects of LLLT in combination with weight-loss strategies such as resistance training have also been investigated, following a preclinical study which demonstrated that a combination of exercise and LLLT improved lipid markers and total body fat in excess of that produced by exercise alone in rats consuming a high-fat diet.115 In a study of 36 young, obese women randomized to receive physical training plus postexercise LLLT (at 808 nm) or sham control, the LLLT cohort exhibited significantly higher postexercise levels of the adipokine adiponectin and significantly reduced proinflammatory cytokine (IL-6) levels and neck circumference.116 They further demonstrated greater improvements in cardiometabolic risk factors including overall percentage body fat, waist and hip circumference, and relatively greater improvements in insulin resistance.117,118 LED-based LLLT has also been studied for use with a phosphatidylcholine-based anticellulite gel with some evidence of improved cellulite in 8 of the 9 patients treated with the active combination, compared with none treated with LED-based LLLT alone.119 Finally, LLLT is a component of a number of proprietary body contouring systems which also use ultrasound, radiofrequency, suction, and massage.120
Discussion
Photobiomodulation is essentially a function of energy absorption by a target photoacceptor and the subsequent cascade of biochemical events manifesting as desirable outcomes at a tissue level. The majority of clinical evidence in support of LLLT is based on laser light at the red and/or near-infrared end of the visible spectrum. Although there is some evidence to suggest that the physiologic responses induced by red and near-infrared light are not the same, LLLT based on light in the wavelength range of around 620 to 810 nm is often considered to be a single clinical entity. Blue light has dissimilar photobiomodulatory effects—a fact that can be exploited for the management of acne vulgaris. Beyond this, the role of blue light (in isolation) appears limited. However, it may be hypothesized that the biochemical cascades initiated by a single monochromatic (or near-monochromatic) light source may be augmented by the addition of a second light source of a different wavelength and energy density. One study found that a combination of blue and red light was more effective in the management of mild to moderate acne vulgaris than blue light alone,70 and it is intriguing to speculate that appropriately targeted combination therapy might yield further clinical benefits. From a commercial point of view, combination light therapy with LEDs emitting light of different wavelengths has proved versatile and attractive. However, most of the evidence in support of this commercial strategy is extrapolated.
The issue of enduring interest is whether laser and LED-based light induce equivalent physiologic responses. Although there is evidence to support the role of LED-based LLLT in the management of facial rhytids in particular, for certain conditions, including dyschromias, acne vulgaris, wound healing, and body contouring, most of the plausible peer-reviewed evidence uses laser as the light source and hence we need to understand the role of coherence, polarity, pulsatility, and the speckle phenomenon on the physiologic cascade. Together, these features may explain experimental evidence to suggest that, when all other factors are controlled for, laser light is more clinically effective in deeper target tissue,121 to the extent that one of the randomized controlled trials of LLLT for body contouring used LED-based light at a similar wavelength in the control arm of the study.108 Because blue light is absorbed superficially, the speckle phenomenon is less relevant here. Thus, a combination of blue LED light and red or near-infrared laser light might offer unique advantages. Moreover, as insufficient energy density is clinically ineffective and excessive density initiates the mitochondrial apoptotic pathway, further clinical optimization of the energy parameters for each indication is required.122
Although the physiologic bases of LLLT for facial rejuvenation, acne vulgaris, and wound healing are easy enough to understand within the paradigm of mitochondrial stimulation, ATP production, increased cell metabolism, and the maintenance of a constitutively anti-inflammatory dermal microenvironment, the roles LLLT play in body contouring and the treatment of alopecia deserves special consideration because they are incompletely understood. Several plausible explanations have been put forward to explain how LLLT interacts with adipocytes. The physiologic basis for the use of LLLT in the management of alopecia may or may not invoke ATP production and upregulated cellular metabolism. Either way, there is work to be done for the science to catch up with clinical demand.
This paper has a number of limitations. This study represents an attempt to cover a huge breadth of the clinical LLLT literature in a single digestible resource. As such, many of the nuances and developmental contexts have been neglected in favor of a strict focus on the best of the clinical evidence. Although some of the pertinent preclinical data have been included to provide context, the preclinical examination is by no means comprehensive. To put it simply, a vast body of preclinical work exists to inform our understanding of LLLT and a comprehensive overview of these data would overwhelm the focused clinical message. Secondly, the clinical evidence is simply too disparate to submit to any meaningful meta-analysis; hence the narrative structure of this review. Moreover, the quality of the source studies varied, but methodologic flaws, including controls, blinding, numbers needed to treat, confounding variables, and industry funding, are common features of the studies we have at our disposal in attempting to draw meaningful conclusions. Additionally, for some of the therapeutic applications (eg, vitiligo), the supporting clinical evidence is provided by a small pool of researchers and it would be more reassuring if these findings were replicated by others. Finally, the proprietary nature of the LLLT devices used means that we are seldom comparing like with like when we use the umbrella term LLLT.
LLLT and the FDA
In the United States, the FDA has regulatory jurisdiction over medical devices and classifies them by risk into class I, II, and III, where class I devices represent the lowest risk. Class III devices require FDA approval, which is a rigorous process requiring proof of efficacy and safety, whereas class I and II devices, which encompass both LED and laser-based LLLT devices,123 may apply for premarket approval (PMA), which also requires proof of efficacy and safety or premarket notification, otherwise known as “510(k) clearance” on account of section 510(k) of the Food, Drug and Cosmetic Act of 1938, which is merely an acknowledgment that the device is substantially equivalent to a similar legally marketed device and does not require these proofs.124 LLLT devices for body contouring were subject to 510(k) clearance in 2011. As of 2019, 47 devices had FDA 510(k) clearance to be marketed for the treatment of androgenic alopecia.125 Although clinical and commercial sources alike refer to “FDA approval” in the context of LLLT for aesthetic indications, they do, in fact, mean FDA clearance.
Conclusions
Photobiomodulation by the nonthermal irradiation of tissue with laser or LED-derived light is backed by enough experimental and clinical evidence that it is here to stay. Red/near-infrared light exhibits the potential to rejuvenate the skin, reduce focal adiposity, heal cutaneous wounds, and induce hair (re)growth by upregulating cellular metabolic processes with enhanced mitochondrial ATP synthesis, differential gene expression, and the maintenance of a constitutively anti-inflammatory dermal and pilosebaceous microenvironment. The addition of blue light induces bactericidal oxidation of porphyrins synthesized by P. acnes residing within the pilosebaceous unit. Although clinical trials provide some evidence for efficacy, especially with regards to body contouring and skin rejuvenation, the clinical literature lags well behind the commercial exploitation. Well-designed, adequately powered, independent clinical trials will help us answer some of the unresolved questions.
Acknowledgments
The author would like to thank Ms Anushka Hardas and Mr Abdul Sulaiman, librarians at Sidra Medicine, for their invaluable help in performing literature searches and sourcing the papers used to compile this study.
Disclosures
Dr Glass is a consultant for Lymalife (London, United Kingdom), a dietary supplement and wellness company.
Funding
The author received no financial support for the research, authorship, and publication of this article.
References
.
FDA.
FDA.



