-
PDF
- Split View
-
Views
-
Cite
Cite
Bing Cai, Rong Yuan, Guo-Zhang Zhu, Wen-Feng Zhan, Cheng-En Luo, Xiang-Xue Kong, Sheng-Kang Luo, Deployment of the Ophthalmic and Facial Angiosomes in the Upper Nose Overlaying the Nasal Bones, Aesthetic Surgery Journal, Volume 41, Issue 12, December 2021, Pages NP1975–NP1985, https://doi.org/10.1093/asj/sjab003
Close - Share Icon Share
Abstract
Nasal filler placement is associated with a high risk of blindness. The arterial supply to the upper nose overlaying the nasal bones is poorly understood.
The aim of this study was to visualize and analyze the deployment of the ophthalmic and facial angiosomes in the upper nose to help prevent blindness following nasal filler injections.
The arterial systems of 62 cadaveric heads were filled with lead oxide contrast agent, and computed tomography (CT) images were acquired and reconstructed in 3 dimensions.
Twenty-six of the cadaveric noses examined demonstrated clear CT images of the facial and ophthalmic angiosomes in the upper nose. The Type 1 upper nose (15.4%) is supplied by 2 independent ophthalmic angiosomes that communicate indirectly through a choke anastomosis. The Type 2 upper nose (38.5%) is supplied by 2 ophthalmic angiosomes with a true anastomosis between them. The Type 3 upper nose (46.1%) is supplied by both ophthalmic and facial angiosomes with true anastomoses across the dorsal midline. These true anastomoses are mediated by the radix arcade in 46% of the noses and involve the dorsal nasal artery in 65% of the cases. The anastomoses all cross the upper dorsal midline and are directly linked to the ophthalmic angiosome.
The deployment and anastomosis of the facial and ophthalmic angiosomes in the upper nose fall into 3 major patterns. About 85% of the noses have true anastomotic arteries that cross the upper dorsal midline and are directly linked to the ophthalmic circulation. Dorsum filler injection poses a significant risk of blindness.
Nasal filler injection is associated with the highest risk of blindness of all facial filler placements,1 and there is currently no effective rescue treatment.2 Loss of vision occurs due to a retrograde embolism of the arteries perfusing the ocular structures.3 An accidental arterial puncture allows filler to enter a perinasal artery, after which injection pressure pushes the filler into the ophthalmic circulation in the opposite direction to the blood flow. When the injection ends, the blood flow carries the filler particles downstream to embolize arteries of the ocular structures.
In order to design evidence-based safer filler injection protocols, it is essential to understand nasal arterial anatomy. Perinasal arteries come from the ophthalmic and facial angiosomes and the infraorbital microangiosome,4-6 and the vasculature of the middle and lower noses has been well studied.7,8 However, the arterial supply to the upper nose overlaying the nasal bones remains poorly understood. Understanding the nature of these arteries is particularly important in light of the fact that the concave radix of the upper nose is considered the riskiest region for filler injection by standard cannulation techniques.9-12
The dorsal nasal artery (DNA), which originates from the ophthalmic angiosome and runs in the subcutaneous plane overlaying the fibromuscular layer, is the only vessel in the upper nose that has been well studied.9-11,13 However, very little is known about the transverse anastomotic arteries that cross the dorsum of the upper nose and thus affect filler injection safety.
Saban et al14 were the first to introduce the concepts of the radix arcade and radix artery, but they only briefly noted that the radix artery originates from the ophthalmic angiosome, and runs transversely at the radix as the starting segment of the DNA. Tansatit et al10 subsequently observed a small anastomotic artery crossing the rhinion midline, and Choi et al11 also reported a small communication branch that connects bilateral DNAs at the nasal radix. Recently, Taylor et al15 noted the frequent presence of true anastomoses between bilateral ophthalmic angiosomes across the nasal dorsum, and between the terminal angular branches of the facial artery and the external nasal branches of the ophthalmic angiosome. Although these studies have examined the arterial structure of the upper nose,10,11,14,15 none of them has discerned the exact identities of these transverse arteries, or profiled the patterns of their true anastomoses across the upper dorsal midline.
In order to clearly define the upper nasal arteries, particularly the arteries that communicate across the upper dorsal midline, we visualized and profiled the deployment and anastomosis of the facial and ophthalmic angiosomes in the upper nose by performing postmortem contrast computed tomography (CT) with 3-dimensional (3D) reconstruction.5,6 We determined the identities of the anastomotic arteries across the upper nasal midline and estimated their potential impact on the risk of blindness associated with filler injection. These findings should help decipher the embolic pathway to vision loss and provide the basis for developing safer filler injection protocols.
METHODS
This study was conducted on 62 fresh-frozen cadaveric heads voluntarily donated to Guang Dong Second Provincial General Hospital (Guang Zhou City, China) between January 2016 and March 2019. They were all structurally intact and of Chinese Han ethnicity, with no history of head trauma or neoplasm. Cadavers were handled professionally and ethically in accordance with the China Ministry of Health Cadaver Dissection Regulation.
The arterial system of each specimen was first filled with a CT contrast agent, which was prepared by mixing 40 grams of lead oxide (Guang Hua Chemical Company, Shan Tou City, China) with 5 mL red dye and 100 mL of latex with constant stirring.6 The suspension was subsequently filtered to remove residual lead oxide particles.
Following contrast agent injection, a CT scan of the head was acquired for each specimen with the same 64-row spiral CT scanner (Philips Brilliance 64 scanner; Philips, Cleveland, OH) under identical conditions: 120 kV tube voltage, 250 mA effective tube current, data acquisition triggering at 140 Hounsfield units above baseline, 500 mm × 600 mm field of view, and 1024 × 1024 pixels slice size. The section thickness was 0.8 mm with an increment of 0.4 mm.6
Finally, the CT images of all specimens were volume-rendered into 3D space on a Philips IntelliSpace workstation with default settings. The CT images of 38 specimens were reconstructed in 3D with the surface-rendering function of Mimics version 14.0 software (Materialise, Leuven, Belgium),5,6 and the facial and ophthalmic angiosomes were highlighted with different colors. Five plastic surgeons reached a consensus on the results of 3D reconstruction, and a board-certified CT radiologist reviewed and confirmed all image-reconstruction procedures.
The contrast agent injection protocol evolved as the study progressed. For the first 28 specimens, the agent was infused into the bilateral external carotid arteries (ECAs), with additional agent infused into the internal carotid arteries in 7 of the specimens. Contrast infusion was maintained until the cadaveric lip and conjunctival mucosa turned pink, and red latex dropped from the vertebral artery stumps, consistent with adequate arterial filling. Subsequent CT suggested that this protocol visualized all arteries of the face, but the presence of numerous superficial terminal arteries in the image sometimes interfered with visualization of the deep arteries. Therefore, in the next 32 specimens we chose to selectively cannulate and inject a limited amount of contrast agent into the bilateral facial artery (FA) and the superficial temporal artery (STA) for better image clarity. We also experimented with contrast agent injection into bilateral FAs and ECAs in one specimen, and bilateral FAs, STAs, and ophthalmic arteries in another specimen.
RESULTS
We obtained 3D reconstructed CT images for 62 specimens, although the images of 36 noses failed to completely and clearly visualize all perinasal arteries (for reasons detailed in the Discussion). These images were excluded from the subsequent angiosome analysis, and the remaining 26 noses with good-quality CT images were used as the final analytic dataset. These specimens represent 15 females and 11 males, with a mean [standard deviation] age of 33 [9] years (range, 20-56 years).
The deployment and anastomosis of the ophthalmic and facial angiosomes in the upper nose overlaying the nasal bones clearly fall into 3 major patterns (Table 1). The Type 1 upper nose (15.4% prevalence) and the Type 2 upper nose (38.5% prevalence) are supplied exclusively by bilateral ophthalmic angiosomes, whereas the Type 3 upper nose (46.1% prevalence) is supplied by both facial and ophthalmic angiosomes. There are true anastomoses across the upper dorsal midline in both the Type 2 and Type 3 upper noses, with a combined prevalence of 84.6%. The communication modality across the upper dorsal midline of the Type 1 upper nose is indirect in nature, and is mediated by choke vessels of dramatically reduced caliber. The Type 2 and Type 3 upper noses were further classified into subcategories that reflect the various structural components of their anastomoses.
| Type (percentage) . | Source of blood supply . | Nature of anastomosis . |
|---|---|---|
| Type 1 (15.4%) | Bilateral ophthalmic angiosomes | Choke anastomosis between bilateral ophthalmic angiosomes (Figure 1) |
| Type 2 (38.5%) | Bilateral ophthalmic angiosomes | True anastomosis between bilateral ophthalmic angiosomes |
| Subtype 2A | Bilateral ophthalmic angiosomes | True anastomosis via a radix arcade formed by bilateral radix arteries (Figure 2) |
| Subtype 2B | True anastomosis via a radix arcade formed by a radix artery and a contralateral DNA (Figure 3) | |
| Subtype 2C | True anastomosis via bilateral DNAs that are directly connected (Figures 4, 5) | |
| Type 3 (46.1%) | Ophthalmic and facial angiosomes | True anastomosis between facial and ophthalmic angiosomes |
| Subtype 3A | Ophthalmic and facial angiosomes | True anastomosis via a radix arcade (Figure 6) |
| Subtype 3B | True anastomosis via reverse DNAs (Figure 7) | |
| Subtype 3C | Multiple true anastomoses among facial and ophthalmic angiosomes |
| Type (percentage) . | Source of blood supply . | Nature of anastomosis . |
|---|---|---|
| Type 1 (15.4%) | Bilateral ophthalmic angiosomes | Choke anastomosis between bilateral ophthalmic angiosomes (Figure 1) |
| Type 2 (38.5%) | Bilateral ophthalmic angiosomes | True anastomosis between bilateral ophthalmic angiosomes |
| Subtype 2A | Bilateral ophthalmic angiosomes | True anastomosis via a radix arcade formed by bilateral radix arteries (Figure 2) |
| Subtype 2B | True anastomosis via a radix arcade formed by a radix artery and a contralateral DNA (Figure 3) | |
| Subtype 2C | True anastomosis via bilateral DNAs that are directly connected (Figures 4, 5) | |
| Type 3 (46.1%) | Ophthalmic and facial angiosomes | True anastomosis between facial and ophthalmic angiosomes |
| Subtype 3A | Ophthalmic and facial angiosomes | True anastomosis via a radix arcade (Figure 6) |
| Subtype 3B | True anastomosis via reverse DNAs (Figure 7) | |
| Subtype 3C | Multiple true anastomoses among facial and ophthalmic angiosomes |
DNA, dorsal nasal artery.
| Type (percentage) . | Source of blood supply . | Nature of anastomosis . |
|---|---|---|
| Type 1 (15.4%) | Bilateral ophthalmic angiosomes | Choke anastomosis between bilateral ophthalmic angiosomes (Figure 1) |
| Type 2 (38.5%) | Bilateral ophthalmic angiosomes | True anastomosis between bilateral ophthalmic angiosomes |
| Subtype 2A | Bilateral ophthalmic angiosomes | True anastomosis via a radix arcade formed by bilateral radix arteries (Figure 2) |
| Subtype 2B | True anastomosis via a radix arcade formed by a radix artery and a contralateral DNA (Figure 3) | |
| Subtype 2C | True anastomosis via bilateral DNAs that are directly connected (Figures 4, 5) | |
| Type 3 (46.1%) | Ophthalmic and facial angiosomes | True anastomosis between facial and ophthalmic angiosomes |
| Subtype 3A | Ophthalmic and facial angiosomes | True anastomosis via a radix arcade (Figure 6) |
| Subtype 3B | True anastomosis via reverse DNAs (Figure 7) | |
| Subtype 3C | Multiple true anastomoses among facial and ophthalmic angiosomes |
| Type (percentage) . | Source of blood supply . | Nature of anastomosis . |
|---|---|---|
| Type 1 (15.4%) | Bilateral ophthalmic angiosomes | Choke anastomosis between bilateral ophthalmic angiosomes (Figure 1) |
| Type 2 (38.5%) | Bilateral ophthalmic angiosomes | True anastomosis between bilateral ophthalmic angiosomes |
| Subtype 2A | Bilateral ophthalmic angiosomes | True anastomosis via a radix arcade formed by bilateral radix arteries (Figure 2) |
| Subtype 2B | True anastomosis via a radix arcade formed by a radix artery and a contralateral DNA (Figure 3) | |
| Subtype 2C | True anastomosis via bilateral DNAs that are directly connected (Figures 4, 5) | |
| Type 3 (46.1%) | Ophthalmic and facial angiosomes | True anastomosis between facial and ophthalmic angiosomes |
| Subtype 3A | Ophthalmic and facial angiosomes | True anastomosis via a radix arcade (Figure 6) |
| Subtype 3B | True anastomosis via reverse DNAs (Figure 7) | |
| Subtype 3C | Multiple true anastomoses among facial and ophthalmic angiosomes |
DNA, dorsal nasal artery.
The Type 1 Upper Nose
The Type 1 upper nose (Figure 1) features 2 anatomically independent ophthalmic angiosomes that are loosely connected through a choke anastomosis. Both of these ophthalmic angiosomes lie exclusively lateral to the dorsal midline. There is no sizable artery running transversely in the upper nose, except for some very minute and often interrupted choke vessels. The diameters of the choke vessels are a small fraction of those of any artery in the ophthalmic angiosomes.
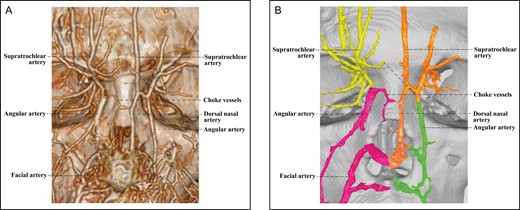
The 3D reconstructed CT of Cadaver No 32, a 26-year-old female, with contrast agent infusion of the facial artery and the superficial temporal artery, demonstrating a Type 1 upper nose with independent bilateral ophthalmic angiosomes that communicate indirectly via a choke vessel. Note that the diameter of the choke vessel is very small compared with that of the dorsal nasal artery of the left ophthalmic angiosome. (A) CT reconstructed with the Philips workstation. (B) CT reconstructed with the Mimics algorithm. CT, computed tomography; 3D, three-dimensional.
The Type 2 Upper Nose
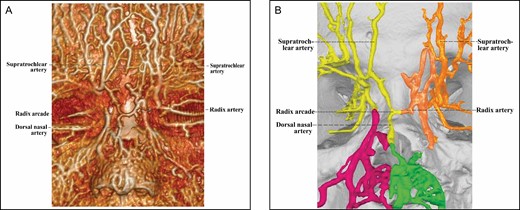
The arterial supply of the Type 2 upper nose (Figures 2-5) comes from bilateral ophthalmic angiosomes with a true anastomosis between them. The true anastomosis is built upon the radix artery, the DNA, or both. The Type 2 upper nose is further classified into 3 subcategories by the constituent of the true anastomosis.
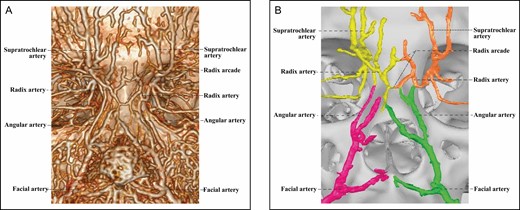
The 3D reconstructed CT of Cadaver No 20, a 23-year-old male, with contrast agent infusion of the bilateral external carotid artery, demonstrating a Type 2A upper nose supplied by bilateral ophthalmic angiosomes with a true anastomosis via 2 radix arcades formed by bilateral radix arteries. (A) CT reconstructed with the Philips workstation. (B) CT reconstructed with the Mimics algorithm. CT, computed tomography; 3D, three-dimensional.
The 3D reconstructed CT of Cadaver No 32, a 26-year-old female, with contrast agent infusion of the facial artery and the superficial temporal artery, demonstrating a Type 1 upper nose with independent bilateral ophthalmic angiosomes that communicate indirectly via a choke vessel. Note that the diameter of the choke vessel is very small compared with that of the dorsal nasal artery of the left ophthalmic angiosome. (A) CT reconstructed with the Philips workstation. (B) CT reconstructed with the Mimics algorithm. CT, computed tomography; 3D, three-dimensional.
The 3D CT of Cadaver No 28, a 41-year-old male, with contrast agent infusions of the bilateral facial artery and superficial temporal artery, demonstrating a Type 2C upper nose supplied by bilateral ophthalmic angiosomes with a true anastomosis via the converged and then merged bilateral dorsal nasal arteries. (A) CT reconstructed with the Philips workstation. (B) CT reconstructed with the Mimics algorithm. CT, computed tomography; 3D, three-dimensional.
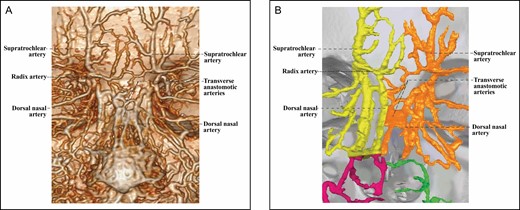
The 3D reconstructed CT of Cadaver No 44, a 40-year-old female, with contrast agent infusion of the external carotid artery, demonstrating a Type 2C upper nose supplied by bilateral ophthalmic angiosomes with a true anastomosis via transversely connected bilateral dorsal nasal arteries. (A) CT reconstructed with the Philips workstation. (B) CT reconstructed with the Mimics algorithm. CT, computed tomography; 3D, three-dimensional.
In the 2 Type 2A upper noses, the true anastomosis between bilateral ophthalmic angiosomes is mediated by 1 or more radix arcades (Figure 2), which are formed by head-to-head anastomosis between 2 bilateral radix arteries. In some noses, multiple interconnected radix arcades demonstrate a network of true anastomoses across the upper dorsal midline.
In the 5 Type 2B upper noses, the true anastomosis between bilateral ophthalmic angiosomes also relies on the radix arcade, although this is generated by a head-to-side anastomosis between a radix artery and the transverse starting segment of a contralateral DNA (Figure 3). After joining the radix artery to form the radix arcade across the upper dorsal midline, the DNA continues its course caudally toward the nasal tip.
There are 3 Type 2C upper noses with well-developed bilateral DNAs, which are directly connected to form the true anastomosis between bilateral ophthalmic angiosomes. These bilateral DNAs may gradually converge and eventually join with each other to form the true anastomosis between ophthalmic angiosomes (Figure 4). The bilateral DNAs may be linked by multiple transverse arteries to relay the true anastomosis between the ophthalmic angiosomes (Figure 5).
The Type 3 Upper Nose
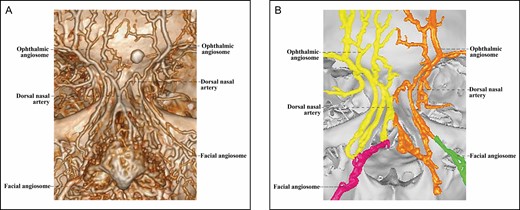
The Type 3 upper nose is supplied by both facial and ophthalmic angiosomes (Figures 6-8). Because the facial angiosome contributes to the upper nasal arterial supply in 3 ways, the Type 3 upper nose is classified into 3 subcategories.
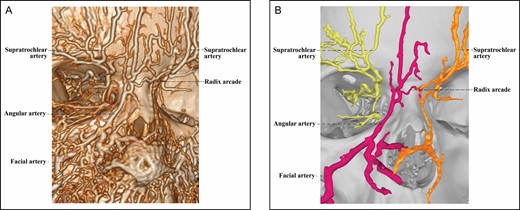
The 3D reconstructed CT of Cadaver No 27, a 35-year-old female, with contrast agent infusions of the bilateral facial artery and superficial temporal artery, demonstrating a Type 3A upper nose supplied by the right facial and left ophthalmic angiosomes with a true anastomosis via a radix arcade. The radix arcade is formed by the right radix artery from the angular artery of the facial angiosome and the left radix artery from the ophthalmic angiosome. (A) CT reconstructed with the Philips workstation. (B) CT reconstructed with the Mimics algorithm. CT, computed tomography; 3D, three-dimensional.
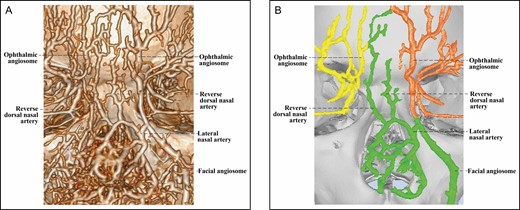
The 3D reconstructed CT of Cadaver No 9, a 25-year-old male, with contrast agent infusions of the bilateral external carotid artery, demonstrating a Type 3B upper nose in which the lateral nasal artery of the right facial angiosome branches off a reverse dorsal nasal artery running cranially to form a true anastomosis with the supratrochlear artery of the left ophthalmic angiosome. (A) CT reconstructed with the Phillips workstation. (B) CT reconstructed with the Mimics algorithm. CT, computed tomography; 3D, three-dimensional.
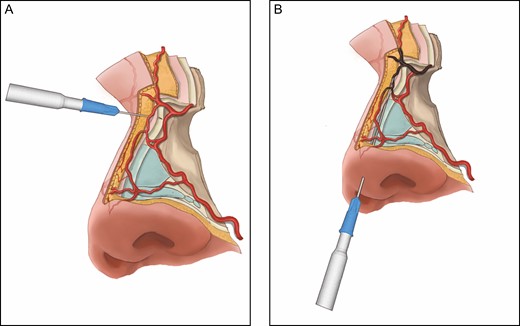
Recommended technique for safe upper nasal dorsum filler injection. (A) Filler for concave nasal radix can be safely injected with a new 27G needle, which allows a reliable aspiration test. The direction of the needle should be perpendicular to the bony surfaces of the midline dorsum, and the filler should be injected into the nasal periosteum. (B) Filler for dorsum augmentation can also be safely injected with a blunt cannula by “hugging” the midline nasal periosteum/perichondrium; however, cannula injection of the filler at the concave nasal radix carries a particularly high risk of blindness, because the tip of the cannula is unable to stay on the radix periosteum due to the concavity. Instead, the tip of the cannula may penetrate the radical soft tissue where there are true anastomotic arteries (highlighted in brownish gray) directly linked to the ophthalmic angiosomes.
The Type 3A upper nose, found in 3 specimens, features an angular artery from a facial angiosome (Figure 6), which forms a true anastomosis with the contralateral ophthalmic angiosome. True anastomosis is mediated by a radix arcade formed by a radix artery with a contralateral radix artery or DNA.
The Type 3B upper nose is unique in that the lateral nasal artery (LNA) of its facial angiosome sends 1 or more atypical DNAs that travel cranially to supply the upper dorsal nose (Figure 7). In addition, there is at least one atypical DNA that forms a true anastomosis with the ophthalmic angiosome. This novel artery is found in 7 noses. Judging from the transition of contour, we believe the direction of its course is cranial, and thus we tentatively call it “reverse DNA.” Supplemental Figure 1 shows a further example of this artery.
The Type 3C upper noses (2 cases) are supplied by bilateral facial and ophthalmic angiosomes with true anastomoses across the dorsal midline between multiple angiosomes (Supplemental Figure 2).
The Structural Components of the True Anastomoses
True anastomosis across the upper dorsal midline is found in 84.6% of the noses studied, and is mediated by the radix arcade in 46% (12/26) of the cases. The DNA is involved is generating true anastomoses in 65% (17/26) of the noses studied.
The radix artery is found in 54% (14/26) of the noses, and originates from the ophthalmic angiosome in 11 noses and from the facial angiosome in 3 noses. The DNA is found in 92% (24/26) of the noses studied, including 16 noses with regular DNA, and 8 noses with reverse DNA. The DNA is bilateral in 14 cases, and unilateral in 10 cases. The radix artery and DNA rarely coexist in the same hemiface.
DISCUSSION
Filler injection is frequently practiced for dorsum augmentation in the East or for the correction of dorsal hump in the West. In general, filler injection into the nasal perichondrium or periosteum is performed either with a sharp needle perpendicular to the dorsum or a blunt cannula parallel to the dorsum (Figure 8).10-12,16 Even taking due precautions, however, nasal filler injection remains a risky procedure.1-3 To complicate matters, little is known about the upper nasal arteries that cross the dorsum and thus may be injured or cannulated by the injection needle/cannula, which can result in feeding filler particles into the ophthalmic circulation, causing vision loss.9-11,13-15
In this anatomic study, we visualized and profiled the deployment of the facial and ophthalmic angiosomes in the upper nose by postmortem CT with 3D reconstruction. We identified some true anastomotic arteries that are directly linked to the ophthalmic angiosomes across the upper dorsal midline, and estimated the risk of blindness for each type of upper nasal artery supply during dorsal filler injection. Our findings shed light on the pathways through which filler particles migrate into the ophthalmic angiosomes and cause blindness. The results from this study should help physicians choose a safe technique for nasal filler injection.
Risk of Blindness During Upper Dorsum Filler Injection
Taylor and Palmer pioneered the angiosome theory in 1987, and further defined choke vs true anastomoses in 1992.17,18 Choke vessels are characterized by anatomically reduced calibers, and by being able to physiologically restrict the flow between the vascular territories of neighboring angiosomes. In contrast, a true anastomotic artery shows no apparent diameter reduction, and is a “super free highway” between neighboring angiosomes. Therefore, anastomosis between 2 adjacent angiosomes represents an anatomic boundary, and the modality of the anastomosis functionally controls the nature of communication and blood flow between adjacent angiosomes.19
In the Type 1 upper nose (15%, Figure 1), choke anastomoses are the only communication channels between bilateral ophthalmic angiosomes. Neither of the ophthalmic angiosomes extends its footprint across the upper dorsal midline. The choke vessels are of dramatically reduced caliber and are thus expected to functionally impede the blood flow between bilateral ophthalmic angiosomes under physiologic conditions.19 It is likely that choke anastomoses in the Type 1 upper nose would strictly prevent any filler particles that have been injected along the dorsal midline from passing through and then entering the ophthalmic circulation. Therefore, the risk of vision loss should be minimal from injecting filler into Type 1 upper noses with a perpendicular needle or parallel cannula.
However, in the approximately 85% of the noses that are not Type 1, there is at least 1 true anastomosis that is directly connected to an ophthalmic angiosome across the upper dorsal midline. These true anastomoses link the bilateral ophthalmic angiosomes in the Type 2 upper nose (38.5%, Figures 2-5), and connect the facial angiosome(s) to the ophthalmic angiosome(s) in the Type 3 upper nose (46.1%, Figures 6 and 7). The diameters of the true anastomotic arteries are similar to those of the branches of the angiosomes in direct anastomoses. These findings are consistent with the recent angiographic observations by Taylor et al that true anastomoses are very prevalent between bilateral ophthalmic angiosomes across the nasal dorsum, and between the terminal angular branches of the facial artery and the external nasal branches of the ophthalmic angiosome.15
According to the functional angiosome theory,17-19 the inner lumen of these true anastomoses crossing the upper dorsal midline are very likely to be large enough to allow filler particles to pass through. If any of these anastomotic arteries is injured or cannulated by a needle or cannula during the placing of filler on the dorsum, filler particles may be fed into the ophthalmic circulation, which could cause vision compromise. Thus, there is a substantial risk of blindness in Type 2 and Type 3 upper noses, especially if a large amount of filler is injected into the artery under a high pressure.
The Nature of the Radix Artery and Radix Arcade
The nature of the radix artery has long been poorly defined. Saban et al14 first reported that the radix artery is a branch of the ophthalmic artery running transversely at the radix of all 20 cadavers that they dissected. They found that this artery usually “culminates” in the DNA, suggesting that it is the transverse starting segment of the DNA, and they also proposed that 2 radix arteries might connect to form a radix arcade. In contrast, in a study of 50 cadaveric noses, Tansatit et al10 noted that when bilateral DNAs are absent, the radix artery enters the middle nose as a minute branch of the angular artery or the LNA. A histology study by this group found sizable (0.4-0.9 mm diameter) anastomotic arteries crossing the rhinion midline in 64.4% of 45 specimens,9 which could be the radix arcade, although the authors did not comment on this.
Similar to the findings of the Tansatit group, in 54% of the noses, we found a radix artery, which is often a branch of the angular artery. This artery may originate from the ophthalmic angiosome (Figures 2 and 3) or from the facial angiosome (Figure 6). Few radix arteries are present in the same hemiface with the DNA. These data suggest the radix artery is an independent arterial entity.
We observed the radix arcade in 46% of the noses. Although the radix artery is not the transverse starting segment of the DNA, as Saban et al previously thought,14 a unilateral radix artery may anastomose with the transverse starting segment of the contralateral DNA to form a radix arcade (Figure 3). Two bilateral radix arteries can also be linked together to form 1 or more radix arcades (Figure 2). All radix arcades transversely cross the upper dorsal midline as an important anastomotic pathway. Each radix arcade poses the risk of blindness during filler injection in the dorsum.
The Contribution of the DNA and Reverse DNA to True Anastomosis
Multiple studies suggest that the DNA is a terminal branch of the ophthalmic artery, and is involved in arterial anastomosis across the dorsal midline.10,11,13 Tansatit et al10 observed the DNA in 62% of noses, and found that in 20% of noses, the DNA anastomoses with the contralateral LNA or courses along the dorsal midline. Choi et al11 found bilateral DNAs in all noses, and noted connections between the DNAs in 82.4% of the specimens. Similarly, we identified the DNA in 92% of the noses. In 65% of the noses, the DNA is involved in true anastomosis with the ophthalmic angiosome. Because the true anastomosis approaches or crosses the upper dorsal midline, it is an important source of blindness risk associated with dorsal filler injection.
We also discovered reverse DNA, which is a branch of the LNA from the facial angiosome, running cranially to supply the upper nasal dorsum (Figure 7). We speculate that the reverse DNA is functionally equivalent to a regular DNA coursing caudally from the radix to the nasal tip. Reverse DNA is found in 31% of the noses studied and, in each case, there is 1 reverse DNA directly linked to the ophthalmic angiosome, which jeopardizes the safety of dorsum filler injection.
Recommended Technique for Safe Upper Nasal Dorsum Filler Injection
To minimize the risk of blindness when placing filler in the dorsum of the upper nose, the physician must consistently maintain the tip of the injection needle or cannula on the surface of the nasal periosteum or perichondrium throughout the injection process. Although our studies were unable to determine the exact plane in which an artery travels in the face,6 multiple studies suggest that the external nasal arteries run in the subcutaneous plane overlaying the fibromuscular layer.9-11,13,14 There is a consensus in the literature that the areolar plane overlaying the nasal periosteum and periosteum is the safest plane for filler injection.9-11
To inject filler into the dorsum with a sharp needle (Figure 8A),16 the thumb and index fingers of the nondominant hand should pinch and lift the soft tissue at the dorsal midline. This procedure can increase the space in the areolar plane to better accommodate the injected filler and elevate the anastomotic arteries in the soft tissue away from the nasal periosteum/perichondrium surface where the tip of the sharp needle is located. In order to minimize potential needle cannulation of any anastomotic artery, it is critical that the direction of the injection needle be perpendicular to the bony or cartilaginous surfaces at the midline dorsum. The inner lumen of the needle should be at least 27G in diameter and be free of any viscous filler material to allow an effective aspiration test.20,21 The dorsal midline is augmented with a series of depot injections and finally gently massaged for contour purposes.
To inject filler into the nasal dorsum with a blunt cannula, the tip of the cannula must be continuously “hugging” and sliding on the surface of nasal perichondrium or periosteum (Figure 8B). In addition, the physician should pinch and raise the soft tissue at the dorsal midline with the fingers of the nondominant hand. This can lift the anastomotic arteries in the soft tissue away from the bony or cartilaginous surface where the cannula tip is moving and injecting filler, and thus help minimize accidental intravascular cannulation and injection. Tansatit et al10 found the mean [standard deviation] diameter of a single DNA to be 0.7 (0.3) mm, but it may be up to 1.2 mm, which is much larger than that of a 22G cannula (0.7 mm). Therefore, it remains unclear which cannula size is safest for nasal filler injection.
The deep concavity at the radix region of the nasal bones deserves special attention. Multiple studies suggest that injecting filler at that point with a cannula parallel to the nasal dorsum carries a particularly high risk of blindness (Figure 8B).10-12 Jung et al noted that there is a 10.2° angle on average between the dorsal skin and the nasal bone, and as a result there is a 1.9 (0.3) mm mean (standard deviation) distance from the cannula tip to the depressed nasal bone.12 Although the body of the injection cannula is “hugging” the bony and cartilaginous surfaces of the nasal dorsum, the tip of the cannula is unable to stay on the radix periosteum because of the radical concavity. Instead, there is the risk of penetrating the radical soft tissue, where our study and others9-11,15 have identified true anastomotic arteries directly linked to the ophthalmic angiosomes. A cannula tip may potentially injure or cannulate the anastomotic arteries and unknowingly feed filler particles into the ophthalmic circulation: an aspiration test is not reliable for a long cannula with viscous filler material inside the lumen.
In contrast, the aspiration test is reliable for a fresh size 27G or below needle with no filler material inside its lumen.20,21 Radical filler injection on the nasal bones is commenced only after a confirmed negative aspiration test. Each depot filler injection is made with a new 27G needle. If a positive aspiration test is confirmed during nasal dorsum filler injection, the injection is aborted for safety reasons, or filler is injected in other segments of the nose. In general, we agree with Tansatit, Choi, and Jung that concave radix filler injection is probably safer with the perpendicular needle technique, whereas other parts of the dorsum should be injected with a blunt cannula inserted through an infratip needle puncture.10-12 With the advent of high-resolution ultrasound, all external nasal arteries can be visualized in real time, which could prevent all injection-related visual compromises.22
Limitations of This Study
This study is limited by its sample size, the reliance on only Chinese specimens, and the selection bias that 58% of the specimens were excluded from the final data analysis due to poor perinasal artery image quality. Therefore, the percentages obtained in this study may not represent the true prevalence in the general population. This high screening failure is probably caused by the fact that perinasal arteries with a luminal diameter less than 0.4 mm cannot be definitively demonstrated by postmortem CT technology.23 Tansatit et al10 reported that the DNAs were very small in 38% of the 50 noses that they examined. In addition, some terminal arteries of the nose might be clogged by thrombus that could not be completely flushed out before the infusion of CT contrast agent. This study is also limited by the relatively young ages of the cadavers (20-56 years) included in our data analysis. Therefore, the results cannot be used to determine if there is any association of advanced age with the robustness of true anastomoses among facial and ophthalmic angiosomes deployed in the upper nose.
CONCLUSIONS
The deployment and anastomosis of the facial and ophthalmic angiosomes in the upper nose fall into 3 major patterns. In about 85% of noses, there are true anastomoses with ophthalmic angiosomes across upper dorsal midline, which pose the risk of blindness during filler injection. The structural components of the true anastomoses are the radix artery, the DNA, and the radix arcade. The facial angiosome may send reverse DNAs to supply the upper nose. On the basis of these results and prior studies, we recommend that nasal filler injection be made with a combination of a needle and a cannula.
Acknowledgments
Drs Cai and Yuan contributed equally to the article as co-first authors.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES



