-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Fiala, Abdominal Laser Lipolysis Using a Microprocessor-Controlled Robotic Arm With Noncontact Heating and Cooling, Aesthetic Surgery Journal, Volume 41, Issue 12, December 2021, Pages NP1951–NP1961, https://doi.org/10.1093/asj/sjab206
Close - Share Icon Share
Abstract
A novel FDA-cleared device uses a 1064-nm laser to noninvasively induce apoptosis for lipolysis of subcutaneous abdominal fat while maintaining comfortable skin temperatures with a proprietary jet cooling system (eon; Dominion Aesthetic Technologies, Inc., San Antonio, TX). A programmable articulated robotic arm moves the treatment head without any subject contact, maintaining an appropriate 3-dimensional treatment path, compensating for patient movement.
The goal of this prospective, single center, open-label study was to demonstrate the safety and effectiveness of this device for reducing subcutaneous abdominal fat when operated with an updated power delivery curve.
Male and female subjects with Fitzpatrick skin types I to VI (N = 26) were treated. Four abdominal zones up to 150 cm2 each, customized in size and location for body habitus, were treated. Each zone underwent a single 20-minute treatment session. Follow-up visits occurred after 6 and 12 weeks. A standardized protocol was used to obtain ultrasound measurement of subcutaneous abdominal fat thickness, abdominal circumference, reported patient satisfaction and digital images.
The mean treatment area was 378.5 cm2. At Week 12, there was a 21.6% or 6.3 mm mean reduction in abdominal subcutaneous fat thickness and a 4.1-cm (1.6-inch) mean reduction in abdominal circumference. Most subjects (84.6%) were satisfied or very satisfied with their results. The mean pain score was 2.5 on an 11-point ordinal scale. There were no nonresponders. Only 2 adverse events were noted: mild transient erythema (n = 1, 3.8%) and localized subcutaneous firmness (n = 1, 3.8%) which resolved without intervention within 12 weeks.
This contact-free device is safe and effective for reducing subcutaneous abdominal fat and represents an improvement on the prior treatment protocol.
Liposuction is one of the most common aesthetic surgical procedures performed in the United States with more than 270,000 performed in 2019.1 Although tumescent liposuction remains the gold standard for fat reduction, disadvantages include postoperative pain and swelling, slow recovery, and the frequent use of general anesthesia.2 Although rare, potential serious complications can occur, including bleeding requiring transfusion, infection, damage to internal organs, or pulmonary thromboemoblism.3
In recent years, less invasive techniques for body contouring have grown in popularity. These devices include cryotherapy,4 high-intensity focal ultrasound,5 low-level lasers,6 and radiofrequency.7 Currently, the use of these nonsurgical devices exceeds that of liposuction.1
An FDA-cleared 1064-nm laser system was used for external noninvasive subcutaneous lipolysis of the abdomen in this study (eon; Dominion Aesthetic Technologies, Inc., San Antonio, TX). As no part of it directly touches the patient, it is unique in its class of body-contouring laser devices. There are no disposables or treatment applicators. The treatment head of the device combines a laser, which heats subcutaneous adipose tissue to a temperature of approximately 42 to 51°C to induce apoptosis, with a jet-inspired impingement cooling system, which protects the skin and provides patient comfort. The treatment head is mobile and is driven by a proprietary, microprocessor-controlled articulated robotic arm which delivers consistently reproducible passes of the treatment head over the designated treatment area.
Since its FDA clearance, additional research has allowed the power delivery curve of the device to be optimized, with the total amount of applied laser energy increased by 60%, while still maintaining FDA-approved lasing parameters, and keeping surface skin and deep tissue temperatures in a desirable safe range (data on file). The aim of the current study was to objectively demonstrate the safety, effectiveness, and patient acceptance of this 1064-nm laser system for performing noncontact subcutaneous abdominal lipolysis with the newer power delivery algorithm, closely repeating the design of the original 12-week open-label FDA clinical study, so as to allow direct comparison. The 12-week study duration was selected in order to allow direct comparison to the prior pivotal FDA study data on this device, and also to allow comparison to a previously published 12-week study on a competitive laser lipolysis device, which uses a contact laser system for fat reduction. Further longer-term studies are planned.
METHODS
Study Subjects
Healthy male and female subjects >18 years old who were seeking treatment of unwanted subcutaneous adipose tissue of the abdomen were enrolled. Subjects were required to have a depth of adipose tissue ≥20 mm on the proposed treatment area and were asked not to make any intentional changes to their dietary or exercise regimens. Female subjects of childbearing potential agreed to use a medically acceptable form of birth control. Subjects were recruited through the use of written flyers about the study which were distributed to a number of gyms and offices in the region. Potential candidates were preliminarily screened to check for appropriate inclusion or exclusion criteria, and then final approval was given by the investigator. We attempted to enroll both male and female subjects, with a variety of skin types. All subjects were compensated $500 USD for participation in the study, paid in installments by the study sponsor (Dominion Aesthetic Technologies, Inc.) at the 6- and 12-week checkups. There were no patient charges for the treatment.
Exclusion criteria included: a prior aesthetic fat reduction procedure in the planned treatment area during the previous year; unstable weight (±3%) during the previous 6 months; presence of an infection, dermatitis, or a rash in the treatment area; tattoos or jewelry in the treatment area or within the digital imaging frame; history of keloid scarring, hypertrophic scarring, or abnormal wound healing; history of immune deficiency disorders or current use of immunosuppressive medications; known bleeding disorder; photosensitivity to the study laser wavelength or use of photosensitizing medications; collagen-vascular diseases; unhealed surgical procedure in the treatment area; significant concurrent illness; use of systemic chemotherapy for cancer; steroid, psychotropic, or gold therapy; participation in another investigational device or drug study within 3 months of enrollment or during the study; and any other condition that might put the subject at risk or jeopardize the study objectives.
Study Device
The treatment device uses proprietary software to control an articulated motorized robotic treatment arm which moves a 1064-nm laser treatment head over a preprogrammed path 1 to 3 cm above the surface of the skin. Proximity sensors in the treatment head detect and automatically adjust the treatment head motion to accommodate for respiratory and other subject movements. A “top-hat” square laser pattern was used to maintain uniform tissue heating. Skin temperature sensors continuously recorded data during treatment and ensured that no skin overheating occurred. A proprietary jet-impingement cooling mechanism transfers cold air into multiple high velocity jets to create efficient skin cooling, maintaining safe and comfortable skin temperatures.
Procedures
The laser treatment was performed after completing the screening visit. All treatments were performed at a single center, between August 24, 2019 and December 21, 2019. A professional photographer obtained consistent pretreatment images with a digital single-lens reflex camera, standardized lighting and included front, oblique, and side views of the abdomen. For consistency, patients were positioned with arms crossed in front of the chest, and images were obtained with the abdomen relaxed, immediately after end-expiration. Following examination by the treating physician, areas for laser lipolysis treatment were marked on the anterior abdomen according to one of several treatment templates. The number and size of the treated areas were chosen at the sole discretion of the physician. In general, 2 treatment areas were performed in the infraumbilical abdomen, and 1 or 2 areas were performed in the upper abdomen, depending on the subject’s adipose distribution and body size. Ultrasound measurements were obtained at the geometric center of each treatment area while in the supine position. To ensure consistency, the same certified ultrasonographer completed each ultrasound measurement throughout the study, with the same ultrasound machine (Eco 3 Expert; Chison USA, Inc., Bellevue, WA) and linear transducer (L7M-4, 10 MHz). Only sufficient pressure needed to ensure proper acoustic contact of the ultrasound transducer with the skin was applied, in order to minimize compression inaccuracies during each measurement. Notations and measurements were retained for accurate identification of the correct measurement locations at follow-up visits.
Abdominal circumferential measurements were recorded both 3.75 cm (1.5 inch) above and below the center of the umbilicus in the standing position, at end-expiration, with the abdomen relaxed, giving a tension-free measurement. A mean abdominal circumference data point was then generated for each patient by calculating an average of these 2 numbers, at each of the time periods.
Each subject then had the designated areas up to 150 cm2 each (≤600 cm2) treated with the laser device for 20 minutes in each zone. The treating physician double-checked the machine placement and scan pattern, prior to initiation of the lasing treatment. For this clinical trial, the laser was supervised by a company-supplied laser technician, according to the physician’s specific instructions. The laser technician did not make any adjustments to the treatment parameters. A registered nurse monitored the subjects throughout the laser procedure with the physician immediately available on-site and checking on the subject periodically. All lased areas were checked by the physician prior to discharge and if there were any concerns by subject or staff during treatment. Eye-shielding precautions were taken for the subject and all personnel in the room. No analgesics were provided to or needed by any subjects, although the patients were encouraged to communicate any subjective pain or other sensations during the treatment.
Subjects agreed to return to the clinic for postprocedure follow-up visits at approximately 6 and 12 weeks after treatment. During this period, subjects were again instructed to maintain their current diet and exercise habits, specifically not to intentionally lose or gain weight; weight changes were not used to exclude patients from subsequent analysis. Assessments during each visit included body weight, posttreatment digital images, circumferential abdominal measurements, ultrasound measurements of the treatment areas, and a written ad hoc subject satisfaction questionnaire, which asked about subject comfort, and at 12 weeks, about satisfaction with their appearance and results (Appendices A and B, available online at http://www.aestheticsurgeryjournal.com).
Efficacy Endpoints
The primary measures of treatment effectiveness were any changes from the pretreatment baseline abdominal subcutaneous adipose tissue thickness, based on ultrasound assessment by a qualified experienced independent ultrasound technician. Measurements were taken at the center of each treatment zone. Secondary assessments included the results of a subject satisfaction survey and patient measurements.
Safety Endpoint
Subject safety was assessed by the occurrence of specific predetermined, protocol-specified, adverse events, in particular: erythema, skin irritation, prolonged pain (arbitrarily defined as pain lasting 14 or more days), swelling, blistering, burns, hypopigmentation, hyperpigmentation, scarring, or subcutaneous nodules/panniculitis.
Statistical Analysis
For the primary endpoint, a biostatistician used data from a previous study to determine that a sample size of 26 subjects had 86% power to reject the null hypothesis of 15% fat reduction at Week 12 against the expected alternative hypothesis to achieve 20% fat reduction at Week 12 under the prospective assumption of an 8% standard deviation for the change from baseline for a 2-sided paired t test. Analyses of percentage fat reduction were performed based on measurements both above and below the navel with results averaged per patient to obtain the stated percentage fat reductions at Weeks 6 and 12. Continuous endpoints for the change from baseline were calculated from 2-sided paired t tests.
Ethics
This study protocol and related materials were approved by a commercial institutional review board (IntegReview IRB, Austin, TX). Each subject provided written informed consent prior to participating in any study-related activity. The study was conducted in compliance with the Declaration of Helsinki as amended,8 US Title 21CFR Part 50,9 and ICH guidance E6.10
RESULTS
Subject Demographics
Among the enrolled subjects (N = 26), most were female (20 females, 6 males). The median age was 42 years (range, 21-61 years), and mean baseline body mass index (BMI) was 25.1 kg/m2 (range, 17.8-32.2 kg/m2). The baseline mean abdominal circumference was 90.7 cm (range, 77.8-112.3 cm). A variety of Fitzpatrick skin types were treated, with most rated III to VI (n = 17, 65.4%). The distribution of Fitzpatrick skin types is shown in Table 1. The mean area treated was 378.5 cm2 (range, 220 to 600 cm2) during a single treatment session. Subjects returned to the clinic for postprocedure follow-up visits after 6 ± 1 weeks and 12 ± 1 weeks.
| Skin type . | n (%) . |
|---|---|
| I | 1 (4) |
| II | 8 (31) |
| III | 14 (54) |
| IV | 0 |
| V | 1 (4) |
| VI | 2 (8) |
| Skin type . | n (%) . |
|---|---|
| I | 1 (4) |
| II | 8 (31) |
| III | 14 (54) |
| IV | 0 |
| V | 1 (4) |
| VI | 2 (8) |
| Skin type . | n (%) . |
|---|---|
| I | 1 (4) |
| II | 8 (31) |
| III | 14 (54) |
| IV | 0 |
| V | 1 (4) |
| VI | 2 (8) |
| Skin type . | n (%) . |
|---|---|
| I | 1 (4) |
| II | 8 (31) |
| III | 14 (54) |
| IV | 0 |
| V | 1 (4) |
| VI | 2 (8) |
Efficacy Results
Ultrasound measurements were made at the center of each treatment zone and compared with baseline measurements, to calculate a percentage of reduction of the subcutaneous fat. A series of calculations was then performed to generate mean values for subcutaneous fat thickness change in the upper abdomen, lower abdomen and for all treatment zones. The mean (standard deviation [SD]; 1-sided 97.5% confidence interval [CI]) percentage subcutaneous fat reduction for all treated areas was 11.8% (8.5; –8.5, –25.3) at Week 6 and 21.6% (8.8; –18.1, –24.1) at Week 12 (Table 2). There was a substantially greater decrease in adipose thickness in tissue below the umbilicus (–25.6%) vs above the umbilicus (–16.4%) (Table 3).
| . | Mean percentage change in adipose tissue thickness . | . | Mean change in abdominal circumference, inches . | . |
|---|---|---|---|---|
| . | Week 6, N = 25 . | Week 12, N = 26 . | Week 6, N = 25 . | Week 12, N = 26 . |
| Mean [SD] | –11.8 [8.5] | –21.6 [8.8] | –0.86 [0.82] | –1.60 [1.10] |
| 95% CI | –8.27, –15.30 | –18.96, –25.14 | –0.53, –1.20 | –1.16, –2.05 |
| Significancea | P < 0.0000001 | P < 0.0000002 |
| . | Mean percentage change in adipose tissue thickness . | . | Mean change in abdominal circumference, inches . | . |
|---|---|---|---|---|
| . | Week 6, N = 25 . | Week 12, N = 26 . | Week 6, N = 25 . | Week 12, N = 26 . |
| Mean [SD] | –11.8 [8.5] | –21.6 [8.8] | –0.86 [0.82] | –1.60 [1.10] |
| 95% CI | –8.27, –15.30 | –18.96, –25.14 | –0.53, –1.20 | –1.16, –2.05 |
| Significancea | P < 0.0000001 | P < 0.0000002 |
CI, confidence interval; SD, standard deviation. aTwo-sided paired t test.
| . | Mean percentage change in adipose tissue thickness . | . | Mean change in abdominal circumference, inches . | . |
|---|---|---|---|---|
| . | Week 6, N = 25 . | Week 12, N = 26 . | Week 6, N = 25 . | Week 12, N = 26 . |
| Mean [SD] | –11.8 [8.5] | –21.6 [8.8] | –0.86 [0.82] | –1.60 [1.10] |
| 95% CI | –8.27, –15.30 | –18.96, –25.14 | –0.53, –1.20 | –1.16, –2.05 |
| Significancea | P < 0.0000001 | P < 0.0000002 |
| . | Mean percentage change in adipose tissue thickness . | . | Mean change in abdominal circumference, inches . | . |
|---|---|---|---|---|
| . | Week 6, N = 25 . | Week 12, N = 26 . | Week 6, N = 25 . | Week 12, N = 26 . |
| Mean [SD] | –11.8 [8.5] | –21.6 [8.8] | –0.86 [0.82] | –1.60 [1.10] |
| 95% CI | –8.27, –15.30 | –18.96, –25.14 | –0.53, –1.20 | –1.16, –2.05 |
| Significancea | P < 0.0000001 | P < 0.0000002 |
CI, confidence interval; SD, standard deviation. aTwo-sided paired t test.
| . | Week 6 . | . | . | Week 12 . | . | . |
|---|---|---|---|---|---|---|
| . | Above umbilicus . | Below umbilicus . | Overall mean . | Above umbilicus . | Below umbilicus . | Overall mean . |
| . | N = 22 . | N = 25 . | N = 25 . | N = 23 . | N = 26 . | N = 26 . |
| Mean [SD] | –7.7 (8.1) | –13.8 (10.2) | –11.8 (8.5) | –16.4 (9.3) | –25.3 (10.8) | –21.6 (8.8) |
| 95% CI | –4.1, –11.3 | –9.6, –18.0 | –8.5, –25.3 | –12.4, –20.4 | –20.9, –29.6 | –18.1, –24.1 |
| . | Week 6 . | . | . | Week 12 . | . | . |
|---|---|---|---|---|---|---|
| . | Above umbilicus . | Below umbilicus . | Overall mean . | Above umbilicus . | Below umbilicus . | Overall mean . |
| . | N = 22 . | N = 25 . | N = 25 . | N = 23 . | N = 26 . | N = 26 . |
| Mean [SD] | –7.7 (8.1) | –13.8 (10.2) | –11.8 (8.5) | –16.4 (9.3) | –25.3 (10.8) | –21.6 (8.8) |
| 95% CI | –4.1, –11.3 | –9.6, –18.0 | –8.5, –25.3 | –12.4, –20.4 | –20.9, –29.6 | –18.1, –24.1 |
CI, confidence interval; SD, standard deviation.
| . | Week 6 . | . | . | Week 12 . | . | . |
|---|---|---|---|---|---|---|
| . | Above umbilicus . | Below umbilicus . | Overall mean . | Above umbilicus . | Below umbilicus . | Overall mean . |
| . | N = 22 . | N = 25 . | N = 25 . | N = 23 . | N = 26 . | N = 26 . |
| Mean [SD] | –7.7 (8.1) | –13.8 (10.2) | –11.8 (8.5) | –16.4 (9.3) | –25.3 (10.8) | –21.6 (8.8) |
| 95% CI | –4.1, –11.3 | –9.6, –18.0 | –8.5, –25.3 | –12.4, –20.4 | –20.9, –29.6 | –18.1, –24.1 |
| . | Week 6 . | . | . | Week 12 . | . | . |
|---|---|---|---|---|---|---|
| . | Above umbilicus . | Below umbilicus . | Overall mean . | Above umbilicus . | Below umbilicus . | Overall mean . |
| . | N = 22 . | N = 25 . | N = 25 . | N = 23 . | N = 26 . | N = 26 . |
| Mean [SD] | –7.7 (8.1) | –13.8 (10.2) | –11.8 (8.5) | –16.4 (9.3) | –25.3 (10.8) | –21.6 (8.8) |
| 95% CI | –4.1, –11.3 | –9.6, –18.0 | –8.5, –25.3 | –12.4, –20.4 | –20.9, –29.6 | –18.1, –24.1 |
CI, confidence interval; SD, standard deviation.
One-half of treated subjects (50%) achieved >20% subcutaneous fat reduction at Week 12 vs the baseline ultrasound thickness measurement (Table 3). The reduction in mean abdominal circumference was 4.1 cm (range, 0.5-12.0 cm) at Week 12 with 57.7% experiencing a ≥2.5 cm reduction in this measurement vs the baseline mean circumference. There were no nonresponders. Representative pre- and posttreatment digital images for a male and a female subject are provided in Figures 1 and 2, along with representative ultrasound images for each patient, and a treatment area orientation photograph. Two additional patient treatment photograph sets are also included (Supplemental Figures 1 and 2, available online at http://www.aestheticsurgeryjournal.com).
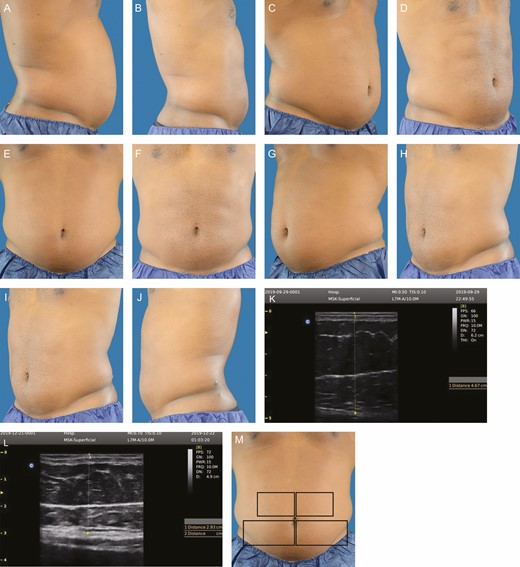
This 30 year-old male subject weighed 96.6 kg prior to treatment. At 12 weeks posttreatment, he weighed 96.8 kg, his mean abdominal fat thickness decreased 49.0%, and his circumference decreased 6 cm. A representative ultrasound image is shown, pretreatment and 12 weeks posttreatment. An orientation diagram of approximate treatment locations is shown for reader orientation. Pretreatment and posttreatment images at 12 weeks following 1 treatment: (A, B) right lateral, (C, D) right oblique, (E, F) frontal, (G, H) left oblique, (I, J) left lateral, (M) orientation view of treatment areas, (K, L) representative pretreatment ultrasound.
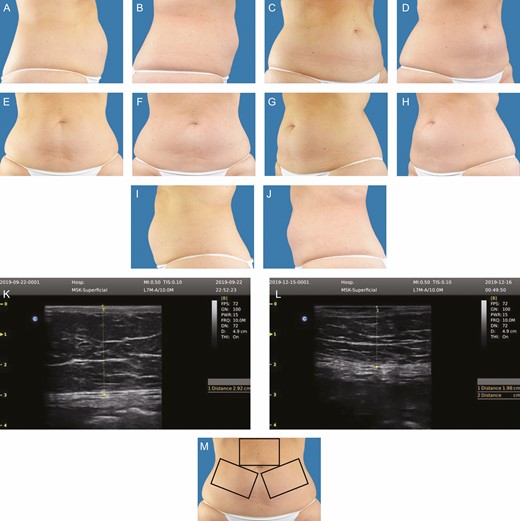
This 48-year-old female subject weighed 83.6 kg prior to treatment. At 12 weeks posttreatment, she weighed 82.5 kg, her abdominal fat thickness decreased 28.6%, and her circumference decreased 8 cm. A representative ultrasound image is shown, pretreatment and 12 weeks posttreatment. An orientation diagram of approximate treatment locations is shown for reader orientation. Pretreatment and posttreatment images at 12 weeks following 1 treatment: (A, B) right lateral, (C, D) right oblique, (E, F) frontal, (G, H) left oblique, (I, J) left lateral, (M) orientation view of treatment areas, (K, L) representative pretreatment ultrasound.
The results of the satisfaction survey indicated that most subjects rated their appearance as improved (84.6%), were satisfied with their improved appearance (88.5%), were satisfied with the amount of fat reduction (92.3%), and were satisfied with the procedure comfort (100%).
Additional post hoc analyses determined there were no gender differences for the changes in abdominal adipose tissue thickness and circumference at Week 12. Younger subject age and higher baseline BMI were correlated with a larger reduction in adipose tissue thickness but not circumference at Week 12. There was no correlation between the size of the treatment area and changes in adipose tissue thickness at Week 12; however, there was a significant change in adipose thickness (P < 0.0000001) and change in circumference (P < 0.000002) from Week 6 to 12 (Table 2). Although the overall mean percentage change in subcutaneous adipose thickness was –21.6%, there was a substantially greater decrease in adipose thickness in tissue below the umbilicus (–25.6%) vs above the umbilicus (–-16.4%) (Table 3).
Safety Results
An 11-point (0-10) scale was used to assess pain severity. Among subjects reporting a pain score during the procedure (n = 25), the mean score was 2.5 (maximum, 4.0). At 30 minutes postprocedure (n = 4) the mean pain score was 0.17 (maximum, 1.5), at Week 6 (n = 6) it was 0.44 (maximum, 2) and at Week 12 one subject reported a score of 1.
There were no serious adverse events or unexpected device-related events. Mild adverse events were reported by 2 subjects (7.7%), as follows: 1 subject (n = 1, 3.8%) developed moderate erythema on the treatment area during the session which prompted the author to shorten the other treatment segments from 20 to 15 minutes for this patient only. The erythema had fully resolved by the time the subject left the clinic, and there were no subsequent issues. The other subject (n = 1, 3.8%) had a single small area of localized subcutaneous firmness, diagnosed as a localized panniculitis, which was reported at Week 6 but was fully resolved at Week 12 without any treatment or intervention, as confirmed by clinical examination and ultrasound scans. There were no occurrences of any other listed adverse effects (Appendix C, available online at http://www.aestheticsurgeryjournal.com).
Discussion
The results of this study confirm the deliverability, safety, efficacy, and tolerability of this new noncontact 1064-nm laser system for performing abdominal lipolysis. After 12 weeks, subjects achieved an overall 21.6% reduction in abdominal subcutaneous fat, with half achieving >20% fat reduction at Week 12 after a single treatment session. The mean absolute thickness reduction in abdominal subcutaneous fat was 6.3 mm. The mean overall reduction in circumference was 4.1 cm (1.6 inches), with more than half experiencing a ≥2.54 cm (1-inch) reduction in their mean abdominal circumference. Subject satisfaction was high.
Changes to the original treatment protocol included an increase in the treatment session from 15 to 20 minutes, and a longer duration of treatment at higher power levels while still maintaining surface skin and deep tissue temperatures in a desirable and safe range (data on file). As a result, the aesthetic improvements achieved in this study exceed those seen previously in our prior study and subsequent FDA 510(k) application (Table 4). In that earlier study, which used the earlier power-delivery curve, subjects achieved a mean reduction in adiposity of 15% and >25% of subjects had a >20% subcutaneous fat reduction as measured with ultrasound.11
| Parameter . | Bass and Doherty, 201830 . | Waibel et al, 202012 . | Current study . |
|---|---|---|---|
| . | (FDA clearance) . | (FDA clearance) . | . |
| Device type | Contact 1060-nm laser | Noncontact 1064-nm laser | Noncontact 1064-nm laser |
| Cooling system | Contact, chilled water | Noncontact air jet | Noncontact air jet |
| Temperature control system | Manual, patient feedback | Automatic | Automatic |
| Sample size | 35 | 36 | 26 |
| Mean treatment time per segment, minutes | 25 | 15 | 20 |
| Week 12 mean fat reduction, mm | –2.65 | –3.4 | –6.3 |
| Overall subject satisfaction | 83% | 89% | 96% |
| Subjects with nodules, n (%) | 8 (23) | 4 (11.1) | 1 (3.9) |
| Parameter . | Bass and Doherty, 201830 . | Waibel et al, 202012 . | Current study . |
|---|---|---|---|
| . | (FDA clearance) . | (FDA clearance) . | . |
| Device type | Contact 1060-nm laser | Noncontact 1064-nm laser | Noncontact 1064-nm laser |
| Cooling system | Contact, chilled water | Noncontact air jet | Noncontact air jet |
| Temperature control system | Manual, patient feedback | Automatic | Automatic |
| Sample size | 35 | 36 | 26 |
| Mean treatment time per segment, minutes | 25 | 15 | 20 |
| Week 12 mean fat reduction, mm | –2.65 | –3.4 | –6.3 |
| Overall subject satisfaction | 83% | 89% | 96% |
| Subjects with nodules, n (%) | 8 (23) | 4 (11.1) | 1 (3.9) |
| Parameter . | Bass and Doherty, 201830 . | Waibel et al, 202012 . | Current study . |
|---|---|---|---|
| . | (FDA clearance) . | (FDA clearance) . | . |
| Device type | Contact 1060-nm laser | Noncontact 1064-nm laser | Noncontact 1064-nm laser |
| Cooling system | Contact, chilled water | Noncontact air jet | Noncontact air jet |
| Temperature control system | Manual, patient feedback | Automatic | Automatic |
| Sample size | 35 | 36 | 26 |
| Mean treatment time per segment, minutes | 25 | 15 | 20 |
| Week 12 mean fat reduction, mm | –2.65 | –3.4 | –6.3 |
| Overall subject satisfaction | 83% | 89% | 96% |
| Subjects with nodules, n (%) | 8 (23) | 4 (11.1) | 1 (3.9) |
| Parameter . | Bass and Doherty, 201830 . | Waibel et al, 202012 . | Current study . |
|---|---|---|---|
| . | (FDA clearance) . | (FDA clearance) . | . |
| Device type | Contact 1060-nm laser | Noncontact 1064-nm laser | Noncontact 1064-nm laser |
| Cooling system | Contact, chilled water | Noncontact air jet | Noncontact air jet |
| Temperature control system | Manual, patient feedback | Automatic | Automatic |
| Sample size | 35 | 36 | 26 |
| Mean treatment time per segment, minutes | 25 | 15 | 20 |
| Week 12 mean fat reduction, mm | –2.65 | –3.4 | –6.3 |
| Overall subject satisfaction | 83% | 89% | 96% |
| Subjects with nodules, n (%) | 8 (23) | 4 (11.1) | 1 (3.9) |
Despite heating subcutaneous adipose tissue to apoptotic temperatures,11 the noncontact cooling system maintained subject comfort throughout treatment. The observation that a greater amount of subcutaneous adipose tissue reduction occurred below the umbilicus was an important observation, as most individuals have thicker fat deposits in that region. Additionally, in our experience, the lower abdomen is the most popular area for non-surgical body contouring. Katz et al noted a similar finding, with better response in the subumbilical abdomen than the upper abdomen, in their study of 33 patients treated with a high-intensity focused electromagnetic device.12
Although a number of energy-based treatments are commercially available and approved for adipose reduction, the laser device described in this study holds several advantages. For example, cryolipolysis reduces subcutaneous adipose tissue by localized cooling which also causes adipocyte apoptosis; 13 however, most patients undergoing cryotherapy report transient erythema, numbness, edema, and tingling immediately following treatment.14 Although uncommon, cases of paradoxic adipose hyperplasia have been reported.15 The applicators require a number of disposable products, adding to cost and set-up time.
High-intensity focused ultrasound HIFU quickly raises local fat tissue temperature, resulting in instantaneous cell death via coagulative necrosis. Although a single treatment with HIFU can achieve a significant decrease in abdominal circumference,16,17 reported adverse events include prolonged tenderness, ecchymosis, hard lumps, edema, and pain.16,18 In 1 study, 11.8% of HIFU-treated subjects reported adverse events.18 Radiofrequency also causes tissue ablation of subcutaneous adipose tissue with heat19 but may require multiple treatment sessions, especially with bipolar devices.20 Expected adverse effects are temporary erythema, temporary discomfort, or minor swelling. Pain and discomfort is usually reported as “tolerable.” 21 Low-level laser therapy is claimed to work by opening pores in subcutaneous fat cells allowing lipids to leak out.22 Although it has a remarkable safety profile, not all authors have been able to duplicate results claimed in the literature.23
Another method of subcutaneous fat reduction involves use of radiofrequency energy. One multipolar radiofrequency device (Vanquish; BTL Industries, Boston, MA) also uses a noncontact design. Fajkošová et al used a four treatment protocol (30 minutes each treatment) with this device in a group of 35 patients, and found a 4.9-cm decrease in abdominal circumference.24 Three patients had no response to the treatment, and transient posttreatment erythema was common. Moradi and Palm used the same radiofrequency device in a protocol which used a series of four 45-minute treatments for 4 consecutive weeks and looked at abdominal contouring.25 They found a similar 4.2-cm decrease in waist circumference at 12 weeks, despite the longer treatment time. Self-limiting “tissue tenderness/inflammation” was reported in 7 of the 96 treatments. The machine was updated in 2016 to a higherpower version, and a 4-week study of 36 patients with a BMI under 30 kg/m2 showed improved early results.26 It should be noted that, as is common with radiofrequency technology, the investigators reported only a 4°C differential between skin temperature and the temperature of the subcutaneous fat with this newer design. Additionally, these treatments take 4 treatments and a longer total treatment time to achieve their results, rather than the 1 treatment with the current device.
The 1060-nm wavelength light from diode lasers has become well known for performing noninvasive lipolysis, with studies dating back to 2014.27 At that time, a direct-contact system used a power output designed to raise subcutaneous temperatures to between 42 and 47°C and was the first to demonstrate effective, noninvasive fat reduction with a 1060 nm diode system 12-weeks after treatment. Several investigators subsequently demonstrated the ability of the 1060-nm diode to heat subcutaneous fat evenly and safely treat all skin types due to low thermal absorption in the dermis and epidermis.12,28,29
One of those studies that investigated a contact 1060-nm diode laser system for abdominal subcutaneous fat reduction was the pivotal clinical trial that led to FDA-approval of the first noninvasive direct-contact laser lipolysis unit (Sculpsure; Cynosure, Westford, MA).28 That device used up to four 4 × 6 cm laser panels placed directly onto the treatment area and employed a 15°C water-cooled system to prevent skin overheating. The treatment heads remain fixed in position during the treatment with a belt-like harness. Laser energy densities ranged between 0.9 and 1.4 W/cm2 during the 25-minute treatment. The energy could be increased or decreased based on the operator’s subjective assessment of patient comfort.
In contrast, the device used in the present study features a noncontact laser treatment system to achieve noninvasive lipolysis. Similar to previous studies, a 1064-nm diode laser was used for deep heating of subcutaneous adipose tissue but differs by the addition of a unique microprocessor-controlled robotic arm with a sophisticated treatment head which combines sensing, heating, and cooling functions. The treatment head never touches the patient. When the robotic arm deploys, the treatment head scans the patient topography and software calculates a treatment pattern, currently up to 150 cm2 in area, which cycles the treatment head over a 3-dimensional treatment path during the subsequent laser treatment.
During the 20-minute treatment cycle, the robot arm moves the treatment head smoothly. Multiple proximity and temperature sensors in the treatment head communicate with the system computer with treatment progress data. Skin cooling of the treatment zone is provided by multiple continuous cold-air jets at 11 to 12°C which are built into the treatment head and are connected to powerful chillers in the base of the device. Unlike contact cooling, the jet system can cool treatment areas within skin folds and depressions. Appropriate spacing between the treatment head and the skin surface is maintained by the computer, which immediately responds to patient movement.
The noncontact laser device also has features to ensure patient safety. Laser energy densities are similar in magnitude to previous devices but are computer-controlled by a proprietary algorithm and are modified by continuous feedback from the patient’s skin temperature measurements, rather than being under manual control. In particular, the system is programmed to automatically switch to a cooling mode if skin temperatures are detected above a predetermined threshold, until skin temperatures return to desired levels. Although the operator may activate additional cool-down cycles or select a lower-power mode for sensitive patients, there is little need with this device to rely on subjective power adjustments. At the completion of the 20-minute treatment cycle, a cool-down mode is automatically initiated. These features may provide additional measures of safety and patient comfort and are believed to contribute to a lower incidence of posttreatment subcutaneous nodules.
The unique robotic arm of the device also deserves comment. This technology is similar to the robotic devices used in automated manufacturing and assembly lines, where their precisely controlled motions can perform repetitive tasks with higher reliability and more stamina than the human arm. The International Space Station also uses a large robot arm, known as the Remote Manipulator System, to manipulate payloads and satellites with dexterity and precision.30 In the laser device used in the present study, the articulated joints of the robotic arm permit the treatment head to be moved with 6 degrees of freedom, bringing deep heating of the subcutaneous fat, skin surface cooling, and an array of sensors to the desired treatment location. The smooth translational motion of the laser treatment arm is a result of many hours of testing and reprogramming of the internal firmware by the development team and is proprietary to this machine. Although a prior low-level visible-light laser device was produced with a noncontact set-up,21,22 the treatment arm of that device was fixed, with simple rotational motion, was not microprocessor-controlled, did not have active skin cooling, and did not utilize proximity-sensing or temperature-sensing technology.
Examining the results obtained in earlier studies with the 1060-nm contact laser device, 1 group achieved a mean 6.5% reduction of the abdominal subcutaneous fat layer thickness at 6 weeks and 11.5% at 12 weeks after 1 treatment with their device, based on ultrasound measurements.28 s the same device and protocol to treat the flank area, another group achieved a mean 9% reduction at 6 weeks, increasing to 13% at 12 weeks.12 In the present study, subjects were followed for a similar 12-week period after a single treatment and standardized ultrasound measurements were also used to determine the thickness of the subcutaneous fat. The results with this noncontact system were a mean 11.8% reduction at 6 weeks, and a 21.6% mean reduction at 12 weeks. Although not a head-to-head comparison, the differences in subcutaneous fat reduction between the direct-contact and noncontact laser devices are substantial and warrant further investigation.
Previous studies with the contact 1060-nm laser device were associated with a greater incidence of posttreatment adverse events. In 1 study on the abdomen, 8 subjects (23%) reported subcutaneous nodules which persisted for a mean of 70 days (range, 35-139 days).28 When used on the flank area, Katz and Doherty reported 6 patients (12%) with subcutaneous nodules which persisted for 38 to 138 days, and another 3 patients (6%) with subcutaneous hardness lasting a mean of 32 days.12 In the current study, just 1 subject (3.8%) reported a subcutaneous nodule at Week 6 which had fully resolved prior to the Week 12 follow-up visit. We believe the lower rate of subcutaneous firmness may relate to the combination of effective surface cooling combined with the device’s automated power-delivery curve with feedback temperature control, but further investigations are required to test this hypothesis.
Limitations of the current study include a relatively small sample at a single treatment site and a 12-week follow-up period. As there were substantial improvements in the percentage change in subcutaneous adipose tissue thickness and mean abdominal circumference between 6 and 12 weeks posttreatment, additional follow-up beyond 12 weeks may have demonstrated additional improvements. Further longer-term follow-up studies beyond 12 weeks are planned.
Other limitations of this study include the errors inevitably associated with manual ultrasound measurements and abdominal circumference measurements. Although every reasonable precaution was taken when obtaining these data, ultrasound is subject to variability due to the exact positioning and angle of the transducer, and the pressure applied during imaging. Direct measurement with a tape measure is subject to similar variation. Tattooing, or other semipermanent skin markings, may have permitted higher accuracy in locating the exact location of the ultrasound measurement sites. Other study methods include the use of computed tomography or MRI to obtain abdominal subcutaneous thickness measurements, but these are much higher in cost, and are more inconvenient for study participants.
Our posttreatment questionnaire was a simple ad hoc design; it has not been separately verified or tested. Our surveys were done on paper and were performed at the time of the clinic visits. The first survey, immediately after treatment, centered primarily on the comfort of the procedure and the patient’s experience. The second, at 6 weeks, asked about discomfort after the treatment. At the 12-week checkup, patients were asked to rate their satisfaction with their posttreatment appearance, the amount of fat reduction, and the comfort of the procedure. Although the questionnaires were filled out privately, in a separate room without influence from the investigator or staff, this method admittedly lacks the anonymity of an online questionnaire, and the in-person distribution system of the survey could certainly be prone to bias. In addition, the patients received $500 USD compensation for their time and participation in the study, and therefore the possibility of a biased response to the questionnaire always exists, compared to noncompensated research subjects. As some subjects were recruited from gyms, they might be more body-image conscious than an average subject group, which could introduce differences in the survey responses or their posttreatment fitness regimen. Also, the timing of the study, with patient follow-up measurements performed during the traditional Thanksgiving to pre-Christmas holiday season, may have negatively influenced results.
Despite being asked to maintain their pretreatment diet and exercise patterns, a few subjects did lose weight. It is unknown whether this weight loss is related to the treatment, or to the Hawthorne effect.31 Weight loss over the course of the study would, by definition, improve the observed results and measurements. We did not reject subjects who had gained or lost weight during our data analysis—all were included.
Similarly, although we diligently tried to standardize all photographs for muscle tightness and diaphragm position by taking the photographs at end-expiration, and asking the subject to relax their abdomen fully, it was not always possible to judge full subject cooperation with these instructions or eliminate a subject’s behavior to “pose” for a photograph.
In terms of the study design, there was no “sham treatment” performed as a control group. A study with a “cold air only” control group that would receive no active lasering, for example, would be more scientifically rigorous. It was felt, however, that because the device has already received FDA clearance and has been shown in prior work to have a significant effect in the reduction of subcutaneous fat, such a study design was unnecessary, and would double the size and cost of the project.
Finally, despite the advanced automation of this system, the author recommends that in actual clinical practice, patient selection and pretreatment planning, double-checking of the machine-recommended scan pattern, and posttreatment examination should always be performed by the treating physician. Depending on state or other local regulations, the actual laser treatment portion is highly automated, and the machine can be run by a mid-level provider or suitably qualified laser technician, provided the treating physician is onsite and available. The system design is not meant for the patient to be left unsupervised.
Conclusions
The results of this prospective, open-label study confirm the deliverability, safety, and effectiveness of this novel noncontact 1064-nm laser device for reducing subcutaneous abdominal fat, and validate the new power-delivery curve, vs the pivotal FDA study for this device. The procedure is well tolerated with a high degree of subject satisfaction.
Acknowledgments
The author wishes to thank Camelia Alawadhi, RDMS, for ultrasound imaging and image acquisition, Mark Harris for professional-quality subject photographs, Heather Rogers for patient care and recruitment, and Philip Lavin, PhD, for biostatistical analysis. The author acknowledges the editorial assistance of Carl S. Hornfeldt, PhD, Apothekon, Inc. (Saint Paul, MN), during the preparation of this manuscript and appreciates the input of Drs Jill Waibel and Suzanne Kilmer during the initial planning of the predicate study, upon which this present work was based. This study was sponsored by Dominion Aesthetic Technologies, Inc., San Antonio, TX.
Disclosures
The author is an unpaid member of the Scientific Advisory Council for Dominion Aesthetic Technologies, Inc., San Antonio, TX. The author has previously received stock options from the company, unrelated to the outcome of this project. The company provided the use of the laser for this study.
Funding
The author received no financial support for the research, authorship, and publication of this article.
References



