-
PDF
- Split View
-
Views
-
Cite
Cite
Marshall E Kadin, John Morgan, Haiying Xu, Caroline Glicksman, David Sieber, William P Adams, Pat McGuire, Mark W Clemens, Archana Thakur, Lawrence G Lum, Granzyme B Is a Biomarker for Suspicion of Malignant Seromas Around Breast Implants, Aesthetic Surgery Journal, Volume 41, Issue 12, December 2021, Pages 1359–1364, https://doi.org/10.1093/asj/sjaa302
Close - Share Icon Share
Abstract
Granzyme B (GrB) is a serine protease secreted, along with pore-forming perforin, by cytotoxic lymphocytes to mediate apoptosis in target cells. GrB has been detected in tumor cells associated with systemic and breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) but its potential use for detection of early BIA-ALCL has not been fully investigated.
Prompted by the increased incidence of BIA-ALCL, the aim of this study was to assess GrB as a new biomarker to detect early disease in malignant seromas and to better understand the nature of the neoplastic cell.
A Human XL Cytokine Discovery Magnetic Luminex 45-plex Fixed Panel Performance Assay was used to compare cytokine levels in cell culture supernatants of BIA-ALCL and other T-cell lymphomas, as well as malignant and benign seromas surrounding breast implants. Immunohistochemistry was employed to localize GrB to cells in seromas and capsular infiltrates.
Differences in GrB concentrations between malignant and benign seromas were significant (P < 0.001). GrB was found in and around apoptotic tumor cells, suggesting that the protease may be involved in tumor cell death.
GrB is a useful marker for early detection of malignant seromas and to identify tumor cells in seromas and capsular infiltrates. Because there is an overlap between the lowest concentrations of soluble GrB in malignant seromas and the highest concentrations of GrB in benign seromas, it is recommended that GrB be used only as part of a panel of biomarkers for the screening and early detection of BIA-ALCL.
Granzyme B (GrB) is a serine protease most commonly found in the granules of natural killer (NK) cells and cytotoxic T cells.1 GrB is secreted, along with the pore-forming protein perforin, by these cells to mediate apoptosis in target cells. Upon contact with target cells, GrB is directionally exocytosed and, with the assistance of perforin, enters the target cell. Because of its substrate specificity for the aspartic acid residue at the P1 site of its substrates, GrB processes and activates various procaspases, inducing apoptosis in target cells. GrB expression is regulated at both transcriptional and translational levels, and is influenced by many factors that stimulate immune cell activation. Transcriptional activation of GrB within T lymphocytes involves activation of the T-cell receptor and costimulation with cytokines.2
Granzymes are inactivated by serine protease inhibitors collectively known as SEPRPINs.3 Cytotoxic lymphocytes are protected from GrB by the specific nuclear-cytoplasmic protease inhibitor SERPINB9.3 SERPINB9 is the only known human protein that specifically inhibits GrB.4 Cytoplasmic GrB is inactivated when SERPINB9 associates with GrB-containing granules to form intracellular complexes. The in vivo distribution of SERPINB9 suggests that this enzyme protects against unwanted GrB-mediated destruction.
GrB can be expressed by CD4+ T cells, mast cells, activated macrophages, neutrophils, basophils, dendritic cells, T regulatory cells, and some nonimmune cells. GrB is also characteristic of some human lymphomas, notably NK/T-cell lymphomas.5 Of special interest, the majority of systemic anaplastic large cell lymphomas (ALCLs) express GrB and other cytotoxic proteins (perforin, TIA-1), and their expression may have prognostic significance.6,7 GrB is the only granzyme expressed at significant levels in ALK+ ALCL cell lines and its expression sensitizes these cell lines to drug-induced apoptosis.8 Recently, GrB was detected by immunohistochemistry in breast implant–associated anaplastic large cell lymphoma (BIA-ALCL).9 However, GrB has not been evaluated as a significant biomarker of seromas around breast implants. Here we examine the differential expression of GrB in malignant and benign seromas around breast implants and supernatants of cell lines derived from BIA-ALCL.
METHODS
This study was performed between January 2018 and December 2019 following approval by the Roger Williams Medical Center Institutional Review Board.
Cytokine/Chemokine Profiling
The Human XL Cytokine Discovery Magnetic Luminex 45-plex Fixed Panel Performance Assay (R&D Systems, Minneapolis, MN) was adopted to simultaneously analyze the concentrations of 45 different cytokines in the supernatants of 20 late seromas (8 malignant and 12 benign) and of 9 T-cell lymphoma lines following the manufacturer’s recommendations, in a Luminex Instrument system (Bio-Rad Laboratories, Hercules, CA). Samples were run in duplicate diluted 1:2 and 1:10 in phosphate-buffered saline (PBS) and compared against a standard curve. The limit of detection for these assays is <10 pg/mL based on a detectable signal of >2-fold above background. Cytokine concentrations were automatically calculated by BioPlex Manager software (Bio-Rad).
Cell Lines
Malignant T-cell lines were seeded at a density of 106 cells/mL and incubated in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum for 48 h, then centrifuged and cell-conditioned media harvested for analysis. BIA-ALCL cell lines (TLBR1-4)10 were generously provided by Alan Epstein, USC Keck School of Medicine; cutaneous ALCL lines Mac-1 and Mac-2A11 were developed by one of the authors (M.E.K.); H9 is a cutaneous T-cell lymphoma line obtained from the American Tissue Culture Collection; ALK+ ALCL line Karpas 299 was obtained from the German Micro-organism and Tissue Culture Collection, Braunschweig, Germany.
Clinical Specimens
Seromas were aspirated if there was a suspicion of ALCL, infection, contracture, or implant rupture (Table 1). Unused portions of seromas were collected with informed consent and sent by overnight mail to our central laboratory where they were frozen immediately at –80°C and stored in 100-μL aliquots until analysis. Prior to freezing, 1-mL samples were used to prepare cytospins stained with Giemsa for cell morphology and immunocytochemistry. Seromas were examined together with cell culture supernatants.
| Malignant sample number . | Date of specimen . | Clinical stage of BIA-ALCL . | Treatment . |
|---|---|---|---|
| 1 | June 27, 2017 | 1C | En bloc capsulectomy |
| 2 | March 22, 2017 | 1A | En bloc capsulectomy |
| 3 | May 15, 2014 | 2A | En bloc capsulectomy |
| 4 | January 23, 2017 | 1B | En bloc capsulectomy |
| 5 | June 14, 2016 | 1C | En bloc capsulectomy |
| 6 | December 1, 2016 | 1C | En bloc capsulectomy |
| 7 | April 1, 2017 | 3 | En bloc capsulectomy, systemic chemotherapy |
| 8 | August 17, 2018 | 2Aa | En bloc capsulectomy, systemic brentuximab chemotherapy |
| Malignant sample number . | Date of specimen . | Clinical stage of BIA-ALCL . | Treatment . |
|---|---|---|---|
| 1 | June 27, 2017 | 1C | En bloc capsulectomy |
| 2 | March 22, 2017 | 1A | En bloc capsulectomy |
| 3 | May 15, 2014 | 2A | En bloc capsulectomy |
| 4 | January 23, 2017 | 1B | En bloc capsulectomy |
| 5 | June 14, 2016 | 1C | En bloc capsulectomy |
| 6 | December 1, 2016 | 1C | En bloc capsulectomy |
| 7 | April 1, 2017 | 3 | En bloc capsulectomy, systemic chemotherapy |
| 8 | August 17, 2018 | 2Aa | En bloc capsulectomy, systemic brentuximab chemotherapy |
aRecurrent disease.
| Malignant sample number . | Date of specimen . | Clinical stage of BIA-ALCL . | Treatment . |
|---|---|---|---|
| 1 | June 27, 2017 | 1C | En bloc capsulectomy |
| 2 | March 22, 2017 | 1A | En bloc capsulectomy |
| 3 | May 15, 2014 | 2A | En bloc capsulectomy |
| 4 | January 23, 2017 | 1B | En bloc capsulectomy |
| 5 | June 14, 2016 | 1C | En bloc capsulectomy |
| 6 | December 1, 2016 | 1C | En bloc capsulectomy |
| 7 | April 1, 2017 | 3 | En bloc capsulectomy, systemic chemotherapy |
| 8 | August 17, 2018 | 2Aa | En bloc capsulectomy, systemic brentuximab chemotherapy |
| Malignant sample number . | Date of specimen . | Clinical stage of BIA-ALCL . | Treatment . |
|---|---|---|---|
| 1 | June 27, 2017 | 1C | En bloc capsulectomy |
| 2 | March 22, 2017 | 1A | En bloc capsulectomy |
| 3 | May 15, 2014 | 2A | En bloc capsulectomy |
| 4 | January 23, 2017 | 1B | En bloc capsulectomy |
| 5 | June 14, 2016 | 1C | En bloc capsulectomy |
| 6 | December 1, 2016 | 1C | En bloc capsulectomy |
| 7 | April 1, 2017 | 3 | En bloc capsulectomy, systemic chemotherapy |
| 8 | August 17, 2018 | 2Aa | En bloc capsulectomy, systemic brentuximab chemotherapy |
aRecurrent disease.
Immunohistochemistry
Immunohistochemistry was done on acetone-fixed cytospins of TLBR cell lines and clinical seromas and formalin-fixed paraffin embedded tissue samples of focal and infiltrative disease. Abcam rabbit anti-GrB antibody ab134933 was used after antigen retrieval at 90°C at a 1:250 dilution. Antibody staining was detected with kits from R&D Systems. Primary antibodies were omitted in negative controls.
Statistical Analysis
Quantitative data are presented as the mean [standard deviation]. Differences between groups were tested via an unpaired, 2-tailed Mann-Whitney test. For each test, P < 0.05 was considered statistically significant. Analyses were performed with GraphPad Prism 8 software.
RESULTS
Soluble GrB was detected in supernatants of BIA-ALCL lines TLBR1 and TLBR2 as well as the ALK+ ALCL line Karpas 299 (Figure 1A). All but 1 of 8 malignant seromas and fewer than one-half of benign seromas had detectable GrB. Malignant seromas showed higher levels of GrB (mean, 5761 pg/mL; range, 280.6-14,624 pg/mL) than found in benign seromas (mean, 314 pg/mL; range, 87.8-947 pg/mL). The differences in concentration of GrB between malignant and benign seromas were significant (P < 0.001) (Figure 1B). However, 2 of 8 malignant cases had soluble GrB levels that fell within the highest range of benign cases. One case which could have been overlooked as being benign (false negative) was malignant case 3, which appears to be an outlier for other reliable markers of malignancy, ie, IL-10 and IL-13, raising the question of accurate diagnosis or artifact in that case. Conversely 4 of 12 benign seromas might have raised a question of malignancy due to GrB levels above the lowest range of malignant seromas.
![(A) Heatmap comparing gene expression of cell line supernatants; malignant and benign seromas are demarcated by black vertical lines. (B) Comparison of granzyme B concentration in malignant vs benign seromas. Quantitative data are presented as mean [standard deviation]. Differences between groups were tested via an unpaired, 2-tailed Mann-Whitney test.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/asj/41/12/10.1093_asj_sjaa302/3/m_sjaa302_fig1.jpeg?Expires=1748242570&Signature=GgdKj3iNAtq0L7Fr4hbWjP6E4y1D3AB0VT7Am3bsbcH8X~VOvb8ClAbhvkvp6OjGcrm2X9~RXidcFrzsXPi3ZIgvbOQ8zORpKwSrUTX4YErjBctV7keweB9zc6ngmLx6hzU94dixFd-ejfjo~EleC5c-Wczq3FUe~tEkKMjiQaVo9UhLk51Nyi0m8QBvkLGk3-aYAH7BgHVR~ESrhbBjNO5iSRaNk1QqjsSGPjdsxaPWYt6hp4meL2fGBr6tFDRmkBZ5feSAWUOnNf7zlnsFl1UUTRhUJwXAFfroxJm~ZWpeWra3Q6exEo2N8UHP2OtCko9wZp1kWwncQdL1p0HXXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
(A) Heatmap comparing gene expression of cell line supernatants; malignant and benign seromas are demarcated by black vertical lines. (B) Comparison of granzyme B concentration in malignant vs benign seromas. Quantitative data are presented as mean [standard deviation]. Differences between groups were tested via an unpaired, 2-tailed Mann-Whitney test.
Immunohistochemistry confirmed that GrB was produced by anaplastic cells in cell line TLBR1, malignant seromas, and capsular infiltrates of BIA-ALCL (Figure 2). GrB staining was often found in and around apoptotic tumor cells (Figure 3), suggesting that GrB may be involved in tumor cell death.
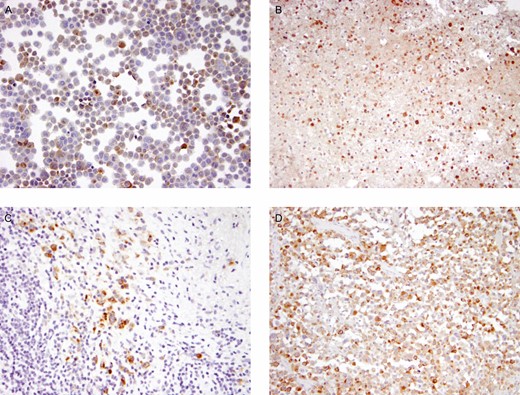
Immunohistochemistry detecting granzyme B as a brown reaction product in anaplastic tumor cells of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) line TLBR1 (A), a malignant seroma (B), and TNM stage T2 (C) and T3 (D) capsular infiltrates of BIA-ALCL.
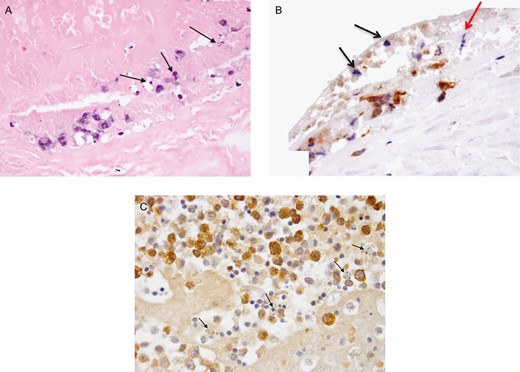
(A) Malignant seroma 8-apoptosis bodies at luminal surface of capsule (hematoxylin and eosin). Adjacent area stained for granzyme B (GrB). (B) Apoptotic bodies (black arrows) associated with GrB (brown reaction product) at luminal side of capsule; a mitotic figure at metaphase is also shown (red arrow, ×400). (C) Malignant seroma 4-GrB+ tumor cells (brown reaction product) and adjacent apoptotic bodies (black arrows, ×600).
DISCUSSION
We have shown that the mean concentration of GrB is significantly higher in malignant than in benign seromas of patients with breast implants. However, overlap between the lowest concentrations of soluble GrB in malignant seromas and the highest concentrations of GrB in benign seromas indicates that GrB should be used as a screening test for malignant seromas only in conjunction with other markers, eg, CD30, IL-9, IL-10, and IL-13.12,13
Within malignant seromas, most nonlymphoid cells capable of secreting GrB are present in smaller numbers than malignant cells, suggesting that GrB in seromas is derived mainly from malignant cells. This view is supported by the detection of high levels of GrB in supernatants of BIA-ALCL lines TLBR-1 and TLBR-2 and within malignant cells by immunohistochemistry. Generally lower levels of GrB in some benign seromas could be due to release from activated macrophages, neutrophils, and possibly from benign precursors to malignant cells.14
Apoptosis is a common feature of tumor cells in BIA-ALCL.9 GrB leakage–induced apoptosis has been shown to be a mechanism of cell death in nasal type NK/T-cell lymphomas.15 Thus, we speculate that apoptosis in BIA-ALCL may result in part from release of GrB and other cytotoxic proteins via a mechanism of fratricide. This may contribute to the usual indolent behavior of in situ (TNM stage 1) BIA-ALCL.
GrB can provide additional clues to the pathogenesis of BIA-ALCL. Among nonmalignant lymphoid cells, GrB is most often attributed to CD56+ NK cells and cytotoxic T lymphocytes. An NK phenotype (CD56+) was observed in only 2 of 21 cases studied by Laurent et al,9 who found consistent expression of GrB and other cytotoxic proteins in BIA-ALCL. Most BIA-ALCLs have a CD4+ phenotype instead of a CD8+ phenotype, which is usually associated with cytotoxic T lymphocytes.16 However, CD4+ T lymphocytes with cytotoxic activity have been observed in various immune responses. These cells are characterized by their ability to secrete GrB and perforin and to kill target cells in a major histocompatibility complex class II–restricted fashion.17 In this regard, BIA-ALCL cells do express major histocompatibility complex class II antigens.10 CD4+ cytotoxic T lymphocytes can develop from Th0, Th1, Th2, Th17, and Treg effector subsets.17 We found a Th2 skewing profile among most BIA-ALCLs.18 Th2 cytotoxic lymphocytes express GATA3, which we found to be a frequent characteristic of tumor cells in BIA-ALCL.19 However, BIA-ALCL cell lines TLBR1 and TLBR3 secrete Th1 cytokine interferon-γ as well as GrB, suggesting partial or complete Th1 polarization.20 Thus, GrB can be a useful biomarker of BIA-ALCL across different cell types of origin.
GrB has a normal concentration of 20-40 pg/mL in human plasma while retaining 70% activity. Elevated concentrations of GrB are found in a number of inflammatory disease states, eg, rheumatoid arthritis.21,22 Plasma levels of GrB in patients with breast implants with or without ALCL have not yet been reported to the best of our knowledge. Because BIA-ALCL is a localized process, elevated plasma levels are unlikely.
CONCLUSIONS
Because GrB is demonstrated within anaplastic cells of different Th-cell lineages in malignant seromas and infiltrative disease, it appears to be a useful biomarker of BIA-ALCL. The mean concentration of GrB is significantly increased in malignant compared with benign seromas. However, because of the overlap between the lowest concentrations of soluble GrB in malignant seromas and the highest concentrations of GrB in benign seromas, GrB should be used only in a panel of biomarkers for early detection of BIA-ALCL.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
Dr Kadin’s research was supported by the Aesthetic Education and Research Foundation (ASERF). Dr Lum and Dr Thakur are supported in part by NIH (R01 CA182526 and P30 CA044579).
REFERENCES
Author notes
Dr Sieber is a plastic surgeon in private practice in San Francisco, CA, USA
- apoptosis
- cytokine
- cell culture techniques
- immunohistochemistry
- biological markers
- cell death
- endopeptidases
- lymphocytes
- ki-1+ anaplastic large cell lymphoma
- t-cell lymphoma
- breast implants
- neoplasms
- leptocytes
- tumor cells
- peptide hydrolases
- granzyme b
- perforin
- infiltrates
- levels of evidence
- early diagnosis
- serine proteases
- breast implant–associated anaplastic large cell lymphoma



