-
PDF
- Split View
-
Views
-
Cite
Cite
Michel Alain Danino, Johnny I Efanov, Cyril Awaida, David Benarous, Laurence Paek, Commentary on: In Vitro Evaluation of Common Antimicrobial Solutions Used for Breast Implant Soaking and Breast Pocket Irrigation—Parts 1 and 2, Aesthetic Surgery Journal, Volume 41, Issue 11, November 2021, Pages 1263–1265, https://doi.org/10.1093/asj/sjaa413
Close - Share Icon Share
The potential link between bacterial contamination at the time of breast implant insertion, chronic inflammation related to biofilm formation, and various complications, including double capsule, capsular contracture and breast implant–associated anaplastic large cell lymphoma, has prompted the perioperative use of antimicrobial agents for both implant soaking and breast pocket irrigation.1 These agents include several antibiotics (eg, cefuroxime, cefazolin, gentamicin, bacitracin) and antiseptics (eg, povidone-iodine [PI]).2 Several controversies arose in the early 2000s, leading the US Food and Drug Administration (FDA) to recommend against any direct contact of PI with implants.3 In 2017, Jewell and Adams4 reported that the FDA had approved a request by a breast implant manufacturer for removal of the warning regarding the use of PI dilute solution. However, this past recommendation continues to affect the daily practice of many plastic surgeons because many scientific societies, regulatory authorities, and manufacturers continue to recommend against the use of PI solution in breast implant surgery.
We would like to congratulate the authors of “In Vitro Evaluation of Common Antimicrobial Solutions Used for Breast Pocket Irrigation—Part 2: Efficacy Against Biofilm-Associated Bacteria” for shining further light on the complex topic of breast implant biofilm and the role of antimicrobial solutions in its prevention.5,6 In this well-designed in vitro study, triple antibiotic (TAB) and PI solutions were compared at different dilutions in order to determine their effectiveness in preventing proliferation of the 5 most common bacterial species involved in biofilm formation. The authors demonstrated that TAB alone failed to eradicate biofilm-related bacteria at any of the 5 time points evaluated (maximum 30 minutes). On the other hand, PI solutions produced a significant log reduction in biofilm-associated bacteria at 5 minutes. Similar findings have recently been documented by Ngaage et al7 on different strains of methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis, demonstrating better efficacy with PI than TAB.
Given recent trends of diluting PI in order to mitigate cytotoxic damage to healthy cells and elastomer surfaces, it is pertinent that the authors evaluated different concentrations in their study. They found that quarter-strength (25%) PI provides adequate efficacy against all biofilm-associated bacteria in their model. Whereas some advocate the use of “tea-colored” PI, Figure 3F in this article clearly demonstrates that this subjective dilution approach more likely corresponds to a 2.5% to 5% PI concentration. Such concentrations were not found to be sufficient in reducing all biofilm-associated bacteria studied in this paper. A seminal article by Adams et al8 in 2000 demonstrated similar results; the minimum effective concentration required to eradicate S. epidermidis in breast pocket irrigation was 12.5%. The color of such a dilution is far from “tea-colored,” further underlying the importance of not overdiluting PI solutions employed in breast implant surgery.
Although only briefly mentioned in this article, surface texturization of silicone implants may be an important factor in the true efficacy of various antimicrobials solutions in preventing both biofilm and subsequent clinical infection. Over the last 20 years, our group has been conducting studies on implant-capsule interactions. For much of this research, patients with breast cancer undergoing 2-stage implant-based breast reconstruction were included as subjects. Prophylactic antibiotics were administered at induction (first-generation cephalosporin or, if allergic, clindamycin), skin preparation involved the standard solution of chlorhexidine with alcohol, total submuscular coverage was obtained, and implants and breast pockets were bathed and irrigated, respectively, with antibiotic solution. Over the years, we were able to assemble a large tissue bank of implant and capsule samples while documenting associated patient profiles and expander implant types. In our previously reported data on 7 patients with double capsules associated with macrotextured expanders,9 it was found that biofilm burden was significantly higher at the prosthetic interface than within the intercapsular space (considered “sterile,” hence formed several weeks/months after implant placement) despite all breast pockets being irrigated with Adam’s TAB solution in the cohort. Our results not only support those in this in vitro study, where TAB alone is suboptimal against biofilm-forming bacteria, but also suggest that texturization of implants is paramount in the severity of biofilm formation. Otherwise, one would expect a similar density of colonies at the prosthetic interface and intercapsular spaces.
It is important to underline the relatively short incubation time used for biofilm formation (48 hours) in the authors’ work. Indeed, TAB solution alone failed to eradicate biofilm-producing bacteria in these specimens, and it is safe to assume that similar results would be obtained had the biofilms matured for weeks to months in vitro given the resultant stronger polysaccharide envelopes. Can the same be concluded about PI solutions? A further avenue of research could be to investigate whether these antimicrobial solutions differ in efficacy when faced with more mature biofilms, thereby more closely reflecting the timelines typically seen in clinical practice where implant exchange as well as capsulotomy or capsulectomy may occur months to years following initial implant placement. Over time, biofilms develop a more robust extracellular polymeric substance, a stronger antimicrobial tolerance, and cellular mutations which may protect against molecular targets of iodine.
In Canada, the Canadian Agency for Drugs and Technologies in Health does not currently provide any evidence-based guidelines for the use of PI in implant soaking, but recognizes the effectiveness of pocket irrigation for reduction of capsular contracture (while acknowledging the necessity for higher-quality studies).2 As such, our group typically employs traditional TAB solutions for implant soaking and pocket irrigation in breast surgery. In previous studies, our group has analyzed implants from patients without untoward clinical manifestations, pathologic capsular contractures, or double capsules; significantly, biofilm formation was nonetheless detected on the majority of analyzed specimens.
In order to further comment on the excellent work presented by the authors, we went back to analyze our specimens from a previous study on the impact of postoperative expansion initiation timing on breast expander capsular characteristics.10 In this study, all the specimens were collected on patients not presenting signs of infection. Under scanning electron microscopy magnification, all specimens demonstrated varying degrees of bacterial and biofilm presence, particularly with macrotextured expanders (Figures 1, 2).

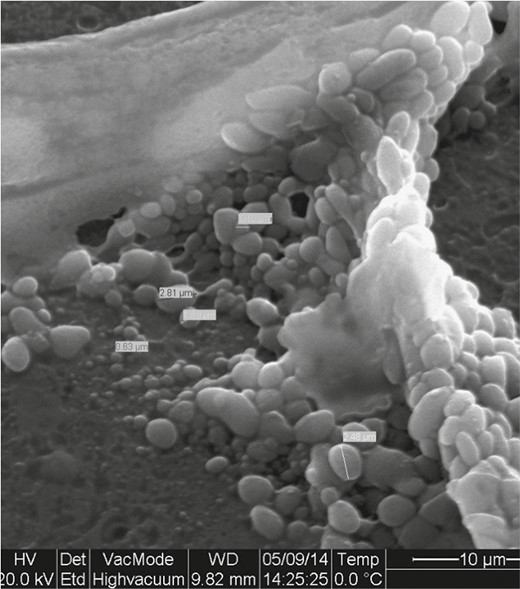
Heterogeneity of bacteria size. This indicates different phenotypes, and therefore different invasive properties and antibiotic resistance.
Although we agree that a multitude of factors can contribute to biofilm formation, this study is nonetheless an empiric in vivo demonstration; as such, the findings of the article should only be applied judiciously. Furthermore, the underwhelming effects of TAB in this study model do not necessarily correlate clinically. After reading this article, one also has to be careful about drawing conclusions based on either the presence or absence of bacteria after the use of antimicrobial solutions. Presence of bacteria from biofilm, which does not necessarily imply pathologic consequences, can be found in over 20% of asymptomatic and supple capsules1. The authors make a good argument that any level of bioburden left on the implant can contribute to subsequent biofilm formation and that the aim of the ideal antimicrobial solution is less about “sterilization” of the implant but rather reduction in the number of bacteria in order to limit potential biofilm development. We applaud the authors for this important contribution to the scientific body of evidence relating to the use of antimicrobial solutions in breast implant surgery and encourage others to apply their work in this crucial subject matter from bench to bedside.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES



