-
PDF
- Split View
-
Views
-
Cite
Cite
Fernanda Marchetti, Ayelén Mariana Distéfano, Maximiliano Cainzos, Nicolás Setzes, Milagros Cascallares, Gabriel Alejandro López, Eduardo Zabaleta, Gabriela Carolina Pagnussat, Cell death in bryophytes: emerging models to study core regulatory modules and conserved pathways, Annals of Botany, Volume 134, Issue 3, 1 September 2024, Pages 367–384, https://doi.org/10.1093/aob/mcae081
Close - Share Icon Share
Abstract
This review summarizes recent progress in our current understanding of the mechanisms underlying the cell death pathways in bryophytes, focusing on conserved pathways and particularities in comparison to angiosperms. Regulated cell death (RCD) plays key roles during essential processes along the plant life cycle. It is part of specific developmental programmes and maintains homeostasis of the organism in response to unfavourable environments. Bryophytes could provide valuable models to study developmental RCD processes as well as those triggered by biotic and abiotic stresses. Some pathways analogous to those present in angiosperms occur in the gametophytic haploid generation of bryophytes, allowing direct genetic studies. In this review, we focus on such RCD programmes, identifying core conserved mechanisms and raising new key questions to analyse RCD from an evolutionary perspective.
INTRODUCTION
Regulated cell death in plants
Regulated cell death (RCD) is an active and controlled process that allows the selective elimination of specific cells through the activation of molecular mechanisms and pathways. It was first observed in 1842 by Karl Vogt, who detected dying cells in toads. However, it was not until the 1960s when RCD research led to the idea of molecular programmes directing cell death (Zakeri and Lockshin, 2008). Even by then, cell death in multicellular metazoans was understood as a strategy to remove unwanted cells during tissue remodelling and differentiation. The following expansion of cell death research has established the occurrence of diverse cellular programmes across different species (Tang et al., 2019; Krasovec et al., 2024).
Although RCD processes are a key part of essential events along the plant life cycle, the mechanisms governing plant cell death are still largely unknown. Most of the studies have been performed in angiosperms, in which RCD is triggered in response to developmental or environmental cues. RCD drives specific developmental programmes that are essential for plant life and reproduction and takes place in both sporophytic and gametophytic cells. Examples of processes occurring in the sporophytic generation that involve RCD include megasporogenesis, differentiation of tracheary elements, embryogenesis and the remodelling of leaf shape, among others (Gunawardena, 2008; Courtois-Moreau et al., 2009; Drews and Koltunow, 2011; Bollhöner et al., 2012; Choi, 2013; Xie et al., 2014; Jiang et al., 2021). RCD also takes place in response to critical stressful situations, resulting in the removal of damaged tissues. Among the abiotic stresses, salinity, drought, UV radiation, heavy metals and extreme temperatures have been reported to trigger RCD (Barnabás et al., 2008; Liu et al., 2009; Petrov et al., 2015; Distéfano et al., 2017; Srivastava et al., 2018; Chua et al., 2019). RCD is also critical to protect plants against pathogens. As we will explain later in this review, the hypersensitive response (HR), a localized type of cell death, takes place in sporophytic cells and is essential to limit the spread of infection (Kiba et al., 2006; Coll et al., 2010; García-Marcos et al., 2013; Dangol et al., 2019; Noman et al., 2020). RCD events are also part of developmental programmes that occur in the gametophytic generation (Ali et al., 2024; Doll and Nowak, 2024). For instance, synergid cells in angiosperms undergo RCD upon pollen tube arrival/fertilization. In Arabidopsis, antipodal cells also die by a still unknown mechanism soon after fertilization.
Because of their critical position in the embryophyte phylogeny and of their key vegetative and reproductive innovations, the study of bryophytes is relevant to understand the evolution of molecular processes in land plants. Bryophytes radiated in land 470–551 million years ago (de Vries et al., 2018a). This transition from water to land went along with adaptations to the novel environmental conditions, which included tolerance to radiation, extreme temperatures and drought (Fürst-Jansen et al., 2020). Although the last common ancestor of embryophytes shared defence responses with algae, early land plants developed a highly specialized mechanism for plant defence (de Vries et al., 2018a, 2018b). Even today, bryophytes are able to inhabit environments where vascular plants are disadvantaged (Wang et al., 2017). For these reasons, the study of bryophytes represents a promising resource for biotechnology, as it might reveal the occurrence of simple but still effective mechanisms that allow survival during adverse conditions. Various bryophyte species, primarily mosses and liverworts, have emerged as interesting study models due to several advantages: they can be cultivated in controlled environments, their genomes have been fully sequenced, and obtaining knockout mutant plants in one generation is easily facilitated by the dominance of the haploid gametophytic generation. Because of their habits in terrestrial life, bryophytes acquired a significant morphological and reproductive complexity in comparison with their aquatic green algal ancestors. Remarkably, a study using the moss Funaria hygrometrica and Arabidopsis thaliana to compare gene expression profiles of sporophytic and gametophytic generations between bryophytes and angiosperms suggested that differentiation in gene expression might be of a less extent in bryophytes than in angiosperms (Szövényi et al., 2011). This is in agreement with the idea that alternating generations derived from a pure-haploid life cycle, and also with a basal phylogenetic position for bryophytes. Besides, in both F. hygrometrica and A. thaliana there are genes that show shared sporophyte-biased expression, which appears to be related to critical molecular pathways associated with adaptations to terrestrial life (Szövényi et al., 2011). It is suggested that most biological pathways might be conserved between gametophytic and sporophytic generations. This implies that the molecular mechanisms and pathways observed in current angiosperm sporophytes might have their origin in conserved gametophytic bryophyte programmes. Indeed, several genes implicated in cell death programmes are conserved in angiosperms and bryophytes despite the phase in which they seem to be active. Examples include transcription factors (TFs) related to the auxin response that are present in the transmitting tract (sporophyte) in angiosperms and the archegonial canal (gametophyte) in bryophytes which are involved in RCD processes (explained below).
In this review, we focus our attention on those RCD programmes that take place in bryophytes during development and in response to different stresses. By comparing known RCD pathways and genes involved in cell death mechanisms in angiosperms and bryophytes, we aim to identify core conserved mechanisms and key questions to analyse RCD from an evolutionary perspective.
Regulated cell death in the development of bryophytes
The initial phase of the life cycle in the bryophyte model Physcomitrium patens commences with the germination of a haploid gametophytic spore, leading to the formation of a filamentous protonema. Occasionally, side branches of the protonema develop into gametophores, giving rise to apical male and female reproductive organs. The male reproductive organ, known as the antheridium, consists of a sterile single-cell layer called the jacket, enveloping a mass of spermatogenous cells that differentiate into spermatids and eventually into sperm. By contrast, the female archegonium comprises a neck with an inner canal that terminates in a cavity filled with mucilage, housing the egg cell. Upon contact with water, the mature archegonium opens at its tip, allowing flagellated sperm, released by the antheridium, to swim through the canal and fertilize the egg cell. The resultant diploid zygote matures into a sporophyte, ultimately generating haploid spores (Fig. 1).
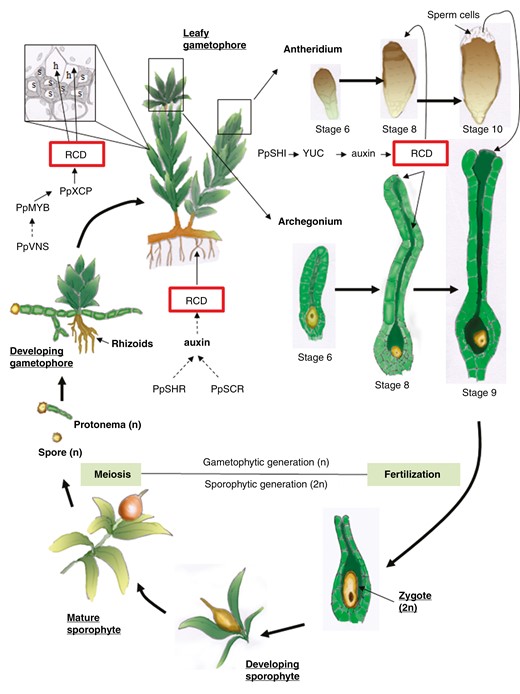
Scheme representing the life cycle of a bryophyte (Physcomitrella patens) focusing on analogous RCD processes observed in bryophytes and angiosperms. Spores (n) develop into a protonema (n) which eventually develops into leafy gametophores with rhizoids. Within the gametophores, water-conducting elements develop in the aerial part depending on PpVNS (VND-, NST/SND-, SMB -SOMBRERO-related protein) that might induce PpMYB transcription factors. Downstream genes include PpXCP (XYLEM CYSTEINE PROTEASES) among others. In the rhizoids, PpSHR (SHORT ROOTS) and PpSCR (SCARECROW), together with auxin signalling (AUX1 homologue), might trigger RCD to form water-conducting elements. Within the gametophore develops the archegonium (where female gametes mature) and the antheridium (where motile male gametes mature). In the archegonium, PpSHI1 and PpSHI2 trigger RCD through a signalling pathway that involves YUCCA genes and an auxin maximum in canal cells, the upper basal cell and apical cells. In the antheridium, PpSHI might be involved in the burst of the apical cells (an RCD event) allowing the release of motile sperm cells. In the presence of water, these flagellated sperm cells will reach the open mature archegonium and moving through the dead canal they will encounter the female gamete (egg cell-n). Fertilization takes place giving rise to the zygote (2n) which develops into the sporophyte on the leafy gametophyte. Once the sporophyte matures in a sporangium, mother cells undergo meiosis and all four haploid spores survive. The sporangium eventually collapses (or opens depending on the species) and haploid spores are released.
While in bryophytes all four haploid spores resulting from meiosis survive and are released to form free-living gametophytes (true spores), in most angiosperm species three of the four megaspores are destined to die, leaving one living megaspore (the functional megaspore) per ovule. In Arabidopsis, an arabinogalactan protein (AGP18) was suggested to be involved in determining the fate of megaspores (Demesa-Arévalo and Vielle-Calzada, 2013; Bartels et al., 2017). Although there are reports of AGPs present in bryophytes (Bartels et al., 2017; Happ and Classen, 2019), the absence of an analogous process for the selection of surviving spores in bryophytes limits comparisons.
In Arabidopsis, apart from AGPs, there are other proteins involved in the selection of the functional megaspore (FM), such as cyclin-dependent kinase inhibitors encoded by seven genes called ICK/KRP [interactor/inhibitor of cyclin-dependent kinase (CDK)/Kip-related proteins] (Cao et al., 2018). Mutations in these genes affect the number and position of surviving megaspores, suggesting that the positional clues involved in the determination of the FM are affected in these mutants. Consistently with a role in megaspore cell death, ICK4 was shown to be strongly expressed in degenerative megaspores but excluded from the FM. ICK/KRP genes are present in the genomes of all seed plants examined so far (Torres Acosta et al., 2011). It was recently reported that KRPs function upstream of RBR1 (RETINOBLASTOMA RELATED 1), which is involved in the repression of CDK1, in the germline initiation in plants (Miao et al., 2024). In this sense, RBR1 is essential to the switch from mitosis to meiosis in Arabidopsis and rice (Zhao et al., 2017; Miao et al., 2024). These reports suggest that the specification of the megaspore mother cell (MMC) is related to the ICK/KRP cell cycle regulation pathways by RBR1. Although KRP genes present low homology in P. patens and Marchantia polymorpha (Ishikawa et al., 2011; Bowman et al., 2017; Peramuna et al., 2023), it is still unknown if these inhibitors are expressed differentially among spores or spore mother cells. As this gene family appeared after plant adaptation to life on land, it has been proposed that their function could have served the needs related to transition from water and of more complex multi-tissue plants (Torres Acosta et al., 2011), but their relation with cell death has not yet been studied to our knowledge.
Concerning RCD mechanisms related to sporogenesis and megagametophyte development, they are associated only with monosporic and bisporic models of embryo sac formation (originated from one or two remaining megaspores, respectively). In the bisporic model, cytokinesis is specifically suppressed after meiosis I, resulting in two functional megaspores that form a female gametophyte. In the case of tetrasporic development, all four megaspores survive and cytokinesis is suppressed after meiosis I and II, resulting in a syncytium and the formation of a chimeric embryo sac (Haig, 1990; see Haig, 2020 for review on this topic). Following meiosis, tetrads can exhibit two patterns: tetrahedral or linear spore tetrad shape. The former is predominant in bryophytes (mosses, hornworts and most hepatics) and ferns, and is considered an ancient trait with all four spores surviving and released as free-living gametophytes. The latter is most commonly found in vascular plants, where only one megaspore survives. The origin of this characteristic in vascular plants may have taken place early during evolution, with retained megaspores, giving rise to gametophytes retained in megasporangia. Although the advantages of RCD in the selection of only a functional megaspore are still in discussion, competition for nutrients is fairly well-accepted as the mechanism by which megaspore elimination was selected in many angiosperm lineages. Functional megaspores are at the chalazal pole, which is close to where nutrients arrive at the nucellus. Additionally, it was found that megaspore selection depends on chalazal cytokinin signalling (Cheng et al., 2013). As dying megaspores show callose deposition in their walls, while the surviving megaspore shows active callose degradation, it is clear that an active isolation mechanism takes part of this process, which could in turn prevent additional cells from receiving either nutrient supplies or other signals from surrounding tissues.
Genetic and hormone pathways present in the angiosperm gametophytes are expected to be conserved in bryophyte gametophytes. The molecular basis of the recruitment of ancestral gametophytic programmes in angiosperm sporophytes is not clear, even though aspects of the initiation, morphogenesis and function of reproductive organs share striking similarities (Landberg et al., 2013; Bowman et al., 2017). Auxin signalling pathways and response are also present in mosses. Genes encoding angiosperm homologous proteins involved in auxin synthesis, transport, perception and signalling were reported in P. patens (Rensing et al., 2008; Paponov et al., 2009; Eklund et al., 2010; Prigge et al., 2010; reviewed in Thelander et al., 2018; Suzuki et al., 2021; Carrillo-Carrasco et al., 2023). SHORT INTERNODE/STYLISH (SHI/STY) is a family of TFs that control the expression of YUCCA (YUC) genes, which are involved in auxin synthesis in Arabidopsis (Cheng et al., 2006). These TFs were reported to be essential in reproductive organ development in angiosperms as well as in mosses (Kuusk et al., 2006; Eklund et al., 2010). Concordantly, P. patens SHI/STY homologues (PpSHI1 and PpSHI2) are also required not only for auxin biosynthesis, but also for vegetative and reproductive development in mosses (Eklund et al., 2010; Landberg et al., 2013). Malformations of the archegonium were observed in Ppshi knockout mutants (Landberg et al., 2013). PpSHI genes are expressed during the early stages of reproductive primordia, suggesting that they might be essential for organ outgrowth or patterning as was shown in Arabidopsis (Landberg et al., 2013). In addition, PpSHI genes are also expressed during later stages of both male and female gametophyte development (Landberg et al., 2013). In the anteridium, apical cells burst allowing the release of flagellated sperm cells (Fig. 1). Even though RCD mechanisms have not been directly associated with this process, it was suggested that PpSHI genes might be involved, as PpSHI knockout lines failed to open the antheridial apex (Landberg et al., 2013). These lines also failed to open the archegonium apical tip, suggesting an ancestral function for SHI/STY proteins (Landberg et al., 2013). In addition to being essential for organ morphogenesis and differentiation, PpSHI genes also appear to be involved in auxin-regulated cell death pathways (Landberg et al., 2013, 2013). PpSHI genes and the auxin response reporter are active in the archegonial canal cells, the upper basal cell and the apical cells, all of which appear to undergo an RCD process. This suggests that auxin biosynthesis in these cells might directly or indirectly induce their terminal differentiation and cell death (Landberg et al., 2013). In agreement with a role inducing RCD, PpSHI triggered a cell death pathway when ectopically expressed in leaf cells (Eklund et al., 2010). Auxin biosynthesis in apical stem cells has been proposed as an ancestral mechanism to control focal growth (Landberg et al., 2013), and the presence of SHI genes seems to correlate with the appearance of auxin receptor TIR/AFB and the auxin response factor ARF genes in embryophytes (Lau et al., 2009).
Remarkably, a role for auxins in inducing genetic processes leading to RCD has been previously suggested in angiosperms. For instance, Crawford and Yanofsky (2011) provided data indicating that the auxin response factors ARF6 and ARF8 indirectly induce RCD of the transmitting tract of Arabidopsis gynoecia by upregulating the expression of HALF FILLED (HAF), which encodes a basic-helix-loop-helix (bHLH) TF (Crawford and Yanofsky, 2011; Di Marzo et al., 2020). In M. polymorpha, MpbHLH43/MpLRL (LOTUS JAPONICUS ROOTHAIRLESS1-LIKE homologue) encoded by the Mpzg01410 gene (Breuninger et al., 2016) has been related to ectopic rhizoid formation (Breuninger et al., 2016) in response to exogenous auxin (Flores-Sandoval et al., 2018). However, to our knowledge, there is no information about the role of these TFs in RCD of bryophytes. HAF TFs act redundantly with BRASSINOSTEROID ENHANCED EXPRESSION1 and 3 (BEE1 and 3) to specify the transmitting tract tissue and to finally induce RCD in order to facilitate pollen tube growth and successful fertilization. This indicates that the transmitting tract and the archegonial canal might constitute an example of analogous structures in the angiosperm sporophyte and in the moss gametophyte, sharing similar underlying mechanisms of RCD (Parish and Li, 2010).
In vascular plants, the differentiation of water-conducting cells (WCCs), known as tracheary elements, involves a RCD process. WCC development is controlled by VND genes in tracheophytes. Members of the NAC family of TFs including the VND/NST/SND subclade (VASCULAR RELATED NAC-DOMAIN –VND-/NAC SECONDARY WALL THICKENING PROMOTING FACTOR – NST-/SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN – SND-) function as master regulators for xylem fibre differentiation by orchestrating gene expression in angiosperms (Zhong et al., 2006, 2010; Mitsuda et al., 2007). Since orthologues of VND control hydroid development in P. patens (Xu et al., 2014), an ancestral role for these has been suggested controlling the development of the vascular system (Woudenberg et al., 2022; Ligrone et al., 2000; Xu et al., 2014; detailed review in Lu et al., 2024).
In mosses, water is internally conducted through specialized cells named hydroid cells, which share several characteristics with xylem vessels from vascular plants, such as an elongated shape and the absence of cellular contents (Hébant, 1977). Unlike xylem vessels, hydroid cells lack lignification (Zhong et al., 2010). However, it is thought that another cell type, named stereid cells, might constitute a supporting tissue as they present secondary thickened cells which have a structural function (Woudenberg et al., 2022). In vascular plants, xylem vessels and fibre cells are formed only in the sporophytic generation, which makes the evolutionary connection unclear (Xu et al., 2014). Interestingly, P. patens also contains VND-related NAC genes with roles in cell wall thickening. Eight similar genes have been identified in the P. patens genome and were named PpVNS (VND-, NST/SND-, SMB -SOMBRERO-related protein) (Xu et al., 2014). These discoveries show that VNS genes are present in pre-vascular plants. At least seven of these genes are expressed in protonema and are more abundant in leafy gametophores of P. patens, preferentially in the central region, where hydroid and stereid cells are differentiated. Triple mutants for PpVNS genes form abnormal water-conducting (hydroid) and supporting (stereid) cells with deficient water transport, as well as malformed sporophyte cells. Overexpression of PpVNS genes promotes the expression of genes supporting water-conducting cell formation, leading to ectopic cell wall thickening and RCD in both P. patens (protonema and gametophore) and A. thaliana (Xu et al., 2014). In P. patens, this RCD process is characterized by degradation of chloroplasts, shrinkage of the protoplasm away from the cell wall and loss of plasma membrane integrity. Moreover, several conserved genes between Arabidopsis and P. patens were up-regulated when PpVNS genes were overexpressed. These included: MYB46/MYB83 TFs that function as master regulators in secondary cell wall formation (Ko et al., 2014); XYLEM CYSTEINE PROTEASE XCP1 and XCP2, known to regulate post-mortem cell clearance during xylem formation (Avci et al., 2008; Xu et al., 2014); the master regulators KNOTTED ARABIDOPSIS THALIANA7 (KNAT7) and IRREGULAR XYLEM (IRX)11, and IRX7. Together with FRAGILE FIBRE8 (FRA8), IRX11 and IRX7 are involved in the synthesis of the hemicellulose glucuronoxylan, a major component of secondary cell walls (Kumar and Turner, 2015). Altogether, these reports suggest that the PpVNS family plays an important role in RCD in P. patens and suggest conservation of the molecular mechanisms underlying cell differentiation of water-conducting cells between mosses and angiosperms (Fukuda and Ohashi-Ito, 2019).
The Marchantia genome harbours a single copy of a VNS gene (Bowman et al., 2017), known as MpNAC5/MpVNS (Lu et al., 2024). Additionally, it also has the core set of VNS-downstream genes that might be able to direct cell death and secondary wall thickenings (Xu et al., 2014; Bowman et al., 2017). Marchantialean or complex thalloid liverworts are characterized by the occurrence of smooth and pegged rhizoids. While mature smooth rhizoids are alive, pegged rhizoids are dead at maturity. However, it was recently suggested that the regulatory elements controlling RCD of pegged rhizoids are the result of a convergent evolution, as such molecular networks were independently recruited to promote RCD in liverwort rhizoids and in flowering plant seeds (Lu et al., 2024). Pegged rhizoid differentiation in Marchantia is controlled by the bHLH TFs ZHOUPI (ZOU) and INDUCER OF CBF EXPRESSION 1 (ICE1). Those genes are orthologues of the Arabidopsis ZHOUPI and ICE genes, respectively, that are involved in endosperm cell death. MpZOU and MpICE1 are required in the regulation of genes involved in cell death and cell wall modifications in pegged rhizoids (Lu et al., 2024). Interestingly, pegged rhizoid formation is not affected by mutation of the MpNAC5 gene (Lu et al., 2024), suggesting no association of MpVNS in the differentiation of pegged rizhoids. Apart from pegged rizhoids, VNS-downstream genes are related to secondary cell wall thickening in elaters (Bowman et al., 2017), which are sporophytic cells derived from archesporial tissue in the capsule of liverworts and hornworts. Elaters are dead at maturity, but the direct relationship between the single MpVNS gene with elater RCD has not yet been demonstrated to our knowledge. Recently, a novel bHLH TF, MpbHLH37, expressed in the elaters and the capsule wall was identified in M. polymorpha sporophytes. Since both structures might function as a tapetum in liverworts, MpbHLH37 is suggested to be involved in RCD (Levins et al., 2024).
Cell death in response to abiotic stresses
As sessile organisms, plants have developed complex physiological, morphological and molecular strategies to cope with fluctuating environmental conditions. If those changes are extreme, plant cell homeostasis can be disrupted, which leads to the induction of an RCD process. This actively controlled mechanism allows the isolation and removal of damaged tissues, ensuring plant acclimation and survival. In this sense, RCD plays a central role in response to abiotic stresses. Different environmental stresses are reported to trigger RCD (Petrov et al., 2015; Burke et al., 2020), such as cold, drought, heat, high light, salt and osmotic stress, among others.
Although there are several reports describing stress tolerance in bryophytes (reviewed in Wang et al., 2008; Ćosić et al., 2019), only a few refer to RCD processes as an adaptive response. The study of RCD mechanisms in halophytes in comparison to glycophytes reveals that plants can trigger cell death in response to different salt concentrations, depending on their specific salt tolerance levels. Halophytes are able to mitigate the effect of salt stress accumulating high levels of ascorbate that reduce the oxidative stress caused by NaCl. Halophytes are also more efficient in managing the cytoplasmic Na+ accumulation through non-selective cation channels (NSCCs), contributing to the reduction of reactive oxygen species (ROS) accumulation, avoiding cell death (Hamed-Laouti et al., 2016). In glycophytes, the entry of Na+ through NSCCs increases the levels of cytosolic Na+, which results in membrane depolarization (Monetti et al., 2014; Hamed-Laouti et al., 2016). Increased levels of cytosolic Na+ also lead to Ca2+ accumulation (Lin et al., 2005), activating Ca2+-dependent NADPH oxidases (RBOHs) that increase ROS concentration (Ma et al., 2012; Monetti et al., 2014). In addition, electrolyte leakage occurs through the activation of the plasma-membrane hydroxyl radical activated K+ channel (AtGORK) through calcium-dependent protein kinases (CPKs) (Demidchik et al., 2010, 2014; Van Kleeff et al., 2018). In Arabidopsis, tobacco and some other angiosperm species, several RCD markers are observed upon high salt stress. The opening of the mitochondrial permeability transition pore (MPTP) that occurs under salt stress leads to a decrease of the mitochondrial membrane potential and release of cytochrome c (Lin et al., 2006; Li et al., 2007; Wang et al., 2010). Salt ions also affect the photosynthetic activity by breaking off thylakoid membranes and disrupting the electron transport chain in chloroplasts (Chaves et al., 2008). An increase of caspase-like activities (Keyster et al., 2013) and degradation of nuclear DNA (Katsuhara and Kawasaki, 1996; Muramoto et al., 1999; Liu et al., 2000; Richardson et al., 2001; Lin et al., 2006; Demidchik et al., 2010; Wang et al., 2010; Roy et al., 2013) are also observed. A similar response was reported in bryophytes. In P. patens, salt stress triggers a burst of ROS and the degradation of nuclear DNA (Zvanarou et al., 2020). This DNA degradation, which was observed through single- or double-strand breaks, is affected by NaCl concentration from 0.3 to 0.6 m (Zvanarou et al., 2020). In M. polymorpha, thalli exposed to salt stress also showed localized cell death and ionic leakage when submitted to salt treatments (Godinez-Vidal et al., 2020). Reduced growth of thalli is observed at 40 mm NaCl while the death of plants is observed when exposed to concentrations higher than 200 mm (Tan et al., 2023). Even though recent advances in studying the conservation of gene families between model plant groups were reported, the mechanisms underlying salt stress-induced cell death in bryophytes are still elusive.
High temperatures are also known to trigger cell death (Petrov et al., 2015). Heat stress-induced pathways are associated with oxidative bursts, mitochondrial membrane depolarization and the activation of caspase-like activities (Vacca et al., 2006). Additionally, ferroptosis, an oxidative cell death pathway that depends on iron and lipid peroxidation, has been associated with heat stress in Arabidopsis (Distéfano et al., 2017). Heat stress also promotes cell death in Marchantia. Extensive cell death is observed in M. polymorpha gemmae after 24 h of heat treatment at 36 or 37 °C, which is accompanied by the induction of some RCD marker genes which have been identified through transcriptomic studies (Olvera-Carrillo et al., 2015; Gimenez-Ibanez et al., 2019; Tan et al., 2023). Exposure to high temperatures in plants is generally associated with an increase of cytosolic Ca2+, which is linked to the activation of cyclic nucleotide gated channels (CNGCs) both in bryophytes and angiosperms (Finka et al., 2012; Finka and Goloubinoff, 2014). An increase in ROS triggered by heat stress is also observed in chlorophytes and angiosperms, suggesting a conserved mechanism for algae and plants (Zuppini et al., 2007; Lee et al., 2014).
Interestingly, a fine balance between the activation of pro-survival genes and genes promoting cell death has been proposed both in Arabidopsis and P. patens in response to heat stress (Elzanati et al., 2020). In this sense, plant-acquired thermotolerance is central to the adaptation of land plants to temperature fluctuations during the day. Heat shock proteins (HSPs) have an important role in maintaining cell functions at extreme temperatures. Recently, three HSP20 genes (HSP17.4A, HSP17.7 and HSP17.8) were identified to be involved in the acquired thermotolerance response in P. patens (Guihur et al., 2021). In M. polymorpha it was shown that MpHSP17.8A1 (MARPO_0076s0004) gene expression was notably induced upon heat treatment (Nishihama et al., 2016), suggesting that this gene might have a role in the regulation of the heat stress response. Based on phylogenetic studies of heat shock TFs (HSFs) and the role of HSFs in Marchantia, Wu et al. (2022) proposed that HSF diversification is linked to the expansion of the heat stress response. Although it requires further confirmation, activation of heat-shock-related proteins and the repression of genes associated with RCD may assist in acclimation under sub-lethal temperatures. This represents an adaptive strategy for plants to overcome fluctuations in environmental temperatures. Notably, dehydrins (DHNs) are reported as one of the most important factors involved in the plant response against abiotic stress (Szlachtowska and Rurek, 2023). These genes are found from cyanobacteria to vascular plants, and phylogenetic studies revealed homologous genes in liverworts (Melgar and Zelada, 2021) and mosses (Agarwal et al., 2017), specifically corresponding to a particular segment of this gene family known as the K-segment, suggesting that their evolutionary origin is associated with their role in bryophytes (Melgar and Zelada, 2021).
Remarkably, while most vascular plants undergo RCD in response to drought stress, bryophytes are extremely tolerant to desiccation (Singh et al., 2015). The latter are recognized for their ability to thrive in a diverse range of terrestrial environments, spanning from cold and hot deserts to tropical regions. Alongside lichens, bryophytes constitute a significant group that frequently employs desiccation tolerance as a successful life strategy (Morales-Sánchez et al., 2022). This tolerance is considered an ancestral trait and is found in cyanobacteria, bryophytes and pteridophytes, while among vascular plants it is only found in orthodox seeds and resurrection plants. The process through which bryophytes are able to tolerate extreme desiccation is called anhydrobiosis. During this process, the plants dehydrate and accumulate proteins and non-reducing disaccharides that result in vitrification of the internal cellular environment (Singh et al., 2015). The wild moss Bryum argenteum is known as a fully drought-tolerant bryophyte. Transcriptomic analysis revealed the specific TFs involved during the different stages of the dehydration and rehydration process, which are responsible for the desiccation tolerance in the moss (Gao et al., 2017). Accumulation of LEA (Late Embryogenesis Abundant), HSP and HSP-like gene products against dehydration stress was early proposed as a main component of the drought tolerance for vegetative tissue in bryophytes which is considered a common response in comparison with land plants (Proctor et al., 2007; Yang et al., 2023). Recently, genomic analyses of the emerging model moss Syntricia ruralis revealed a conserved regulator of desiccation that was previously unknown in flowering plants, the TF MYB55 (Zhang et al., 2024). Under stress, MYB55 acts as a negative regulator of an abscisic acid (ABA)-dependent response, indicating that desiccation tolerance in mosses might share similarities with flowering plants (Cutler et al., 2010). So far, it is still unknown whether there is a direct connection between RCD mechanisms and anhydrobiosis in bryophytes. It would be interesting to further investigate possible scenarios across plant groups, such as the lack of drought-induced RCD programmes or their active inhibition through unidentified TFs to date.
Freezing is also a stress that was reported to trigger cell death in bryophytes. Exposing P. patens gametophytes to −4 °C leads to cell death through a process characterized by oxidative damage and ion leakage. Interestingly, cell death is also associated with a marked accumulation of malondialdehyde (MDA), a marker of lipid peroxidation that has also been associated with plant ferroptosis (Distéfano et al., 2017, 2022; Tan et al., 2017), suggesting that this process might also be part of this response.
PDCD4 (PROGRAMMED CELL DEATH 4) has been shown to be involved in ethylene signalling and abiotic stress responses. In animal cells, PDCD4 is induced during apoptosis, having an inhibitory role. Even though PDCD4 appears in many lower eukaryotes and in plant genomes, in higher plants PDCD4 contains four MA3 domains (which are involved in the interaction with proteins for translation) in comparison to only two found in the moss P. patens and the lycophyte Selaginella moellendorffii. Evolutionary comparisons suggest that it may represent a possible adaptation of an existing protein, which was involved in programmed cell death, acquiring a role in abiotic stress responses mediated by hormone signalling (Cheng et al., 2013).
Cell death in response to biotic stresses
In response to pathogen attacks, plants activate a first response that relies on the activation of cell surface receptor proteins. These receptors are known as pattern-recognition receptors (PRRs), and detect extracellular molecules produced by pathogens (pathogen-associated molecular patterns, PAMPs), which triggers a PAMP-triggered immunity (PTI) pathway. Typical PTI responses include callose deposition, transient cytosolic Ca2+ accumulation, ROS production and the expression of specific genes (Zhang and Zhou, 2010; Yu et al., 2017).
A second layer of defence detects pathogen virulence factors (effectors) through the activity of intracellular immune receptor proteins, which in general are nucleotide-binding site leucine-rich repeat (NB-LRR or NLR) proteins. These proteins activate an immune response that amplifies ROS production and sustained cytosolic Ca2+ accumulation, and induce the expression of specific effector-triggered immunity (ETI) marker genes and a type of plant regulated cell death named the hypersensitive response (HR) (Jones and Dangl, 2006; Tsuda and Katagiri, 2010). The HR is a localized cell death that is rapidly activated to restrict pathogen growth at the site of infection. These responses are genetically determined. Pathogen avirulence (Avr) genes code for species/strain-specific effector proteins (Avr proteins). Once in the host cells, Avr proteins can suppress PTI responses resulting in disease (Badel et al., 2006). However, in specific interactions, plants can also recognize pathogen Avr effector proteins through cytoplasmic resistance (R) protein receptors (NBS-LRR proteins), activating an ETI response and thus limiting pathogen spread (reviewed in Balint-Kurti, 2019). However, while HR cell death contributes to resistance to biotrophic pathogens, it might increase plant susceptibility to necrotrophic pathogens. In fact, biotrophic pathogens such as Pseudomonas syringae and Xanthomonas campestris actively deliver effectors into host cells to suppress PTI and ETI responses, and eventually HR cell death. On the other hand, necrotrophic pathogens (such as Botrytis cinerea) produce phytotoxic metabolites that contribute to cell death, stimulating the HR (Glazebrook, 2005).
In bryophytes, plant–pathogen interactions have been well studied in mosses. Similarly to angiosperms, defence responses are also activated in P. patens by the perception of pathogen effectors (Currah and Davey, 2006), which leads to an HR-like response. This is usually accompanied by other responses that include reinforcement of the cell wall, increase of ROS production, reorientation of chloroplasts, and specific changes in hormonal levels and in the expression of genes related to plant defence (Ponce de León et al., 2007; Ponce De León et al., 2012; Ponce de León and Montesano, 2013; Otero-Blanca et al., 2021; Jeong et al., 2023). Additionally, infections with Pythium and B. cinerea and treatment with Erwinia carotovora elicitors have been reported to induce cell death, particularly in cells colonized by fungal hyphae (Ponce de León et al., 2007; Oliver et al., 2009). Plant–pathogen interactions have been studied in P. patens gametophytes infected with the oomycete Pythium ultimum and the fungi Thyronectria hyperantartica, Tephrocybe palustris, Bryoscyphus dicrani, Scleroconidioma sphagnicola, Acrospermum adeanum, Arrhenia retiruga, Lizonia baldinii and Atradidymella muscivora (Currah and Davey, 2006; Davey et al., 2009). Interestingly, these pathogens that affect mosses in their natural environment can also infect crop plants (Ponce de León, 2011). Research data related to defence responses and cell death mechanisms upon pathogen attacks are scarce in other groups of bryophytes such as hornworts and liverworts (reviewed in Carella and Schornack, 2018). Studies in Marchantia thalli and gemmae indicated that conidia from the non-host fungus Erysiphe trifoliorum are destroyed on the surface of both structures (Takikawa et al., 2014). Although the molecular mechanism underlying this process is still unknown, a hydrophobic layered structure in the surface of Marchantia has been postulated to act specifically on non-host fungi cell membranes (Takikawa et al., 2014). The specificity of such a response points towards the activation of a mechanism that relies on pathogen recognition. This idea is also in agreement with results showing that Phytophthora infestans spores are unable to infect Marchantia thalli, while thalli inoculated with Phytophthora palmivora zoospores were highly susceptible (Carella et al., 2018). While phytophthora RXLR effectors are expressed during the biotrophic phase of plant colonization, they are not up-regulated in incompatible interactions (Carella et al., 2018). In addition, thalli inoculated with Phytophthora infestans showed no significant differences in oomycete biomass (Carella et al., 2018). A similar response is observed when Arabidopsis leaves are inoculated with Phytophthora infestans cysts. Although Phytophthora infestans biomass increases during the first 24 h post-inoculation, this is followed by an HR (Huitema et al., 2003). Although more studies are needed to dissect the events behind incompatible interactions in Marchantia and other bryophytes, the main components involved in defence programmes seem to be conserved, suggesting an early appearance of such responses during land plant evolution (Carella and Schorlack, 2018).
HR cell death hallmarks are found in pathogen-infected mosses and in elicitor-treated moss tissues. These processes include chloroplast breakdown, cytoplasmic shrinkage, nucleus fragmentation and increase of nuclease activities (Ponce de León et al., 2007, 2012; Lawton and Saidasan, 2009). Cell death in Physcomitrium in response to E. carotovora elicitor treatments and B. cinerea inoculation is preceded by the expression of defence-related genes. These genes encode lipid oxygenases, enzymes involved in salicylic acid synthesis, phenylalanine ammonia lyase (PAL) and chalcone synthase (CHS), among others, all proteins known to play a role during the defence response in angiosperms.
The cell-surface receptor kinase BRI1-associated receptor kinase 1 (BAK1), a member of the somatic embryogenesis receptor kinases (SERKs), belongs to the LRR-RLK II group of receptor-like kinases. BAK1 was originally described in A. thaliana as a key component of the brassinosteroid (BR) signalling pathway. However, current data indicate that BAK1 can function as a co-receptor regulating a plethora of processes that include BR-dependent development but also flagellin-sensitive 2 (FLS2)-dependent PTI responses and other cell death processes involved in immunity and defence (Chinchilla et al., 2009; Ma et al., 2016). Recently, it was shown that the FLS2–BAK1–BIR complex triggers the activation of RBOH through phosphorylation, leading to the accumulation of ROS upon the presence of flg22 (Lee et al., 2020). Although the P. patens genome lacks close homologues to FLS2 receptors (Bressendorff et al., 2016), it encodes for three SERK homologues, which closely resemble SERKs from angiosperms, suggesting that SERKs have been conserved throughout evolution (Aan den Toorn et al., 2015). Their role in cell death processes occurring in bryophytes upon biotic stress, however, is still to be elucidated. Functional characterization of these proteins using knockout mutants and rescue assays on flowering plant mutants with these putative bryophyte orthologues might help to reveal if their role has been conserved during land plant evolution.
ROS production and accumulation occurs soon after pathogen recognition. ROS generation plays several roles during this response. Besides being toxic to the attacking pathogens, ROS are involved in cell wall reinforcement in Arabidopsis (O’Brien et al., 2012; Kärkönen and Kuchitsu, 2015) and also in bryophytes (Ponce de León and Montesano, 2013; Carella et al., 2018). ROS have also been implicated in the signalling pathway that leads to specific gene expression in response to the stress. HR-cell death induction strongly depends on ROS generation. Analyses of P. patens tissues infected with B. cinerea show that the production of ROS occurs in single cells as a rapid response after hyphal contact, which finally leads to cell death (Ponce de León et al., 2012). Besides, genes related to programmed cell death such as subtilisin-like proteases, metacaspase and the E3 ligase BOI were reported as being up-regulated during B. cinerea infection of P. patens (Reboledo et al., 2021), which were previously identified in angiosperms as suppressors of pathogen-induced cell death (Luo et al., 2010). Together with ROS production, there are other HR cell death hallmarks that are observed in P. patens, such as protoplast shrinkage, nuclei fragmentation and chloroplast breakdown (Ponce de León et al., 2007; Fig. 2). In addition, ROS accumulation is observed during the colonization of Marchantia thalli with Phytophthora palmivora, which is also accompanied by the up-regulation of PRX (peroxidase) and DIR (dirigent-like) transcripts (Carella et al., 2018). As ROS accumulation is an essential hallmark for RCD pathways, more studies are required to understand the source and regulation of ROS production in this process. In higher plants, multiple sources of ROS were identified, such as chloroplasts, mitochondria, RBOH proteins, cell wall peroxidases, etc. Concordantly, and although data on bryophytes are scarce, Chu et al. (2023) showed that RBOH1 is required for chitin-induced ROS production in Marchantia.
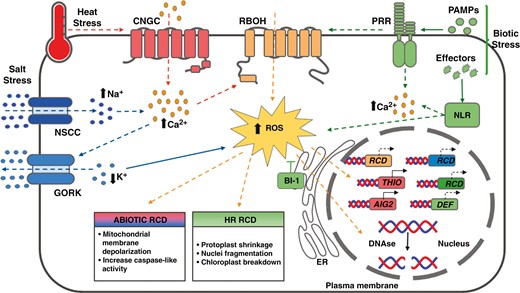
Stress-triggered RCD pathways proposed in bryophytes. Heat stress is known to enhance the influx of calcium (Ca2+) through the activation of cyclic nucleotide gated channels (CNGCs). Likewise, under salt stress, the influx of sodium (Na+) through non-selective cation channels (NSCCs) is proposed to increase the concentration of cytosolic Ca2+. This might lead to the activation of Ca2+-dependent NADPH oxidases (RBOH) resulting in the accumulation of reactive oxygen species (ROS). Under salt stress, ROS accumulation is thought to activate the hydroxyl radical-activated K+ channel (GORK) which results in K+ efflux. ROS accumulation is followed by mitochondrial depolarization and the activation of caspase-like activities and the induction of regulated cell death (RCD) markers such as THIO and AIG. Pathogen attacks also activate RCD mechanisms in bryophytes. PAMP-triggered immunity is activated through pattern-recognition receptors (PRRs). Pathogen virulence factors (effectors) are detected through the activity of intracellular immune receptor proteins, which in general are nucleotide-binding site leucine-rich repeat (NLR) proteins. This recognition results in ROS and Ca2+ accumulation, which in turn induces the expression of specific effector-triggered immunity (ETI) markers and of genes related to pathogen responses (DEF). Also, DNA degradation takes place through the activity of nucleases (DNAse). The endoplasmic reticulum (ER) protein BAX Inhibitor-1 (BI-1) acts as a suppressor of RCD. Dashed lines indicate proposed pathways based on evolutionary conservation while full lines indicate experimentally demonstrated mechanisms in Marchantia polymorpha or Physcomitrium patens. Red lines and boxes are associated with heat stress. Blue lines, blue boxes and blue captions are related to salt stress responses. The green lines, green boxes and green captions are related to biotic stress. The orange lines, orange boxes and orange captions are common to both biotic and abiotic stresses.
The transmembrane BAX inhibitor motif containing protein superfamily (TMBIM) constitutes an important group of cell death regulators. This group of proteins comprises the BAX Inhibitor (BI) family, also referred to as BAX Inhibitor-1 (BI-1) proteins, which were named after their ability to inhibit BAX-induced RCD in human cells (Xu and Reed, 1998) and the Lifeguard (LFG) proteins, a group of conserved cytoprotective cell death regulators. Recent studies in comparative genomics and synteny networks revealed that the BI and LFG families evolved independently in plants (Gamboa-Tuz et al., 2018). BI-1 has been related to endoplasmic reticulum (ER) stress-triggered pathways involved in response to different types of stress signals. Specifically, ER stress signalling pathways have been associated with pathogenicity. In Arabidopsis, BI-1 plays a central role in ER stress-mediated cell death. Plants impaired in AtBI-1 show an increased sensitivity to the ER-stress inducer tunicamycin (TM), while overexpressing AtBI-1 has an opposite effect (Watanabe and Lam, 2008). Importantly, BI-1 plays important roles in responses to biotic stresses (Watanabe and Lam, 2009). BI-1 overexpression has been shown to confer resistance to infection in diverse plant species, such as rice (Matsumura et al., 2003; Ishikawa et al., 2010), Arabidopsis (Kawai-Yamada et al., 2004) and barley (Babaeizad et al., 2009). Similarly, overexpression of BI-1 in the moss P. patens inhibits cell death and confers more resistance to the infection with necrotrophic fungal pathogens (Lawton and Saidasan, 2009; Ponce de León and Montesano, 2013), suggesting a conserved role for BI-1 in bryophytes.
Several studies in M. polymorpha indicate that the mechanisms underlying plant–pathogen recognition/interactions are also conserved in liverworts (Gimenez-Ibanez et al., 2019). By studying the interactions between Marchantia and Phytophthora syringae/Phytophthora cucumerina, the authors showed that Marchantia activates typical hallmarks of PTI in response to pathogen recognition. In addition, the M. polymorpha genome codes for about 200 surface-localized RK-like proteins, including a BAK1 orthologue. Furthermore, M. polymorpha also possesses a CERK1 orthologue, which is a lysin motif (LysM)-RK that might mediate the perception of peptidoglycans (PGNs) and chitin. Additionally, another report showed that M. polymorpha activates a core of conserved plant defence response during colonization by the pathogenic oomycete Phytophthora palmivora (Carella et al., 2018). This response was characterized by the induction of a number of gene families associated with defence pathways, such as PR proteins, stress-associated enzymes, specific TFs and cellular trafficking-related proteins. In this sense, it has been reported that the N-terminal NLR domain has an important role in orchestrating the plant immune response (Chia and Carella, 2023).
Jasmonic acid (JA) belongs to the jasmonate plant hormone family and plays unique functions in wound responses and plant defence mainly against herbivores and necrotrophs (Creelman et al., 1992; Farmer and Ryan, 1992; Albrecht et al., 1993; Avdiushko et al., 1995; Reinbothe et al., 2009; Monte, 2023). The main known precursor of JAs is OPDA (oxylipin 12-oxo-phytodienoic acid). It was recently reported that dn-iso-OPDA (isomer of the dinor-cis-12 oxo-phytodienoic acid, dn-OPDA) acts as the hormone signal for the orthologous COI1/JAZ receptor in bryophytes and lycophytes (Chini et al., 2023). The synthesis of OPDA in Marchantia is induced by wounding, analogous to what is known in higher plants (Aleman et al., 2022). Studies in Marchantia and in the moss P. patens provide solid evidence that OPDA-dependent responses are independent of the JA and JA-Ile pathways. JA-Ile participates in regulating cell death in plants (Reinbothe et al., 2009). In Marchantia, loss of OPDA production was reported due to the disruption of the allene oxide synthase MpAOS1/2 genes (encoded by the Mp3g21350 and Mp5g16260 genes), which results in reduced defence against spider mites Tetranuchus urticae (Koeduka et al., 2022), as reported in vascular plants (Aleman et al., 2022). Furthermore, it was demonstrated that salicylic acid (SA) treatment of M. polymorpha promotes the Irpex lacteus fungus infection, which is suppressed by the bioactive jasmonate dn-OPDA (Matsui et al., 2019). Since SA accumulation is associated with HR cell death, this result suggests that the ETI programme might be conserved in Marchantia as well. In addition, it also indicates that the antagonistic interactions between SA and oxylipin pathways during plant–pathogen interactions might have an ancient origin and were already established in liverworts (Matsui et al., 2019).
Autophagy in bryophytes
Autophagy is a complex catabolic process highly conserved in eukaryotes, through which intracellular components, from molecules to organelles, could be degraded and recycled. Generally, it is divided into macro- and micro-autophagy. While in micro-autophagy uptake of cellular contents occurs directly at the limiting membrane of the lysosome or vacuole, macro-autophagy refers to a process in which uptake of the cargo takes place away from lysosomes or vacuoles. It involves the synthesis of double-membrane vesicles (autophagosomes) that subsequently transport cellular contents to the lysosome or vacuole. Although less studied than macro-autophagy, micro-autophagy has been well reported in yeast species (Müller et al., 2000; Uttenweiler et al., 2005; Oku and Sakai, 2018). Recent studies demonstrated the existence of micro-autophagy in mammalian cells, where it was shown that lysosomal membranes are able to directly engulf endosomes, lipid droplets and organelles. In Arabidopsis, anthocyanin aggregates in the cytosol are captured and transported into the vacuole by micro-autophagy (Mijaljica et al., 2011; Schuck, 2020; Sieńko et al., 2020). However, as research has been mainly focused on macro-autophagy, it is usually directly referred to as autophagy. Contrary to common belief, macro-autophagy (hereafter autophagy) could be highly selective and according to the type of substrate degraded can be named as aggrephagy (degradation of protein aggregates), xenophagy (selective autophagy that eliminates pathogens), reticulophagy (degradation of ER components), among others (Marshall and Vierstra, 2018). Although autophagy is a process that is generally activated to keep cellular homeostasis, different stresses can activate autophagy allowing the cells to adapt to new conditions while avoiding potential damage (reviewed in Das et al., 2012; Marshall and Vierstra, 2018; Liao and Bassham, 2020).
In angiosperms, changes in autophagy flux occur during different stages of plant development (e.g. senescence) and upon biotic and abiotic stresses (Signorelli et al., 2019; Su et al., 2020). Several autophagy mutants display accelerated senescence and are less resistant to different stresses, highlighting the pro-survival role of autophagy in plants (reviewed in Marshall and Vierstra, 2018; Su et al., 2020). However, autophagy could also lead to cell death, as reported in specific cell types of the Arabidopsis root cap (Feng et al., 2022), in the HR (Hofius et al., 2009) or during degradation of the integumentary tapetum, which is critical for embryo pattern formation (Zhao et al., 2024). Interestingly, the activation of autophagy under stressful conditions might have very different outcomes depending on the duration and intensity of the adverse conditions. During the first stages of starvation and ER stress autophagy is up-regulated, recycling nutrients and degrading modified proteins to prevent toxicity. However, if stressful conditions persist over time, there is a transition from adaptive autophagic activities to autophagic cell death (Contento et al., 2004; Srivastava et al., 2018).
Even though there are only a few reports about autophagy in bryophytes, its role has been reported during male gamete differentiation (Minamino et al., 2017; Sanchez-Vera et al., 2017; Norizuki and Ueda, 2022; Norizuki et al., 2023), hydrogen peroxide-induced cell death (Sakil et al., 2023), senescence and starvation (Mukae et al., 2015; Norizuki et al., 2019; Chen et al., 2020).
Autophagy mainly relies on autophagy-related genes (ATGs), which are highly conserved among eukaryotes (Marshall and Vierstra, 2018; Chung, 2019). ATGs encode proteins required for autophagosome biogenesis and fusion with the vacuole. Analysis of ATG genes in charophytes and Marchantia shows low redundancy compared with higher plants or even with Physcomitrium, suggesting that multiplication of ATGs occurred during land plant evolution (Norizuki et al., 2019). ATG proteins can be classified into four core functional groups: (1) the ATG1/ATG13 kinase complex that initiates autophagosome assembly; (2) the ATG6 complex, which is commonly known as the phosphatidylinositol 3-kinase (PI3K) complex; (3) the ATG9 complex, which promotes autophagosome expansion; and (4) the ATG8/ATG12 ubiquitin-like conjugation systems, which act during phagophore expansion and maturation, and are essential for cargo recognition (Marshall and Vierstra, 2018).
Even though the core ATG genes are present in bryophytes (Norizuki et al., 2019), there is little information about their physiological role. Bryophyte ATG knockout mutants display early senescence and are more sensitive to nutrient stress, as reported for ATG mutants from other species (Hanaoka et al., 2002; Li et al., 2015; Wada et al., 2015). These studies include the ATG2, ATG5 and ATG7 knockout mutants in Marchantia (Norizuki et al., 2019) and Physcomitrium knockout mutants for ATG5 (Mukae et al., 2015) and ATG3 (Chen et al., 2020). In addition, a large set of regulatory pathways (such as chlorophyll biosynthesis, lipid metabolism and ROS) is affected in PpATG3 knockout mutants (Chen et al., 2020).
In a recent study, senescence and cell death were observed in PpATG5 and PpATG7 mutants exposed to abiotic stress conditions. Although the authors reported that neither of these ATG genes is essential for the moss, experiments conducted under optimal and nutrient-deprived growth conditions revealed a significant contribution of autophagy to growth and development (Pettinari et al., 2022), possibly associated with cell death prevention in P. patens.
Among ATGs, ATG8 is one of the most well studied. As it remains attached to the phagosome even during vacuole fusion, it has been used as a tool to follow autophagy flux in many species (Thompson et al., 2005; Izumi et al., 2015; Li et al., 2015). Interestingly, reporter lines for MpATG8 and PpATG8e were developed to follow autophagic flux in Marchantia and Physcomitrium respectively (Sanchez-Vera et al., 2017; Norizuki et al., 2019). Through a phylogenetic study, Kellner et al. (2017) hypothesized that a large expansion of the ATG8 family occurred in plants via multiple whole-genome duplications. While yeasts and algae genomes encode only one ATG8 gene (Nakatogawa et al., 2007; Kellner et al., 2017), Marchantia has two (Norizuki et al., 2019), Physcomitrium has six, and higher plants code for 5–11 ATG8s (Bu et al., 2020). These events might have been advantageous to cope with diverse and complex environmental conditions and resulted in the diversity of selective autophagy observed in plants today. Recent bioinformatic studies have revealed the regulation of ATG8 via the plant homologue of AMPK, SnRK1 (SNF1-Related Protein Kinase 1) in bryophytes. The SnRK1-FLZ module interacted with the FCD-like zinc finger protein during plant evolution, forming a regulatory axis that initially appears in gymnosperms and exhibits high conservation in seed plants. While direct evidence of cell death is lacking to date, autophagy mechanisms demonstrate positive regulation of SnRK1 signalling, suggesting a role in plant adaptation to stressful environmental conditions (Yang et al., 2023).
AtNBR1 was the first selective autophagy receptor identified in plants. It interacts with several ATG8 proteins through a specific motif (Svenning et al., 2011). Remarkably, AtNBR1 was associated with plant immunity, as it was shown to directly bind to viral protein CaMV P4, a protein from the cauliflower mosaic virus (CaMV) capsid, mediating their selective autophagic degradation (Hafrén et al., 2017, 2018). Homologues of AtNBR1 have been identified in tobacco (Zientara-Rytter et al., 2011), tomato (Zhou et al., 2014), P. patens (Svenning et al., 2011) and Marchantia (Stephani and Dagdas, 2020). However, AtNBR1 has not been related to the HR or to other cell death processes. NBR1 also targets protein aggregates (Jung et al., 2020) and could modulate the cross-talk between autophagy and the ABA signalling pathway (Tarnowski et al., 2020). As autophagy could induce cell death during ER stress, which induces protein aggregation, it would be interesting to study the role of NBR1 in bryophytes in such a scenario. Interestingly, a recent report showed that during ER stress, a specific protein named C53 functions as a conserved receptor for ER autophagy in human culture cells, Arabidopsis and Marchantia (Stephani et al., 2020). Additionally, the evolutionary conserved serine/threonine kinase Target of Rapamycin (TOR) has an essential role in the induction of autophagy. TOR forms complexes that are tightly regulated in response to specific stimuli (Marshall and Vierstra, 2018; Janse van Rensburg et al., 2019). TOR complexes are found in mammals, yeasts and plants. In mammals and yeasts, TOR forms at least two distinct protein complexes (TORC1 and TORC2). Although the precise compositions of TOR kinase complexes in plants have not been characterized, it is currently accepted that plants only harbour TORC1 complexes. Gene orthologues for TOR, Raptor and LST8 could be identified while neither Rictor nor mSIN1 orthologues were found in available plant genomes (Fu et al., 2020). While showing conserved functions in terms of regulation of nutrient status, plants have evolved a more complex system, as TORC1 is also regulated in response to stress, light and phytohormones (reviewed in Jamsheer et al., 2019). The antagonistic kinase of TOR, 5ʹ AMP-Activated Protein Kinase (AMPK), is activated during carbon starvation and represses TOR activity. The core TOR-AMPK is highly conserved in all eukaryotes with the exception of some intracellular parasites (Jamsheer et al., 2019). However, SnRK1 shows considerable divergence in key aspects such as activation, subunit composition and signalling mechanisms (Jamsheer et al., 2019). Green plants show high variation in the copy number of SnRK1α kinase subunits and, remarkably, great expansion of α subunits is observed in bryophytes such as P. patens and Sphagnum falla (Jamsheer et al., 2019). Furthermore, SnRK2 and SnRK3, which originated from SnRK1, are also involved in controlling stress responses and nutrient homeostasis (Jamsheer et al., 2019). In SnRK2, there are four genes in the P. patens genome coding for PpSnRK2A/2B/2C/2D proteins. It was demonstrated that the quadruple mutant shows severe insensitivity to ABA and reduced tolerance to osmotic stress, a response that is conserved for these SnRK2s (subclass III) against abiotic stress between bryophytes and angiosperms (Shinozawa et al., 2019).
Interestingly, TOR overexpression inhibits autophagy triggered by nutrient starvation, salt and osmotic stress, but does not affect autophagy triggered by oxidative stress or ER stress, indicating the occurrence of a TOR-independent pathway for autophagy regulation (Pu et al., 2017). Apart from phylogenetic studies, we were not able to find specific references for physiological roles of TOR in bryophytes. Although there are studies on P. patens PpSNF1a and PpSNF1b knockout mutants, coding for two redundant SnRK1 proteins (Thelander et al., 2004), their role in autophagy or cell death was not studied.
Despite a growing number of studies on plant autophagy, our understanding of autophagic cell death is incomplete in higher plants and almost unexplored in bryophytes. Since the core components of the autophagy process seem to be conserved in bryophytes, it might be interesting to know if autophagic cell death also takes place in these plants. Several environmental stresses might trigger autophagic cell death. In particular, autophagy has been related to the immune response in plants. As HR-related cell death has been described in Physcomitrium (Ponce de León et al., 2007, 2012; Reboledo et al., 2015), studying the role of autophagy during pathogen attacks seem a plausible starting point to disclose a putative involvement of this process in bryophyte cell death.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
RCD is a complex process that involves several pathways and subcellular compartments. The cascade of events triggered during RCD are synchronized and controlled, so cell death is restricted. Despite the functional conservation of RCD among evolutionarily distant species, our understanding of the molecular mechanisms underpinning this fundamental process in plants remains largely elusive. This knowledge is even scarcer in bryophytes. In this review, we have summarized the information available about known triggers and mechanisms of RCD in bryophytes from an evolutionary perspective. Based on the signalling pathways and cell death-related genes identified in bryophytes, it appears that there is a core of components that are conserved between bryophytes and higher plants. An example constitutes the development of the archegonial canal in mosses, which shares similar RCD pathways with the transmitting tract of the angiosperm sporophyte. Interestingly, as auxin response reporters are active in the archegonial cells that undergo RCD, it has been proposed that auxin signalling in these cells might regulate this process, as was also suggested in angiosperms (Crawford and Yanofsky, 2011; Landberg et al., 2013; Fig. 1). However, the role of auxins and other hormones regulating cell death mechanisms in bryophytes is still a relatively unexplored field and much research is required to reveal their function in these plants. Some similarities could be attributed to convergent evolution, and further in-depth studies of the underlying mechanisms in bryophytes are still needed. Furthermore, autophagy is related to cell death associated with ER stress, which induces the aggregation of proteins. Since NBR1 participates in the formation of protein aggregates and in the cross-talk between autophagy–ABA signalling pathways, it would be interesting to elucidate the role of the AtNBR1 homologues found in P. patens and M. polymorpha during cell death-associated autophagy processes in relation to ER stress.
Additionally, we have summarized specific factors influencing the fate of megaspores in angiosperms, contrasting with the absence of spore selection in bryophytes. Additionally, we highlight differences in AGPs and the presence or differential expression of cyclin kinase inhibitors (ICK/KRP) between angiosperms and bryophytes. The shape of resulting spores, positional effects and genetic regulation of nucellar cells emerge as key factors determining the final spore fate that may help to explain differences between vascular and non-vascular plants, such as bryophytes.
On the other hand, the role of autophagy in bryophyte cell death remains elusive. A recent report showed that hydrogen peroxide induced cell death in a pathway dependent on ATG5 and ATG7 in P. patens (Sakil et al., 2023). However, there are several reports that link autophagy with nutrient stress and senescence (Hanaoka et al., 2002; Li et al., 2015; Mukae et al., 2015; Wada et al., 2015; Norizuki et al., 2019; Chen et al., 2020). As ATG5 mutants display susceptibility to fungus infection in P. patens, autophagy might play a role in the RCD processes that take place in response to pathogen attacks as occurs in angiosperms (Bressendorff, 2012). However, this idea is still preliminary and more studies are needed to uncover a putative role for autophagy in this process. Nevertheless, it seems that HR cell death features are conserved in bryophytes (Ponce de León et al., 2012) (Fig. 2). These features include ROS production, protoplast shrinkage, nuclei fragmentation and chloroplast breakdown, which have been observed in P. patens (Ponce de León et al., 2007) but seem to be also conserved in M. polymorpha (Gimenez-Ibanez et al., 2019).
Abiotic stresses are also able to trigger cell death in bryophytes, although the underlying molecular mechanisms remain to be elucidated. Interestingly, cell death triggered by freezing temperatures led to the accumulation of MDA, a marker of lipid peroxidation that has also been associated with plant ferroptosis (Distéfano et al., 2017; Tan et al., 2017). Other responses related to cell death mechanisms in angiosperms are also observed in bryophytes, such as ROS accumulation and the induction of specific cell death marker genes (Fig. 2). However, the source of ROS and the regulatory mechanisms underlying ROS accumulation during these processes are still a matter of investigation in bryophytes.
There are several understudied fields in bryophytes that would benefit from further experiments in order to elucidate the mechanisms underlying RCD. Several common pathways have been reported to be shared with vascular plants, which could shed light on conserved processes. In particular, the elucidation of autophagy in bryophyte cell death, which seems to involve homologous genes, requires further exploration. Additionally, ROS accumulation is another common hallmark in the RCD pathways reported for vascular plants and bryophytes. However, the sources of ROS and the regulatory mechanisms governing ROS accumulation during cell death processes in bryophytes are still unknown, as are the downstream targets and molecular programmes involved.
More genetic and molecular approaches to manipulate gene expression and study their impact on cell death are needed. The role of hormones is also intriguing, as they seem to activate analogous developmental processes and responses.
Since bryophytes show a remarkable tolerance to extreme environmental conditions, it is thought that cell death-promoting mechanisms are tightly controlled in favour of pro-survival responses (Elzanati et al., 2020). Although much research is required to further demonstrate this idea, it is a prospective field for exploration. This aspect is not only important from a basic research perspective, but also might be of great interest for possible agronomical and biotechnological applications.
ACKNOWLEDGEMENTS
We acknowledge the International Centre for Genetic Engineering and Biotechnology (ICGEB) and The Argentinean Agency for the promotion of Science and Technology (ANPCyT) for financial support (ICGB-CRP/19/020 grant to GCP; ANPCyT PICTs 2017-00201 to GCP; PICT 2016-00382 to AMD and PICT 2020-00013 to EZ). GAL is an ANPCyT doctoral fellow; FM is a CONICET post-doctoral fellow; NS, MC and MC are CONICET fellows; AMD, EZ and GCP are CONICET researchers.
AUTHOR CONTRIBUTIONS
Conceptualization: AMD, FM, EZ and GCP; Funding Acquisition: GCP, EZ and AMD; Project Administration: GCP and AMD; Supervision: AMD and GCP; Writing: AMD, FM, EZ and GCP; Writing – Review & Editing: AMD, GAL, NS, FM, MC, MC and EZ.
DATA AVAILABILITY
All data supporting the findings of this study are available within the paper and within its supplementary materials published online.
LITERATURE CITED
Author notes
Fernanda Marchetti and Ayelén Mariana Distéfano Contributed equally to this work.
Present address of Maximiliano Cainzos: Department of Plant Physiology, Umea Plant Science Centre, Umea University, Umea, Sweden



